Abstract
The incidence of esophageal adenocarcinoma (EA) and its precursor condition, Barrett’s esophagus, has risen rapidly in the United States for reasons that are not fully understood. Therefore, we evaluated the association between use of supplemental vitamins and minerals and risk of neoplastic progression of Barrett’s esophagus and EA. The Seattle Barrett’s Esophagus Program is a prospective study based on 339 men and women with histologically confirmed Barrett’s esophagus. Participants underwent baseline and periodic follow-up exams, which included endoscopy and self-administered questionnaires on diet, supplement use, and lifestyle characteristics. Use of multivitamins and 4 individual supplements was calculated using time-weighted averages of reported use over the observational period. Cox proportional-hazards models were used to calculate hazard ratios (HR) for each endpoint: EA, tetraploidy, and aneuploidy. During a mean follow-up of 5 yr, there were 37 cases of EA, 42 cases of tetraploidy, and 34 cases of aneuploidy. After controlling for multiple covariates including diet, nonsteroidal anti-inflammatory drug use, obesity, and smoking, participants who took 1 or more multivitamin pills/day had a significantly decreased risk of tetraploidy [HR = 0.19; 95% confidence interval (CI) = 0.08-0.47) and EA (HR = 0.38; 95% CI = 0.15-0.99] compared to those not taking multivitamins. Significant inverse associations were also observed between risk of EA and supplemental vitamin C (≥250 mg vs. none: HR = 0.25; 95% 0.25; 95% CI = 0.11-0.58) and vitamin E (≥ 180 mg vs. none: HR = 0.25; 95% CI = 0.10-0.60). In this cohort study, use of multivitamins and single antioxidant supplements was associated with a significantly reduced risk of EA and markers of neoplastic progression among individuals with Barrett’s esophagus.
INTRODUCTION
Esophageal cancer is the seventh leading cause of cancer death among men in the United States, causing an estimated 13,770 deaths in 2006 (1,2). The incidence of esophageal adenocarcinoma (EA), now the most common histological type of esophageal cancer, has increased dramatically in the United States, Western Europe, Australia, and other developed countries over the past 30 yr for reasons that are not fully understood (3-5). The rapid change in incidence suggests that environmental and lifestyle factors, such as diet and obesity, may be responsible (6-12).
EA is typically preceded by the premalignant metaplastic condition, Barrett’s esophagus, in which the normal squamous epithelium in the distal esophagus is replaced with a specialized, metaplastic columnar epithelium as a result of chronic gastroesophageal reflux disease (GERD) (13-15). The number of cases of Barrett’s esophagus in the United States has been estimated to be around 1 million, although most remain undetected (16). Approximately 0.5% of individuals with Barrett’s esophagus develop EA every year (17-19). Given the increasing incidence rate of EA combined with the low survival rate (5-yr survival rate of 15% among all races (2), there is a need to monitor and determine the proper clinical management of patients with Barrett’s esophagus. Previous studies have identified risk factors associated with EA such as low fruit and vegetable intake (6,20,21), obesity (9-11), and GERD (22,23). However, it remains essential to identify measures that can help prevent progression of Barrett’s esophagus to EA.
Over the past several decades, the use of dietary supplements has grown rapidly in the United States (24,25). Supplements may have a role in chronic disease prevention because they are an easily modifiable behavior and a high-dose source of nutrients with antioxidative properties. Several epidemiologic studies have supported the relationship between dietary factors and EA. So far, only a small number of studies have reported on multivitamin or dietary supplement use (20,26-30), and a few have suggested a potential inverse association (20,27,28); however, all studies have been conducted as case-control studies and thus may be subject to recall bias.
The development of EA in persons with Barrett’s esophagus is a well-established multistep process, which involves an accumulation and clonal expansion of somatic mutations in the esophageal epithelium. Progression of Barrett’s esophagus is marked by certain characteristics that can be assessed with DNA content flow cytometry, such as tetraploidy and aneuploidy, or by evaluation of mucosal biopsies. DNA content abnormalities have been validated as being highly predictive of subsequent cancer development (31,32) and mechanistically related to the progression of Barrett’s esophagus to EA (33-5). Because EA is rare and may take many years to develop, intermediate markers of progression can also be used as endpoints to study the relationship between potential risk factors and EA.
The aim of this study was to evaluate the association of dietary supplement use with risk of neoplastic progression and EA in a prospective cohort of individuals with Barrett’s esophagus. We hypothesized that vitamins with antioxidative properties might be beneficial in the prevention of EA because the development and progression of Barrett’s esophagus involves oxidative damage.
METHOD
Participants
This study included participants from the Seattle Barrett’s Esophagus Program—a dynamic cohort study that began in 1983. All participants in this study were recruited from this continuing program of cancer surveillance in which participants undergo periodic endoscopy and multiple biopsies following a standard protocol (31,32). Beginning in 1995, the study was expanded to include an extensive personal interview and anthropometric measurements that most cohort members agreed to undergo (36). Of our cohort, 30% were previously under surveillance (prior to 1995) and thus had preexisting knowledge of their condition when they were recruited to this study, whereas the remaining proportion of participants entered into the cohort at the same time as their diagnosis of Barrett’s esophagus. Participants underwent a baseline assessment, which included a standard endoscopic protocol for collecting esophageal mucosal biopsies as well as a personal interview performed by the study team at their first visit on or after February 1, 1995. On follow-up visits, the biopsy protocol was repeated, and baseline data, including diet and supplement use, were updated. This study was conducted at a specialty research and referral center, and thus our cohort is considered a high-risk patient population. The cohort is typical for gender, age, and Barrett’s segment length compared to other specialty research centers (37-40).
All research participants were counseled concerning risks and benefits of endoscopic biopsy surveillance for Barrett’s esophagus. Patients with high-grade dysplasia were also counseled concerning risks and benefits of esophagectomy and endoscopic therapies. If, after fully informed consent, participants with high-grade dysplasia opted for endoscopic biopsy surveillance reserving intervention for cancer if detected, they were evaluated for coexisting cancer by an intensive protocol performed at closely timed intervals during the first 4 mo of the study after which endoscopies were typically repeated approximately every 6 mo. Patients without high-grade dysplasia were typically followed up about every 2-3 yr. According to pathology reports, 47 (14%) participants included in this analysis were diagnosed with high-grade dysplasia at baseline entry into the cohort.
The present report includes persons with a baseline and at least one follow-up assessment performed between February 1, 1995, and July 1, 2004, and no prior history of esophageal cancer (n = 350). Participants were excluded from our analyses if 1) they were diagnosed with cancer within the first 3 mo of baseline (n = 4), 2) tetraploidy or aneuploidy was detected within the first 3 mo of baseline in their respective analyses (n = 35 and n = 38), or 3) over half of their submitted food frequency questionnaires (FFQs) were considered invalid as determined by sex-specific energy cutoffs (Males: 800 < kcals ≤ 5,000; Females: 700 < kcals ≤ 4,000; n = 8). If a questionnaire was considered invalid, it was not used to calculate food and nutrient intake for the individual. After these exclusions, which are not mutually exclusive, the analytic cohort consisted of 339 men and women. The study was approved by the institutional review boards of the University of Washington, Seattle, WA, and the Fred Hutchinson Cancer Research Center, Seattle, WA. All participants gave written informed consent.
