Abstract
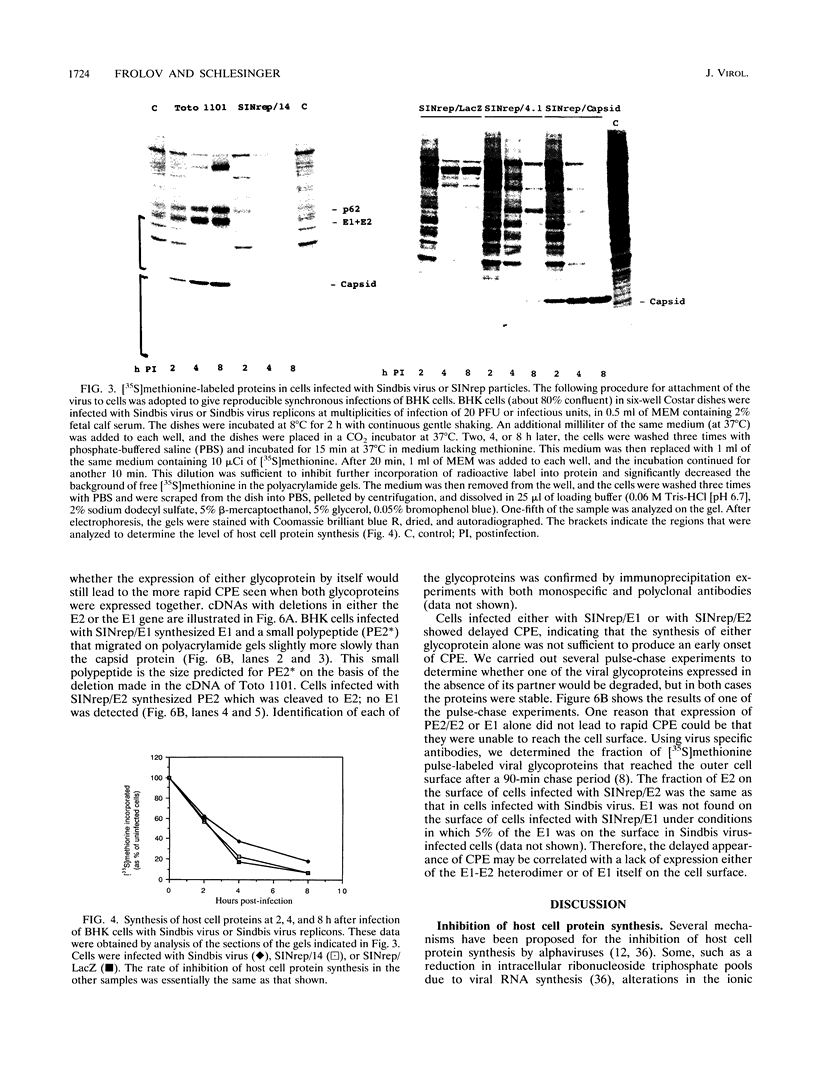
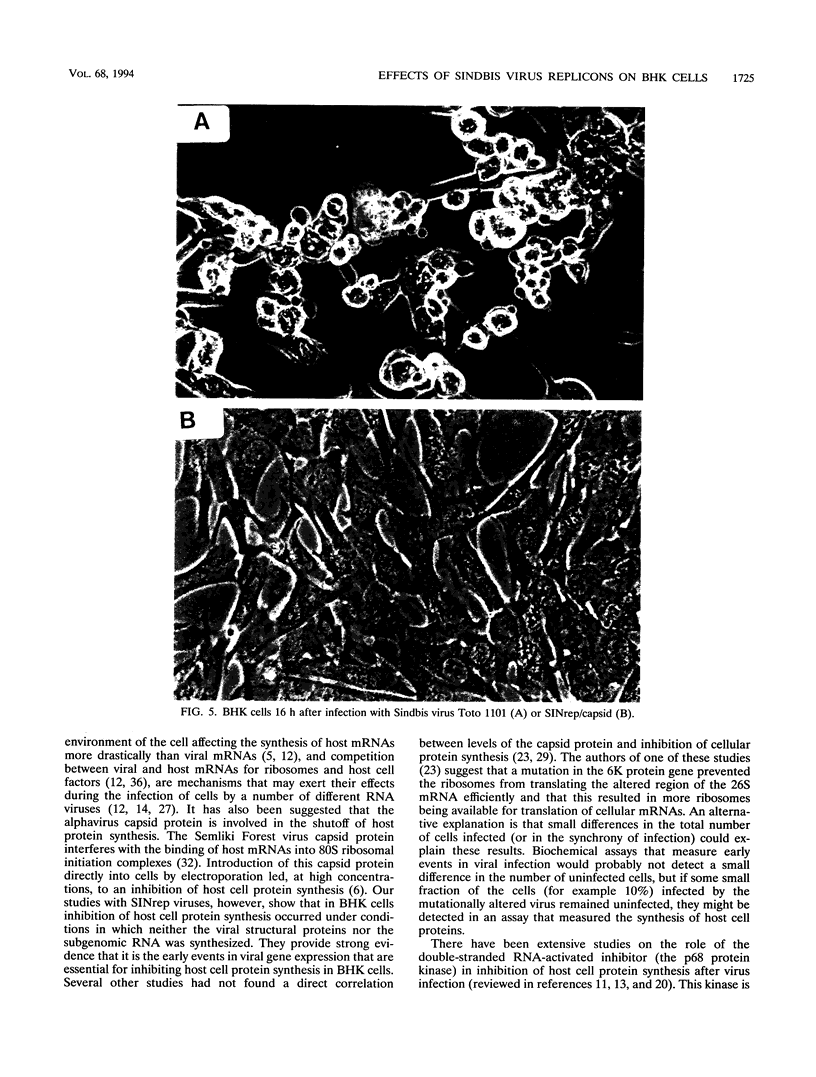
Infection of BHK cells by Sindbis virus leads to rapid inhibition of host cell protein synthesis and cytopathic effects (CPE). We have been studying these events to determine whether the expression of a specific viral gene is required and, in the present study, have focused our attention on the role of the structural proteins--the capsid protein and the two membrane glycoproteins. We tested a variety of Sindbis viruses and Sindbis virus replicons (virus particles containing an RNA that is self-replicating but with some or all of the viral structural protein genes deleted) for their abilities to inhibit host cell protein synthesis and cause CPE in infected BHK cells. Our results show that shutoff of host cell protein synthesis occurred in infected BHK cells when no viral structural proteins were synthesized and also under conditions in which the level of the viral subgenomic RNA was too low to be detected. These results support the conclusion that the early steps in viral gene expression are the ones required for the inhibition of host cell protein synthesis in BHK cells. In contrast, the Sindbis viruses and Sindbis virus replicons were clearly distinguished by the time at which CPE became evident. Viruses that synthesized high levels of the two membrane glycoproteins on the surface of the infected cells caused a rapid (12 to 16 h postinfection) appearance of CPE, and those that did not synthesize the glycoprotein spikes showed delayed (30 to 40 h) CPE.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berben-Bloemheuvel G., Kasperaitis M. A., van Heugten H., Thomas A. A., van Steeg H., Voorma H. O. Interaction of initiation factors with the cap structure of chimaeric mRNA containing the 5'-untranslated regions of Semliki Forest virus RNA is related to translational efficiency. Eur J Biochem. 1992 Sep 15;208(3):581–587. doi: 10.1111/j.1432-1033.1992.tb17222.x. [DOI] [PubMed] [Google Scholar]
