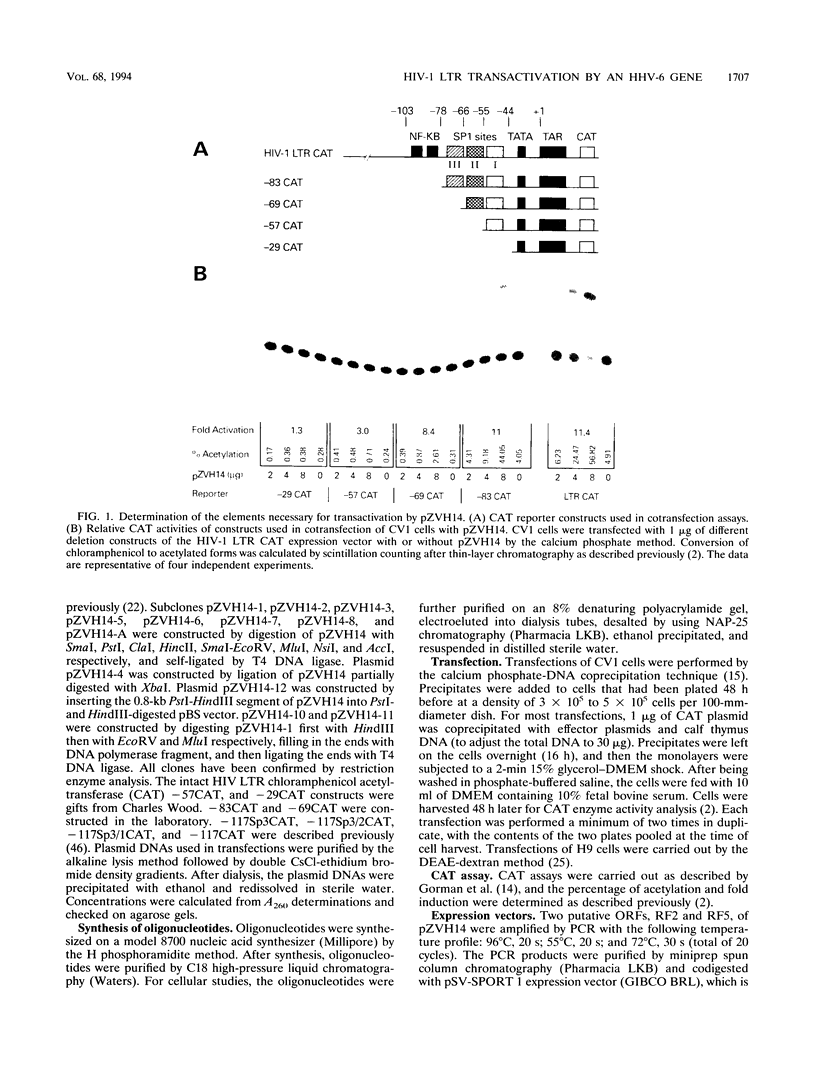
Abstract
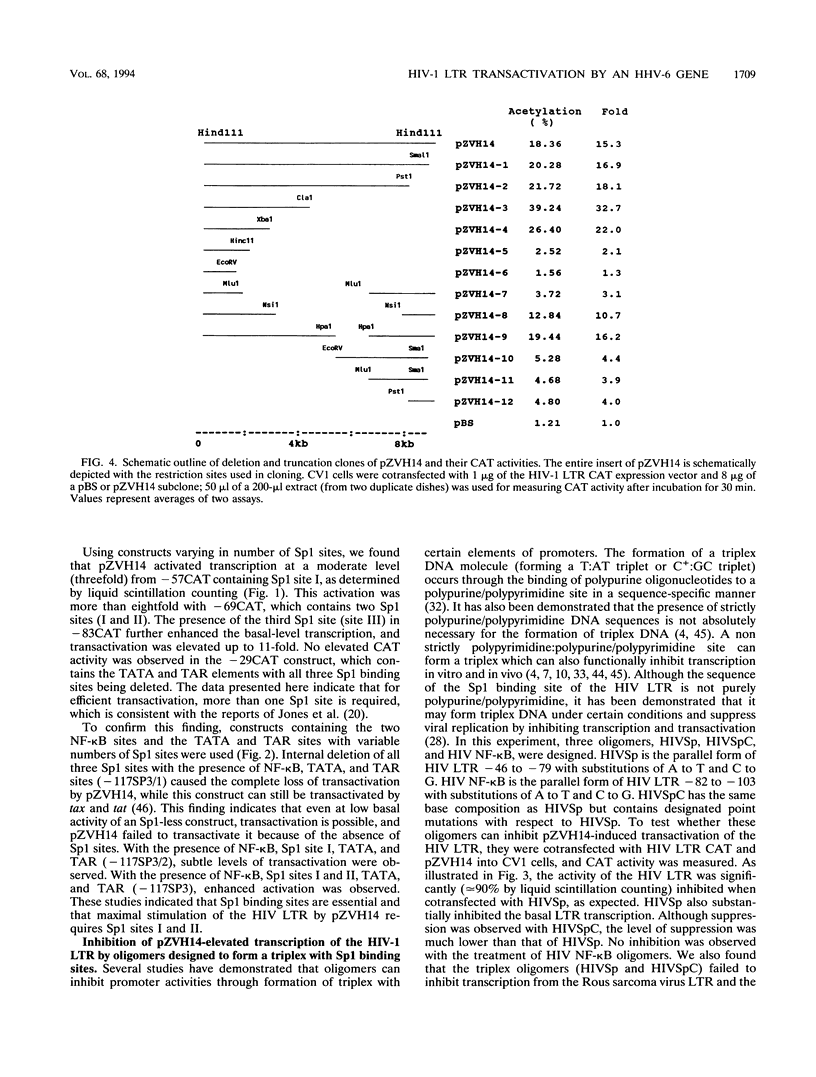
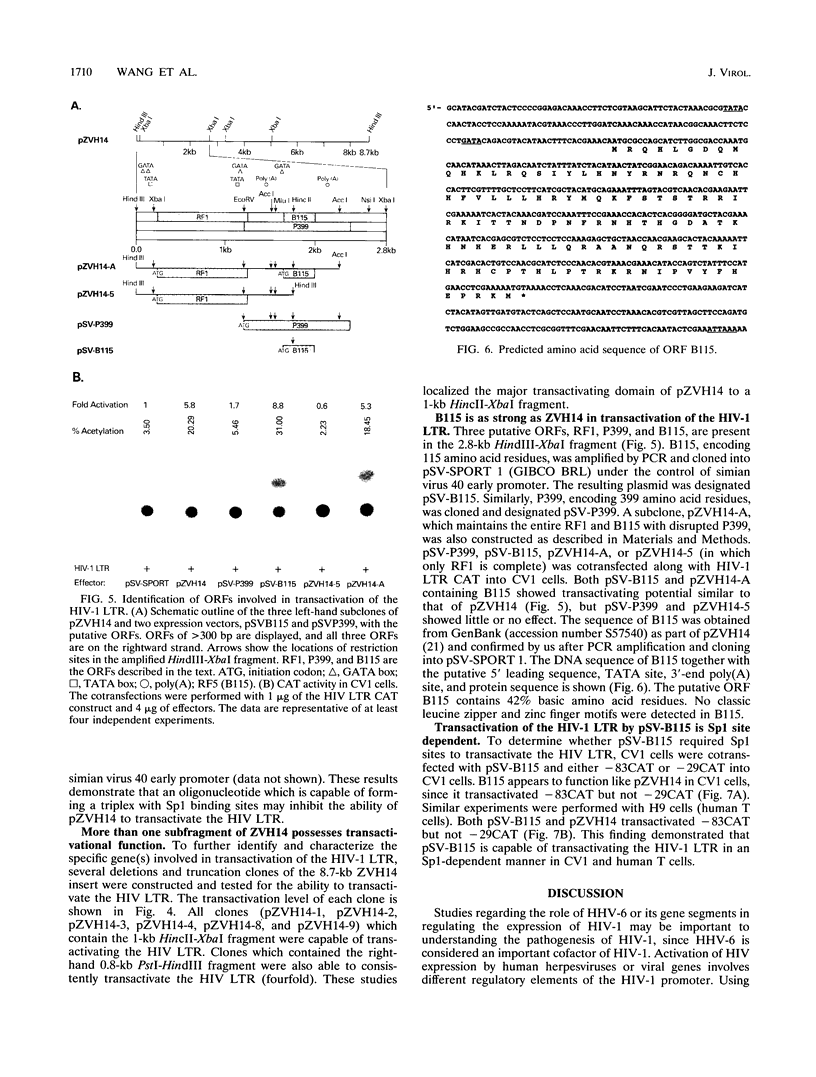
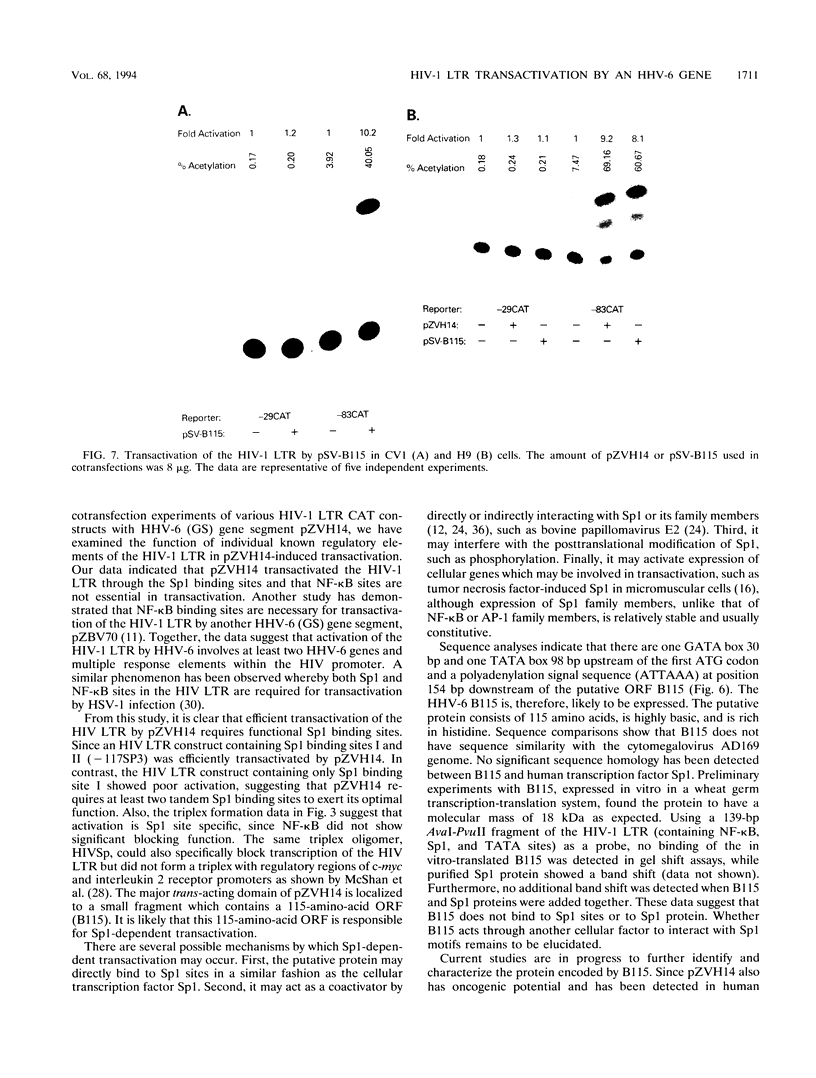
The human immunodeficiency virus type 1 (HIV-1) long terminal repeat (LTR) is transactivated by various extracellular signals and viral cofactors that include human herpesviruses. These transactivators are capable of transactivating the HIV-1 LTR through the transactivation response element, NF-kappa B, or other regulatory binding elements. Human herpesvirus 6 (HHV-6) is a potential cofactor of HIV-1. Here, we report that an HHV-6 gene segment, ZVH14, which can neoplastically transform NIH 3T3 and human keratinocytes, is capable of transactivating HIV-1 LTR chloramphenicol acetyltransferase constructs in an Sp1 binding site-dependent manner. Transactivation increased synergistically in the presence of multiple Sp1 sites and was dramatically reduced by cotransfection with oligomers designed to form triplex structures with HIV-1 LTR Sp1 binding sites. HIV-1 LTR NF-kappa B sites were not essential for ZVH14-mediated transactivation. A putative open reading frame in ZVH14, B115, which may encode a highly basic peptide consisting of 115 amino acid residues, showed transactivation capacity similar to that of ZVH14. This open reading frame also transactivated the HIV-1 LTR in an Sp1 site-dependent fashion in African green monkey kidney cells and human T cells. These data suggest that HHV-6 may stimulate HIV-1 replication via transactivation of Sp1 binding sites present in the HIV-1 promoter.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano Y., Yoshikawa T., Suga S., Yazaki T., Hata T., Nagai T., Kajita Y., Ozaki T., Yoshida S. Viremia and neutralizing antibody response in infants with exanthem subitum. J Pediatr. 1989 Apr;114(4 Pt 1):535–539. doi: 10.1016/s0022-3476(89)80689-4. [DOI] [PubMed] [Google Scholar]
- Barry P. A., Pratt-Lowe E., Peterlin B. M., Luciw P. A. Cytomegalovirus activates transcription directed by the long terminal repeat of human immunodeficiency virus type 1. J Virol. 1990 Jun;64(6):2932–2940. doi: 10.1128/jvi.64.6.2932-2940.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal P. A., Dervan P. B. Second structural motif for recognition of DNA by oligonucleotide-directed triple-helix formation. Science. 1991 Mar 15;251(4999):1360–1363. doi: 10.1126/science.2003222. [DOI] [PubMed] [Google Scholar]
