Abstract
The present report presents the findings in 8 countries at the end of the first 5 years of an international investigation into the possible relationship between acute persisting spinal paralysis and the use of oral poliomyelitis vaccine.
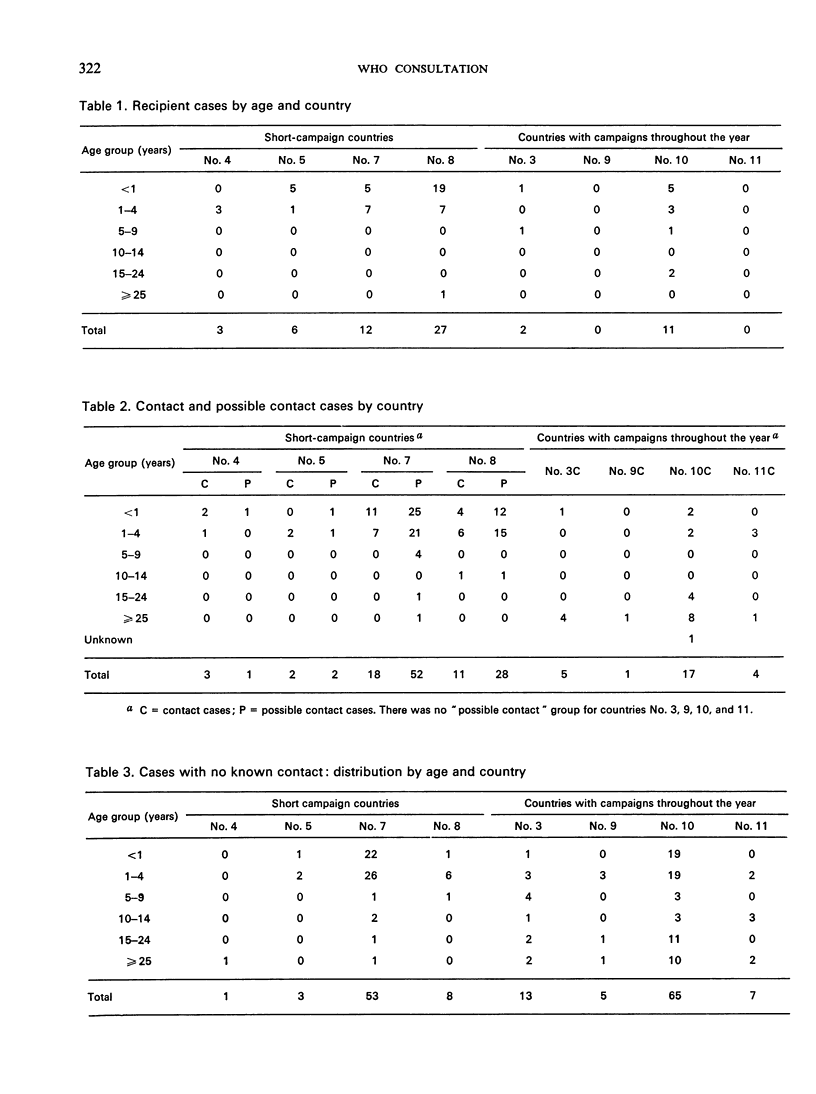
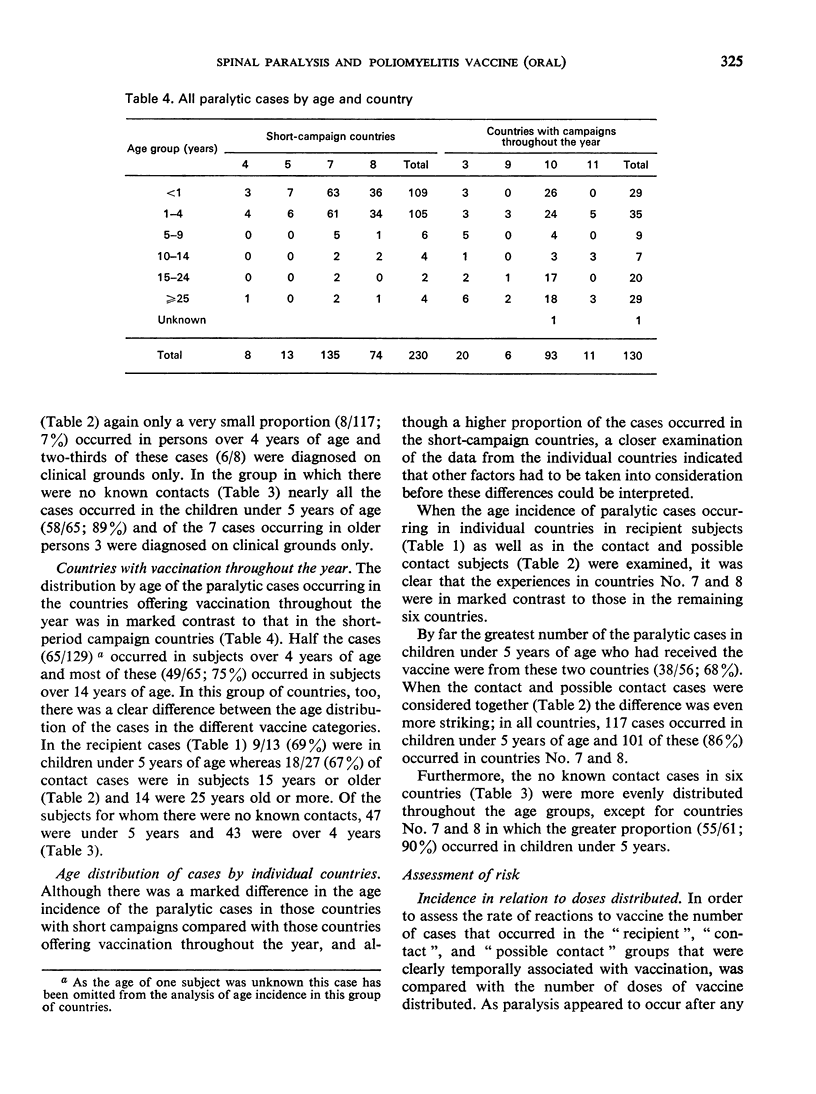
The most striking finding was the high association with type 3 virus in the recipient cases and with type 2 virus in the “contacts” and “possible contacts”. Most of the cases in the recipient groups