Abstract
Peroxisome proliferator-activated receptors (PPAR) are involved in the pathogenesis of insulin resistance, diabetes, and related complications. Consequently, the identification of PPAR subtypes and the potential for their activation provides promising therapeutic targets for the management of type 2 diabetes mellitus. Available data from rodent carcinogenicity studies, however, demonstrate that PPAR agonists can be tumorigenic in one or more species of rodents at multiple sites. In 2005, the Health and Environmental Sciences Institute (HESI) PPAR Agonist Project Committee was established by a group of pharmaceutical companies to advance research on and to understand the modes of action and human relevance of this emerging rodent tumor data for PPAR agonists. Since the most commonly observed tumor types reported in rodents are hemangiosarcomas, fibrosarcomas and liposarcomas, the PPAR Agonist Project Committee approved a Pathology Working Group (PWG) to develop consensus of morphologic criteria for tumor diagnoses and consistency of diagnoses across multiple studies for hemangiosarcomas in mice and hamsters and liposarcomas/fibrosarcomas in rats. Therefore, the focus of the PWG review was to establish consistent tumor diagnostic criteria, to assess evidence of potentially preneoplastic changes and to identify distinguishing morphologic differences which may exist between spontaneous changes present in control animals with similar changes from treated animals. Specific diagnostic criteria and nomenclature are recommended for the classification of proliferative vascular lesions which may be present in mice or hamsters and for proliferative mesenchymal changes in rats in studies that are conducted with PPAR agonists.
Keywords: PPAR agonists, hemangiosarcoma, liposarcoma, fibrosarcoma, rodent, carcinogenesis, diagnostic criteria
INTRODUCTION
Peroxisome proliferator-activated receptors (PPAR) are nuclear receptors involved in regulating lipids and the effects of insulin (Yki-Järvinen, 2004). Three major receptors have been identified: 1) alpha (α), 2) gamma (γ), and 3) delta (δ), which sometimes is referred to as beta (β), with differing tissue distributions and biological effects. Agonists have been developed for each of these receptors, with differing pharmacologic and toxicologic effects within and across laboratory animal species.
PPARα are present in liver, brown fat, kidney, heart, skeletal muscle, immune system, intestine, and retina. PPARα agonists have effects on maintenance of lipid levels, especially triglycerides and high-density-lipoproteins (HDL) (Klaunig et al., 2003). The toxicological effects of PPARα agonists (peroxisome proliferators) have been extensively investigated, including carcinogenic effects in rodents. They may induce liver tumors in rats and mice and frequently induce pancreatic acinar cell and testicular Leydig cell tumors in rats. The modes of action for these tumors have been identified and generally been found not to be relevant to humans (at least quantitatively) (Klaunig et al., 2003).
PPARδ are ubiquitous, whereas PPARγ (are present in adipose tissue, endothelial cells, tissues of the immune system, and several epithelial tissues, such as urothelium and intestine (Yki-Järvinen, 2004). Agonists to these receptors, either alone or having combination effects on more than one PPAR, are being developed for clinical use in treating lipid disorders and diabetes. The carcinogenic effects of PPARδ agonists in rodents have not been extensively investigated, but have already produced varying and conflicting results. PPARγ agonists and dual PPARγ/α agonists have been more extensively investigated. Although various tumors have been produced by specific agonists, the most common types of tumors observed so far have been rat urinary bladder urothelial tumors and various mesenchymal tissue tumors (especially in subcutaneous adipose tissue) in rats, mice, and hamsters (El-Hage, 2005).
In mice and hamsters, both PPARγ and dual γ/α agonists increase the incidence of vascular tumors. Drug-induced increases in vascular tumors have been observed in male and female CD-1 mice, B6C3F1 mice, or hamsters with 8 of 11 compounds (γ3γ, 5 dual γ/α agonists) tested. For most compounds, the increased incidence of hemangiosarcomas occurs in multiple tissues consistent with the sites of spontaneous tumor formation (e.g., liver, spleen, skin, bone marrow, and adipose tissue). Morphologic changes diagnosed as angiomatous hyperplasia and angiectasis have been described with several compounds in rodents and nonrodents. In rats, increases in benign and malignant neoplasms have been observed in 5 of 6 studies (3γ, 3 dual γ/α agonists) (El-Hage, 2005).
Based on findings in these initial studies, the FDA decided to require sponsors to complete 2-year rodent carcinogenicity studies and submit draft reports for agency review prior to clinical studies longer than 6 months in duration (El-Hage, 2005).
In 2005, the Health and Environmental Sciences Institute (HESI) PPAR Agonist Project Committee was established by a group of pharmaceutical companies to advance research on and to understand the modes of action and human relevance of this emerging rodent tumor data for PPAR agonists. The most commonly observed tumor types reported in rodents are hemangiosarcomas, fibrosarcomas and liposarcomas. In 2006, the PPAR Agonist Project Committee approved a Pathology Working Group (PWG) to develop consensus on tumor diagnoses and consistency of diagnoses across multiple studies for hemangiosarcomas in mice and hamsters and liposarcomas/fibrosarcomas in rats. Therefore the focus of the PWG review was to establish consistent tumor diagnostic criteria to assess evidence of potentially preneoplastic changes.
MATERIALS AND METHODS
All materials reviewed during the PWG were provided by the sponsoring companies. For studies conducted in mice and hamsters in which proliferative vascular changes were of interest, 2 unstained slides were submitted to EPL. Six unstained slides were submitted from studies conducted in rats, since it was anticipated that either special histochemical or immunohistochemical stains might be helpful in the diagnosis. There were a total of 420 cases submitted from studies conducted in mice, 10 cases submitted from hamsters, and 99 cases submitted from rats.
It was critical that the origin of the slides being reviewed by the PWG be kept confidential. Individuals involved in organizing and conducting the PWG and the panel members did not know the company, study, or compound for any of the slides being reviewed. In order to achieve this degree of confidentiality, EPL provided glassware to be used to submit the unstained tissue sections. All glass slides were purchased from the same vendor and were the same style and color. The slides had preprinted numbers. The slides were initially numbered consecutively, and then were arranged in a random order according to a computer-generated list of random numbers.
The randomized slides were submitted to HESI for distribution to the participating companies. Upon request, the number of slides requested was sent by HESI to each of the companies making submissions. The slides were labeled with only the random number. No other information appeared on the glass slide. When submitting the material, the company also submitted a log containing the random number, animal number, species, tissue of origin, and indicated if it was a control or treated animal and if it was sacrificed or died spontaneously. If the animal died or was euthanized prior to the scheduled sacrifice, the log also included the weeks on study or the number of weeks on treatment and the probable cause of death if available. All slides were first submitted to HESI from the companies participating in the project. As submissions were received by HESI, the slides and the complete log were repackaged and shipped to EPL to be used for the PWG without any accompanying information concerning the origin of the slides.
When received at EPL, the randomized slides were rearranged in the original numerical order. All studies were combined, thus precluding the association of a particular slide with a particular study. Since all the slides with random numbers were not used for the PWG, the slides used for the PWG were assigned ascending consecutive identification numbers (ID#). This provided a third level of coding of the slides (triple blind) and made the review of the slides by the PWG easier since they only had to be concerned about a single consecutive number when recording their findings. This approach ensured the complete confidentiality of the materials being submitted.
EPL stained one slide from each case submitted with hematoxylin and eosin (H&E). The remaining slides were available for diagnostic histochemical or immunohistochemical staining that might help in the definitive classification of selected lesions. Prior to convening the PWG, the Chairperson reviewed all cases submitted and requested representative special stains that could be used in addition to the routine H&E stained slides. The histochemical stains which were used included: Masson’s trichrome, alcian blue/periodic acid Schiff (PAS), and Mallory’s phosphotungstic acid hematoxylin (PTAH). Immunohistochemical stains that were applied to selected cases included: S-100 (liposarcoma), Desmin (muscle differentiation), and Uncoupling Protein-1 (UCP-1) (Hibernoma).
The Pathology Working Group (PWG) meeting, consisting of independent consulting pathologists with expertise in toxicologic pathology, with specific emphasis on rodent carcinogenicity studies, was held in Herndon, Virginia, January 16-18, 2007. The purpose of the PWG was to develop a consensus for tumor diagnoses and consistency of diagnoses across multiple studies for hemangiosarcomas in mice or hamsters and liposarcomas/fibrosarcomas in rats. The focus of the PWG review was to establish consistent tumor diagnostic criteria, assess evidence of preneoplastic changes, and evaluate morphologic differences between spontaneous changes examined from controls with similar changes from treated animals.
The PWG Chairperson (JFH) organized the meeting and was responsible for conducting the meeting, leading the discussion, and preparing a report of the PWG’s findings. The PWG participants included board-certified medical (PG) and veterinary pathologists (MRE, HE, DEM, PCM, and PAT). Several interested scientists from the HESI PPAR Agonist Project Committee or from sponsoring member companies were also in attendance as observers. At the beginning of the PWG meeting, the PWG Chairperson provided the panel with a review of the issues to be addressed and an outline of the approach to be followed. The PWG examined coded slides without knowledge of the specific test article or the origin of the case selected for their review.
