Abstract
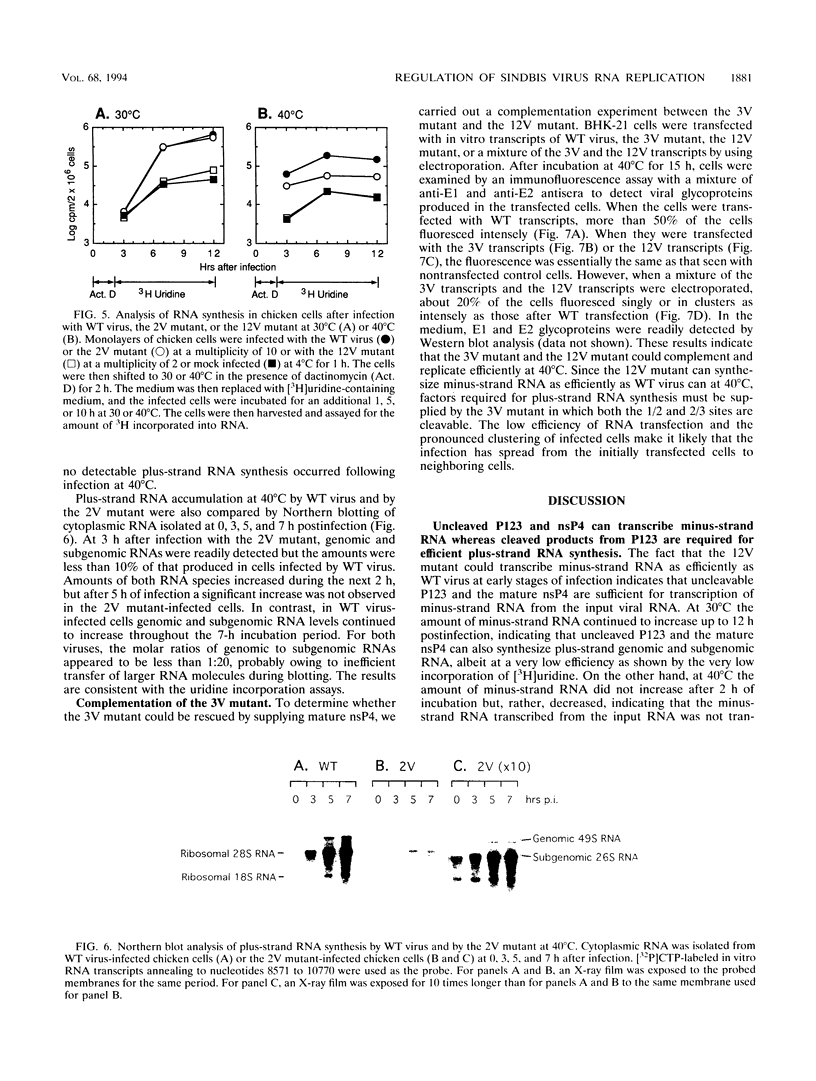
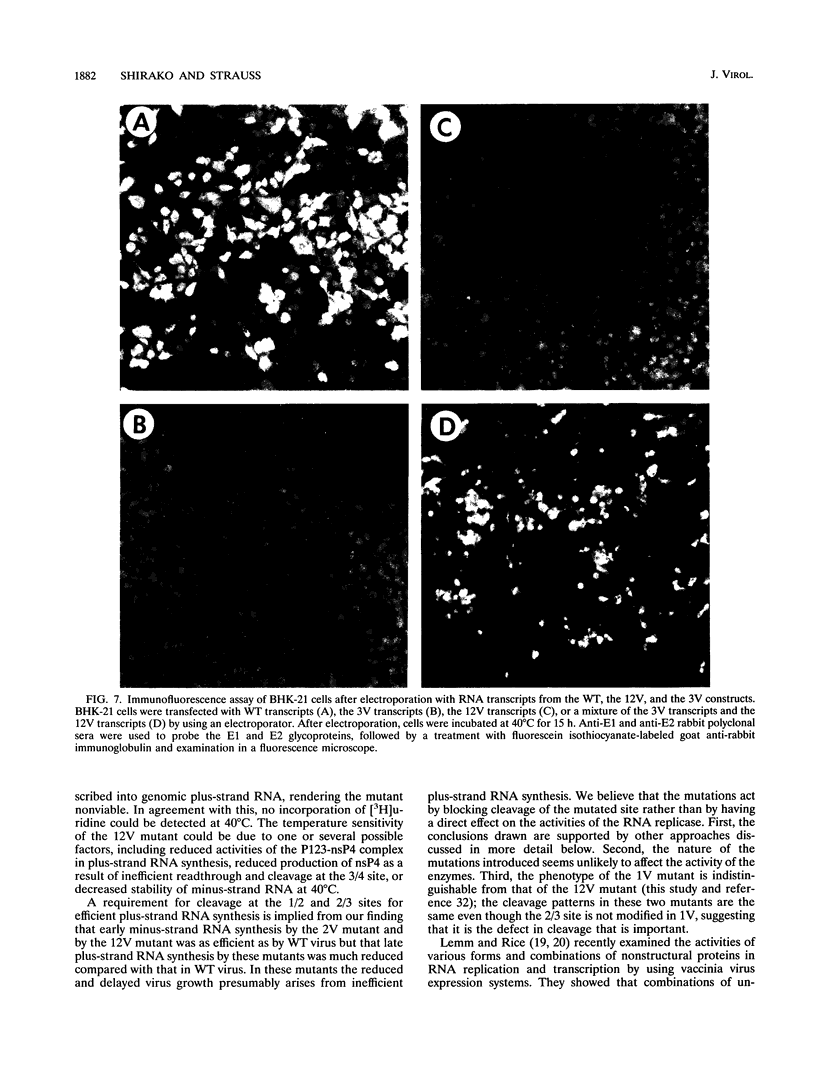
Nonstructural proteins of Sindbis virus, nsP1, nsP2, nsP3, and nsP4, as well as intermediate polyproteins, are produced from two precursor polyproteins, P123 and P1234, by a proteolytic enzyme encoded in the C-terminal half of nsP2. We studied the requirements for and the functions of the intermediate and mature processing products for Sindbis virus RNA synthesis by using site-directed mutants which have a defect(s) in processing the 1/2, 2/3, or 3/4 cleavage sites either singly or in various combinations. A mutant defective in cleaving both the 1/2 and 2/3 sites, which makes only uncleavable P123 and mature nsP4 as final products, produced 10(-3) as much virus as did the wild-type virus after 10 h at 30 degrees C and was nonviable at 40 degrees C. A mutant defective in processing the 2/3 site, which makes nsP1, nsP4, and P23 as well as precursor P123, grew 10(-1) as efficiently as wild-type virus at 30 degrees C and 10(-3) as efficiently at 40 degrees C. Early minus-strand RNA synthesis by these mutants was as efficient as that by wild-type virus, whereas plus-strand RNA synthesis was substantially decreased compared with that by wild-type virus. A mutant defective in processing the 3/4 site was nonviable at either 30 or 40 degrees C. The 3/4 site mutant could be complemented by the mutant unable to cleave either the 1/2 or 2/3 site, which can provide mature nsP4. We interpret these results to signify that (i) mature nsP4 is required for RNA replication, (ii) nsP4 and uncleaved P123 function in minus-strand RNA synthesis, and (iii) cleavage of P123 is required for efficient plus-strand RNA synthesis. We propose that Sindbis virus RNA replication is regulated by differential proteolysis of P123. Early in infection, nsP4 and uncleaved P123 form transient minus-strand RNA replication complexes which vanish upon cleavage of P123. Later in infection, an elevated level of viral proteinase activity eliminates de novo synthesis of P123, and no further synthesis of minus-strand RNA is possible. In contrast, nsP4 and cleavage products from P123 form plus-strand RNA replication complexes which are stable and remain active throughout the infection cycle.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P., Strauss E. G., Rice C. M., Strauss J. H., Haseloff J., Zimmern D. Sindbis virus proteins nsP1 and nsP2 contain homology to nonstructural proteins from several RNA plant viruses. J Virol. 1985 Feb;53(2):536–542. doi: 10.1128/jvi.53.2.536-542.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton D. J., Sawicki S. G., Sawicki D. L. Demonstration in vitro of temperature-sensitive elongation of RNA in Sindbis virus mutant ts6. J Virol. 