Determination of Endpoints
The methods used for endoscopy, biopsy, and flow cytometry have been described previously (31,32,41). Briefly, 4-quadrant biopsies for histology, plus one biopsy for flow cytometry, were obtained from every alternate centimeter throughout the Barrett’s segment for most participants. For patients with a history of high-grade dysplasia, biopsies were taken from every centimeter of Barrett’s mucosa. Biopsies were interpreted by pathologists who were blinded to patient identity as well as the flow cytometry and genetic analysis findings. Participants were classified, histologically, into no dysplasia, indefinite for dysplasia, or positive for dysplasia (low and high grade) according to previously published criteria (41).
Flow-cytometry histograms were interpreted by P. S. Rabinovitch and C. A. Sanchez without knowledge of histological or genetic results at the time of the reading. A diagnosis of aneuploidy was established if discrete peaks were recorded on the histogram, showing aneuploid and diploid cell populations, and if the aneuploid peak included at least 2.5% of cells in the biopsy sample (31,32,42). An abnormal tetraploid (i.e., 4N) fraction was defined as more than 6% of cells with a DNA content between 3.85 and 4·10N (31). A patient was classified as having aneuploidy or tetraploidy if the abnormality was detected in more than one biopsy from a given endoscopy. Although DNA abnormalities tend to occur concurrently, this is not always true, and a previous study of ours has demonstrated that aneuploidy and tetraploidy are independent predictors of EA risk (43). Hence, we chose to look at aneuploidy and tetraploidy as independent secondary outcomes in our analyses.
Diet, Supplement Use, and Other Health-Related Data
All participants underwent structured interviews at baseline by trained staff. Interviews usually occurred in the clinic before endoscopy or occasionally in the participant’s home. The baseline interview took approximately 45 min to complete and obtained data on diet and supplement use, medical history, family history of cancer and gastrointestinal disorders, past and current tobacco and alcohol use, past and current use of medications, current occupation, and demographic characteristics. Height, weight, and anthropometric measurements were taken at baseline and at follow-up by use of a standard protocol described previously (44).
Diet and supplement use was assessed through self-administered questionnaires at baseline and subsequent follow-up exams. Diet was assessed with a 122-item self-administered FFQ (45). The nutrient database for the FFQ was based on the University of Minnesota Nutrient Data System (46). Dietary supplement use was assessed with a separate questionnaire that was administered concurrently with the FFQ. Two versions of the questionnaire were used throughout follow-up. The earlier version of the questionnaire was used through 2002 in which participants were asked to report their use of vitamin and mineral supplements over the past year including multivitamins, antioxidant complexes, vitamins C and E, β-carotene, and selenium. Amount and frequency of use were recorded for single supplements taken at least once a week over the past year. The later version of the supplement questionnaire was more comprehensive and updated to include questions about multivitamin brand name. This version of the questionnaire was designed and validated in the VITamins And Lifestyle (VITAL) cohort (47). Multivitamin composition was based on the Physicians Desk Reference for Non-Prescription Drugs and Dietary Supplements [1993-2002 (48)] and data provided by manufacturers. If no brand name was provided, Centrum® and Protegra Antioxidant, which were commonly used supplements at that time, were used as the multivitamin and antioxidant defaults. Total supplement intake of individual nutrients was calculated by summing the amounts contributed from both single supplements and multivitamins.
Using all eligible questionnaire data collected during follow-up, we calculated a time-weighted average dose of each supplement using reports collected until either the time of the event or the last follow-up visit. The time interval between follow-up visits was determined, and doses were associated with half the time interval before and half the time interval after the visit the dose was reported. Supplement doses were analyzed as categorical variables that separated “no use” from the amounts usually contained in multivitamins and the much higher doses in single supplements. The distribution of observed values was used to define these cut points.
Statistical Methods
Cox proportional hazard models were used to calculate hazard ratios (HR) and 95% confidence intervals (95% CIs) for supplement use and each outcome. Known and suspected risk factors for EA were included as covariates in the model including baseline measurements—age, sex, waist:hip ratio (tertiles), and cigarette smoking (0, <20, 20-40, ≥40 pack-yr)— and measurements repeated during follow-up: nonsteroidal anti-inflammatory drug (NSAID) use (current, intermittent, never) (49), fruit and vegetable consumption, and percent energy from fat. Supplement use was calculated as a time-weighted average dose as described previously. Dietary covariates were averages based on values from all repeat assessments. Multiple indicators of healthy behavior were included in the model to adjust for potential confounding. Waist:hip ratio was more strongly associated with our outcomes than body mass index and was thus chosen for our models (44). Tests for linear trend were evaluated by assigning an ordinal score to the categories and treating it as a continuous variable in the statistical model.
Follow-up time was assessed separately for each outcome: EA, tetraploidy, and aneuploidy. Time of entry was defined as the date of the baseline interview date. Time of exit was defined as either the date of the endoscopic diagnosis for the outcome of interest or the date of the last follow-up visit, whichever came first. Sensitivity analyses to evaluate whether our results persist within subsets of our population were conducted by stratifying on factors that may influence risk or detection of EA: NSAID use (none vs. any use), grade of dysplasia at baseline (high vs. others), and length of follow-up (entered cohort before/in 1995 vs. after 1995). Because subjects with low fruit and vegetable intake may receive added benefits from supplements due to low dietary nutrient intake and because smoking causes oxidative stress, we also conducted stratified analyses by fruit and vegetable consumption (above vs. below the median value of 3.3 servings) and smoking status (never vs. ever) to evaluate potential effect modification. All statistical analyses were performed with SAS software (version 9.1; SAS Institute, Inc., Cary, NC).
RESULTS
Among the 339 participants eligible for analyses, 37 developed EA, 42 tetraploidy, and 34 aneuploidy. The mean period of follow-up for the EA endpoint was 5 yr (range = 0.3-8.9 yr). On average, participants underwent 5 endoscopies (range = 2-21) and submitted 3 valid diet and supplement use questionnaires (range = 1-8). Older participants and those who had smoked previously had a higher incidence of tetraploidy, aneuploidy, and EA (Table 1). Males had a higher incidence of EA compared to females but a lower incidence of tetraploidy and aneuploidy. Current NSAID use (49), high consumption of fruits and vegetables, and low fat intake were associated with a lower incidence of all 3 outcomes. Participants who took a multivitamin regularly had a lower incidence of all 3 outcomes compared with those who did not consume a multivitamin. There was a similar trend of decreasing incidence of all 3 outcomes with increasing use of supplemental vitamin C, vitamin E, β-carotene, or selenium. Differences in demographic and health-related characteristics between multivitamin users and nonusers were small (Table 2). However, participants who reported taking 1 or more multivitamin pills/day were more likely to be current users of NSAIDs and had diets higher in fruits and vegetables and lower in fat intake than nonusers.