The PWG examined H&E stained slides from all rats (99 cases) for which lesions were submitted. If the diagnosis using only the H&E stained sections was uncertain, selected histochemical and immunohistochemical stains were available for some cases for PWG review to aid in classification of tumors. Due to the large number of cases submitted for the mouse portion of the PWG, the PWG Chairperson selected representative cases to include the entire spectrum of non-neoplastic changes and benign and malignant neoplasms available from the slides submitted. Since there were only 10 cases submitted from the hamster study, the PWG examined all slides from hamsters. No histochemically or immunohistochemically stained slides were prepared for the examination of vascular lesions in mice or hamsters.
Each participant recorded their diagnoses and comments on worksheets which were prepared by the PWG Chairperson. The PWG participants recorded either a morphologic diagnosis of a proliferative change or made an observation of “no proliferative lesion,” if no significant hyperplastic or neoplastic changes were identified. The diagnosis for each case examined was discussed by the group, reexamined if necessary, and the final opinion was recorded by the Chairperson.
During the review, the PWG participants developed recommended nomenclature and diagnostic criteria for each of the changes discussed. Following the PWG, an illustrated lexicon was prepared in the form of an interactive CD-ROM which included the nomenclature and diagnostic criteria recommended by the Pathology Working Group and representative photomicrographs of each of the changes including both non-neoplastic proliferative lesions and neoplasms and also a list of important references related to the project. A copy of this illustrated lexicon is present in the back cover of this issue of the journal.
RESULTS
Liposarcomas/Fibrosarcomas In Rats
There were 99 cases submitted to be used for the Pathology Working Group. These included 8 slides submitted from control animals and 91 slides submitted from treated animals. The most frequently submitted tissues included skin with subcutaneous tissue and adipose tissue. The remaining slides included a variety of tissues including intestinal tract, skeletal muscle, spleen, kidney, liver and lung.
All of the slides submitted from control animals contained neoplastic lesions. Two were classified as benign and 6 as malignant. Both of the benign neoplasms were diagnosed as lipomas and were characterized by discrete masses composed of well-differentiated adipocytes. The malignant tumors included one liposarcoma and five fibrosarcomas.
A broader spectrum of lesions was submitted from the treated rats. These changes ranged from nonneoplastic proliferative changes classified as hyperplasia of the adipose tissue to benign and malignant neoplasms involving mesenchymal tissues in various sites and organs. Most of these involved masses present in the skin, subcutaneous tissue or white adipose tissue. The majority of the benign tumors were classified as lipomas and were located at several sites including the white adipose tissue, skin and subcutaneous tissue, abdominal cavity, and wall of the cecum. The malignant neoplasms were classified primarily as fibrosarcomas or liposarcomas. A few other malignant neoplasms diagnosed by the PWG, included fibrosarcoma, pleomorphic type (malignant fibrous histiocytoma), malignant schwannoma, and sarcoma, NOS (not otherwise specified).
The lipomas in treated and control rats were similar in appearance with few distinguishing morphologic differences (Figure 1). The lipomas were grossly observed lesions forming a distinct nodule or mass. They consisted of expansile proliferations of well-differentiated adipocytes and often were lobulated with distinct fibrous bands separating the lobules. Lipomas were differentiated from proliferative changes classified as adipose hyperplasia which consisted of aggregates of diffuse increased numbers of adipocytes which were non-expansile and did not result in compression or distortion of the surrounding tissue.
FIGURE 1.
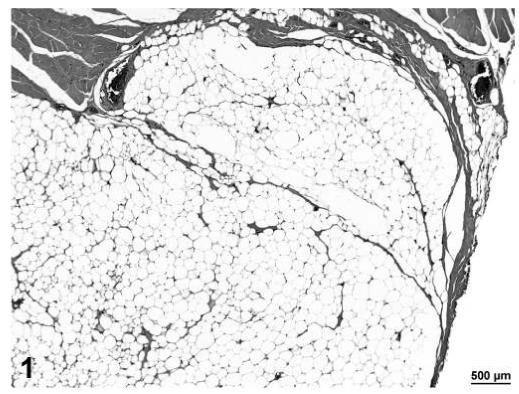
Lipoma, adipose tissue, H&E, 6.4×.
Malignant tumors (liposarcoma and fibrosarcoma) from control animals were composed of a more uniform population of cells. Unlike liposarcomas in treated rats, the single liposarcoma observed in a control rat had no evidence of fibroblastic spindle cell proliferation within the tumor (Figure 2). The fibrosarcomas in control animals were comprised of well-differentiated spindle cells producing a distinct collagen matrix. Unlike fibrosarcomas observed in treated rats, there was no evidence of vacuolation or myxomatous change observed in the fibrosarcomas of control rats (Figures 3 and 4).
FIGURE 2.
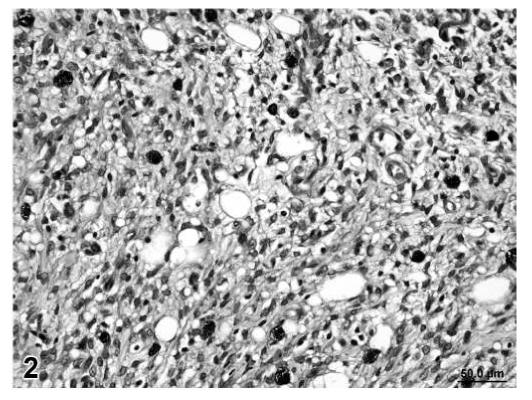
Liposarcoma, skin/subcutaneous tissue, control rat, H&E, 40×.
FIGURE 3.
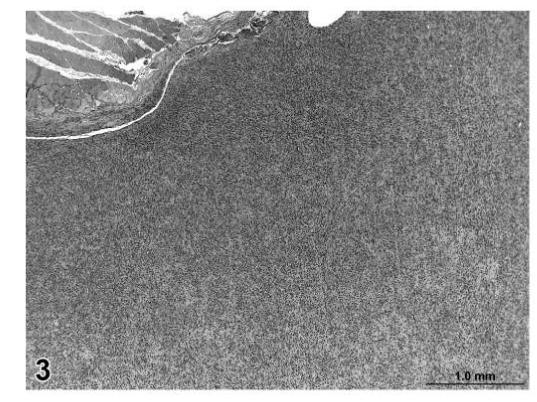
Fibrosarcoma, skin/subcutaneous tissue, control rat, H&E, 4×.
Liposarcomas in treated rats were variable in appearance but all were characterized by the presence of malignant lipoblasts. In addition, some of the liposarcomas contained areas of poorly differentiated fibroblastic cells arranged in interlacing bundles as well as more pleomorphic tumor cells (Figure 5). Fibrosarcomas in treated rats were characterized by a similar pattern of poorly differentiated fibroblastic and pleomorphic cells but without evidence of lipoblastic differentiation (Figures 6).
FIGURE 5.
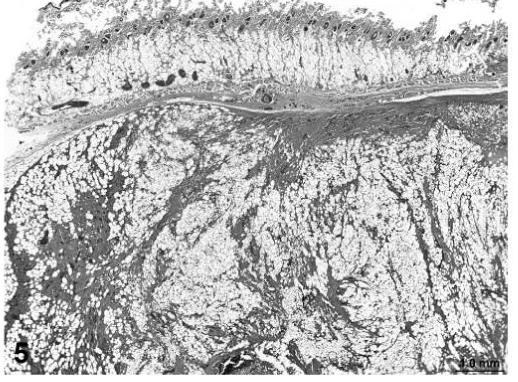
Liposarcoma, skin, treated rat, H&E, 2×.
FIGURE 6.

Fibrosarcoma, skin, treated rat, H&E, 3.2×.
The nomenclature and diagnostic criteria developed by the PWG was based on previously published recommendations by the Society of Toxicologic Pathology (STP) (Greaves, et al., 1992, 2004). The PWG noted that the nomenclature and diagnostic criteria presented in these 2 publications were developed primarily to address the diagnosis of spontaneous proliferative lesions. They were not specifically intended to address the nomenclature and diagnostic criteria that may be necessary to properly classify more typical or unique treatment-related neoplasms. For many of the lesions examined during the PWG, the published nomenclature and diagnostic criteria were sufficient to accurately classify the proliferative change. However, a few of the liposarcomas, primarily in treated rats, had morphologic features that were more complex, consisting of a proliferation of poorly differentiated adipocytes with focal areas of interlacing bundles of fibroblastic cells and/or myxomatous change within the mass (Figures 7 and 8).
FIGURE 7.
