Abstract
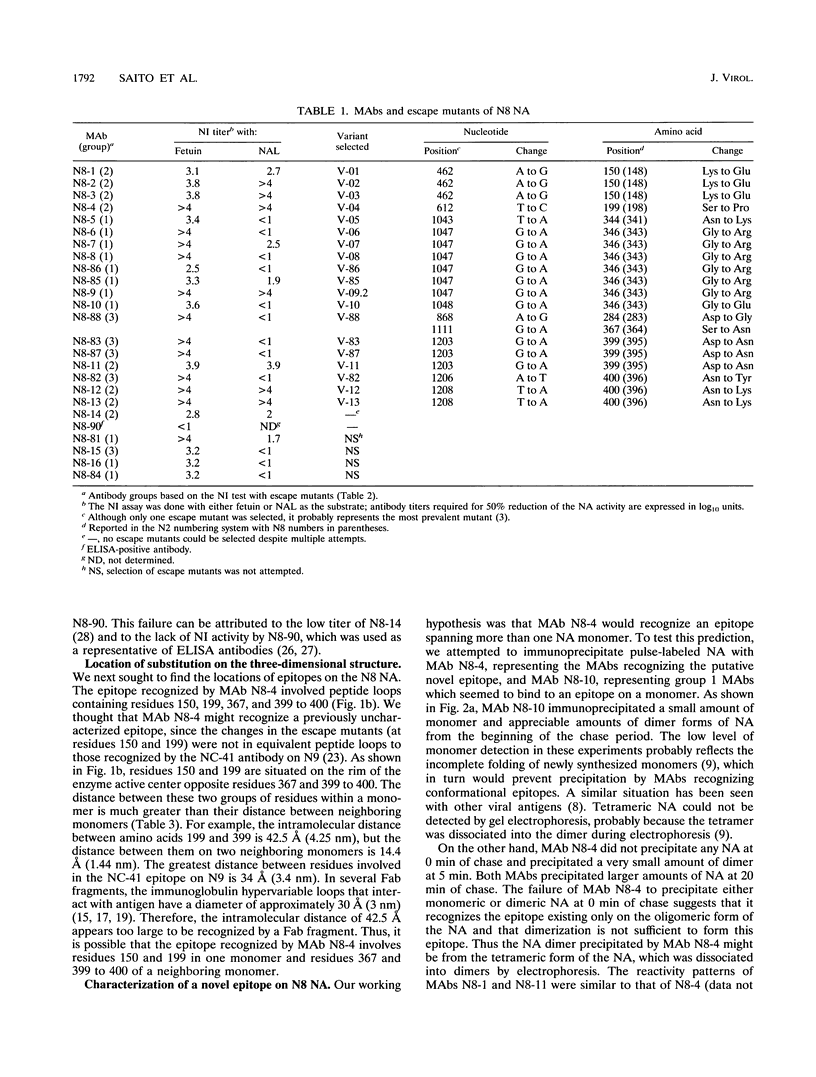
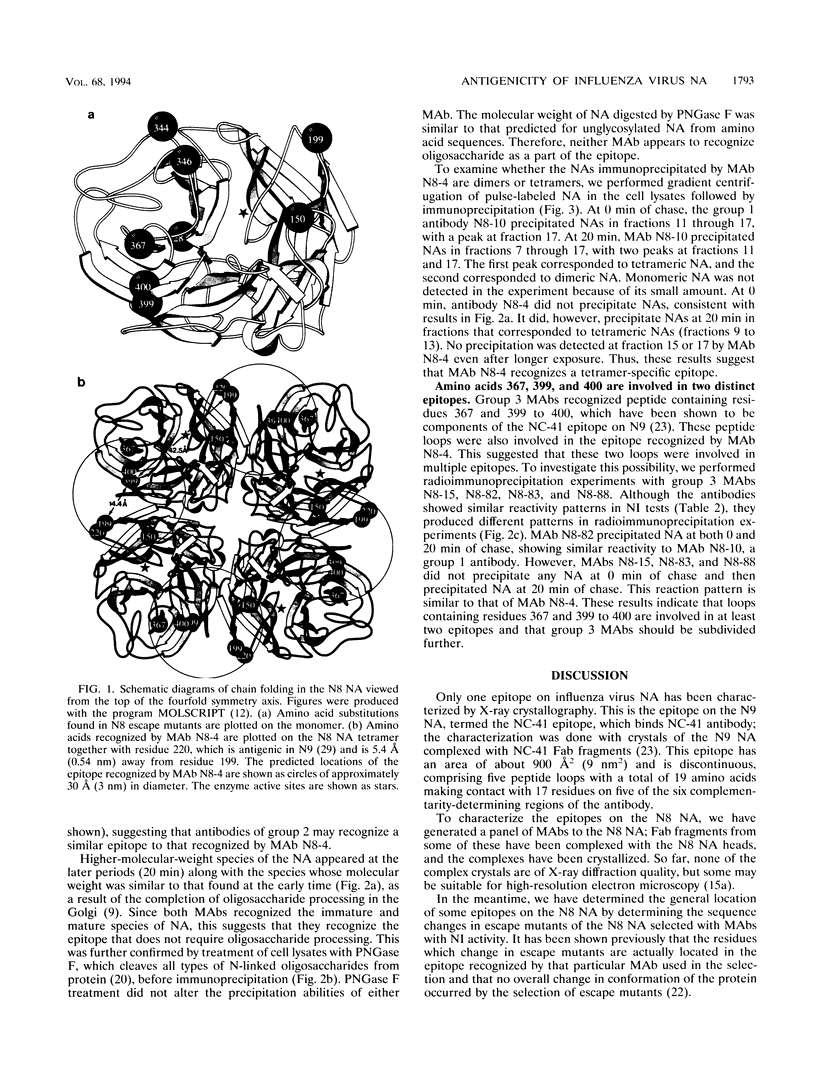
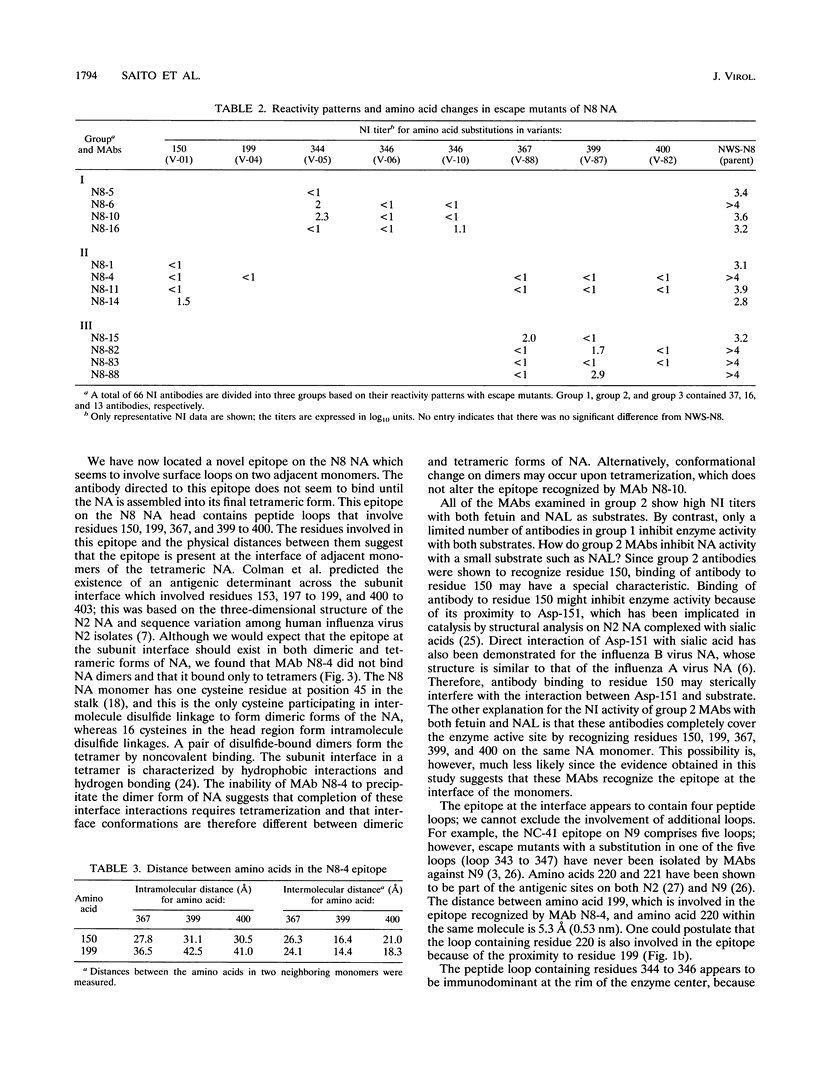
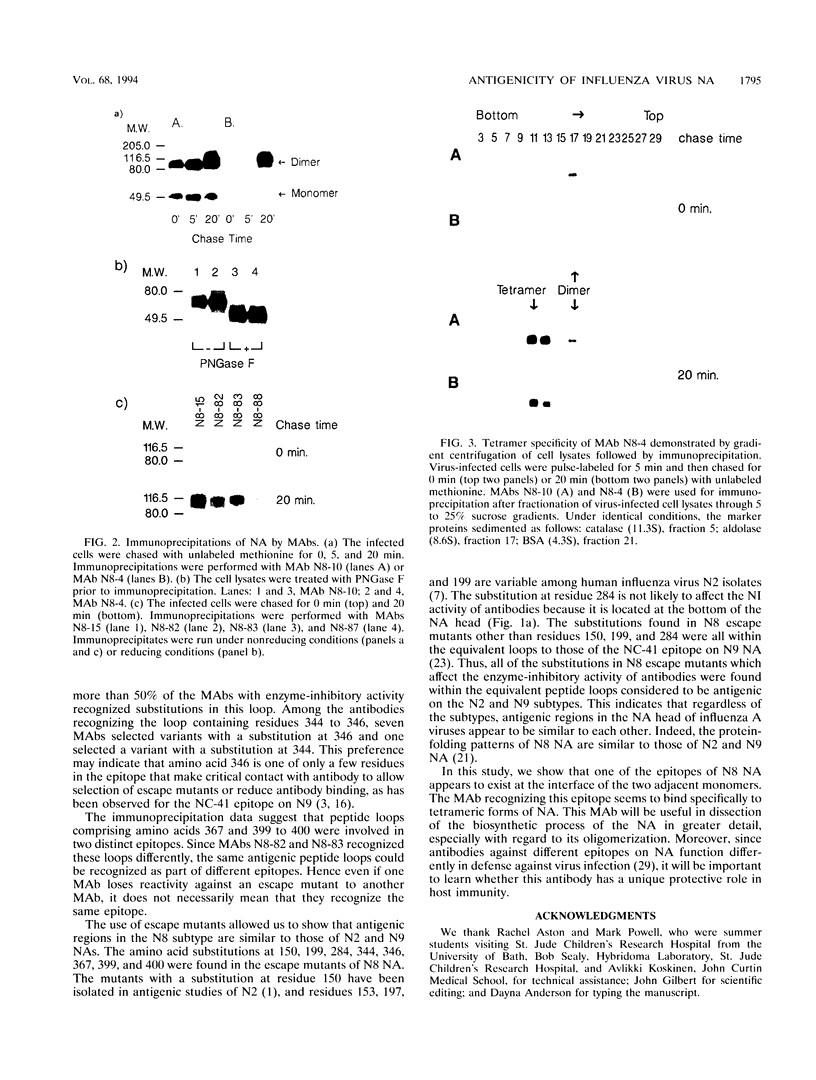
To locate antigenic epitopes on the N8 neuraminidase (NA), we generated a panel of 97 monoclonal antibodies (MAbs), 66 of which inhibited NA activity (NI antibodies). Three groups of NI MAbs were identified from their different reactivities with escape mutants. Group 1 antibodies recognized the peptide loop containing residues 344 to 346, which appears to be an immunodominant region on the rim of the enzyme center of the N8 NA. Group 2 antibodies recognized a novel epitope containing residues 150, 199, 367, 399, and 400 (N2 numbering). From the location of these residues on the three-dimensional structure of the N8 NA, the epitope appears to be located at the interface of two adjacent monomers in the tetrameric NA, one contributing residues 150 and 199 and the other contributing residues 367 and 399 to 400. The available evidence indicates that the MAbs of this group react with the NA only after it is fully assembled. The third group of antibodies recognized the peptide loops containing residues 367 and 399 to 400. All of the amino acid substitutions in N8 escape mutants which affect the NI activity of antibodies were located in the peptide loops known to form epitopes in the N2 and N9 subtypes, indicating that antigenic regions in the NA head inducing NI antibodies appear to be similar among different subtypes of influenza A viruses. The MAbs used in this study will be valuable in studying the role of each N8 NA epitope in host immune defense systems and in the kinetics analysis of the biosynthesis of the enzyme.