TABLE 1.
Incidence rates by demographic and health-related characteristics of the study participantsa
| Tetraploidy |
Aneuploidy |
Esophageal Adenocarcinoma |
|||||
|---|---|---|---|---|---|---|---|
| Characteristic | Cohort n (%)b | Person Years | Ratec | Person Years | Ratec | Person Years | Ratec |
| Age, yr | |||||||
| 30-54 | 103 (30.4) | 519 | 13.5 | 530 | 18.9 | 560 | 14.3 |
| 55-69 | 144 (42.5) | 651 | 29.2 | 646 | 18.6 | 729 | 20.6 |
| ≥70 | 92 (27.1) | 321 | 49.8 | 355 | 33.8 | 407 | 34.4 |
| Gender | |||||||
| Male | 275 (81.1) | 1,219 | 26.3 | 1,256 | 20.7 | 1,401 | 22.8 |
| Female | 64 (18.9) | 273 | 36.7 | 275 | 29.1 | 296 | 16.9 |
| NSAID use | |||||||
| Never | 77 (22.7) | 316 | 41.1 | 310 | 41.9 | 342 | 49.7 |
| Former | 27 (8.0) | 89 | 56.3 | 96 | 41.8 | 100 | 39.9 |
| Current | 235 (69.3) | 1,086 | 22.1 | 1,125 | 15.1 | 1,254 | 12.8 |
| Smoking status | |||||||
| Never | 117 (34.5) | 549 | 21.9 | 575 | 13.9 | 602 | 11.6 |
| Ever | 222 (65.5) | 942 | 31.8 | 956 | 27.2 | 1,095 | 27.4 |
| Fruit and vegetable intake | |||||||
| <5 a day | 256 (75.5) | 1,133 | 29.1 | 1,148 | 22.7 | 1,280 | 23.4 |
| 5+ a day | 83 (24.5) | 358 | 25.1 | 383 | 20.9 | 417 | 16.8 |
| Percent of calories as fat | |||||||
| <30% | 65 (19.2) | 310 | 19.4 | 307 | 16.3 | 322 | 15.5 |
| 30%+ | 274 (80.8) | 1,181 | 30.5 | 1,224 | 23.7 | 1,375 | 23.3 |
| Multivitamin use | |||||||
| No multivitamin use | 80 (23.6) | 291 | 58.4 | 289 | 31.1 | 335 | 38.8 |
| <1 pill/day | 149 (44.0) | 636 | 28.3 | 712 | 23.9 | 801 | 21.2 |
| 1 or more pills/day | 110 (32.4) | 564 | 12.4 | 529 | 15.1 | 560 | 12.5 |
| Vitamin C, mg | |||||||
| 0 | 61 (18.0) | 185 | 59.4 | 195 | 40.9 | 235 | 55.3 |
| <250 | 141 (41.6) | 636 | 17.3 | 679 | 17.7 | 736 | 16.3 |
| ≥250 | 137 (40.4) | 670 | 29.9 | 657 | 21.3 | 725 | 16.5 |
| Vitamin E, mg | |||||||
| 0 | 61 (18.0) | 182 | 76.8 | 197 | 40.7 | 226 | 53.1 |
| <180 | 145 (42.8) | 659 | 16.7 | 704 | 21.3 | 777 | 19.3 |
| ≥180 | 133 (39.2) | 650 | 26.2 | 630 | 17.5 | 694 | 14.4 |
| β-carotene, μg | |||||||
| 0 | 89 (26.3) | 328 | 45.7 | 323 | 31.0 | 378 | 34.4 |
| <1,800 | 212 (62.5) | 995 | 21.1 | 1,040 | 22.1 | 1,128 | 16.8 |
| ≥1,800 | 38 (11.2) | 167 | 35.9 | 168 | 5.9 | 191 | 26.2 |
| Selenium, μg | |||||||
| 0 | 88 (26.0) | 322 | 52.8 | 320 | 31.2 | 374 | 34.7 |
| <50 | 219 (64.6) | 1,006 | 21.9 | 1,049 | 21.9 | 1,150 | 20.0 |
| ≥50 | 32 (9.4) | 163 | 18.4 | 161 | 6.2 | 172 | 5.8 |
Abbreviation is as follows: NSAID, nonsteroidal anti-inflammatory drug.
N shown for entire cohort (N = 339). Number of participants included in analysis for each outcome were as follows: tetraploidy, n = 307; aneuploidy, n = 308; and esophageal adenocarcinoma, n = 339.
Incidence rate per 1,000 person yr.
TABLE 2.
Demographic and health-related characteristics of the study participants by use of multivitaminsa
| Multivitamin use |
|||
|---|---|---|---|
| Characteristic | No Use, % (n = 80) | <1 Pill/Day, % (n = 149) | 1 or more Pill/Day, % (n = 110) |
| Age | |||
| 30–54 yr | 32.5 | 24.2 | 27.3 |
| 55–69 yr | 38.7 | 38.9 | 50.0 |
| ≥70 yr | 28.8 | 36.9 | 22.7 |
| Female | 16.2 | 19.5 | 20.0 |
| NSAID use | |||
| Never | 26.3 | 25.9 | 11.8 |
| Former | 7.5 | 10.1 | 5.5 |
| Current | 66.2 | 61.1 | 82.7 |
| Ever smoker | 68.8 | 61.1 | 69.1 |
| 5 + servings/day of fruits and vegetables | 25.0 | 18.1 | 32.7 |
| <30% of calories as fat | 12.5 | 20.1 | 22.7 |
Abbreviation is as follows: NSAID, nonsteroidal anti-inflammatory drug.
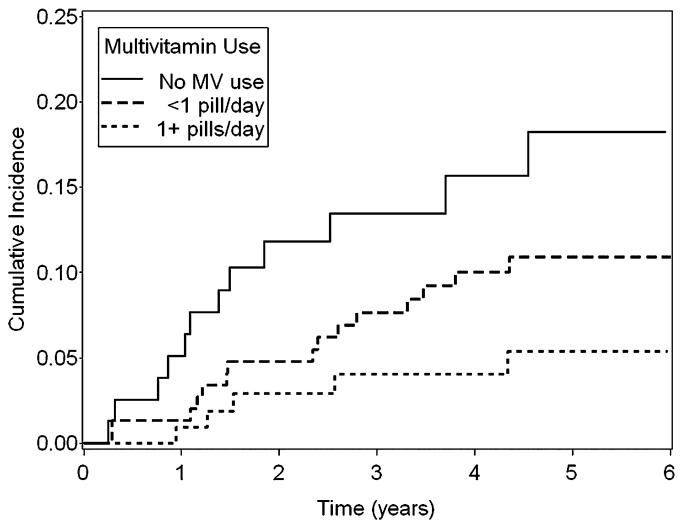
Compared with nonusers, use of one or more multivitamin pills/day was associated with a statistically significant 81% decreased risk of tetraploidy (95% CI 53-92%, P for trend < 0.001) and a 62% decreased risk of EA (95% CI 1-85%, P for trend = 0.04; Table 3). As shown in Fig. 1, participants who took 1 or more multivitamin pills/day had a consistently lower cumulative incidence of EA compared to either those who took multivitamins less regularly or never took multivitamins.