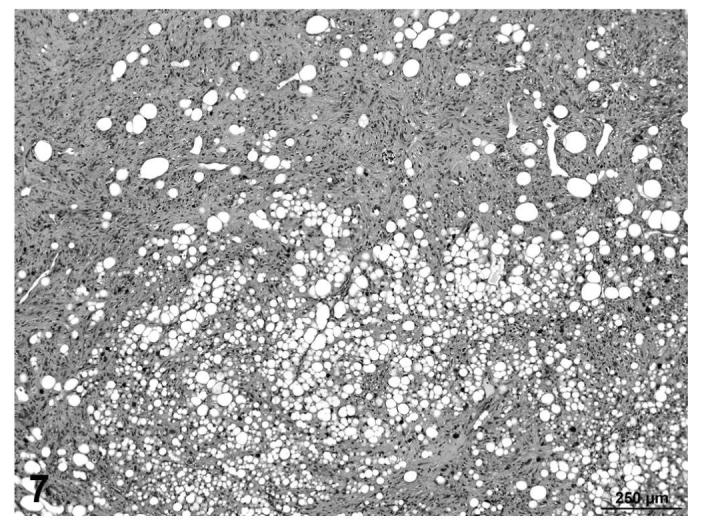
Liposarcoma, with poorly differentiated adipocytes, interlacing bundles of fibroblastic cells and myxomatous change, skin, treated rat, H&E, 10×.
FIGURE 8.
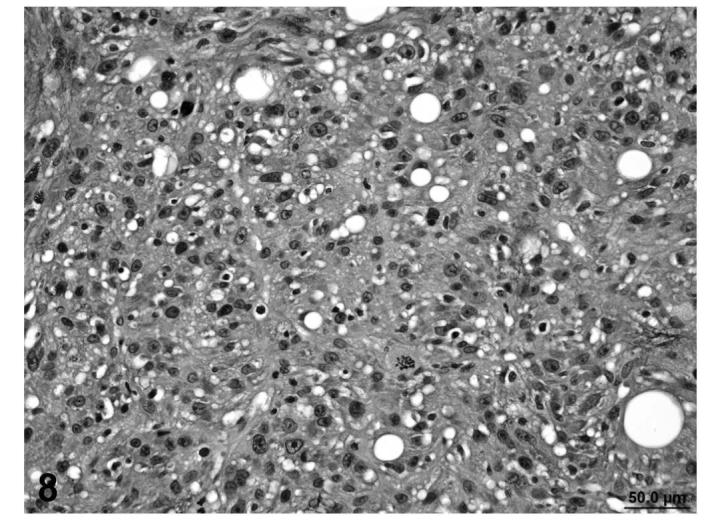
Liposarcoma, with poorly differentiated adipocytes, interlacing bundles of fibroblastic cells and myxomatous change, skin, treated rat, H&E, 40×.
In other cases (fibrosarcoma), the majority of the tumor consisted of proliferating poorly differentiated fibrocytes producing varying amounts of collagen with varying degrees of vacuolization within the mass (Figures 9 and 10). In a few cases (liposarcomas), the lipomatous mass was dissected by bands of proliferating fibrous tissue that contained abundant well-differentiated collagen fibers (Figures 11 and 12). Those tumors that contained poorly-differentiated adipocytes were classified as liposarcomas, while tumors that were composed of fibroblastic and pleomorphic cells alone were classified as fibrosarcomas.
FIGURE 9.
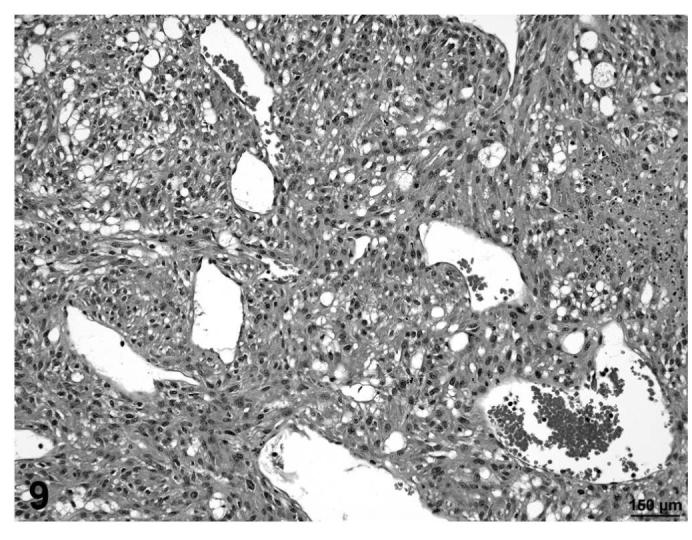
Fibrosarcoma, proliferating undifferentiated fibroblasts with vacuolization, skin, treated rat, H&E, 20×.
FIGURE 10.
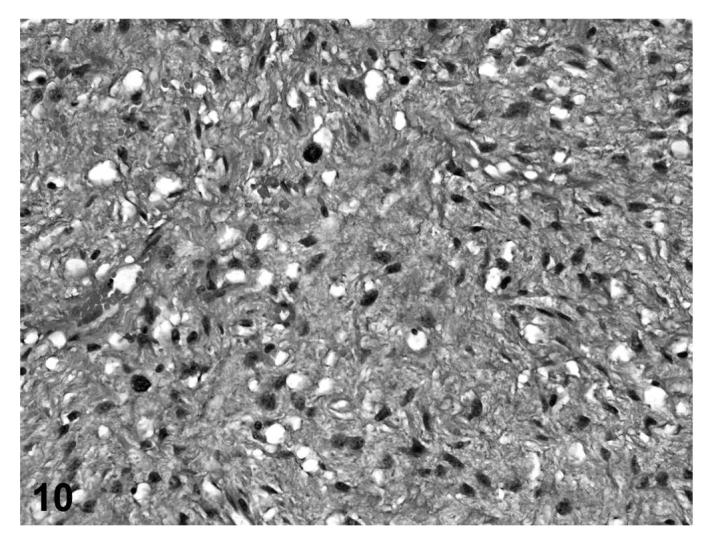
Fibrosarcoma, proliferating undifferentiated fibroblasts with vacuolization, skin, treated rat, H&E, 40×.
FIGURE 11.
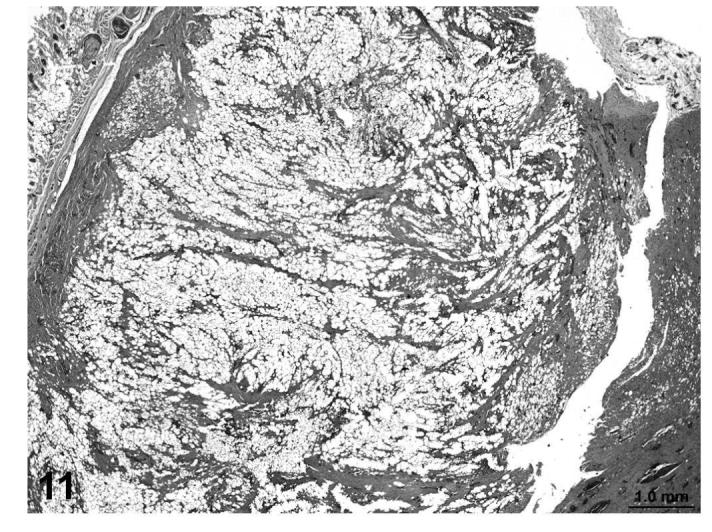
Liposarcoma, lipomatous mass dissected by bands of proliferating fibrous tissue and well-differentiated collagen bundles, skin, treated rat, H&E, 2×.
FIGURE 12.
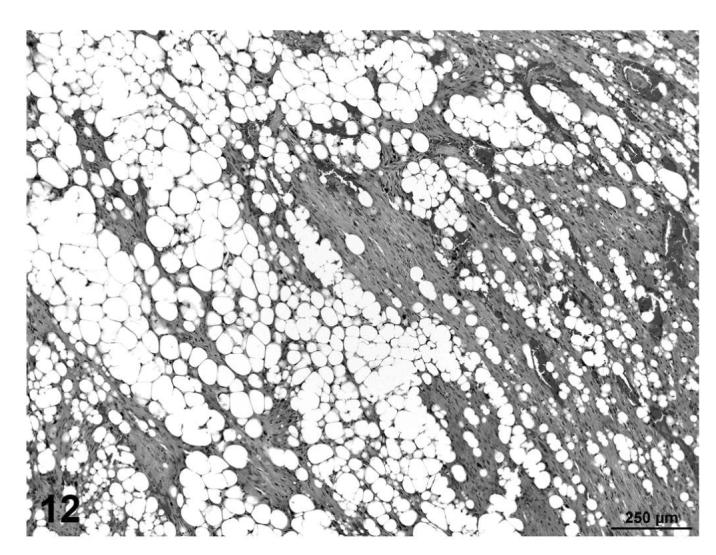
Liposarcoma, lipomatous mass dissected by bands of proliferating fibrous tissue and well-differentiated collagen bundles, skin, treated rat, H&E, 10×.
Neither immunohistochemistry (IHC) for desmin and S-100 nor histochemistry for intratumoral collagen was informative for distinguishing fibrosarcomas from liposarcomas (see Table 1). Sixteen fibrosarcomas and 12 liposarcomas were histochemically stained for collagen (trichrome stain) and immunohistochemically for desmin and/or S-100. All 28 neoplasms (100%) contained a minimal to moderate amount of collagen. Cytoplasmic desmin, a marker of muscle differentiation, was found at minimal to moderate levels in 45% (5/11) of the fibrosarcomas and 25% (2/8) of the liposarcomas. S-100, a marker used to distinguish liposarcomas from other sarcomas, was detected in minimal to moderate levels in the cytoplasm of 50% (5/10) of fibrosarcomas and 82% (9/11) of liposarcomas.