1988 Oct;62(10):3597–3602. doi: 10.1128/jvi.62.10.3597-3602.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bransom K. L., Weiland J. J., Dreher T. W. Proteolytic maturation of the 206-kDa nonstructural protein encoded by turnip yellow mosaic virus RNA. Virology. 1991 Sep;184(1):351–358. doi: 10.1016/0042-6822(91)90851-2. [DOI] [PubMed] [Google Scholar]
- Burge B. W., Pfefferkorn E. R. Isolation and characterization of conditional-lethal mutants of Sindbis virus. Virology. 1966 Oct;30(2):204–213. doi: 10.1016/0042-6822(66)90096-1. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Ding M. X., Schlesinger M. J. Evidence that Sindbis virus NSP2 is an autoprotease which processes the virus nonstructural polyprotein. Virology. 1989 Jul;171(1):280–284. doi: 10.1016/0042-6822(89)90539-4. [DOI] [PubMed] [Google Scholar]
- Gorbalenya A. E., Blinov V. M., Donchenko A. P., Koonin E. V. An NTP-binding motif is the most conserved sequence in a highly diverged monophyletic group of proteins involved in positive strand RNA viral replication. J Mol Evol. 1989 Mar;28(3):256–268. doi: 10.1007/BF02102483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn Y. S., Grakoui A., Rice C. M., Strauss E. G., Strauss J. H. Mapping of RNA- temperature-sensitive mutants of Sindbis virus: complementation group F mutants have lesions in nsP4. J Virol. 1989 Mar;63(3):1194–1202. doi: 10.1128/jvi.63.3.1194-1202.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn Y. S., Strauss E. G., Strauss J. H. Mapping of RNA- temperature-sensitive mutants of Sindbis virus: assignment of complementation groups A, B, and G to nonstructural proteins. J Virol. 1989 Jul;63(7):3142–3150. doi: 10.1128/jvi.63.7.3142-3150.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy W. R., Hahn Y. S., de Groot R. J., Strauss E. G., Strauss J. H. Synthesis and processing of the nonstructural polyproteins of several temperature-sensitive mutants of Sindbis virus. Virology. 1990 Jul;177(1):199–208. doi: 10.1016/0042-6822(90)90473-5. [DOI] [PubMed] [Google Scholar]
- Hardy W. R., Strauss J. H. Processing the nonstructural polyproteins of Sindbis virus: study of the kinetics in vivo by using monospecific antibodies. J Virol. 1988 Mar;62(3):998–1007. doi: 10.1128/jvi.62.3.998-1007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy W. R., Strauss J. H. Processing the nonstructural polyproteins of sindbis virus: nonstructural proteinase is in the C-terminal half of nsP2 and functions both in cis and in trans. J Virol. 1989 Nov;63(11):4653–4664. doi: 10.1128/jvi.63.11.4653-4664.