TABLE 3.
Risk of tetraploidy, aneuploidy, and EA associated with supplement intakea
| Tetraploidy |
Aneuploidy |
EA |
||||||
|---|---|---|---|---|---|---|---|---|
| Supplement Intake | Amount | Nb | HRc | 95% CI | HRc | 95% CI | HRc | 95% CI |
| Multivitamin use | ||||||||
| No multivitamin use | 80 | 1.00 | 1.00 | 1.00 | ||||
| <1 pill/day | 149 | 0.54 | 0.27-1.08 | 0.80 | 0.34-1.87 | 0.49 | 0.22-1.05 | |
| 1 or more pills/day | 110 | 0.19 | 0.08-0.47 | 0.62 | 0.22-1.72 | 0.38 | 0.15-0.99 | |
| P < 0.001 | P = 0.35 | P = 0.04 | ||||||
| Vitamin C, mg | 0 | 61 | 1.00 | 1.00 | 1.00 | |||
| <250 | 141 | 0.32 | 0.13–0.74 | 0.42 | 0.16–1.05 | 0.25 | 0.11–0.57 | |
| ≥250 | 137 | 0.47 | 0.22–1.03 | 0.52 | 0.21–1.30 | 0.25 | 0.11–0.58 | |
| P = 0.17 | P = 0.28 | P = 0.003 | ||||||
| Vitamin E, mg | 0 | 61 | 1.00 | 1.00 | 1.00 | |||
| <180 | 145 | 0.26 | 0.11–0.58 | 0.71 | 0.29–1.78 | 0.36 | 0.16–0.82 | |
| ≥180 | 133 | 0.30 | 0.14–0.64 | 0.58 | 0.22–1.52 | 0.25 | 0.10-0.60 | |
| P = 0.007 | P = 0.28 | P = 0.003 | ||||||
| β-carotene, μg | 0 | 89 | 1.00 | 1.00 | 1.00 | |||
| <1,800 | 212 | 0.46 | 0.23–0.94 | 0.76 | 0.34–1.68 | 0.50 | 0.24–1.06 | |
| ≥1,800 | 38 | 0.61 | 0.22–1.74 | 0.25 | 0.03–2.12 | 0.99 | 0.34–2.94 | |
| P = 0.14 | P = 0.19 | P = 0.45 | ||||||
| Selenium, μg | 0 | 88 | 1.00 | 1.00 | 1.00 | |||
| <50 | 219 | 0.42 | 0.21–0.81 | 0.75 | 0.34–1.67 | 0.58 | 0.28–1.19 | |
| ≥50 | 32 | 0.26 | 0.07–0.99 | 0.22 | 0.03–1.85 | 0.27 | 0.03–2.21 | |
| P = 0.005 | P = 0.16 | P = 0.08 | ||||||
Abbreviations are as follows: EA, esophageal adenocarcinoma; CI, confidence ratio.
N shown for entire cohort (N = 339). Number of participants included in analysis for each outcome were as follows: tetraploidy, n = 307; aneuploidy, n = 308; and EA, n = 339.
Adjusted for age, sex, fruit and vegetable consumption, percent energy from fat, waist:hip ratio, cigarette smoking, and nonsteroidal anti-inflammatory drug use.
FIG. 1.
Cumulative incidence rates associated with levels of multivitamin use and risk of EA.
Supplemental vitamin C intake, calculated as a dose received from both multivitamin and single-supplement sources, was associated with a reduced risk of tetraploidy (HR for ≥250 mg/day vs. none = 0.47; 95% CI = 0.22-1.03, P for trend = 0.17) and EA (HR for ≥250 mg/day vs. none = 0.25; 95% CI = 0.11-0.58, P for trend = 0.003; Table 3). There was a significant inverse association between supplemental vitamin E use and tetraploidy (HR for ≥180 mg/day vs. none = 0.30; 95% CI = 0.14-0.64, P for trend = 0.007) and EA (HR for ≥180 mg/day vs. none = 0.25; 95% CI = 0.10-0.60, P for trend = 0.003). With the exception of supplemental selenium use and tetraploidy, nonsignificant inverse associations between supplemental β-carotene and selenium use were observed for all 3 outcomes. However, a small number of individuals taking the higher supplemental doses limited our ability to fully examine these associations.
We tried to differentiate intake from single supplements from use of multiple supplements; however, multivitamin use was correlated with use of single supplements, as only a small number of individuals took a single supplement alone (Pearson correlations for multivitamin use with vitamin C: r = 0.45; vitamin E: r = 0.43; β-carotene: r = 0.38; and selenium: r = 0.54). In an attempt to distinguish between the effects of multivitamin and single-supplement use, we separated participants into those taking only multivitamins (n = 49), only single supplements (n = 35), or both (n = 210; Table 4). Compared with nonusers of multivitamins or single supplements, those who took only multivitamins had a significant reduced risk of tetraploidy (HR = 0.17; 95% CI = 0.04-0.62) and EA (HR = 0.35; 95% CI = 0.13-0.95). Furthermore, compared to nonsupplement use, use of single supplements (vitamin C, vitamin E, β-carotene, or selenium) and use of multivitamins and single supplements combined were both significantly associated with a reduced risk of all 3 outcomes of a similar magnitude to use of only multivitamins. We could not distinguish between individual single supplements because among the small number of participants who reported taking single supplements only, 43% took multiple types of single supplements (e.g., vitamins C and E).
TABLE 4.
Combined effect of multivitamin and single-supplement use and risk of tetraploidy, aneuploidy, and EAa
| Tetraploidy |
Aneuploidy |
EA |
|||||
|---|---|---|---|---|---|---|---|
| Supplement use | Nb | HRc | 95% CI | HRc | 95% CI | HRc | 95% CI |
| No multivitamin or any single-supplement use | 45 | 1.00 | 1.00 | 1.00 | |||
| Multivitamins only | 49 | 0.17 | 0.04-0.62 | 0.51 | 0.18-1.47 | 0.35 | 0.13-0.95 |
| Single supplements onlyd | 35 | 0.31 | 0.11-0.90 | 0.14 | 0.03-0.72 | 0.10 | 0.02-0.49 |
| Both multivitamin and single-supplement use | 210 | 0.21 | 0.09-0.46 | 0.28 | 0.11-0.72 | 0.17 | 0.07-0.38 |
Abbreviations are as follows: EA, esophageal adenocarcinoma; CI, confidence ratio.