- Biegalke B. J., Geballe A. P. Sequence requirements for activation of the HIV-1 LTR by human cytomegalovirus. Virology. 1991 Jul;183(1):381–385. doi: 10.1016/0042-6822(91)90151-z. [DOI] [PubMed] [Google Scholar]
- Carrigan D. R., Knox K. K., Tapper M. A. Suppression of human immunodeficiency virus type 1 replication by human herpesvirus-6. J Infect Dis. 1990 Oct;162(4):844–851. doi: 10.1093/infdis/162.4.844. [DOI] [PubMed] [Google Scholar]
- Cooney M., Czernuszewicz G., Postel E. H., Flint S. J., Hogan M. E. Site-specific oligonucleotide binding represses transcription of the human c-myc gene in vitro. Science. 1988 Jul 22;241(4864):456–459. doi: 10.1126/science.3293213. [DOI] [PubMed] [Google Scholar]
- Davis M. G., Kenney S. C., Kamine J., Pagano J. S., Huang E. S. Immediate-early gene region of human cytomegalovirus trans-activates the promoter of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8642–8646. doi: 10.1073/pnas.84.23.8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensoli B., Lusso P., Schachter F., Josephs S. F., Rappaport J., Negro F., Gallo R. C., Wong-Staal F. Human herpes virus-6 increases HIV-1 expression in co-infected T cells via nuclear factors binding to the HIV-1 enhancer. EMBO J. 1989 Oct;8(10):3019–3027. doi: 10.1002/j.1460-2075.1989.tb08452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee J. E., Blume S., Snyder R. C., Ray R., Miller D. M. Triplex formation prevents Sp1 binding to the dihydrofolate reductase promoter. J Biol Chem. 1992 Jun 5;267(16):11163–11167. [PubMed] [Google Scholar]
- Geng Y. Q., Chandran B., Josephs S. F., Wood C. Identification and characterization of a human herpesvirus 6 gene segment that trans activates the human immunodeficiency virus type 1 promoter. J Virol. 1992 Mar;66(3):1564–1570. doi: 10.1128/jvi.66.3.1564-1570.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill G., Tjian R. Eukaryotic coactivators associated with the TATA box binding protein. Curr Opin Genet Dev. 1992 Apr;2(2):236–242. doi: 10.1016/s0959-437x(05)80279-5. [DOI] [PubMed] [Google Scholar]
