Abstract
Phospholipids are the major components of cell membranes and are required for cellular growth. We studied membrane phosphatidylcholine (PtdCho) biosynthesis in neuronal cells undergoing neurite outgrowth, by using PC12 cells as a model system. When neurite outgrowth was induced by exposing PC12 cells to nerve growth factor for 2 and 4 days, the amounts of [14C]choline incorporated into [14C]phosphatidylcholine per cell (i.e., per DNA) increased approximately 5- and 10-fold, respectively, as compared with control cells, reflecting increases in the rate of PtdCho biosynthesis. [14C]choline uptake was not affected. Analysis of the three major PtdCho biosynthetic enzymes showed that the activity of CDPcholine:1,2-diacylglycerol cholinephosphotransferase was increased by approximately 50% after nerve growth factor treatment, but the activities of choline kinase or choline-phosphate cytidylyltransferase were unaltered; the cholinephosphotransferase displayed a high Km value (≈1,200 μM) for diacylglycerol. Moreover, free cellular diacylglycerol levels increased by approximately 1.5- and 4-fold on the second and fourth days, respectively. These data indicate that PtdCho biosynthesis is enhanced when PC12 cells sprout neurites, and the enhancement is mediated primarily by changes in cholinephosphotransferase activity and its saturation with diacylglycerol. This suggests a novel regulatory role for diacylglycerol in membrane phospholipid biosynthesis.
Phospholipids are the major constituents of cellular membranes, and their synthesis is essential for cellular growth. These compounds both form the bilayer of membranes in which biologically active proteins are embedded and act as reservoirs for the precursors of such first and second messengers as acetylcholine, eicosanoids, diacylglycerol, and inositol 1,4,5,-trisphosphate. Phosphatidylcholine (PtdCho) and phosphatidylethanolamine primarily are synthesized via the CDPcholine and CDPethanolamine pathways (Kennedy cycle); phosphatidylserine, in contrast, apparently is formed exclusively by base exchange (1). The CDPcholine pathway involves three enzymatic steps, catalyzed by choline kinase (CK), choline-phosphate cytidylyltransferase (CT), and CDPcholine:1,2-diacylglycerol cholinephosphotransferase (CPT). CT, which converts phosphocholine and CTP to CDPcholine and inorganic pyrophosphate, previously has been proposed as rate-limiting in overall PtdCho biosynthesis (1). However, other factors such as the activity of CPT or its saturation with CDPcholine and diacylglycerol also could limit PtdCho synthesis (2).
Axons of neurons contain all three of the PtdCho biosynthetic enzymes and are able to synthesize PtdCho (3). Moreover, PtdCho synthesis appears to be required for axonal growth (4, 5). Little information is available about whether or how membrane phospholipid synthesis is accelerated when axonal growth is occurring (6, 7). In this study, we used rat pheochromocytoma PC12 cells as a model system to investigate this issue. We now report that PtdCho synthesis is enhanced when neurite outgrowth is induced by exposing PC12 cells to nerve growth factor (NGF), and that this enhancement most likely results from activation of the final enzyme in the CDPcholine pathway and an increase in its saturation with diacylglycerol.
MATERIALS AND METHODS
Cell Culture.
PC12 cells were obtained from American Type Culture Collection and maintained in RPMI 1640 medium supplemented with 10% horse serum and 5% fetal bovine serum. Cells were plated on mouse collagen IV-coated dishes (Beckton Dickinson), and the medium was changed to RPMI 1640 with 1% horse serum 1 day after plating. NGF (2.5S; GIBCO-BRL) or basic fibroblast growth factor (bFGF) (Sigma) was added to the medium to induce neurite outgrowth. The medium was changed every 2 days.
Measurement of Total Phospholipids and Choline and Phosphocholine Masses.
Cell phospholipids were extracted as follows: Cells were rinsed with ice-cold PBS twice, and 1 ml of ice-cold methanol was added. The cells were scraped from the dish, and the methanol extract was transferred to test tubes and sonicated. Two milliliters of chloroform and 1 ml of water were added, and the tubes were vortexed. The upper (aqueous) and lower (organic) phases were separated and dried under vacuum. An aliquot of the methanol extract was assayed for DNA content. To measure the amounts of individual phospholipids, extracts were purified by TLC on silica gel G plates (Analtech) by using a mobile phase containing chloroform/ethanol/triethylamine/water (30:34:30:8, vol/vol). The amounts of phospholipids were determined by phosphate assay (8) by using dipalmitoyl PtdCho as standard.