Number of participants included in analysis for each outcome were as follows: tetraploidy, n = 307; aneuploidy, n = 308; and EA, n = 339.
Adjusted for age, sex, fruit and vegetable consumption, percent energy from fat, waist:hip ratio, cigarette smoking, and nonsteroidal anti-inflammatory drug use.
Single-supplement types considered were vitamins C, E, β-carotene, or selenium.
NSAID use was strongly associated with a reduced risk of EA in our cohort (49). To ensure that observed associations with supplement use were not confounded by NSAID use (potentially correlated behavior of “pill poppers”), we adjusted for NSAID use and further restricted analysis to non-NSAID users (n = 77). In this stratified analysis, a decreased risk of EA remained associated with multivitamin use (HR for any multivitamin vs. none = 0.32; 95% CI = 0.10-1.04), supplemental vitamin C (HR for ≥250 mg/day vs. none = 0.08; 95% CI = 0.02-0.42), and supplemental vitamin E (HR for ≥180 mg/day vs. none = 0.13; 95% CI = 0.03-0.67).
Of our cohort, 30% were previously under surveillance. Because these patients had a chance to adopt a healthier lifestyle after Barrett’s diagnosis, we tested whether associations between supplemental use and the 3 outcomes was different for these patients but found little difference between patterns of supplement use. Those who were diagnosed with high-grade dysplasia at baseline were seen more frequently and were at higher risk for EA. Our results were similar among those with high-grade dysplasia at baseline (HR for any multivitamin use = 0.46; 95% CI = 0.16-1.30) and those without high-grade dysplasia at baseline (HR for any multivitamin use = 0.52; 95% CI=0.16-1.69); however, as a small proportion of our cohort (14%) was diagnosed with high-grade dysplasia at baseline, our numbers were too small to fully examine this.
When stratified by smoking status, supplement use associations were limited to ever smokers. Any multivitamin use was significantly associated with a reduced risk of EA among this subset (HR = 0.33; 95% CI = 0.15-0.74). A similar association was not seen among never smokers (HR = 2.53; 95% CI = 0.24-26.6); however, the number of cancer outcomes in this low-risk group was very small (Table 1). Associations observed for supplement use did not vary significantly when stratified by fruit and vegetable consumption. Any multivitamin use among those who had low fruit and vegetable intake was associated with a HR of 0.52 (95% CI = 0.18-1.51), whereas any multivitamin use among those with high fruit and vegetable intake was 0.37 (95% CI = 0.13-1.10).
DISCUSSION
In this prospective study, daily use of multivitamins, vitamin C, and vitamin E were associated with a significantly reduced risk of tetraploidy and EA among individuals with Barrett’s esophagus. We were able to adjust for many important EA risk factors that were also associated with supplement use such as NSAID use, obesity, and diet. Due to the correlated use of multivitamins and single supplements within our cohort, it was difficult to differentiate between associations for specific single nutrients; however, our findings suggest an association between supplement intake and reduced risk of EA.
Diet has been considered an important factor in the etiology of EA in several epidemiologic studies (6,8), but only 4 population-based case-control studies have previously reported specifically on dietary supplement use (20,27-29), and no randomized controlled trial of supplement use and EA has been conducted so far. Of these four case-control studies, 3 reported a suggestive but nonsignificant reduction in risk associated with use of any vitamin supplement, with odds ratios (ORs) ranging from 0.57 to 0.90 (20,27,28). Due to the small proportion of participants consuming individual supplements in these studies, analyses by supplement type were limited and may have resulted in imprecise risk estimates. In the largest study, no association was observed between either regular use of any multivitamin (OR = 1.07; 95% CI = 0.76-1.51) or any single type of supplement (OR = 0.90; 95% CI = 0.63-1.28) and risk of EA (29). Differences between the findings in our cohort study and previously conducted case-control studies may be due to recall bias. If cases recalled their supplement use more accurately, this may lead to a spurious null association in a case-control study. Furthermore, individuals recruited into case-control studies for EA are different from our study population, which consisted of a high-risk cohort of individuals with Barrett’s esophagus. These differences in study populations may also explain the inconsistent findings.
Multivitamin pills consist of varying compositions of vitamins and minerals including several antioxidants, such as vitamin C, vitamin E, β-carotene, and selenium, which have been shown to reduce oxidative stress. Because chronic exposure to acid and bile during reflux is believed to cause oxidative damage (50,51) and inflammation to the esophagus, antioxidants may reduce cancer risk through their ability to scavenge free radicals produced by bile acids and the inflammatory process and thus prevent DNA damage (52,53). The anti-inflammatory effect of antioxidants may also reduce hydroperoxides in esophageal epithelium, which can otherwise activate lipoxygenase and cyclooxygenase, leading to the production of inflammatory prostaglandins and leukotrienes, an important step in the inflammatory process (54,55). The potential importance of antioxidant defenses against excessive freeradical production in the esophageal epithelium can be gathered from studies that have found lower levels of plasma and mucosal vitamin C in the metaplastic epithelium of Barrett’s esophagus samples (56) and complete loss of glutathione peroxidase-3 expression, an antioxidative selenoenzyme, in EA tumors (57). Smoking, another source of oxidative damage, has been associated with a twofold increased risk of EA (58). Past studies have documented lower serum levels of several antioxidants, including vitamins C, E, and β-carotene, in smokers than nonsmokers (59,60). The additional oxidative damage caused by smoking may consequently lead to an increased demand for antioxidants. This supports our finding that multivitamin use among individuals with a smoking history may benefit more from antioxidant supplementation than nonsmokers. An excess of antioxidants possibly impedes the neoplastic progression of Barrett’s esophagus and could explain the potential preventive effect of dietary supplements on the development of EA.
As individuals with Barrett’s esophagus are at high risk of developing esophageal cancer, the continual surveillance of patients in our study is a substantial strength, allowing us to collect data on a large number of exposures and the details of each outcome during an important time of disease progression. To the best of our knowledge, this is the first cohort study to provide results on multivitamin and single-supplement use and neoplastic development of EA. Unlike case-control studies, the prospective nature of our study allowed us to collect exposure data prior to the development of the outcome, potentially avoiding any differential bias in recall between cases and controls. Another important strength of our study is the assessment of supplement use at multiple times throughout the follow-up period. By combining data from several questionnaires collected at different time points, we were able to account for variability over time and presumably provide a more accurate description of long-term supplement use. Furthermore, we created a supplement composition database to calculate exact nutrient intakes for each type of multivitamin, taking into account multivitamin type has been shown to improve the accuracy of estimating supplemental intake (61). In addition, data on known and suspected risk factors for EA have been collected, thus allowing us to control for possible confounding. Reproduction of our findings in stratified and sensitivity analyses strengthened these findings. Our study benefited from the diagnosis of flow cytometric abnormalities that have been validated in predicting subsequent cancer risk (31); and compared to the diagnosis of dysplasia, these markers may be less subjective to interobserver variation (62,63).