- Gimble J. M., Duh E., Ostrove J. M., Gendelman H. E., Max E. E., Rabson A. B. Activation of the human immunodeficiency virus long terminal repeat by herpes simplex virus type 1 is associated with induction of a nuclear factor that binds to the NF-kappa B/core enhancer sequence. J Virol. 1988 Nov;62(11):4104–4112. doi: 10.1128/jvi.62.11.4104-4112.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hamanaka R., Kohno K., Seguchi T., Okamura K., Morimoto A., Ono M., Ogata J., Kuwano M. Induction of low density lipoprotein receptor and a transcription factor SP-1 by tumor necrosis factor in human microvascular endothelial cells. J Biol Chem. 1992 Jul 5;267(19):13160–13165. [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Methods Enzymol. 1983;100:333–342. doi: 10.1016/0076-6879(83)00066-x. [DOI] [PubMed] [Google Scholar]
- Horvat R. T., Wood C., Balachandran N. Transactivation of human immunodeficiency virus promoter by human herpesvirus 6. J Virol. 1989 Feb;63(2):970–973. doi: 10.1128/jvi.63.2.970-973.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvat R. T., Wood C., Josephs S. F., Balachandran N. Transactivation of the human immunodeficiency virus promoter by human herpesvirus 6 (HHV-6) strains GS and Z-29 in primary human T lymphocytes and identification of transactivating HHV-6(GS) gene fragments. J Virol. 1991 Jun;65(6):2895–2902. doi: 10.1128/jvi.65.6.2895-2902.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. A., Kadonaga J. T., Luciw P. A., Tjian R. Activation of the AIDS retrovirus promoter by the cellular transcription factor, Sp1. Science. 1986 May 9;232(4751):755–759. doi: 10.1126/science.3008338. [DOI] [PubMed] [Google Scholar]
- Josephs S. F., Ablashi D. V., Salahuddin S. Z., Jagodzinski L. L., Wong-Staal F., Gallo R. C. Identification of the human herpesvirus 6 glycoprotein H and putative large tegument protein genes. J Virol. 1991 Oct;65(10):5597–5604. doi: 10.1128/jvi.65.10.5597-5604.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs S. F., Buchbinder A., Streicher H. Z., Ablashi D. V., Salahuddin S. Z., Guo H. G., Wong-Staal F., Cossman J., Raffeld M., Sundeen J. Detection of human B-lymphotropic virus (human herpesvirus 6) sequences in B cell lymphoma tissues of three patients. Leukemia. 1988 Mar;2(3):132–135. [PubMed] [Google Scholar]