Choline masses in aqueous extracts were analyzed by an HPLC system (Bioanalytical Systems, West Lafayette, IN) consisting of a polymeric analytical column in series with a choline oxidase column. To measure phosphocholine masses, the samples and phsophocholine standards were treated with alkaline phosphatase (4 units/tube) for 45 min at 37°C and centrifuged with methanol. Supernatant fluids then were dried under vacuum, resuspended in water, and subjected to HPLC analysis for choline. DNA was determined by the method of Labarca and Paigen (9) by using calf thymus DNA as the standard.
Labeling and Extraction of Cell PtdCho and Metabolites.
Cells were cultured as above and labeled with [methyl-14C]choline chloride (0.2 μCi/ml, 0.4 μCi/dish) for 2 hr in the same medium as that used for neurite induction. Phospholipids were extracted and purified by TLC as above, and radioactivity corresponding to PtdCho was counted. Water-soluble choline metabolites in aqueous extracts were separated by HPLC, and their radioactivities were measured by using an on-line detector (10). Analysis of [14C]choline uptake was performed by labeling cells with [14C]choline for 5 min by using choline-free RPMI 1640 medium.
Measurement of PtdCho Biosynthetic Enzyme Activities.
To prepare soluble and membrane fractions, cells were rinsed with cold PBS twice, scraped from culture dishes in homogenization buffer (10 mM Tris/150 mM NaCl/0.2 mM phenylmethylsulfonyl fluoride, pH 7.4), and homogenized on ice. After the homogenates were centrifuged at 105 g for 30 min at 4°C, the supernatant fluids were collected (soluble fraction). The pellet was resuspended in the homogenization buffer and homogenized as above (membrane fraction).
CK was assayed by a method described previously (11). The assay mixture, in a final volume of 100 μl, contained 100 mM glycine-NaOH (pH 9.0), 10 mM ATP, 12 mM MgCl2, 250 μM choline chloride, 0.1 μCi [methyl-14C]choline chloride (54 mCi/mmol), and an aliquot of soluble fraction. The reaction was carried out at 30°C for 5 min and stopped by addition of tetraphenylboron solution (30 mg/ml in butyronitrile). The tubes were centrifuged at 1,000 g for 2 min, and the upper phase was removed. The lower phase was washed three times with the tetraphenylboron solution and transferred to a scintillation vial, and its radioactivity was counted.
CT was assayed in total homogenates, unless otherwise noted, according to a procedure described previously (12, 13). Cells were homogenized on ice in assay buffer (50 mM imidazole/150 mM KCl/2 mM EDTA, pH 7.0) containing 0.2 mM phenylmethylsulfonyl fluoride. The reaction mixture contained 1 mM phosphocholine plus 0.1 μCi [methyl-14C]phosphocholine (50 mCi/mmol), 15 mM magnesium acetate, 3 mM CTP, 6 mM ADP, PtdCho-oleic acid liposomes, and an aliquot of homogenate in a total volume of 100 μl. The reaction was incubated at 37°C for 15 min and stopped by addition of 100 μl of 10% trichloroacetic acid/150 mM phosphocholine. The protein precipitate was removed by centrifugation, and the supernatant fluid was added to 1 ml of 6% charcoal solution. After sitting at room temperature for 30 min, the charcoal was pelleted by centrifugation, and the pellet was washed four times with water. Finally, the charcoal was extracted with 1 ml of water/ethanol/28% NH4OH (116:188:11, vol/vol). The extract was neutralized with glacial acetic acid, and its radioactivity was determined. Data were corrected for the recovery of CDPcholine, which was measured by using [14C]CDPcholine in the assay mixture.