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamer G., Argos P. Primary structural comparison of RNA-dependent polymerases from plant, animal and bacterial viruses. Nucleic Acids Res. 1984 Sep 25;12(18):7269–7282. doi: 10.1093/nar/12.18.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonin E. V. The phylogeny of RNA-dependent RNA polymerases of positive-strand RNA viruses. J Gen Virol. 1991 Sep;72(Pt 9):2197–2206. doi: 10.1099/0022-1317-72-9-2197. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemm J. A., Rice C. M. Assembly of functional Sindbis virus RNA replication complexes: requirement for coexpression of P123 and P34. J Virol. 1993 Apr;67(4):1905–1915. doi: 10.1128/jvi.67.4.1905-1915.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemm J. A., Rice C. M. Roles of nonstructural polyproteins and cleavage products in regulating Sindbis virus RNA replication and transcription. J Virol. 1993 Apr;67(4):1916–1926. doi: 10.1128/jvi.67.4.1916-1926.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G. P., Rice C. M. Mutagenesis of the in-frame opal termination codon preceding nsP4 of Sindbis virus: studies of translational readthrough and its effect on virus replication. J Virol. 1989 Mar;63(3):1326–1337. doi: 10.1128/jvi.63.3.1326-1337.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi S., Durbin R., Huang H. V., Rice C. M., Stollar V. Association of the Sindbis virus RNA methyltransferase activity with the nonstructural protein nsP1. Virology. 1989 Jun;170(2):385–391. doi: 10.1016/0042-6822(89)90429-7. [DOI] [PubMed] [Google Scholar]
- Rice C. M., Levis R., Strauss J. H., Huang H. V. Production of infectious RNA transcripts from Sindbis virus cDNA clones: mapping of lethal mutations, rescue of a temperature-sensitive marker, and in vitro mutagenesis to generate defined mutants. J Virol. 1987 Dec;61(12):3809–3819. doi: 10.1128/jvi.61.12.3809-3819.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki D. L., Sawicki S. G. A second nonstructural protein functions in the regulation of alphavirus negative-strand RNA synthesis. J Virol. 1993 Jun;67(6):3605–3610. doi: 10.1128/jvi.67.6.3605-3610.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki D. L., Sawicki S. G. Functional analysis of the A complementation group mutants of Sindbis HR virus. Virology. 1985 Jul 15;144(1):20–34. doi: 10.1016/0042-6822(85)90301-0. [DOI] [PubMed] [Google Scholar]