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Air G. M., Els M. C., Brown L. E., Laver W. G., Webster R. G. Location of antigenic sites on the three-dimensional structure of the influenza N2 virus neuraminidase. Virology. 1985 Sep;145(2):237–248. doi: 10.1016/0042-6822(85)90157-6. [DOI] [PubMed] [Google Scholar]
- Air G. M., Laver W. G. The neuraminidase of influenza virus. Proteins. 1989;6(4):341–356. doi: 10.1002/prot.340060402. [DOI] [PubMed] [Google Scholar]
- Air G. M., Laver W. G., Webster R. G. Mechanism of antigenic variation in an individual epitope on influenza virus N9 neuraminidase. J Virol. 1990 Dec;64(12):5797–5803. doi: 10.1128/jvi.64.12.5797-5803.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aymard-Henry M., Coleman M. T., Dowdle W. R., Laver W. G., Schild G. C., Webster R. G. Influenzavirus neuraminidase and neuraminidase-inhibition test procedures. Bull World Health Organ. 1973;48(2):199–202. [PMC free article] [PubMed] [Google Scholar]
- Bean W. J., Jr, Sriram G., Webster R. G. Electrophoretic analysis of iodine-labeled influenza virus RNA segments. Anal Biochem. 1980 Feb;102(1):228–232. doi: 10.1016/0003-2697(80)90343-7. [DOI] [PubMed] [Google Scholar]
- Burmeister W. P., Ruigrok R. W., Cusack S. The 2.2 A resolution crystal structure of influenza B neuraminidase and its complex with sialic acid. EMBO J. 1992 Jan;11(1):49–56. doi: 10.1002/j.1460-2075.1992.tb05026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman P. M., Varghese J. N., Laver W. G. Structure of the catalytic and antigenic sites in influenza virus neuraminidase. Nature. 1983 May 5;303(5912):41–44. doi: 10.1038/303041a0. [DOI] [PubMed] [Google Scholar]