CPT was assayed by the method of Cornell (14), with minor modifications. The reaction mixture consisted of 50 mM Tris⋅HCl, pH 8.5/10 mM MgCl2/10 mM EGTA/8 mM sn-1,2-diolein emulsion/0.8 mM CDPcholine/0.02 μCi [14C]CDPcholine (56 mCi/mmol) and an aliquot of membrane fraction in a total volume of 50 μl. The reaction was initiated with CDPcholine and incubated at 37°C in a shaking water bath for 15 min. The reaction was terminated by addition of 1.5 ml methanol/chloroform (2:1, vol/vol). [14C]labeled lipids were extracted by addition of 0.3 ml of water, 0.5 ml of chloroform, and 0.5 ml of water with vortexing after each addition. After centrifugation, the upper layer was removed, and the lower layer was washed with 2 ml of methanol/water/chloroform (48:47:3, vol/vol). The lower layer then was transferred to a scintillation vial, the solvent was evaporated under nitrogen, and the radioactivity was counted.
Protein was determined by the bicinchoninic acid assay (Sigma) or by the method of Lowry (15).
Measurement of Diacylglycerol Mass.
Measurement of sn-1,2-diacylglycerol (DAG) was done by the method of Preiss et al. (16). Cells were scraped from culture dishes and sonicated in 0.4 ml of methanol, and 0.2 ml of chloroform was added. After centrifugation, the supernatant fluid was transferred to a new tube. The pellet again was extracted with 0.3 ml of chloroform/methanol (1:2, vol/vol), and the supernatant fluids were combined. Then 1 ml of chloroform and 1 ml of 1 M NaCl were added, and the phases were separated by centrifugation. The upper layer was aspirated off, and the lower layer was dried under vacuum. The extracted samples and sn-1,2-dioleoylglycerol standards were solubilized in 20 μl of octyl-β-d-glucoside cardiolipin solution. Fifty microliters of 2× reaction buffer (100 mM imidazole⋅HCl/100 mM NaCl/25 mM MgCl2/2 mM EGTA, pH 6.6), 10 μl of 20 mM DTT, and 10 μl of DAG kinase (Lipidex) were added, and the reaction was started by addition of 10 μl of [γ-32P]ATP solution [10 mM ATP plus 1 μCi [γ-32P]ATP (30 Ci/mmol)]. The reaction was incubated at room temperature for 30 min and stopped by addition of 3 ml of chloroform/methanol (1:2, vol/vol) and 0.7 ml of 1% HClO4. Then 1 ml of chloroform and 1 ml of 1% HClO4 were added, and the phases were separated by centrifugation. After washing the lower phase twice with 2 ml of 1% HClO4, a 0.5-ml sample was removed from the chloroform phase and dried under vacuum. The dried sample was dissolved in 100 μl of 5% methanol in chloroform, and 20 μl was spotted on a TLC plate (LK6D, Whatman). Plates were developed with chloroform/methanol/acetic acid (65:15:5, vol/vol) and stained in iodine vapor. The bands corresponding to phosphatidic acid were scraped into a scintillation vial, and their radioactivities were counted.
RESULTS
Evidence That PtdCho Synthesis Increases in Association with Neurite Outgrowth.
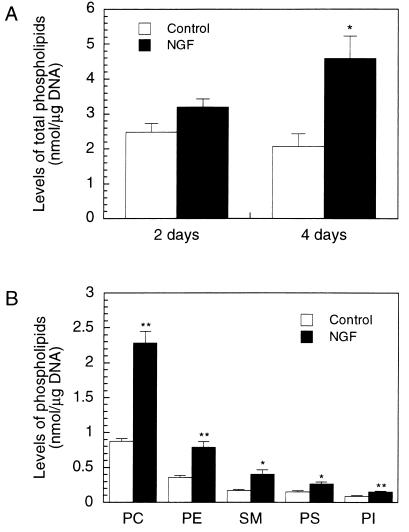
We first compared total levels of phospholipids in untreated PC12 cells and cells stimulated with 50 ng/ml NGF. Exposure of PC12 cells to NGF increased total phospholipid levels, corrected for DNA, by ≈30% and 120% after 2 and 4 days, respectively (Fig. 1A), a period concurrent with continued neurite outgrowth (17, 18). Among the major membrane phospholipids, levels of the Kennedy cycle products PtdCho, phosphatidylethanolamine, and sphingomyelin increased, after 4 days, by ≈160, 120, and 130%, respectively, whereas phosphatidylserine and phosphatidylinositol, products of other biosynthetic pathways, increased by ≈70% (Fig. 1B). We used DNA to normalize our data, both because neurite outgrowth is accompanied by significant increases in protein contents, which complicates interpretation of the data, and because we wanted to estimate changes in phospholipid content per cell.