TABLE 1.
Results of Staining Procedures
| HESI PPAR Agonist Project Selected Histochemical and Immunohistochemical Stains Mesenchymal Neoplasms in Rats | |||||||
|---|---|---|---|---|---|---|---|
| ID# | Tissue | Histologic Diagnoses | Trichrome | Alcian Blue | Desmin | UCP-1 | S100 |
| 1 | Adipose Tissue | Lipoma | +++ | ++ | |||
| 2 | Adipose Tissue | Liposarcoma | + | Negative | ± | ± | |
| 3 | Adipose Tissue | Liposarcoma | + | +++ | ++ | ± | |
| 18 | Cavity, Abdominal | Liposarcoma | ± | Negative | |||
| 29 | Liver | Sarcoma, NOS | + | ++ | + | ||
| 30 | Lung | Liposarcoma | Negative | Negative | + | ||
| 31 | Muscle, Skeletal | Fibrosarcoma, Pleomorphic Type | + | Negative | Negative | ||
| 32 | Muscle, Skeletal | Liposarcoma | + | Negative | Negative | ||
| 37 | Skin/Subcutaneous Tissue | Fibrosarcoma | + | + | Negative | ||
| 38 | Skin/Subcutaneous Tissue | Fibrosarcoma | + | Negative | ± | ||
| 39 | Skin/Subcutaneous Tissue | Liposarcoma | ++ | Negative | ++ | ||
| 40 | Skin/Subcutaneous Tissue | Fibrosarcoma | + | Negative | Negative | ||
| 41 | Subcutaneous Tissue | Fibrosarcoma | ++ | +++ | |||
| 42 | Skin/Subcutaneous Tissue | Lipoma | Negative | Negative | + | ||
| 43 | Skin/Subcutaneous Tissue | Fibroma | +++ | ++ | |||
| 44 | Skin/Subcutaneous Tissue | Lipoma | Negative | Negative | |||
| 45 | Skin/Subcutaneous Tissue | Liposarcoma | ± | Negative | + | ||
| 46 | Skin/Subcutaneous Tissue | Fibrosarcoma | ± | Negative | Negative | ||
| 47 | Skin/Subcutaneous Tissue | Fibrosarcoma | + | Negative | ± | ||
| 48 | Skin/Subcutaneous Tissue | Fibrosarcoma | + | + | Negative | ||
| 49 | Subcutaneous Tissue | Fibrosarcoma | + | ||||
| 50 | Skin/Subcutaneous Tissue | Liposarcoma | ± | Negative | Negative | ||
| 51 | Subcutaneous Tissue | Fibrosarcoma | + | Negative | Negative | ||
| 52 | Skin/Subcutaneous Tissue | Liposarcoma | ± | Negative | + | ||
| 53 | Skin/Subcutaneous Tissue | Fibrosarcoma | ± | Negative | ± | ||
| 54 | Skin/Subcutaneous Tissue | Fibrosarcoma | + | ± | ± | ||
| 55 | Skin/Subcutaneous Tissue | Liposarcoma | + | Negative | ± | ||
| 56 | Skin/Subcutaneous Tissue | Fibrosarcoma | + | + | ± | ||
| 57 | Skin/Subcutaneous Tissue | Fibrosarcoma | ++ | ± | Negative | ||
| 58 | Skin/Subcutaneous Tissue | Fibrosarcoma | + | ||||
| 68 | Spleen | Liposarcoma | ± | + | + | ||
| 69 | Spleen | Fibrosarcoma | ++ | ||||
| 70 | Thoracic Mass | Liposarcoma | ± | Negative | + | ||
Many of the neoplasms expressed both proteins based on IHC results. The findings suggest that for these sarcomas with multiple features of mesenchymal differentiation (lipid, fibroblastic, and muscle) desmin and S-100 are not specific for either tumor type and these neoplasms are best distinguished by morphology. The Masson’s trichrome demonstrated significant collagen production by the fibroblastic cells in the tumors classified as fibrosarcomas. The PWG did not recommend that these stains be used routinely for the classification of these poorly-differentiated sarcomatous neoplasms.
The final recommendations of the PWG consisted of a slight modification of the nomenclature and diagnostic criteria previously recommended by the STP. The nomenclature and diagnostic criteria recommended by the PWG for the diagnoses of proliferative lesions of the soft tissue (including liposarcoma and fibrosarcoma) are presented as follows:
Recommended Nomenclature and Diagnostic Criteria Soft Tissue
Tumors of Fibrous Tissue
Fibroma
Poorly to moderately cellular well-circumscribed nodules or masses.
Fusiform cells with elongated tapered, hyperchromatic or vesicular nuclei with one or more nucleoli.
Mitotic activity is slight or may be absent.
Dense interwoven bands of collagen present.
Most often occur in the dermis and subcutis.
Fibrosarcoma
Composed of monomorphic spindle shaped cells with oval nuclei and basophilic cytoplasm; often arranged in a characteristic “herringbone” cellular pattern.
Variable mitotic activity, mitotic figures can be numerous.
Variable amounts of collagen interspersed between tumor cells.
Areas of fatty infiltration, “trapped adipocytes” or myxomatous change may be present (observed primarily in tumors in treated rats).
Areas of hemorrhage, necrosis and local invasion can be extensive, but metastatic spread is infrequent.
Fibrosarcoma, Pleomorphic Type (Malignant Fibrous Histiocytoma)
Solid fibrous homogeneous mass, large lesions show ulceration of overlying skin.
Variable histology ranging from storiform pattern of uniform plump spindle cells to a highly pleomorphic pattern of spindle cells and tumor giant cells.
Variable mitotic activity.
Variable amount of interstitial collagen and intercellular ground substance.
Widespread infiltration and invasion of local tissues.
Occasional metastases in lung, liver and other organs.
Tumors of Adipose Tissue
Lipoma
Lipomas are nodular, well-demarcated neoplasms capable of compressing surrounding tissues.
Red-brown discoloration and central necrosis may be present particularly if pedunculated.
Composed of mature fat cells, each typically having a single large fat vacuole.
Devoid of mitotic activity, cellular pleomorphism and necrosis.
Hibernoma
Lobulated pale yellowish mass located most often in the intrascapular intrathoracic (mediastinum) or abdominal fat.
Fairly uniform rounded cells with abundant fine cytoplasmic lipid droplets and central, round nuclei.
Mitotic activity and cellular pleomorphism usually low but may be marked and associated with invasion and metastatic spread (malignant hibernoma/liposarcoma).
Liposarcoma
Characterized by the presence of malignant or primitive fat forming cells, termed lipoblasts.
Other cell types occur including brown fat cells, foam cells, giant cells, myxoid cells, spindle-shaped and stellate cells.
The stroma is usually well vascularized; myxoid or fibrocytic areas can occur and sometimes are extensive.
Undifferentiated cells can also be found and mitotic activity can be prominent.
Rapidly growing liposarcomas frequently have areas of necrosis.
Often with extensive local invasion.
Tumors of Striated Muscle
Rhabdomyosarcoma
Rhabdomyosarcomas usually are highly pleomorphic cellular neoplasms composed of mononuclear, polynuclear, polygonal, or elongated spindle shaped cells.
Spindle shaped cells are often arranged in bundles and have abundant eosinophilic cytoplasm.
Eosinophilic strap-like cells, multinucleated giant cells, and round cells with one or two eccentrically placed nuclei are also observed.
Rhabdomyoblasts characterized by eosinophilic fibrillary cytoplasm with myofilaments, cross striations, immunocytochemical staining for desmin and myogenin and ultrastructural evidence of Z bands.
Mitotic figures are common.
Necrosis and hemorrhage are common.
Infiltrative growth and distant metastases occur.
Tumors of Smooth Muscle
Leiomyoma
Spindle cells resembling smooth muscle myocytes arranged in fascicles with some palisading of nuclei.
Nuclei typically blunt-ended.
Cytoplasm contains longitudinal myofilaments, desmin and smooth muscle (α) actin (SMA) reactivity, and perinuclear vacuoles.
Little or no mitotic activity; pleomorphism not a prominent feature.
Leiomyosarcoma
Similar to leiomyoma except it is invasive, has considerable cellular pleomorphism, and increased mitotic activity.
Necrosis, cystic degeneration, mineralization, and distant metastases are also features.
Miscellaneous Tumors
Malignant Schwannoma
Herringbone to storiform pattern, nuclear palisading, and Verocay bodies (Antoni type A pattern).
Loosely textured, edematous area, foci of degeneration with progress to the formation of small cysts (Antoni type B tissue).
Extension from a peripheral nerve fiber sheath may be present.
Often S-100 positive.
Sarcoma, NOS (Not Otherwise Specified)
Mesenchymal tumor without adequate light microscopic features to permit further classification as a specific tumor type.
Ultrastructural differentiation and specific immunohistochemical staining may provide information to further classify some Sarcoma, NOS.
Hemangiosarcomas in Mice and Hamsters
There were a total of 420 cases submitted from studies conducted in mice; 10 cases were submitted from hamsters. The liver was the most frequent organ submitted, followed by the hemolymphoreticular system (various lymph nodes), skin and subcutaneous tissue, spleen, heart, uterus, and white adipose tissue. All slides submitted from the hamster study were liver sections. Slides submitted from other tissues and organs for mice were fewer in number and included other organs normally examined during a carcinogenicity bioassay. The only tissues that were notably missing were the central and peripheral nervous system.