There are several weaknesses in this study. It was not possible to distinguish among associations of a single nutrient because most participants took multivitamins or a combination of supplements. Only 6% of participants reported taking a single supplement. Although we observed an inverse association for selenium, the small number of users with high intake and the fact that selenium was primarily consumed in the form of multivitamins makes it difficult to distinguish the effect of selenium from other multivitamin constituents. Another limitation of our study was the small number of endpoint events. Tetraploidy, aneuploidy, and EA are relatively rare events, which limited our power to perform stratified analyses. Continued follow-up of this cohort will allow us to accumulate more events and determine whether these associations persist. It is also possible that supplement use could change due to symptoms from early esophageal cancer. However, we excluded any patients that were diagnosed within the first 3 mo of surveillance, and in an analysis further extending this duration to 1 yr, study results did not change (data not shown). Similarly, the development of abnormalities on flow cytometry, also used as outcomes in this study, are not to our knowledge associated with symptoms of early esophageal cancer. The presence or severity of reflux symptoms might also affect participants’ supplement use. However, the vast majority of participants were taking acid-decreasing drugs titrated to reduce or eliminate reflux symptoms. This made it impractical to evaluate associations by this factor due to limited variation among participants. Due to the small number of females in our cohort, we were unable to fully examine differences by sex. We were also limited in the number of supplements we could study because the initial version of the questionnaire included few individual supplements. We addressed the issue of confounding by healthy behaviors through controlling for multiple indicators of healthy behavior in our analysis (e.g., NSAID use, low fat intake, and high fruit and vegetable intake); however, it may be possible that residual confounding remains despite statistical adjustment, resulting in a stronger association being observed. We also did not adjust significance levels for multiple comparisons; however, the consistency of our findings across endpoints suggests that findings were not due to chance.
In summary, results from this first prospective study of supplement use and neoplastic progression of Barrett’s esophagus suggest that risk of EA is reduced with daily use of multivitamins and supplements containing vitamins C and E. Whether a specific nutrient on its own is responsible for this observation cannot be determined using current available data. These findings, which require replication, suggest that multivitamin supplement use may be an effective means of prevention progression to cancer in persons with Barrett’s esophagus.
ACKNOWLEDGMENTS
This research was supported in part by National Institute of Health (NIH) Grant P01 CA91955 and NIH R25 CA94880.
Contributor Information
Carissa A. Sanchez, Gastrointestinal Oncology Program, Fred Hutchinson Cancer Research Center, Seattle, Washington, USA
Robert D. Odze, Harvard Medical School and Brigham and Women’s Hospital, Boston, Massachusetts, USA
Kamran Ayub, Department of General Oncology and Hematology, Seattle Cancer Care Alliance, Seattle, Washington, USA.
Brian J. Reid, Gastrointestinal Oncology Program, Fred Hutchinson Cancer Research Center, and Department of Pathology, University of Washington, Seattle, Washington, USA
Thomas L. Vaughan, Epidemiology Program, Fred Hutchinson Cancer Research Center, and Department of Epidemiology, University of Washington, Seattle, Washington, USA
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Ries LAG, Harkins D, Krapcho M, Mariotto A, Miller BA, et al. SEER Cancer Statistics Review, 1975-2003. National Cancer Institute; Bethesda, MD: 2006. [Google Scholar]
- 3.Devesa SS, Blot WJ, Fraumeni JF., Jr Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049–2053. [PubMed] [Google Scholar]
- 4.Botterweck AA, Schouten LJ, Volovics A, Dorant E, van Den Brandt PA. Trends in incidence of adenocarcinoma of the oesophagus and gastric cardia in ten European countries. Int J Epidemiol. 2000;29:645–654. doi: 10.1093/ije/29.4.645. [DOI] [PubMed] [Google Scholar]
- 5.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97:142–146. doi: 10.1093/jnci/dji024. [DOI] [PubMed] [Google Scholar]
- 6.Chainani-Wu N. Diet and oral, pharyngeal, and esophageal cancer. Nutr Cancer. 2002;44:104–126. doi: 10.1207/S15327914NC4402_01. [DOI] [PubMed] [Google Scholar]
- 7.Cheng KK, Day NE. Nutrition and esophageal cancer. Cancer Causes Control. 1996;7:33–40. doi: 10.1007/BF00115636. [DOI] [PubMed] [Google Scholar]
- 8.Mayne ST, Navarro SA. Diet, obesity and reflux in the etiology of adenocarcinomas of the esophagus and gastric cardia in humans. JNutr. 2002;132:3467S–3470S. doi: 10.1093/jn/132.11.3467S. [DOI] [PubMed] [Google Scholar]
- 9.Vaughan TL, Davis S, Kristal A, Thomas DB. Obesity, alcohol, and tobacco as risk factors for cancers of the esophagus and gastric cardia: adenocarcinoma versus squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 1995;4:85–92. [PubMed] [Google Scholar]
- 10.Kubo A, Corley DA. Body mass index and adenocarcinomas of the esophagus or gastric cardia: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:872–878. doi: 10.1158/1055-9965.EPI-05-0860. [DOI] [PubMed] [Google Scholar]
- 11.Chow WH, Blot WJ, Vaughan TL, Risch HA, Gammon MD, et al. Body mass index and risk of adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst. 1998;90:150–155. doi: 10.1093/jnci/90.2.150. [DOI] [PubMed] [Google Scholar]
- 12.Lagergren J, Bergstrom R, Nyren O. Association between body mass and adenocarcinoma of the esophagus and gastric cardia. Ann Intern Med. 1999;130:883–890. doi: 10.7326/0003-4819-130-11-199906010-00003. [DOI] [PubMed] [Google Scholar]