- Bredenbeek P. J., Frolov I., Rice C. M., Schlesinger S. Sindbis virus expression vectors: packaging of RNA replicons by using defective helper RNAs. J Virol. 1993 Nov;67(11):6439–6446. doi: 10.1128/jvi.67.11.6439-6446.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras A., Carrasco L. Selective inhibition of protein synthesis in virus-infected mammalian cells. J Virol. 1979 Jan;29(1):114–122. doi: 10.1128/jvi.29.1.114-122.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgizoli M., Dai Y., Kempf C., Koblet H., Michel M. R. Semliki Forest virus capsid protein acts as a pleiotropic regulator of host cellular protein synthesis. J Virol. 1989 Jul;63(7):2921–2928. doi: 10.1128/jvi.63.7.2921-2928.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaedigk-Nitschko K., Schlesinger M. J. Site-directed mutations in Sindbis virus E2 glycoprotein's cytoplasmic domain and the 6K protein lead to similar defects in virus assembly and budding. Virology. 1991 Jul;183(1):206–214. doi: 10.1016/0042-6822(91)90133-v. [DOI] [PubMed] [Google Scholar]
- Grakoui A., Levis R., Raju R., Huang H. V., Rice C. M. A cis-acting mutation in the Sindbis virus junction region which affects subgenomic RNA synthesis. J Virol. 1989 Dec;63(12):5216–5227. doi: 10.1128/jvi.63.12.5216-5227.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn Y. S., Strauss E. G., Strauss J. H. Mapping of RNA- temperature-sensitive mutants of Sindbis virus: assignment of complementation groups A, B, and G to nonstructural proteins. J Virol. 1989 Jul;63(7):3142–3150. doi: 10.1128/jvi.63.7.3142-3150.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Regulation of translation in eukaryotic systems. Annu Rev Cell Biol. 1992;8:197–225. doi: 10.1146/annurev.cb.08.110192.001213. [DOI] [PubMed] [Google Scholar]
- Käriäinen L., Ranki M. Inhibition of cell functions by RNA-virus infections. Annu Rev Microbiol. 1984;38:91–109. doi: 10.1146/annurev.mi.38.100184.000515. [DOI] [PubMed] [Google Scholar]
- Leavitt R., Schlesinger S., Kornfeld S. Impaired intracellular migration and altered solubility of nonglycosylated glycoproteins of vesicular stomatitis virus and Sindbis virus. J Biol Chem. 1977 Dec 25;252(24):9018–9023. [PubMed] [Google Scholar]
- Levine B., Huang Q., Isaacs J. T., Reed J. C., Griffin D. E., Hardwick J. M. Conversion of lytic to persistent alphavirus infection by the bcl-2 cellular oncogene. Nature. 1993 Feb 25;361(6414):739–742. doi: 10.1038/361739a0. [DOI] [PubMed] [Google Scholar]