There were several morphological changes in various organs that were vascular in nature but were considered to represent nonproliferative changes. These were present in tissues from control and treated mice. The most frequent findings involved congestion, hemorrhage and angiectasis (sinusoidal dilatation) in several organs and tissues including lymph nodes, liver, uterus, and white adipose tissue (Figures 13, 14, and 15). Although atrial thrombosis was the most frequent observation in the heart (Figure 16), hemangiosarcomas involving the ventricular myocardium (Figure 17) were present in a few mice.
FIGURE 13.
Congestion and hemorrhage, skin, treated mouse, H&E, 20×.
FIGURE 14.
Congestion and hemorrhage, lymph node, treated mouse, H&E, 25×.
FIGURE 15.
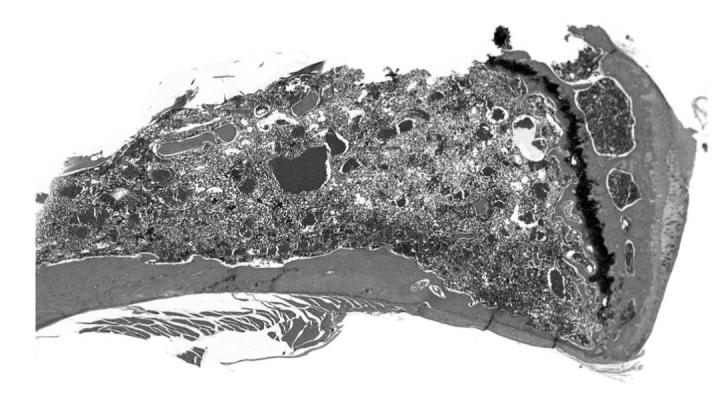
Angiectasis, bone marrow, treated mouse, H&E, 2×.
FIGURE 16.
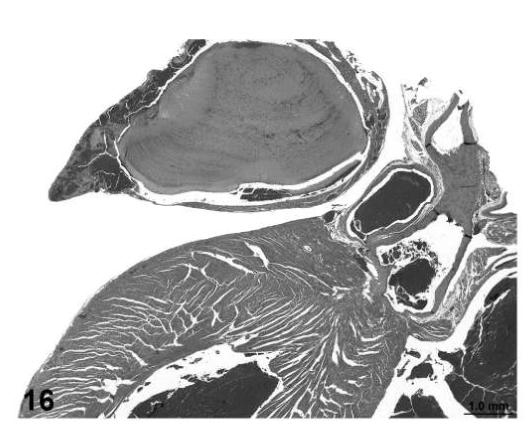
Atrial thrombosis, heart, treated mouse, H&E, 2×.
FIGURE 17.
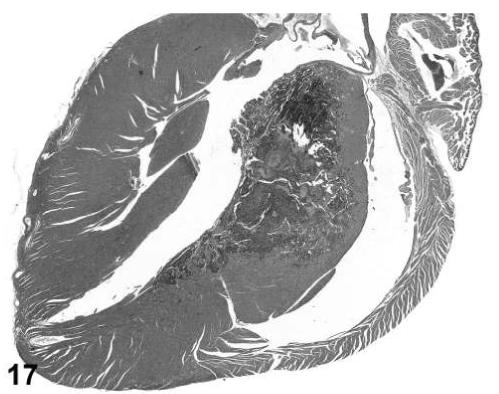
Hemangiosarcoma, ventricular myocardium, heart, treated mouse, H&E, 2×.
Most of the proliferative vascular lesions consisted of benign and malignant endothelial neoplasms which were classified as hemangiomas and hemangiosarcomas by the PWG. With the exception of angiomatous hyperplasia and angiolipoma, the vascular neoplasms were similar in appearance to those described in publications by the STP (Ruben, et al., 1997; Elwell, et al., 2004) and the International Agency for Research on Cancer (IARC) (Ernst et al., 2001). The criteria and nomenclature presented in these publications have been widely reviewed and are generally accepted and used by toxicologic pathologists internationally. It was the opinion of the PWG panel members that similar, slightly modified, diagnostic criteria and nomenclature should be applied to studies conducted with PPAR agonists. A summary of the nomenclature and diagnostic criteria included in these publications as modified by the PWG is presented as follows:
Recommended Nomenclature and Diagnostic Criteria Proliferative and Non-Proliferative Lesions in the Heart and Vasculature
Congestion
Increased prominence (number) of erythrocytes in the capillary bed or larger vessels of an organ.
No appreciable distention (angiectasis) of the vessel lumen.
Diagnosis often correlates with gross observation (e.g., reddened, darkened focus).
Angiectasis
Dilatation (distention) of endothelial-lined channels.
Angiomatous Hyperplasia
Focal, well demarcated, prominent lesion with increased number of capillaries and other vascular structures.
Vascular spaces are usually blood-filled, round to oval in shape, closely spaced, and uniform in size.
Absence of supporting tissue.
Absence of mitotic figures and nuclear atypia.
Angiolipoma
Generally well-circumscribed nodular masses composed of well-differentiated lipocytes with a prominent vascular component.
Prominent pale eosinophilic interstitial matrix often present.
Variability in size of adipocytes.
Vascular structures consisting of irregular-shaped aggregates of vessels often located near periphery of mass but may be located throughout the mass.
Hemangioma
Focal, non-invasive expansion of capillary or sinusoidal structures.
Variably-sized cavernous spaces are lined by a single layer of well-differentiated endothelium.
Endothelial nuclei increased in number and may be slightly enlarged; mitoses are infrequent.
These lesions usually compress surrounding tissue but they are rarely encapsulated.
Focal thrombosis within vascular spaces can occur.
Cavernous hemangiomas are composed principally of large vascular channels surrounded by collagenous stroma and frequently accompanied by a scattering of hemosiderin-laden macrophages.
The capillary hemangioma is composed of a network of capillary-sized vessels surrounded by thickened reticular and/or collagenous fibers.
Hemangiosarcoma
An increase in the number of endothelial cells, many of which have pleomorphic, enlarged, hyperchromatic nuclei. Mitotic figures are usually present.
Discrete to irregular expansive mass with local invasion by vascular structures.
Solid areas with collapsed vascular channels and areas with poorly formed, irregular-shaped vascular spaces are common.
Endothelial cells may be multilayered or form clusters.
Metastases may occur.
Histologic appearance may be similar to hemangiomas although endothelial cells are more prominent and atypical ranging from flat to rounded polygonal or extremely irregular cells with single, multiple, irregular, or lobulated nuclei.
May arise in vessels within angiolipoma.
There were two proliferative vascular lesions diagnosed by the PWG in treated mice that in their experience were considered relatively unique and not expected to occur in untreated control mice. These were angiomatous hyperplasia and angiolipoma. Angiomatous hyperplasia consisted of a focal, well demarcated, prominent increase in the number of capillaries or other vascular channels that were lined by relatively normal appearing endothelial cells. This was most often diagnosed in the subcutaneous adipose tissue, but infrequently located in other tissues.
Angiolipomas consisted of subcutaneous masses that were generally well-circumscribed and were composed of well-differentiated, variably-sized, adipocytes with a prominent vascular component (Figures 18 and 19). In a few tumors that would have otherwise been classified as angiolipoma, regions of the vascular component appeared to be malignant. These neoplasms were diagnosed as hemangiosarcomas with a comment that they were arising in an angiolipoma (Figures 20 and 21). Other neoplasms diagnosed as hemangioma (Figures 22 and 23) and hemangiosarcoma (Figures 24 and 25) were morphologically similar in control and treated mice.
FIGURE 18.
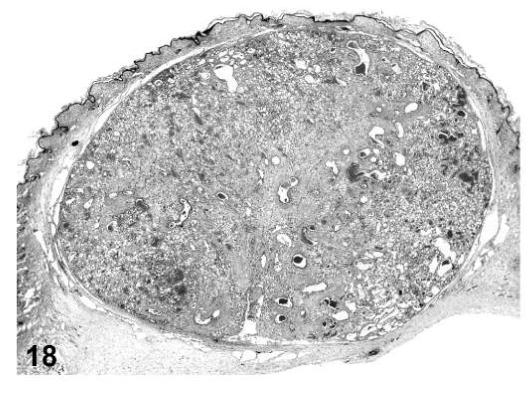
Angiolipoma, skin, treated mouse, H&E, 2.5×.
FIGURE 19.
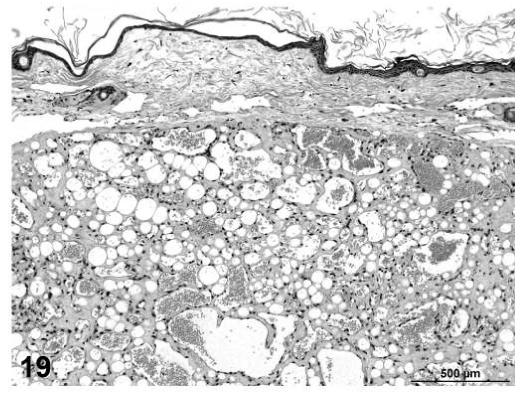
Angiolipoma, skin, treated mouse, H&E, 16×.