Figure 1.
Increases in total and individual phospholipid levels in PC12 cells treated with NGF. PC12 cells were cultured with or without NGF (50 ng/ml) for 2 or 4 days (A) or for 4 days (B). Cell phospholipids were extracted and the amounts of total (A) or individual (B) phospholipids were determined. Results are means ± SEM from three (A) or four (B) independent experiments; duplicate dishes were used for each treatment. ∗ indicate data significantly different from control by unpaired Student’s t test. ∗, P < 0.05; ∗∗, P < 0.01. PC, phosphatidylcholine; PE, phosphatidylethanolamine; SM, sphingomyelin; PS, phosphatidylserine; PI, phosphatidylinositol.
To examine the rate of phospholipid synthesis, we studied [methyl-14C]choline incorporation into choline phospholipids in PC12 cells labeled for 2 hr. The amounts of [14C]PtdCho per μg DNA in NGF-treated cells were approximately 5- and 10-fold those of control cells, on the second and fourth days, respectively (Fig. 2A). (The increases were ≈3- and 6-fold, respectively, when protein levels were used to normalize the data.) The increase in [14C]PtdCho level was dependent on the concentration of NGF used (Fig. 2B); a 5 ng/ml NGF caused both weaker stimulation of neurite outgrowth and a smaller (≈4-fold) rise in [14C]PtdCho levels. bFGF, which induces neurite outgrowth in PC12 cells but with less potency than NGF (19), also had a similar, but smaller, effect on the incorporation of [14C]choline into [14C]PtdCho (Fig. 2A).
Figure 2.
[14C]choline incorporation into [14C]PtdCho increases in PC12 cells stimulated with NGF or bFGF. (A) PC12 cells that had been treated with or without NGF (50 ng/ml) or bFGF (50 ng/ml) for 2 or 4 days were labeled with [methyl-14C]choline for 2 hr, and [14C]choline incorporation into [14C]PtdCho was determined. (B) [14C]choline incorporation was analyzed in PC12 cells treated with the indicated concentrations of NGF for 4 days. Results are means ± SEM from three independent experiments; duplicate dishes were used for each treatment. ∗, P < 0.05; ∗∗, P < 0.01 compared with control. C, control.
Water-soluble [14C]choline metabolites from cells labeled with [14C]choline for 2 hr then were analyzed by HPLC (Table 1). In PC12 cells not treated with NGF, [14C]phosphocholine comprised ≈70% of the total radioactivity, and [14C]choline and [14C]CDPcholine each contributed ≈15%. Exposure of the cells to NGF or bFGF caused no significant changes in cellular levels of [14C]choline nor of [14C]CDPcholine, corrected for DNA. However, it did increase levels of [14C]phosphocholine per DNA (by ≈60% and 30% on the second and fourth days, respectively, of exposure to NGF).
Table 1.
Levels of water-soluble [14C]choline metabolites in PC12 cells treated with NGF or bFGF
| Time | Treatment | [14C]choline | [14C]p-choline | [14C]CDPcholine |
|---|---|---|---|---|
| 2 days | Control | 152 ± 23 | 775 ± 26 | 185 ± 35 |
| NGF | 132 ± 18 | 1,224 ± 72 | 163 ± 25 | |
| bFGF | 140 ± 17 | 1,002 ± 86 | 180 ± 11 | |
| 4 days | Control | 139 ± 1 | 803 ± 63 | 147 ± 11 |
| NGF | 109 ± 21 | 1,023 ± 190 | 153 ± 9 | |
| bFGF | 123 ± 27 | 980 ± 162 | 147 ± 35 |
PC12 cells were cultured and labeled with [methyl-14C]choline as in Fig. 2. Water-soluble [14C]choline metabolites then were extracted, and their radioactivities were measured. Results are means ± SEM (in dpm/μg DNA) from two independent experiments; duplicate dishes were used for each treatment. P-choline, phosphocholine.