- 13.Shaheen N, Ransohoff DF. Gastroesophageal reflux, barrett esophagus, and esophageal cancer: scientific review. JAMA. 2002;287:1972–1981. doi: 10.1001/jama.287.15.1972. [DOI] [PubMed] [Google Scholar]
- 14.Spechler SJ, Goyal RK. The columnar-lined esophagus, intestinal meta-plasia, and Norman Barrett. Gastroenterology. 1996;110:614–621. doi: 10.1053/gast.1996.v110.agast960614. [DOI] [PubMed] [Google Scholar]
- 15.Phillips RW, Wong RK. Barrett’s esophagus: natural history, incidence, etiology, and complications. Gastroenterol Clin North Am. 1991;20:791–816. [PubMed] [Google Scholar]
- 16.Pera M, Manterola C, Vidal O, Grande L. Epidemiology of esophageal adenocarcinoma. J Surg Oncol. 2005;92:151–159. doi: 10.1002/jso.20357. [DOI] [PubMed] [Google Scholar]
- 17.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–2252. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 18.Shaheen NJ, Crosby MA, Bozymski EM, Sandler RS. Is there publication bias in the reporting of cancer risk in Barrett’s esophagus? Gastroenterology. 2000;119:333–338. doi: 10.1053/gast.2000.9302. [DOI] [PubMed] [Google Scholar]
- 19.Paulson TG, Reid BJ. Focus on Barrett’s esophagus and esophageal adenocarcinoma. Cancer Cell. 2004;6:11–16. doi: 10.1016/j.ccr.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 20.Brown LM, Swanson CA, Gridley G, Swanson GM, Schoenberg JB, et al. Adenocarcinoma of the esophagus: role of obesity and diet. J Natl Cancer Inst. 1995;87:104–109. doi: 10.1093/jnci/87.2.104. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez CA, Pera G, Agudo A, Bueno-de-Mesquita HB, Ceroti M, et al. Fruit and vegetable intake and the risk of stomach and oesophagus adenocarcinoma in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) Int J Cancer. 2006;118:2559–2566. doi: 10.1002/ijc.21678. [DOI] [PubMed] [Google Scholar]
- 22.Lagergren J, Bergstrom R, Lindgren A, Nyren O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl JMed. 1999;340:825–831. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 23.Farrow DC, Vaughan TL, Sweeney C, Gammon MD, Chow WH, et al. Gastroesophageal reflux disease, use of H2 receptor antagonists, and risk of esophageal and gastric cancer. Cancer Causes Control. 2000;11:231–238. doi: 10.1023/a:1008913828105. [DOI] [PubMed] [Google Scholar]
- 24.National Institutes of Health State-of-the-science conference statement: multivitamin/mineral supplements and chronic disease prevention. Ann Intern Med. 2007;145:364–371. doi: 10.7326/0003-4819-145-5-200609050-00136. [DOI] [PubMed] [Google Scholar]
- 25.Radimer K, Bindewald B, Hughes J, Ervin B, Swanson C, et al. Dietary supplement use by US adults: data from the National Health and Nutrition Examination Survey, 1999-2000. Am J Epidemiol. 2004;160:339–349. doi: 10.1093/aje/kwh207. [DOI] [PubMed] [Google Scholar]
- 26.Kabat GC, Ng SK, Wynder EL. Tobacco, alcohol intake, and diet in relation to adenocarcinoma of the esophagus and gastric cardia. Cancer Causes Control. 1993;4:123–132. doi: 10.1007/BF00053153. [DOI] [PubMed] [Google Scholar]
- 27.Terry P, Lagergren J, Ye W, Nyren O, Wolk A. Antioxidants and cancers of the esophagus and gastric cardia. Int J Cancer. 2000;87:750–754. [PubMed] [Google Scholar]
- 28.Cheng KK, Sharp L, McKinney PA, Logan RF, Chilvers CE, et al. A case-control study of oesophageal adenocarcinoma in women: a preventable disease. Br J Cancer. 2000;83:127–132. doi: 10.1054/bjoc.2000.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mayne ST, Risch HA, Dubrow R, Chow WH, Gammon MD, et al. Nutrient intake and risk of subtypes of esophageal and gastric cancer. Cancer Epidemiol Biomarkers Prev. 2001;10:1055–1062. [PubMed] [Google Scholar]
- 30.Chen H, Tucker KL, Graubard BI, Heineman EF, Markin RS, et al. Nutrient intakes and adenocarcinoma of the esophagus and distal stomach. Nutr Cancer. 2002;42:33–40. doi: 10.1207/S15327914NC421_5. [DOI] [PubMed] [Google Scholar]
- 31.Rabinovitch PS, Longton G, Blount PL, Levine DS, Reid BJ. Predictors of progression in Barrett’s esophagus III: baseline flow cytometric variables. Am J Gastroenterol. 2001;96:3071–3083. doi: 10.1111/j.1572-0241.2001.05261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reid BJ, Levine DS, Longton G, Blount PL, Rabinovitch PS. Predictors of progression to cancer in Barrett’s esophagus: baseline histology and flow cytometry identify low- and high-risk patient subsets. Am J Gastroenterol. 2000;95:1669–1676. doi: 10.1111/j.1572-0241.2000.02196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barrett MT, Sanchez CA, Prevo LJ, Wong DJ, Galipeau PC, et al. Evolution of neoplastic cell lineages in Barrett oesophagus. Nat Genet. 1999;22:106–109. doi: 10.1038/8816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McManus DT, Olaru A, Meltzer SJ. Biomarkers of esophageal adenocarcinoma and Barrett’s esophagus. Cancer Res. 2004;64:1561–1569. doi: 10.1158/0008-5472.can-03-2438. [DOI] [PubMed] [Google Scholar]
- 35.van Lieshout EM, Jansen JB, Peters WH. Biomarkers in Barrett’s esophagus (review) Int J Oncol. 1998;13:855–864. doi: 10.3892/ijo.13.4.855. [DOI] [PubMed] [Google Scholar]
- 36.Vaughan TL, Kristal AR, Blount PL, Levine DS, Galipeau PC, et al. Non-steroidal anti-inflammatory drug use, body mass index, and anthropometry in relation to genetic and flow cytometric abnormalities in Barrett’s esophagus. Cancer Epidemiol Biomarkers Prev. 2002;11:745–752. [PubMed] [Google Scholar]
- 37.Cameron AJ, Lomboy CT. Barrett’s esophagus: age, prevalence, and extent of columnar epithelium. Gastroenterology. 1992;103:1241–1245. doi: 10.1016/0016-5085(92)91510-b. [DOI] [PubMed] [Google Scholar]
- 38.Conio M, Blanchi S, Lapertosa G, Ferraris R, Sablich R, et al. Long-term endoscopic surveillance of patients with Barrett’s esophagus: incidence of dysplasia and adenocarcinoma: a prospective study. Am J Gastroenterol. 2003;98:1931–1939. doi: 10.1111/j.1572-0241.2003.07666.x. [DOI] [PubMed] [Google Scholar]