FIGURE 20.
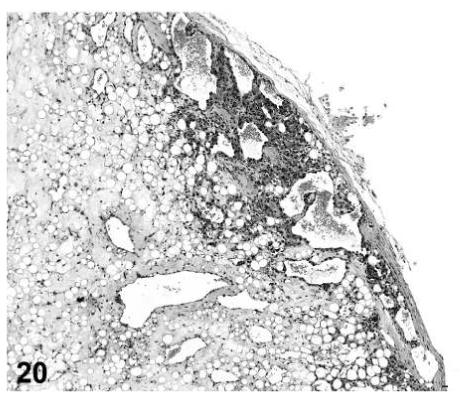
Hemangiosarcoma arising in an angiolipoma, skin, treated mouse, H&E, 10×.
FIGURE 21.
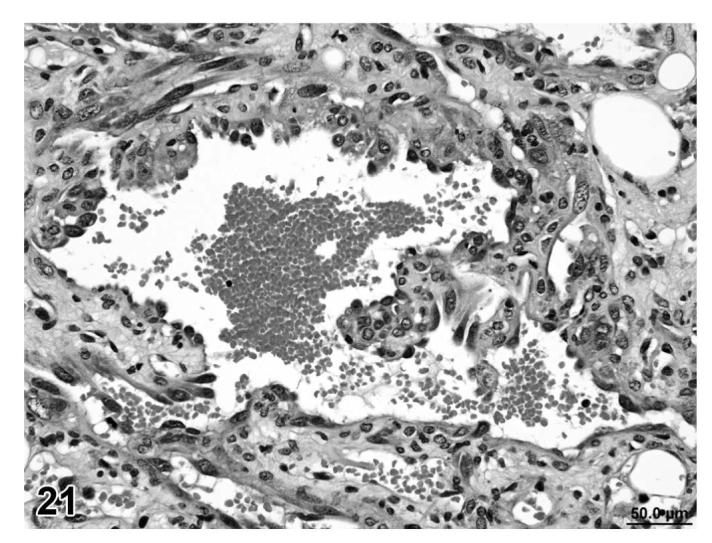
Hemangiosarcoma arising in angiolipoma, skin, treated mouse, H&E, 40×.
FIGURE 22.
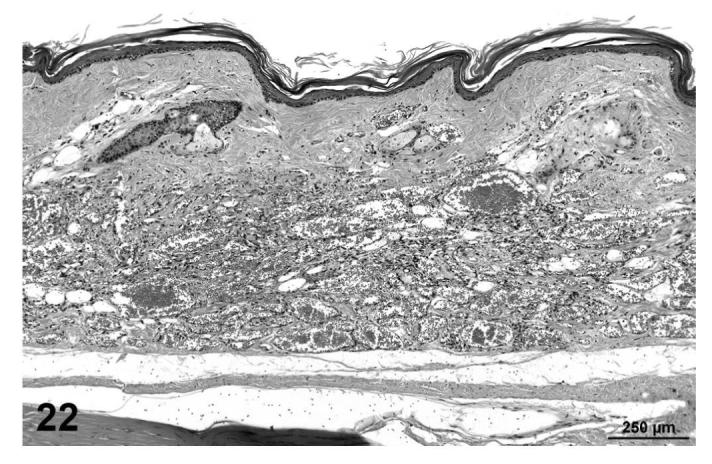
Hemangioma, skin of tail, treated mouse, H&E, 10×.
FIGURE 23.
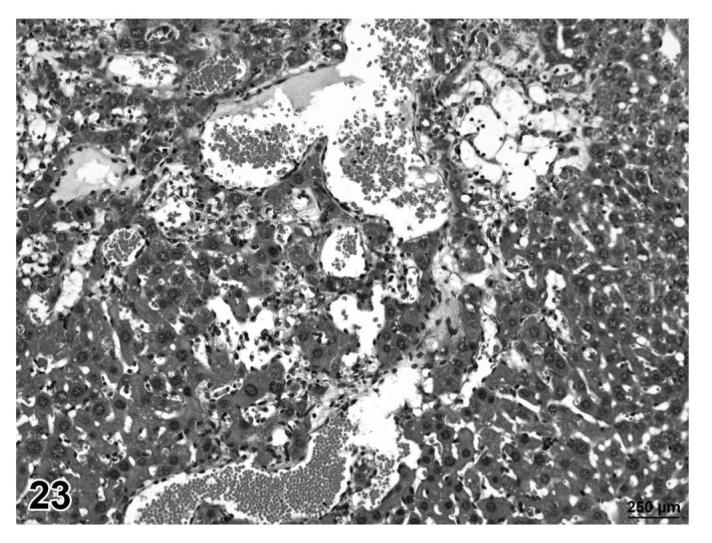
Hemangioma, liver, treated mouse, H&E, 25×.
FIGURE 24.
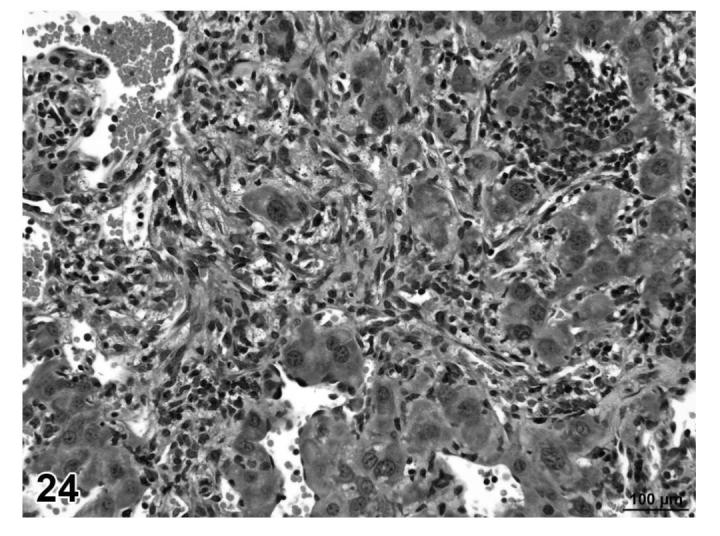
Hemangiosarcoma, liver, treated mouse, H&E, 40×.
FIGURE 25.
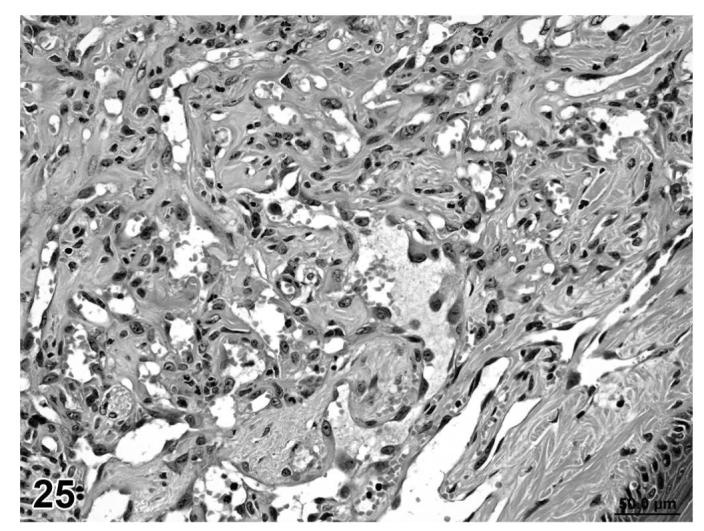
Hemangiosarcoma, skin of tail, treated mouse, H&E, 40×.
Discussion
The 2-year carcinogenesis bioassay in rodents has been routinely used to assess the carcinogenic activity of drugs as part of the hazard identification process in drug development. Results from these bioassays, in combination with other data, are used by toxicologists, statisticians, and regulatory authorities to assess the potential risk for human exposure. Over the last 25 years, there has been a concerted effort for editors and authors to publish textbooks describing the spontaneous and induced diseases and lesions in laboratory animals. Many of these have specifically focused on various species and strains of rats, mice, and hamsters that are commonly used in chronic toxicity/carcinogenicity bioassays conducted in rodents (Greaves and Faccini, 1984; Boorman et al., 1990; Faccini et al., 1990; Turusov and Mohr, 1996; Jones et al., 1989; Jones et al., 1991; Mohr et al., 1992a, 1992b, 1996a, 1996b; Maronpot et al., 1999; Greaves, 2000). The international Societies of Toxicologic Pathology in the United States, Great Britain, Japan, and Europe have provided a concerted effort to establish standardized nomenclature and diagnostic criteria that are internationally harmonized.