- Earl P. L., Moss B., Doms R. W. Folding, interaction with GRP78-BiP, assembly, and transport of the human immunodeficiency virus type 1 envelope protein. J Virol. 1991 Apr;65(4):2047–2055. doi: 10.1128/jvi.65.4.2047-2055.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogue B. G., Nayak D. P. Synthesis and processing of the influenza virus neuraminidase, a type II transmembrane glycoprotein. Virology. 1992 Jun;188(2):510–517. doi: 10.1016/0042-6822(92)90505-j. [DOI] [PubMed] [Google Scholar]
- Kida H., Brown L. E., Webster R. G. Biological activity of monoclonal antibodies to operationally defined antigenic regions on the hemagglutinin molecule of A/Seal/Massachusetts/1/80 (H7N7) influenza virus. Virology. 1982 Oct 15;122(1):38–47. doi: 10.1016/0042-6822(82)90375-0. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Air G. M., Webster R. G., Markoff L. J. Amino acid sequence changes in antigenic variants of type A influenza virus N2 neuraminidase. Virology. 1982 Oct 30;122(2):450–460. doi: 10.1016/0042-6822(82)90244-6. [DOI] [PubMed] [Google Scholar]
- Lentz M. R., Air G. M., Laver W. G., Webster R. G. Sequence of the neuraminidase gene of influenza virus A/Tokyo/3/67 and previously uncharacterized monoclonal variants. Virology. 1984 May;135(1):257–265. doi: 10.1016/0042-6822(84)90135-1. [DOI] [PubMed] [Google Scholar]
- Matsushima M., Marquart M., Jones T. A., Colman P. M., Bartels K., Huber R. Crystal structure of the human Fab fragment Kol and its comparison with the intact Kol molecule. J Mol Biol. 1978 Jun 5;121(4):441–459. doi: 10.1016/0022-2836(78)90393-5. [DOI] [PubMed] [Google Scholar]
- Nuss J. M., Whitaker P. B., Air G. M. Identification of critical contact residues in the NC41 epitope of a subtype N9 influenza virus neuraminidase. Proteins. 1993 Feb;15(2):121–132. doi: 10.1002/prot.340150204. [DOI] [PubMed] [Google Scholar]