- Levis R., Weiss B. G., Tsiang M., Huang H., Schlesinger S. Deletion mapping of Sindbis virus DI RNAs derived from cDNAs defines the sequences essential for replication and packaging. Cell. 1986 Jan 17;44(1):137–145. doi: 10.1016/0092-8674(86)90492-7. [DOI] [PubMed] [Google Scholar]
- Liljeström P., Garoff H. A new generation of animal cell expression vectors based on the Semliki Forest virus replicon. Biotechnology (N Y) 1991 Dec;9(12):1356–1361. doi: 10.1038/nbt1291-1356. [DOI] [PubMed] [Google Scholar]
- Liljeström P., Lusa S., Huylebroeck D., Garoff H. In vitro mutagenesis of a full-length cDNA clone of Semliki Forest virus: the small 6,000-molecular-weight membrane protein modulates virus release. J Virol. 1991 Aug;65(8):4107–4113. doi: 10.1128/jvi.65.8.4107-4113.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivo P. D., Frolov I., Schlesinger S. A cell line that expresses a reporter gene in response to infection by Sindbis virus: a prototype for detection of positive strand RNA viruses. Virology. 1994 Jan;198(1):381–384. doi: 10.1006/viro.1994.1046. [DOI] [PubMed] [Google Scholar]
- Rice C. M., Levis R., Strauss J. H., Huang H. V. Production of infectious RNA transcripts from Sindbis virus cDNA clones: mapping of lethal mutations, rescue of a temperature-sensitive marker, and in vitro mutagenesis to generate defined mutants. J Virol. 1987 Dec;61(12):3809–3819. doi: 10.1128/jvi.61.12.3809-3819.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M. J., London S. D., Ryan C. An in-frame insertion into the Sindbis virus 6K gene leads to defective proteolytic processing of the virus glycoproteins, a trans-dominant negative inhibition of normal virus formation, and interference in virus shut off of host-cell protein synthesis. Virology. 1993 Mar;193(1):424–432. doi: 10.1006/viro.1993.1139. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J., Schlesinger S. Domains of virus glycoproteins. Adv Virus Res. 1987;33:1–44. doi: 10.1016/S0065-3527(08)60315-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger S. Alphaviruses--vectors for the expression of heterologous genes. Trends Biotechnol. 1993 Jan;11(1):18–22. doi: 10.1016/0167-7799(93)90070-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R. J., Shenk T. Impact of virus infection on host cell protein synthesis. Annu Rev Biochem. 1987;56:317–332. doi: 10.1146/annurev.bi.56.070187.001533. [DOI] [PubMed] [Google Scholar]