- 39.Conio M, Cameron AJ, Romero Y, Branch CD, Schleck CD, et al. Secular trends in the epidemiology and outcome of Barrett’s oesophagus in Olmsted County, Minnesota. Gut. 2001;48:304–309. doi: 10.1136/gut.48.3.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Connor JB, Falk GW, Richter JE. The incidence of adenocarcinoma and dysplasia in Barrett’s esophagus: report on the Cleveland Clinic Barrett’s Esophagus Registry. Am J Gastroenterol. 1999;94:2037–2042. doi: 10.1111/j.1572-0241.1999.01275.x. [DOI] [PubMed] [Google Scholar]
- 41.Reid BJ, Blount PL, Rabinovitch PS. Biomarkers in Barrett’s esophagus. Gastrointest Endosc Clin N Am. 2003;13:369–397. doi: 10.1016/s1052-5157(03)00006-0. [DOI] [PubMed] [Google Scholar]
- 42.Reid BJ, Blount PL, Rubin CE, Levine DS, Haggitt RC, et al. Flowcytometric and histological progression to malignancy in Barrett’s esophagus: prospective endoscopic surveillance of a cohort. Gastroenterology. 1992;102:1212–1219. [PubMed] [Google Scholar]
- 43.Galipeau PC, Li X, Blount PL, Maley CC, Sanchez CA, et al. NSAIDs modulate CDKN2A, TP53, and DNA content risk for progression to esophageal adenocarcinoma. PLoS Med. 2007;4:e67. doi: 10.1371/journal.pmed.0040067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moe GL, Kristal AR, Levine DS, Vaughan TL, Reid BJ. Waist-tohip ratio, weight gain, and dietary and serum selenium are associated with DNA content flow cytometry in Barrett’s esophagus. Nutr Cancer. 2000;36:7–13. doi: 10.1207/S15327914NC3601_2. [DOI] [PubMed] [Google Scholar]
- 45.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, et al. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 46.Feskanich D, Sielaff BH, Chong K, Buzzard IM. Computerized collection and analysis of dietary intake information. Comput Methods Programs Biomed. 1989;30:47–57. doi: 10.1016/0169-2607(89)90122-3. [DOI] [PubMed] [Google Scholar]
- 47.Satia-Abouta J, Patterson RE, King IB, Stratton KL, Shattuck AL, et al. Reliability and validity of self-report of vitamin and mineral supplement use in the vitamins and lifestyle study. Am J Epidemiol. 2003;157:944–954. doi: 10.1093/aje/kwg039. [DOI] [PubMed] [Google Scholar]
- 48.Medical Economics Company . Physicians’ Desk Reference for Nonprescription Drugs and Dietary Supplements. Medical Economics Co.; Montvale, NJ: 1999. p. v. [Google Scholar]
- 49.Vaughan TL, Dong LM, Blount PL, Ayub K, Odze RD, et al. Non-steroidal anti-inflammatory drugs and risk of neoplastic progression in Barrett’s oesophagus: a prospective study. Lancet Oncol. 2005;6:945–952. doi: 10.1016/S1470-2045(05)70431-9. [DOI] [PubMed] [Google Scholar]
- 50.Jenkins GJ, D’Souza FR, Suzen HS, Eltahir ZS, James SA, et al. Deoxycholic acid (DCA) at neutral and acid pH, is genotoxic to oesophageal cells through the induction of ROS: the potential role of antioxidants in Barrett’s oesophagus. Carcinogenesis. 2007;28:136–142. doi: 10.1093/carcin/bgl147. [DOI] [PubMed] [Google Scholar]
- 51.Payne CM, Weber C, Crowley-Skillicorn C, Dvorak K, Bernstein H, et al. Deoxycholate induces mitochondrial oxidative stress and activates NF-{kappa}B through multiple mechanisms in HCT-116 colon epithelial cells. Carcinogenesis. 2007;28:215–222. doi: 10.1093/carcin/bgl139. [DOI] [PubMed] [Google Scholar]
- 52.Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem. 2004;266:37–56. doi: 10.1023/b:mcbi.0000049134.69131.89. [DOI] [PubMed] [Google Scholar]
- 53.Wild CP, Hardie LJ. Reflux, Barrett’s oesophagus and adenocarcinoma: burning questions. Nat Rev Cancer. 2003;3:676–684. doi: 10.1038/nrc1166. [DOI] [PubMed] [Google Scholar]
- 54.Spallholz JE, Boylan LM, Larsen HS. Advances in understanding selenium’s role in the immune system. Ann NY Acad Sci. 1990;587:123–139. doi: 10.1111/j.1749-6632.1990.tb00140.x. [DOI] [PubMed] [Google Scholar]
- 55.Villette S, Kyle JA, Brown KM, Pickard K, Milne JS, et al. A novel single nucleotide polymorphism in the 3′ untranslated region of human glutathione peroxidase 4 influences lipoxygenase metabolism. Blood Cells Mol Dis. 2002;29:174–178. doi: 10.1006/bcmd.2002.0556. [DOI] [PubMed] [Google Scholar]
- 56.Fountoulakis A, Martin IG, White KL, Dixon MF, Cade JE, et al. Plasma and esophageal mucosal levels of vitamin C: role in the pathogenesis and neoplastic progression of Barrett’s esophagus. Dig Dis Sci. 2004;49:914–919. doi: 10.1023/b:ddas.0000034548.89117.d6. [DOI] [PubMed] [Google Scholar]
- 57.Lee OJ, Schneider-Stock R, McChesney PA, Kuester D, Roessner A, et al. Hypermethylation and loss of expression of glutathione peroxidase-3 in Barrett’s tumorigenesis. Neoplasia. 2005;7:854–861. doi: 10.1593/neo.05328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gammon MD, Schoenberg JB, Ahsan H, Risch HA, Vaughan TL, et al. Tobacco, alcohol, and socioeconomic status and adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst. 1997;89:1277–1284. doi: 10.1093/jnci/89.17.1277. [DOI] [PubMed] [Google Scholar]
- 59.Wei W, Kim Y, Boudreau N. Association of smoking with serum and dietary levels of antioxidants in adults: NHANES III, 1988-1994. Am J Public Health. 2001;91:258–264. doi: 10.2105/ajph.91.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Galan P, Viteri FE, Bertrais S, Czernichow S, Faure H, et al. Serum concentrations of beta-carotene, vitamins C and E, zinc and selenium are influenced by sex, age, diet, smoking status, alcohol consumption and corpulence in a general French adult population. Eur J Clin Nutr. 2005;59:1181–1190. doi: 10.1038/sj.ejcn.1602230. [DOI] [PubMed] [Google Scholar]
- 61.Park SY, Murphy SP, Wilkens LR, Yamamoto JF, Kolonel LN. Allowing for variations in multivitamin supplement composition improves nutrient intake estimates for epidemiologic studies. J Nutr. 2006;136:1359–1364. doi: 10.1093/jn/136.5.1359. [DOI] [PubMed] [Google Scholar]
- 62.Reid BJ, Haggitt RC, Rubin CE, Roth G, Surawicz CM, et al. Observer variation in the diagnosis of dysplasia in Barrett’s esophagus. Hum Pathol. 1988;19:166–178. doi: 10.1016/s0046-8177(88)80344-7. [DOI] [PubMed] [Google Scholar]
- 63.Sagan C, Flejou JF, Diebold MD, Potet F, Le Bodic MF. Reproducibility of histological criteria of dysplasia in Barrett mucosa. Gastroenterol Clin Biol. 1994;18:D31–D34. [PubMed] [Google Scholar]