Because the apparent increase in [14C]choline incorporation into [14C]PtdCho could have been an artifact resulting from a higher specific radioactivity of PtdCho precursors, we measured total levels of phosphocholine, in cells that had or had not been exposed to NGF, and used this information to estimate its specific activities. Phosphocholine levels per DNA were approximately 40 times those of choline in control cells and were unaffected by exposure to NGF for 2 or 4 days (Table 2). [The total free choline levels per DNA were reduced by ≈20% or 40% in cells exposed to NGF for 2 or 4 days, respectively (Table 2).] The estimated specific activity of phosphocholine for control cells, at the end of labeling, was 2,500 dpm/nmol, and for cells exposed to NGF for 2 and 4 days, 4,500 and 3,700 dpm/nmol, respectively. (Because all of the choline that becomes PtdCho must pass through phosphocholine, the specific activity of phosphocholine provides the best estimate of the specific activity of the PtdCho precursors.) Thus, the increase in [14C]choline incorporation into [14C]PtdCho could not be explained by changes in the specific radioactivity of its precursors, and the bulk of the increase in [14C]choline incorporation appears to reflect actual increases in the rate of PtdCho synthesis. This conclusion was corroborated further by the results of pulse–chase experiments. When cells were labeled with [14C]choline for 1 hr and chased for various times, the rate of disappearance of the label from phosphocholine was faster in NGF-treated cells than in control cells (after 2 hr, the levels of [14C]phosphocholine decreased by ≈50% in control cells, and by ≈70% and 80%, respectively, in cells that had been exposed to NGF for 2 or 4 days). Because the specific activity of phsophocholine was initially the same in all treatment groups, these findings confirm increased conversion of phosphocholine to PtdCho with NGF treatment.
Table 2.
Choline and phosphocholine contents in PC12 cells treated with NGF
| Treatment | Choline, nmol/μg DNA | P-choline, nmol/μg DNA |
|---|---|---|
| Control | 0.0069 | 0.31 |
| NGF 2 days | 0.0056 | 0.27 |
| NGF 4 days | 0.0043 | 0.28 |
PC12 cells were cultured as in Fig. 1. The amounts of choline and phosphocholine (p-choline) in aqueous extracts were determined using an HPLC system with a choline oxidase column. Results represent means of at least two experiments.
We also determined whether choline uptake is altered in NGF-treated PC12 cells by labeling the cells with [methyl-14C]choline for 5 min in a nearly choline-free medium (absolute choline concentration = ≈4 μM). Total amounts of measurable 14C-labeled materials increased almost linearly for up to 5 min, at rates unaffected by previous exposure to NGF for 2 or 4 days (Table 3). The amounts of [14C]choline taken up also were not changed significantly by NGF exposure at higher choline concentrations tested (25 and 100 μM; 25 μM is the concentration of choline in the medium used for labeling experiments). These data indicated that the increased incorporation of [14C]choline into [14C]PtdCho after NGF treatment was not caused by an increase in [14C]choline uptake.
Table 3.
[14C]Choline uptake in PC12 cells treated with NGF
| Treatment | [14C]choline | [14C]p-choline | [14C]PL |
|---|---|---|---|
| Control | 169 ± 22 | 310 ± 49 | ND |
| NGF 2 days | 131 ± 25 | 348 ± 76 | ND |
| NGF 4 days | 109 ± 28 | 329 ± 13 | 37 ± 22 |
PC12 cells were cultured with or without NGF (50 ng/ml) for 2 or 4 days and labeled with [methyl-14C]choline for 5 min using choline-free medium. Water-soluble [14C]choline metabolites and [14C]phospholipids then were extracted, and their radioactivities were measured. [14C]CDPcholine was not included in this table, because it gave no clear peak. Results are means ± SEM (in dpm/5 min per μg DNA) from three independent experiments. P-choline, phosphocholine; PL, phospholipids; ND, not detected.
Activation of CPT and An Increase in DAG Levels Are Involved in the Enhanced PtdCho Synthesis.