- Sawicki D. L., Sawicki S. G., Keränen S., Käriäinen L. Specific Sindbis virus-coded function for minus-strand RNA synthesis. J Virol. 1981 Aug;39(2):348–358. doi: 10.1128/jvi.39.2.348-358.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki D. L., Sawicki S. G. Short-lived minus-strand polymerase for Semliki Forest virus. J Virol. 1980 Apr;34(1):108–118. doi: 10.1128/jvi.34.1.108-118.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki S. G., Sawicki D. L., Käriäinen L., Keränen S. A Sindbis virus mutant temperature-sensitive in the regulation of minus-strand RNA synthesis. Virology. 1981 Nov;115(1):161–172. doi: 10.1016/0042-6822(81)90098-2. [DOI] [PubMed] [Google Scholar]
- Sawicki S. G., Sawicki D. L. The effect of loss of regulation of minus-strand RNA synthesis on Sindbis virus replication. Virology. 1986 Jun;151(2):339–349. doi: 10.1016/0042-6822(86)90054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidel L. M., Stollar V. Mutations that confer resistance to mycophenolic acid and ribavirin on Sindbis virus map to the nonstructural protein nsP1. Virology. 1991 Apr;181(2):490–499. doi: 10.1016/0042-6822(91)90881-b. [DOI] [PubMed] [Google Scholar]
- Shirako Y., Strauss J. H. Cleavage between nsP1 and nsP2 initiates the processing pathway of Sindbis virus nonstructural polyprotein P123. Virology. 1990 Jul;177(1):54–64. doi: 10.1016/0042-6822(90)90459-5. [DOI] [PubMed] [Google Scholar]
- Shirako Y., Wilson T. M. Complete nucleotide sequence and organization of the bipartite RNA genome of soil-borne wheat mosaic virus. Virology. 1993 Jul;195(1):16–32. doi: 10.1006/viro.1993.1342. [DOI] [PubMed] [Google Scholar]
- Strauss E. G., De Groot R. J., Levinson R., Strauss J. H. Identification of the active site residues in the nsP2 proteinase of Sindbis virus. Virology. 1992 Dec;191(2):932–940. doi: 10.1016/0042-6822(92)90268-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E. G., Rice C. M., Strauss J. H. Complete nucleotide sequence of the genomic RNA of Sindbis virus. Virology. 1984 Feb;133(1):92–110. doi: 10.1016/0042-6822(84)90428-8. [DOI] [PubMed] [Google Scholar]
- Wang Y. F., Sawicki S. G., Sawicki D. L. Sindbis virus nsP1 functions in negative-strand RNA synthesis. J Virol. 1991 Feb;65(2):985–988. doi: 10.1128/jvi.65.2.985-988.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot R. J., Hardy W. R., Shirako Y., Strauss J. H. Cleavage-site preferences of Sindbis virus polyproteins containing the non-structural proteinase. Evidence for temporal regulation of polyprotein processing in vivo. EMBO J. 1990 Aug;9(8):2631–2638. doi: 10.1002/j.1460-2075.1990.tb07445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot R. J., Rümenapf T., Kuhn R. J., Strauss E. G., Strauss J. H. Sindbis virus RNA polymerase is degraded by the N-end rule pathway. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8967–8971. doi: 10.1073/pnas.88.20.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]