- Levy J. A., Landay A., Lennette E. T. Human herpesvirus 6 inhibits human immunodeficiency virus type 1 replication in cell culture. J Clin Microbiol. 1990 Oct;28(10):2362–2364. doi: 10.1128/jcm.28.10.2362-2364.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Knight J. D., Jackson S. P., Tjian R., Botchan M. R. Direct interaction between Sp1 and the BPV enhancer E2 protein mediates synergistic activation of transcription. Cell. 1991 May 3;65(3):493–505. doi: 10.1016/0092-8674(91)90467-d. [DOI] [PubMed] [Google Scholar]
- Lopata M. A., Cleveland D. W., Sollner-Webb B. High level transient expression of a chloramphenicol acetyl transferase gene by DEAE-dextran mediated DNA transfection coupled with a dimethyl sulfoxide or glycerol shock treatment. Nucleic Acids Res. 1984 Jul 25;12(14):5707–5717. doi: 10.1093/nar/12.14.5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusso P., De Maria A., Malnati M., Lori F., DeRocco S. E., Baseler M., Gallo R. C. Induction of CD4 and susceptibility to HIV-1 infection in human CD8+ T lymphocytes by human herpesvirus 6. Nature. 1991 Feb 7;349(6309):533–535. doi: 10.1038/349533a0. [DOI] [PubMed] [Google Scholar]
- Lusso P., Ensoli B., Markham P. D., Ablashi D. V., Salahuddin S. Z., Tschachler E., Wong-Staal F., Gallo R. C. Productive dual infection of human CD4+ T lymphocytes by HIV-1 and HHV-6. Nature. 1989 Jan 26;337(6205):370–373. doi: 10.1038/337370a0. [DOI] [PubMed] [Google Scholar]
- McShan W. M., Rossen R. D., Laughter A. H., Trial J., Kessler D. J., Zendegui J. G., Hogan M. E., Orson F. M. Inhibition of transcription of HIV-1 in infected human cells by oligodeoxynucleotides designed to form DNA triple helices. J Biol Chem. 1992 Mar 15;267(8):5712–5721. [PubMed] [Google Scholar]
- Meyaard L., Otto S. A., Jonker R. R., Mijnster M. J., Keet R. P., Miedema F. Programmed death of T cells in HIV-1 infection. Science. 1992 Jul 10;257(5067):217–219. doi: 10.1126/science.1352911. [DOI] [PubMed] [Google Scholar]