- Poljak R. J., Amzel L. M., Avey H. P., Chen B. L., Phizackerley R. P., Saul F. Three-dimensional structure of the Fab' fragment of a human immunoglobulin at 2,8-A resolution. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3305–3310. doi: 10.1073/pnas.70.12.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T., Kawaoka Y., Webster R. G. Phylogenetic analysis of the N8 neuraminidase gene of influenza A viruses. Virology. 1993 Apr;193(2):868–876. doi: 10.1006/viro.1993.1196. [DOI] [PubMed] [Google Scholar]
- Segal D. M., Padlan E. A., Cohen G. H., Rudikoff S., Potter M., Davies D. R. The three-dimensional structure of a phosphorylcholine-binding mouse immunoglobulin Fab and the nature of the antigen binding site. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4298–4302. doi: 10.1073/pnas.71.11.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarentino A. L., Gómez C. M., Plummer T. H., Jr Deglycosylation of asparagine-linked glycans by peptide:N-glycosidase F. Biochemistry. 1985 Aug 13;24(17):4665–4671. doi: 10.1021/bi00338a028. [DOI] [PubMed] [Google Scholar]
- Taylor G., Garman E., Webster R., Saito T., Laver G. Crystallization and preliminary X-ray studies of influenza A virus neuraminidase of subtypes N5, N6, N8 and N9. J Mol Biol. 1993 Mar 5;230(1):345–348. doi: 10.1006/jmbi.1993.1147. [DOI] [PubMed] [Google Scholar]
- Tulip W. R., Varghese J. N., Baker A. T., van Donkelaar A., Laver W. G., Webster R. G., Colman P. M. Refined atomic structures of N9 subtype influenza virus neuraminidase and escape mutants. J Mol Biol. 1991 Sep 20;221(2):487–497. doi: 10.1016/0022-2836(91)80069-7. [DOI] [PubMed] [Google Scholar]