The resulting standardized nomenclature and diagnostic criteria have been developed for each organ system by committees composed of toxicologic pathologists from each of the societies and reviewed by the entire membership of these groups prior to being finalized and published for general application by the scientific community. These have been published as a series of guides for each organ system by the Society of Toxicologic Pathology and those that were prepared for the rat have recently been made available to the public on the STP website (http://www.toxpath.org/ssndc.asp). Similar internationally harmonized nomenclature and diagnostic criteria were published by WHO/IARC in the International Classification of Rodent Tumors, Part 1, The Rat (Mohr, ed., 1992-1997) and Part 2, The Mouse (Mohr, ed., 2001). An extended version of both publications is available via Internet: http://www.goRENI.org to pathologists and colleagues from regulatory agencies world-wide. goRENI (“Global Open RENI”) forms the discussion platform for the INHAND project (“International Harmonization of Nomenclature and Diagnostic Criteria for Lesions in Rats and Mice”). Access is provided free after registration. goRENI includes the (updated) diagnostic criteria (as published by WHO/IARC) additional histopathological images, links from literature references to Medline abstracts, and other useful information for toxicologic pathologists.
Among these classifications, those for proliferative lesions of the mesenchymal tissues and blood vessels have been based on spontaneous lesions in rodents using conventionally stained slides only. However, based on slide examination from this review and previous experience of the panel members, the PWG concluded that with only some minor modifications and expanded morphologic criteria, the previous classifications were applicable to the range of these treatment-related proliferative lesions in rodeuts from studies with the PPAR agonists. The minor modifications addressed variation in collagen and fibrosarcomatous regions in liposarcomas in rats; occurrence of angiolipomas and hemangiosarcomas arising from angiolipomas in mice; regional occurrence of cytoplasmic vacuoles (vacuolization) in some fibrosarcomas in rats; and the occurrence of angiomatous hyperplasia in mice.
In addition to examining routinely stained H&E stained slides, several histochemical and immunohistochemical stains were also evaluated by the PWG for their utility in establishing the histogenesis of these neoplasms. Histochemical stains included Masson’s trichrome (muscle and collagen), PAS-alcian blue (mucopolysaccharides), and Mallory’s PTAH method (muscle) (Coolidge and Howard, 1979; Sheehan and Hrapchak, 1980). Immunohistochemical stains have a well-established place in the diagnosis of human mesenchymal tumors and have also been used in experimental animals to identify specific markers that might assist in establishing the histogenesis of a tumor cell type. However, in experimental models these stains have met with mixed results due to cross reactivity and inconsistent or weak expression of these markers as tumor cells become more undifferentiated.
S-100 protein has been reported as a useful marker in the diagnosis of some tumors based on the presence of this antigen in a restricted number of definite cell types (Cocchia, et al., 1983). Tumors of cartilaginous or white adipose tissue origin stain positive for S-100, while other tumors of mesenchymal origin such as fibrosarcoma and malignant fibrous histiocytoma typically have an absence of S-100 (Cocchia et al., 1983; Hashimoto et al., 1984). S-100 protein has also been reported both in stimulated and inactive brown adipose tissue and it is expressed to a much greater degree in inactive cells.
When brown adipose tissue is active, most of the cells are multilocular and have large mitochondria containing numerous cristae rich in mitochondrial uncoupling protein. In inactive brown adipose tissue found in warm-acclimatized animals, most of the cells are pauci- or unilocular, very similar to white adipose tissue. The multilocular cells are always negative for S-100, whereas the pauci- or unilocular cells were always S-100 positive (Barbatelli, et al., 1993). The mitochondrial uncoupling protein (UCP-1) is a protein essential for the thermogenic properties of brown fat in mammals (Kozak et al., 1988). A polyclonal antibody has been developed that can be used to react with mouse, rat, and human UCP-1 expressed in brown adipose tissue. This immunohistochemical procedure is used for the expression of UCP-1 in tumors suspected to be of brown fat origin. There were too few UCP-1 results in this study to make any conclusions.
In view of these reports (immunohistochemistry), these procedures were also assessed by the PWG. However, it was concluded based on these immunocytochemical stains evaluated by the PWG that they added little to the basic categorization of mesenchymal neoplasms associated with the administration of PPAR agonists. Other immunohistochemical procedures not assessed by the PWG have been used to attempt to determine the histogenesis of mesenchymal neoplasms with varying results. These include vimentin, desmin, cytokeratin (AE1 and AE3), epithelial membrane antigen (EMA), smooth muscle (α) actin, muscle actin (α and γ), skeletal muscle myosin, lymphoid markers, macrophage-specific antigen, CD34, and CD99 (Brown, 1995; Coindre, 2003). In addition, it has also been reported that immunohistochemical stains for Factor VIII Ra, CD-31, CD-34 or Vascular Endothelial Growth Factor Receptor 2 (VEGF-2) may help in confirming the endothelial origin of the tumor cells.
PPARγ is most abundant in adipose tissue in the rat. PPARγ agonist exposure in rodents has been associated with adipogenesis and a sustained proliferation in the subcutaneous fat regions. There are two potential hypotheses for this sustained proliferation. One hypothesis is that the PPARγ agonist exposure has a direct effect on the adipocyte and the second is that the exposure has an indirect effect mediated by cytokine/hormone production by other cell types (Cohen, 2006).
The most likely mode of action for PPARγ agonists is stimulation of adipogenesis with subsequent release of cytokines and growth factors leading to mesenchymal or endothelial cell proliferation, and eventually mesenchymal neoplasia or hemangiosarcoma. This hypothesis is based on: 1) adipocytes are a rich source of cytokines and growth factors, 2) adipogenesis and angiogenesis are coordinated and closely linked in adipose development, and 3) PPARγ agonists induce hemangiosarcomas primarily in adipose tissue and associated structures (Cohen, 2006). Some of the potential growth factors involved in this process include VEGF, FGF, PDGF, and HGF (Herman et al., 2002). These proangiogenic factors could stimulate angiogenesis and endothelial cell proliferation and could potentially also have other vascular effects locally or at distant sites in the body. Hemangiosarcoma induction is generally species selective (mouse and hamster), with mice being predisposed to spontaneous development of hemangiosarcoma.
Although these factors may also be involved in mesenchymal sarcoma development observed in rats, the identification of the exact line of differentiation of the neoplastic cell type is often unclear, even though the distinction between benign neoplasms and malignant neoplasms is rarely a problem. By contrast, the histogenesis of proliferative vascular lesions is usually not questionable; however, the distinction between nonneoplastic proliferative changes and benign neoplasia, or differentiating benign neoplasms and malignant neoplasms of vascular origin is often a challenge for the toxicologic pathologist. Several authors have published information that may be helpful in differentiating nonneoplastic and neoplastic proliferative vascular lesions and aiding in the distinction between benign and malignant neoplasms of vascular origin in mice (Frith and Wiley, 1982; Booth and Sunberg, 1995; Hanahan and Folkman, 1996; Duddy, et al., 1999a; Duddy, et al., 1999b; O’Hara and Nascimento, 2003).
The nomenclature and diagnostic criteria recommended by the PWG for soft tissue tumors of mesenchymal origin in rats and proliferative vascular lesions in mice and hamsters do not include every proliferative change that might be encountered during the evaluation of tissue from the 2-year carcinogenesis studies being conducted with PPAR agonists. However, they should provide a sound basis for the consistent reporting of study results in a manner that allows the scientific community to use this information for both regulatory submissions and for future investigations that may elucidate a mode of action for this class of drugs as it relates to their carcinogenic potential and activity. Use of these criteria does not require the application of special immunocytochemical techniques.
The figures presented in this manuscript included only selected photomicrographs used to illustrate the lesions being described and were printed in black and white. A more extensive list of lesions illustrating the range of changes examined during the PWG review are presented in color in the interactive CD-ROM included as an insert in the back cover of this issue of the Journal of Toxicologic Pathology.
FIGURE 4.
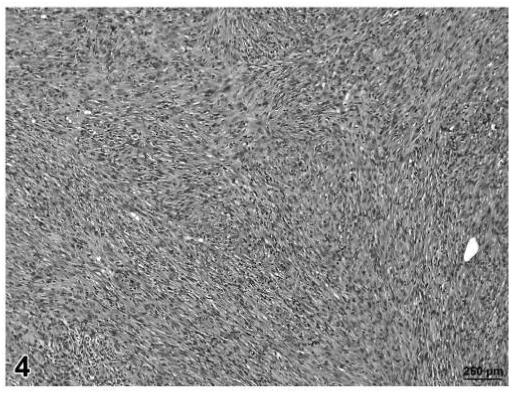
Fibrosarcoma, skin/subcutaneous tissue, control rat, H&E, 12.5×.
Acknowledgments
The work described in this manuscript was supported by the PPAR Agonist Project Committee of the ILSI Health and Environmental Sciences Institute (HESI). The authors are solely responsible for the contents of this paper. All of the authors gratefully acknowledge Emily Singletary for photographic support, David Sabio for computer graphics support and Ann Marie Hauck for technical and clerical assistance during the entire project.