- Mosca J. D., Bednarik D. P., Raj N. B., Rosen C. A., Sodroski J. G., Haseltine W. A., Hayward G. S., Pitha P. M. Activation of human immunodeficiency virus by herpesvirus infection: identification of a region within the long terminal repeat that responds to a trans-acting factor encoded by herpes simplex virus 1. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7408–7412. doi: 10.1073/pnas.84.21.7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosca J. D., Bednarik D. P., Raj N. B., Rosen C. A., Sodroski J. G., Haseltine W. A., Pitha P. M. Herpes simplex virus type-1 can reactivate transcription of latent human immunodeficiency virus. Nature. 1987 Jan 1;325(6099):67–70. doi: 10.1038/325067a0. [DOI] [PubMed] [Google Scholar]
- Moser H. E., Dervan P. B. Sequence-specific cleavage of double helical DNA by triple helix formation. Science. 1987 Oct 30;238(4827):645–650. doi: 10.1126/science.3118463. [DOI] [PubMed] [Google Scholar]
- Orson F. M., Thomas D. W., McShan W. M., Kessler D. J., Hogan M. E. Oligonucleotide inhibition of IL2R alpha mRNA transcription by promoter region collinear triplex formation in lymphocytes. Nucleic Acids Res. 1991 Jun 25;19(12):3435–3441. doi: 10.1093/nar/19.12.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrove J. M., Leonard J., Weck K. E., Rabson A. B., Gendelman H. E. Activation of the human immunodeficiency virus by herpes simplex virus type 1. J Virol. 1987 Dec;61(12):3726–3732. doi: 10.1128/jvi.61.12.3726-3732.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M. How eukaryotic transcriptional activators work. Nature. 1988 Oct 20;335(6192):683–689. doi: 10.1038/335683a0. [DOI] [PubMed] [Google Scholar]
- Razzaque A. Oncogenic potential of human herpesvirus-6 DNA. Oncogene. 1990 Sep;5(9):1365–1370. [PubMed] [Google Scholar]
- Razzaque A., Williams O., Wang J., Rhim J. S. Neoplastic transformation of immortalized human epidermal keratinocytes by two HHV-6 DNA clones. Virology. 1993 Jul;195(1):113–120. doi: 10.1006/viro.1993.1351. [DOI] [PubMed] [Google Scholar]