occurred in children under 5 years of age in all countries, but in the “contact” groups in the countries in which vaccination is offered through the year, many of the cases occurred in the non-immune parents of recently vaccinated infants.
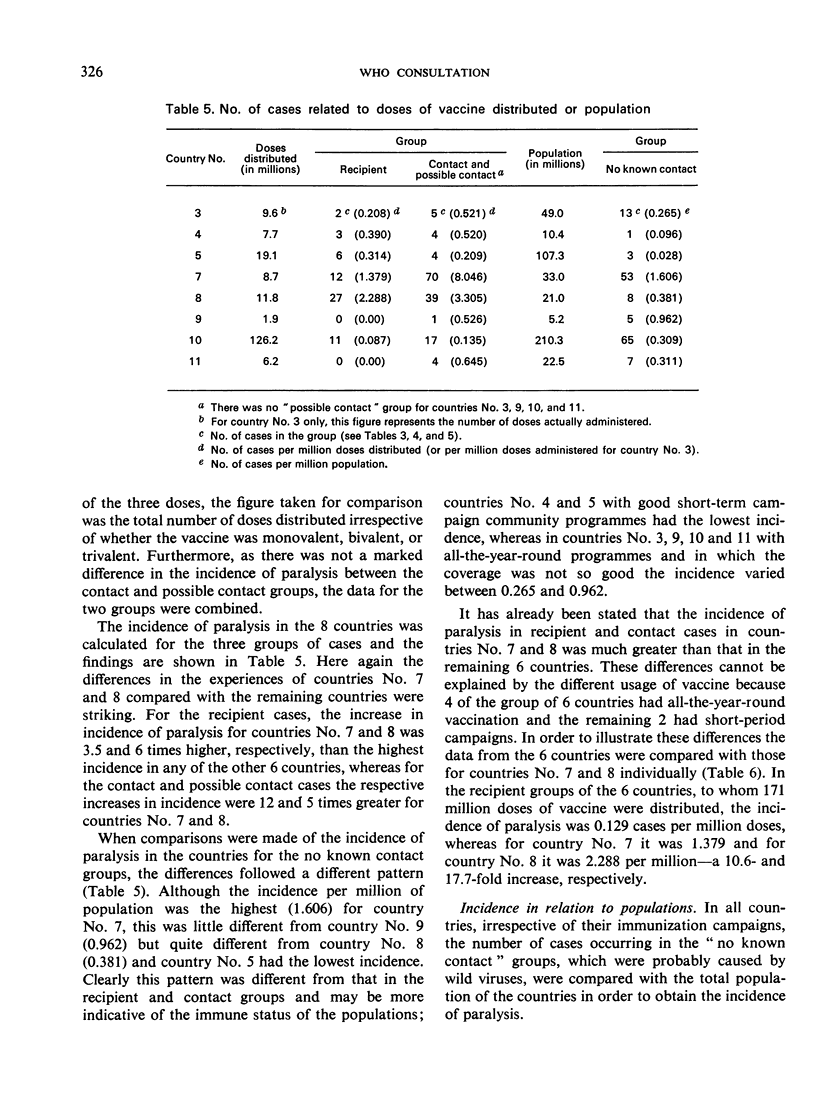
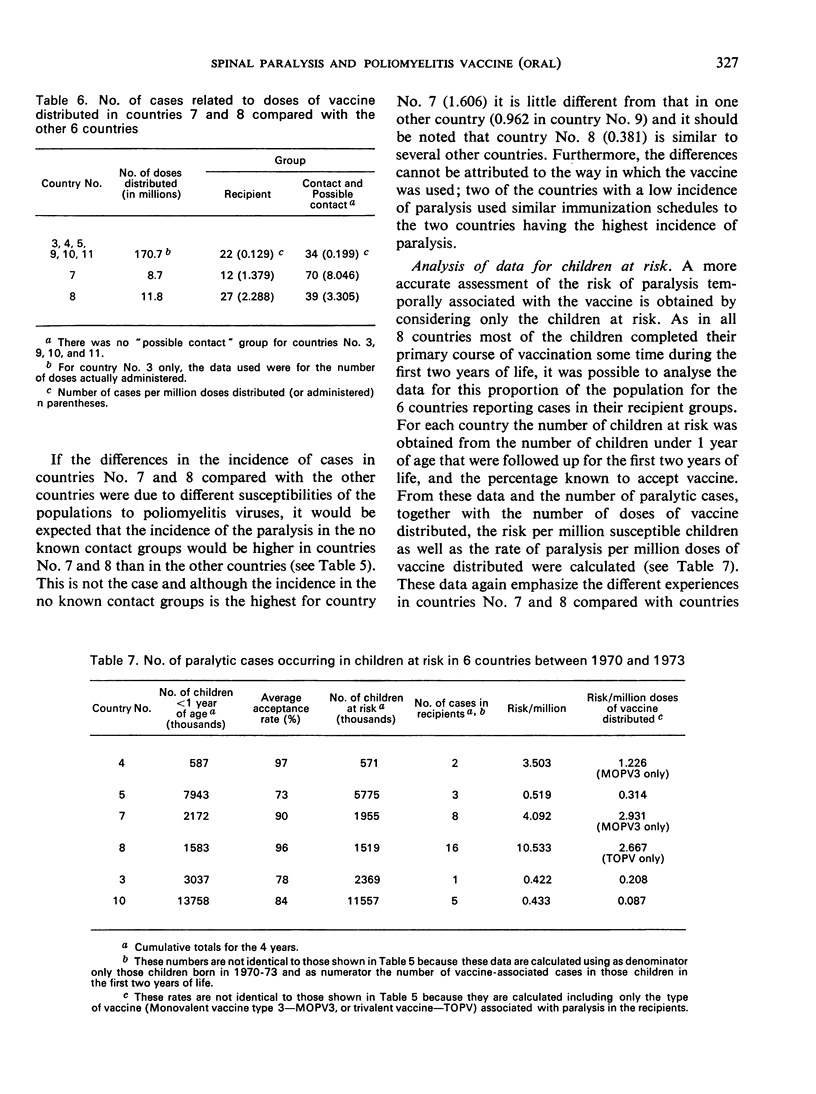
There were marked differences among countries, and it was not possible to pinpoint a single factor as the sole cause. However, the quality of the vaccine clearly played an important role. For some time, and certainly at the beginning of this enquiry, some of the countries were using vaccine from the same source without continuous external control and were using seed viruses at high passage levels. The situation changed during the enquiry and the incidence of paralytic cases decreased. The enquiry will be continued and particular efforts will be made to establish the cause of the associated paralysis. The findings of the enquiry confirm that oral Sabin poliomyelitis vaccines are among the safest vaccines in use today.
Full text
PDF