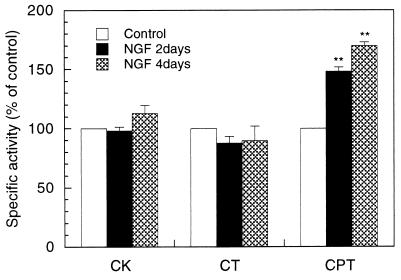
We hypothesized that the enhanced synthesis of PtdCho during neurite sprouting was mediated by an acceleration of one or more of the three enzymatic steps in the CDPcholine pathway. Thus we assayed each of the biosynthetic enzymes. CK and CPT activities were exclusively present in soluble and membrane fractions, respectively; CT activity was predominantly recovered in the soluble fraction. As shown in Fig. 3, CK activities were unchanged after cells were exposed for 2 or 4 days to 50 ng/ml NGF. CT activities in total homogenates as well as those in soluble and membrane fractions were also similar in control and NGF-treated cells. In contrast, CPT activities were ≈50–70% higher after 2 or 4 days of NGF treatment than in control cells. In addition, kinetic studies of CPT, using membrane fractions of control PC12 cells, revealed that its Km values for CDPcholine and DAG were ≈50 μM and ≈1,200 μM, respectively; these Km values did not change significantly after NGF treatment (Table 4).
Figure 3.
Activities of PtdCho biosynthetic enzymes in PC12 cells treated with NGF. PC12 cells were cultured with or without NGF (50 ng/ml) for 2 or 4 days. CK was assayed in soluble fractions, CT in total homogenates, and CPT in membrane fractions. Data are expressed as percentages of control, and represent means ± SEM from at least three experiments. ∗∗, P < 0.01 compared with control. The actual activities in control cells for CK, CT and CPT were 3 ± 0.4, 1.8 ± 0.3, and 7.3 ± 0.6 nmol/min per mg of protein, respectively.
Table 4.
Km values of CPT for CDPcholine or DAG
| Treatment |
Km
|
|
|---|---|---|
| CDP choline, μM | DAG, μM | |
| Control | 50 | 1,200 |
| NGF 2 days | 46 | 1,200 |
| NGF 4 days | 64 | 1,400 |
PC12 cells were cultured as in Fig 3. CPT assay was performed using membrane fractions, and Km values were determined from a double-reciprocal plot of enzyme kinetics. The results were means of two experiments except that the data for 4-day-NGF-treated cells were from a single experiment.
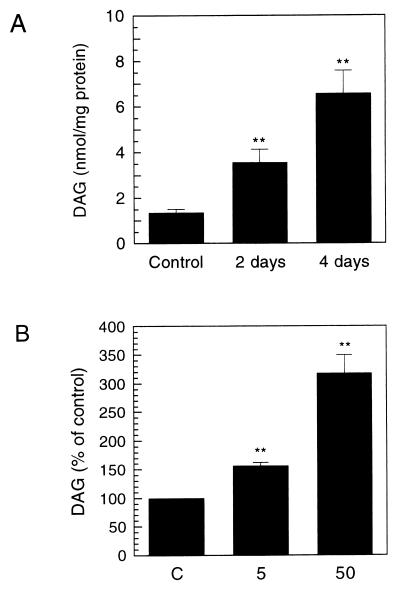
Because the Km of CPT for DAG is thus very high, it seemed possible that the CPT reaction is controlled by concentrations of this substrate. Hence, we measured total DAG levels in the cells. The estimated DAG concentration in control cells was 200–300 μM [calculation was made by assuming that the protein content in control cells is ≈100 μg/106 cells, and their radius is ≈5 μm (20)]. As shown in Fig. 4A, total DAG levels were increased by ≈160% and 390% after 2 and 4 days of exposure to 50 ng/ml NGF, respectively, (protein was used to normalize the data, because DAG was considered as an enzyme substrate). Furthermore, the increase in DAG levels was dependent on the NGF concentrations used (Fig. 4B); at 5 ng/ml, NGF caused only a small (≈60%) increase after 4 days. The time course and dose–response of the increase in DAG levels correlated well with those of the increase in [14C]choline incorporation into [14C]PtdCho. Taken together, these results suggest that the increases in CPT activity and its saturation with DAG are the principal factors underlying the acceleration in PtdCho synthesis seen in PC12 cells stimulated with NGF.
Figure 4.
Increases in total DAG levels in PC12 cells treated with NGF. (A) PC12 cells were cultured with or without NGF (50 ng/ml) for 2 or 4 days. DAG masses in cell extracts were determined by enzymatic assay with DAG kinase. (B) DAG levels were measured in PC12 cells treated with the indicated concentrations of NGF for 4 days. Results are expressed as nmol/mg protein, and represent means ± SEM from four (A) or three (B) experiments. ∗∗, P < 0.01 compared with control. C, control.