- Ulug E. T., Bose H. R., Jr Effect of tunicamycin on the development of the cytopathic effect in Sindbis virus-infected avian fibroblasts. Virology. 1985 Jun;143(2):546–557. doi: 10.1016/0042-6822(85)90393-9. [DOI] [PubMed] [Google Scholar]
- Ulug E. T., Garry R. F., Bose H. R., Jr The role of monovalent cation transport in Sindbis virus maturation and release. Virology. 1989 Sep;172(1):42–50. doi: 10.1016/0042-6822(89)90105-0. [DOI] [PubMed] [Google Scholar]
- Ulug E. T., Garry R. F., Waite M. R., Bose H. R., Jr Alterations in monovalent cation transport in Sindbis virus-infected chick cells. Virology. 1984 Jan 15;132(1):118–130. doi: 10.1016/0042-6822(84)90096-5. [DOI] [PubMed] [Google Scholar]
- Weiss B., Rosenthal R., Schlesinger S. Establishment and maintenance of persistent infection by Sindbis virus in BHK cells. J Virol. 1980 Jan;33(1):463–474. doi: 10.1128/jvi.33.1.463-474.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong C., Levis R., Shen P., Schlesinger S., Rice C. M., Huang H. V. Sindbis virus: an efficient, broad host range vector for gene expression in animal cells. Science. 1989 Mar 3;243(4895):1188–1191. doi: 10.1126/science.2922607. [DOI] [PubMed] [Google Scholar]
- van Steeg H., Kasperaitis M., Voorma H. O., Benne R. Infection of neuroblastoma cells by Semliki Forest virus. The interference of viral capsid protein with the binding of host messenger RNAs into initiation complexes is the cause of the shut-off of host protein synthesis. Eur J Biochem. 1984 Feb 1;138(3):473–478. doi: 10.1111/j.1432-1033.1984.tb07940.x. [DOI] [PubMed] [Google Scholar]
- van Steeg H., Thomas A., Verbeek S., Kasperaitis M., Voorma H. O., Benne R. Shutoff of neuroblastoma cell protein synthesis by Semliki Forest virus: loss of ability of crude initiation factors to recognize early Semliki Forest virus and host mRNA's. J Virol. 1981 May;38(2):728–736. doi: 10.1128/jvi.38.2.728-736.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Steeg H., van Grinsven M., van Mansfeld F., Voorma H. O., Benne R. Initiation of protein synthesis in neuroblastoma cells infected by Semliki Forest Virus. A decreased requirement of late viral mRNA for eIF-4B and cap binding protein. FEBS Lett. 1981 Jun 29;129(1):62–66. doi: 10.1016/0014-5793(81)80756-9. [DOI] [PubMed] [Google Scholar]