- Tulip W. R., Varghese J. N., Laver W. G., Webster R. G., Colman P. M. Refined crystal structure of the influenza virus N9 neuraminidase-NC41 Fab complex. J Mol Biol. 1992 Sep 5;227(1):122–148. doi: 10.1016/0022-2836(92)90687-f. [DOI] [PubMed] [Google Scholar]
- Varghese J. N., Colman P. M. Three-dimensional structure of the neuraminidase of influenza virus A/Tokyo/3/67 at 2.2 A resolution. J Mol Biol. 1991 Sep 20;221(2):473–486. doi: 10.1016/0022-2836(91)80068-6. [DOI] [PubMed] [Google Scholar]
- Varghese J. N., McKimm-Breschkin J. L., Caldwell J. B., Kortt A. A., Colman P. M. The structure of the complex between influenza virus neuraminidase and sialic acid, the viral receptor. Proteins. 1992 Nov;14(3):327–332. doi: 10.1002/prot.340140302. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Air G. M., Metzger D. W., Colman P. M., Varghese J. N., Baker A. T., Laver W. G. Antigenic structure and variation in an influenza virus N9 neuraminidase. J Virol. 1987 Sep;61(9):2910–2916. doi: 10.1128/jvi.61.9.2910-2916.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R. G., Brown L. E., Laver W. G. Antigenic and biological characterization of influenza virus neuraminidase (N2) with monoclonal antibodies. Virology. 1984 May;135(1):30–42. doi: 10.1016/0042-6822(84)90114-4. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Hinshaw V. S., Laver W. G. Selection and analysis of antigenic variants of the neuraminidase of N2 influenza viruses with monoclonal antibodies. Virology. 1982 Feb;117(1):93–104. doi: 10.1016/0042-6822(82)90510-4. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Reay P. A., Laver W. G. Protection against lethal influenza with neuraminidase. Virology. 1988 May;164(1):230–237. doi: 10.1016/0042-6822(88)90640-x. [DOI] [PubMed] [Google Scholar]