DISCUSSION
These observations show that, as expected, PtdCho synthesis increases when neurite outgrowth is induced in PC12 cells by exposure to NGF. The continued increase in PtdCho synthesis in the course of neurite outgrowth induction (0–4 days) probably reflects the demands for additional PtdCho to form new neurites. The magnitude of the increase in PtdCho synthesis correlated with the extent of neurite outgrowth, as shown in Fig. 2, confirming this association. Our data also indicate, perhaps surprisingly, that the enhancement of PtdCho synthesis is primarily the consequence of a major increase in cellular DAG levels, which increases the substrate saturation of the CPT enzyme, which also is activated by NGF treatment. It is likely that changes in cellular DAG levels can control the CPT reaction, because the CPT enzyme appears to be largely unsaturated at resting cellular concentrations of DAG, and only about half-saturated even after 4 days of exposure to NGF. This conclusion is at odds with the hypothesis that the CT step is rate-limiting for PtdCho synthesis (1), but is supported by some previous studies on HeLa cells (2). Activation of CT can occur through translocation of the enzyme from cytosol to membrane or increased expression of the enzyme (1). We found neither an increase in total CT activity nor evidence of CT translocation in PC12 cells exposed to NGF. It thus appears that CT is not the critical step for the enhancement of PtdCho synthesis associated with neurite outgrowth. Accordingly, we suggest that the third step (CPT) in the CDPcholine pathway is limiting under the special circumstance in which PtdCho synthesis is enhanced in association with neurite outgrowth.
Our data also indicate that choline uptake is not increased when PC12 cells are stimulated with NGF. This is consistent with the observation that total phosphocholine levels did not increase after NGF treatment. The decrease in total free choline levels observed in PC12 cells exposed to NGF may reflect an increased conversion of choline to PtdCho without a compensatory increase in choline uptake.
The persistent 5-fold increase in DAG levels observed when neurite outgrowth is induced for 4 days appears to enhance PtdCho synthesis by increasing saturation of CPT. The elevation in DAG levels also may contribute to the increase in phosphatidylethanolamine levels, because DAG also serves as a substrate for CDPethanolamine diacylglycerol ethanolamine-phosphotransferase. Therefore, we propose that endogenous DAG can exert a novel regulatory function by varying the saturation of an enzyme for which it is a substrate, independent of its role as a second messenger. The source of the DAG molecules responsible for DAG’s elevated concentration in NGF-treated PC12 cells remains unresolved. DAG can derive from the recycling of membrane phospholipids via phospholipase C or phospholipase D, or from de novo synthesis. Although NGF transiently induces hydrolysis of phosphoinositides and production of DAG (21), the increase in total phospholipid levels in our PC12 cells treated with NGF suggests that the increased DAG may be supplied principally by de novo synthesis.
The mechanisms responsible for the increase in CPT activity remain unknown, because the molecular properties of mammalian CPT have not been well characterized because of difficulties in its purification (14, 22). Cloning of mammalian CPT will be an important contribution to addressing this issue. Interestingly, in chicken brain, the specific activity of CPT increases during embryonic development in parallel with the quantity of PtdCho (23). This developmental change in CPT activity is consistent with our results and provides additional evidence that PtdCho synthesis in brain is in part regulated by CPT. The Km of CPT for CDPcholine was slightly higher than reported endogenous CDPcholine levels (≈30 μM) (24), hence CDPcholine concentrations also can limit the CPT reaction. Whether NGF-induced neurite outgrowth affects CDPcholine levels remains to be determined.
In conclusion, our study shows that PtdCho synthesis actually increases when PC12 cells develop neurites and produce more membranes. Our search for the regulatory step(s) mediating this enhancement suggests that the CPT step is limiting and that DAG levels can control PtdCho formation.
Acknowledgments
We thank Denise Gallagher and Jeff Breu for excellent technical assistance. This work was supported by the National Institute of Mental Health (MH-28783) and The Center for Brain Sciences and Metabolism Charitable Trust.