- Rosenberg Z. F., Fauci A. S. Immunopathogenic mechanisms of HIV infection: cytokine induction of HIV expression. Immunol Today. 1990 May;11(5):176–180. doi: 10.1016/0167-5699(90)90070-p. [DOI] [PubMed] [Google Scholar]
- Salahuddin S. Z., Ablashi D. V., Markham P. D., Josephs S. F., Sturzenegger S., Kaplan M., Halligan G., Biberfeld P., Wong-Staal F., Kramarsky B. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science. 1986 Oct 31;234(4776):596–601. doi: 10.1126/science.2876520. [DOI] [PubMed] [Google Scholar]
- Seto E., Yen T. S., Peterlin B. M., Ou J. H. Trans-activation of the human immunodeficiency virus long terminal repeat by the hepatitis B virus X protein. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8286–8290. doi: 10.1073/pnas.85.21.8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Sonoda S., Kawakami K., Miyata K., Oki T., Nagata T., Okuno T., Kamanishi K. Human herpesvirus 6 and exanthem subitum. Lancet. 1988 Jun 25;1(8600):1463–1463. doi: 10.1016/s0140-6736(88)92275-1. [DOI] [PubMed] [Google Scholar]
- Tedder R. S., Briggs M., Cameron C. H., Honess R., Robertson D., Whittle H. A novel lymphotropic herpesvirus. Lancet. 1987 Aug 15;2(8555):390–392. doi: 10.1016/s0140-6736(87)92404-4. [DOI] [PubMed] [Google Scholar]
- Wang Z. Y., Lin X. H., Nobyuoshi M., Qui Q. Q., Deuel T. F. Binding of single-stranded oligonucleotides to a non-B-form DNA structure results in loss of promoter activity of the platelet-derived growth factor A-chain gene. J Biol Chem. 1992 Jul 5;267(19):13669–13674. [PubMed] [Google Scholar]
- Yoon K., Hobbs C. A., Koch J., Sardaro M., Kutny R., Weis A. L. Elucidation of the sequence-specific third-strand recognition of four Watson-Crick base pairs in a pyrimidine triple-helix motif: T.AT, C.GC, T.CG, and G.TA. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3840–3844. doi: 10.1073/pnas.89.9.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann K., Dobrovnik M., Ballaun C., Bevec D., Hauber J., Böhnlein E. trans-activation of the HIV-1 LTR by the HIV-1 Tat and HTLV-I Tax proteins is mediated by different cis-acting sequences. Virology. 1991 Jun;182(2):874–878. doi: 10.1016/0042-6822(91)90633-m. [DOI] [PubMed] [Google Scholar]