References
- Barbatelli G, Morroni M, Vinesi P, Cinti S, Michetti F. S-100 protein in rat brown adipose tissue tissue under different functional conditions: a morphological, immunocytochemical, and immunochemical study. Exper Cell Res. 1993;208:226–31. doi: 10.1006/excr.1993.1241. [DOI] [PubMed] [Google Scholar]
- Boorman GA, Eustis SL, Elwell MR, Montgomery CA, MacKenzie WF, editors. Pathology of the Fischer rat, reference and atlas. Academic Press, Inc.; San Diego, California: 1990. [Google Scholar]
- Booth CJ, Sundberg JP. Hemangiomas and hemangiosarcomas in inbred laboratory mice. Lab Anim Sci. 1995;45(5):497–502. [PubMed] [Google Scholar]
- Brown MP. The identification of subcutaneous tumors related to grouphousing in male B6C3F1 mice; Presented at the 2nd International Conference of the IFSTP; Tours, France. 1995. [Google Scholar]
- Cocchia D, Lauriola L, Stolfi VM, Tallini G, Mitchetti F. S-100 antigen labels neoplastic cells in liposarcoma and cartilaginous tumors. Virchows Arch. 1983;402:139–45. doi: 10.1007/BF00695055. Pathol Anat. [DOI] [PubMed] [Google Scholar]
- Cohen S. PPAR agonists and carcinogenesis; Presentation at July 2006 JSOT Meeting; Nagoya, Japan. 2006. [Google Scholar]
- Coindre JM. Immunohistochemistry in the diagnosis of soft tissue tumors. Histopathology. 2003;43:1–16. doi: 10.1046/j.1365-2559.2003.01639.x. [DOI] [PubMed] [Google Scholar]
- Coolidge BS, Howard RM. Animal histology procedures. Pathology Technology Section, Laboratory of Pathology; 1979. NIH Publication No. 80-275. [Google Scholar]
- Duddy SK, Gorospe SM, Bleavins MR, de la Iglesia FA. Spontaneous and thiazolidinedione-induced B6C3F1 mouse hemangiosarcomas exhibit low ras oncogene mutation frequencies. Toxicol Appl Pharmacol. 1999a;16:133–140. doi: 10.1006/taap.1999.8763. [DOI] [PubMed] [Google Scholar]
- Duddy SK, Parker RF, Bleavins MR, Gough AW, Rowse PE, Gorospe S, Dethloff LA, de la Iglesia FA. p53 is not inactivated in B6C3F1 mouse vascular tumors arising spontaneously or associated with long-term administration of the thiazolidinedione troglitazone. Toxicol Appl Pharmacol. 1999b;156:106–12. doi: 10.1006/taap.1999.8631. [DOI] [PubMed] [Google Scholar]
- El-Hage J. Preclinical and clinical safety assessments for PPAR agonists; Presentation at 2005 Winter ToxForum Meeting; Washington, DC. 2005; www.fda.gov/cder/present/DIA2004/Elhage.ppt. [Google Scholar]
- Elwell MR, Mahler JF, Ruecker FA. MCV-1. n: Guides for Toxicologic Pathology. STP/ARP/AFIP; Washington, DC: 2004. Proliferative and non-proliferative lesions in the heart and vasculature in mice. [Google Scholar]
- Ernst H, Carlton WW, Courtney C, Rinke M, Greaves P, Isaacs KR, Krinke G, Konishi Y, Mesfin GM, Sandusky G. Soft tissue and skeletal muscle. In: Mohr U, Capen CC, Dungworth DL, Greaves P, Hardisty JF, Hayashi Y, Ito N, Long PH, Krinke G, editors. International classification of rodent tumors. The mouse. Springer; Berlin, Heidelberg, New York: 2001. pp. 361–88. [Google Scholar]
- Faccini JM, Abbott DP, Paulus GJJ. Mouse histopathology: a glossary for use in toxicity and carcinogenicity studies. Elsevier; Amsterdam, The Netherlands: 1990. [Google Scholar]
- Frith CH, Wiley L. Spontaneous hepatocellular neoplasms and hepatic hemangiosarcomas in several strains of mice. Lab Animal Sci. 1982;32(2):157–62. [PubMed] [Google Scholar]
- Greaves P. Histopathology of preclinical toxicity studies, interpretation and relevance in drug safety evaluation. Second Edition Elsevier; Amsterdam, The Netherlands: 2000. [Google Scholar]
- Greaves P, Faccini JM. Rat histopathology: a glossary for use in toxicity and carcinogenicity studies. Elsevier; Amsterdam, The Netherlands: 1984. [Google Scholar]
- Greaves P, Faccini JM, Courtney CL. Guides for Toxicologic Pathology. STP/ARP/AFIP; Washington, DC: 1992. Proliferative lesions in soft tissues in rats, MST-1. [Google Scholar]
- Greaves P, Carlton WW, Courtney CL, Ernst H, Halm S, Isaacs KR, Konishi Y, Krinke GJ, Mesfin GM, Rinke M, Sandusky G. Guides for Toxicologic Pathology. STP/ARP/AFIP; Washington, DC: 2004. Proliferative and non-proliferative lesions of soft tissues and skeletal muscle in mice. MST-1. [Google Scholar]
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–64. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Daimaru Y, Enjoji M. S-100 protein distribution in liposarcoma an immunoperoxidase study with special reference to the distinction of liposarcoma from myxoid malignant fibrous histiocytoma. Virchows Arch. 1984;405:1–10. doi: 10.1007/BF00694921. Pathol Anat. [DOI] [PubMed] [Google Scholar]
- Herman JR, Dethloff LA, McGuire EJ, Parker RF, Walsh KM, Gough AW, Masuda H, de la Iglesia FA. Rodent carcinogenicity with the thiazolidinedione antidiabetic agent troglitazone. Toxicol Sci. 2002;68(1):226–36. doi: 10.1093/toxsci/68.1.226. [DOI] [PubMed] [Google Scholar]
- Jones TC, Mohr U, Hunt RD, editors. Monographs on pathology of laboratory animals: integument and mammary glands. Springer-Verlag; Heidelberg, Germany: 1989. [Google Scholar]
- Jones TC, Mohr U, Hunt RD, editors. Monographs on pathology of laboratory animals: cardiovascular and musculoskeletal systems. Springer-Verlag; Heidelberg, Germany: 1991. [Google Scholar]
- Klaunig JE, Babich MA, Baetcke KP, Cook JC, Corton JC, David RM, DeLuca JG, Lai DY, McKee RH, Peter JM, Roberts RA, Fenner-Crisp PA. PPAR agonist-induced rodent tumors: modes of action and human relevance. Crit Rev Toxicol. 2003;33(6):655–780. doi: 10.1080/713608372. [DOI] [PubMed] [Google Scholar]
- Kozak LP, Britton JH, Kozak UC, Wells JM. The mitochondrial uncoupling protein gene. Correlation of exon structure to transmembrane domains. J Biol Chem. 1988;263(25):12274–7. [PubMed] [Google Scholar]
- Maronpot RR, Boorman GA, Gaul BW. Pathology of the mouse. Cache River Press; Vienna, Illinois: 1999. [Google Scholar]
- Mohr U, editor. International classification of rodent tumors: The mouse. Springer-Verlag; Heidelberg, Germany: 2001. [Google Scholar]
- Mohr U, Dungworth DL, Capen CC, editors. Pathobiology of the aging rat, volume I: blood and lymphoid, respiratory, urinary, cardiovascular, and reproductive systems. ILSI Press; Washington, DC: 1992a. [Google Scholar]
- Mohr U, Dungworth DL, Capen CC, editors. Pathobiology of the aging rat, volume II: nervous system and special sense organs, endocrine system, digestive system, integumentary system and mammary gland, musculoskeletal and soft tissue, and general aspects. ILSI Press; Washington, DC: 1992b. [Google Scholar]
- Mohr U, Dungworth DL, Capen CC, Carlton WW, Sunberg JP, Ward JM, editors. Pathobiology of the aging mouse, volume I: general aspects, endocrine system, blood and lymphoid system, respiratory system, urinary system, cardiovascular system, and reproductive system. ILSI Press; Washington, DC: 1996a. [Google Scholar]
- Mohr U, Dungworth DL, Capen CC, Carlton WW, Sunberg JP, Ward JM, editors. Pathobiology of the aging rat, volume II: nervous system, special senses; eye, special senses: ear, digestive system, integumentary system and mammary gland, musculoskeletal system. ILSI Press; Washington, DC: 1996b. [Google Scholar]
- O’Hara CD, Nascimento AG. Endothelial lesions of soft tissues: a review of reactive and neoplastic entities with emphasis on low-grade malignant (“borderline”) vascular tumors. Adv Anatom Pathol. 2003;10(2):69–87. doi: 10.1097/00125480-200303000-00002. [DOI] [PubMed] [Google Scholar]
- Ruben Z, Arceo RJ, Bishop SP, Elwell MR, Kerns WD, Mesfin GM, Sandusky GE, Van Vleet JF. Guides for Toxicologic Pathology. STP/ARP/AFIP; Washington, DC: 1997. Proliferative lesions of the heart and vasculature in rats, CV-1. [Google Scholar]
- Sheehan DC, Hrapchak BB. Theory and practice of histotechnology. The C.V. Mosby Company; St. Louis.: 1980. [Google Scholar]
- Turusov V, Mohr U, editors. Pathology of tumors in laboratory animals, volume 3 - tumors of the hamster. second edition. Lyon, France: 1996. IARC Scientific Publications, No. 126. [Google Scholar]
- Yki-Järvinen H. Thiazolidinediones. New Engl J Med. 2004;351:1106–18. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]