ABBREVIATIONS
- CK
choline kinase
- CPT
CDPcholine:1,2-diacylglycerol cholinephosphotransferase
- CT
choline-phosphate cytidylyltransferase
- DAG
sn-1,2-diacylglycerol
- NGF
nerve growth factor
- PtdCho
phosphatidylcholine
- bFGF
basic fibroblast growth factor
References
- 1.Vance D E. In: Biochemistry of Lipids, Lipoproteins, and Membranes. Vance D E, Vance J E, editors. New York: Elsevier; 1996. pp. 153–181. [Google Scholar]
- 2.Lim P, Cornell R, Vance D E. Biochem Cell Biol. 1986;64:692–698. doi: 10.1139/o86-095. [DOI] [PubMed] [Google Scholar]
- 3.Vance J E, Pan D, Campenot R B, Bussiere M, Vance D E. J Neurochem. 1994;62:329–337. doi: 10.1046/j.1471-4159.1994.62010329.x. [DOI] [PubMed] [Google Scholar]
- 4.De Chaves E P, Vance D E, Campenot R B, Vance J E. J Cell Biol. 1995;128:913–918. doi: 10.1083/jcb.128.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Chaves E P, Vance D E, Campenot R B, Vance J E. Biochem J. 1995;312:411–417. doi: 10.1042/bj3120411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wurtman R J. Trends Neurosci. 1992;15:117–122. doi: 10.1016/0166-2236(92)90351-8. [DOI] [PubMed] [Google Scholar]
- 7.Futerman A H, Banker G A. Trends Neurosci. 1996;19:144–149. doi: 10.1016/s0166-2236(96)80025-7. [DOI] [PubMed] [Google Scholar]
- 8.Svanborg A, Svennerholm L. Acta Med Scand. 1961;169:43–49. [Google Scholar]
- 9.Labarca C, Paigen K. Anal Biochem. 1980;102:344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- 10.Lopes G, Coviella I, Wurtman R J. J Neurochem. 1992;59:338–343. doi: 10.1111/j.1471-4159.1992.tb08909.x. [DOI] [PubMed] [Google Scholar]
- 11.Uchida T, Yamashita S. Methods Enzymol. 1992;209:147–153. doi: 10.1016/0076-6879(92)09018-x. [DOI] [PubMed] [Google Scholar]
- 12.Weinhold P A, Feldman D A. Methods Enzymol. 1992;209:248–258. doi: 10.1016/0076-6879(92)09031-w. [DOI] [PubMed] [Google Scholar]
- 13.Shiratori Y, Okwu A K, Tabas I. J Biol Chem. 1994;269:11337–11348. [PubMed] [Google Scholar]
- 14.Cornell R B. Methods Enzymol. 1992;209:267–272. doi: 10.1016/0076-6879(92)09033-y. [DOI] [PubMed] [Google Scholar]
- 15.Lowry O H, Rosebrough N J, Farr A L, Randall R J. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 16.Preiss J E, Loomis C R, Bell R M, Niedel J E. Methods Enzymol. 1987;141:294–300. doi: 10.1016/0076-6879(87)41077-x. [DOI] [PubMed] [Google Scholar]
- 17.Greene L A, Tischler A S. Proc Natl Acad Sci USA. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tischler A S, Greene L A. Lab Invest. 1978;39:77–89. [PubMed] [Google Scholar]
- 19.Rydel R E, Greene L A. J Neurosci. 1987;7:3639–3653. doi: 10.1523/JNEUROSCI.07-11-03639.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watanabe O, Torda M, Meldolesi J. Neuroscience. 1983;10:1011–1024. doi: 10.1016/0306-4522(83)90239-7. [DOI] [PubMed] [Google Scholar]
- 21.Altin J G, Bradshaw R A. J Neurochem. 1990;54:1666–1676. doi: 10.1111/j.1471-4159.1990.tb01220.x. [DOI] [PubMed] [Google Scholar]
- 22.O K, Choy P C. Lipids. 1990;25:122–124. doi: 10.1007/BF02562217. [DOI] [PubMed] [Google Scholar]
- 23.Freysz L, Lastennet A, Mandel P. J Neurochem. 1972;19:2599–2605. doi: 10.1111/j.1471-4159.1972.tb01318.x. [DOI] [PubMed] [Google Scholar]
- 24.Vance D E, Trip E M, Paddon H B. J Biol Chem. 1980;255:1064–1069. [PubMed] [Google Scholar]