Abstract
The cytoplasmic region of Fas, a mammalian death factor receptor, shares a limited homology with reaper, an apoptosis-inducing protein in Drosophila. Expression of either the Fas cytoplasmic region (FasC) or of reaper in Drosophila cells caused cell death. The death process induced by FasC or reaper was inhibited by crmA or p35, suggesting that its death process is mediated by caspase-like proteases. Both Ac-YVAD aldehyde and Ac-DEVD aldehyde, specific inhibitors of caspase 1- and caspase 3-like proteases, respectively, inhibited the FasC-induced death of Drosophila cells. However, the cell death induced by reaper was inhibited by Ac-DEVD aldehyde, but not by Ac-YVAD aldehyde. A caspase 1-like protease activity that preferentially recognizes the YVAD sequence gradually increased in the cytosolic fraction of the FasC-activated cells, whereas the caspase 3-like protease activity recognizing the DEVD sequence was observed in the reaper-activated cells. Partial purification and biochemical characterization of the proteases indicated that there are at least three distinct caspase-like proteases in Drosophila cells, which are differentially activated by FasC and reaper. The conservation of the Fas-death signaling pathway in Drosophila cells, which is distinct from that for reaper, may indicate that cell death in Drosophila is controlled not only by the reaper suicide gene, but also by a Fas-like killer gene.
Fas, also called APO-1 or CD95, is a type I membrane protein belonging to the tumor necrosis factor (TNF) receptor family, and is expressed widely in a variety of mammalian cells (1). The Fas ligand, a member of the TNF family, is predominantly expressed in activated T cells and natural killer (NK) cells, plays an important role in down-regulating the immune response, and works as an effector molecule for cytotoxic T cells and NK cells (1).
Binding of the Fas ligand to Fas induces trimerization of the Fas receptor and activates a cascade of caspases, cysteine proteases related to interleukin 1β-converting enzyme (ICE), which in turn kill the Fas-bearing cells by inducing apoptosis (1). Mutational analysis of the Fas cytoplasmic region (FasC) identified a 68-amino acid domain that is responsible for the apoptotic signal transduction (2). A similar domain has been identified in the TNF type I receptor, which also transduces the apoptotic signal (3). These regions with apoptosis-inducing activity are known as the “death domain.” In the current model for Fas-induced apoptosis, the trimerized Fas receptor is bound by a death-domain-containing adaptor called FADD or MORT1, through which a member of the caspase family of proteases (caspase 8) is recruited to the receptor to trigger the caspase cascade (1, 4–8). The Fas-mediated apoptotic system appears to be conserved among mammals, because the Fas gene has been identified in various mammalian species, including human, mouse, rat, bovine, and cat. However, no homologues for Fas or Fas ligand have been identified in an invertebrate.
Reaper is a 65-amino acid protein that is expressed in Drosophila cells and has a tendency to self-aggregate (9). Flies carrying a deletion of a region containing genes for reaper, hid, and grim exhibit no apoptotic cell death during embryogenesis. The exogenous expression of reaper in Drosophila eyes as a transgene or in Drosophila cell lines causes cell death (10, 11). On the basis of these results, the reaper gene has been identified as a suicide gene that regulates programmed cell death in Drosophila (12). As with the Fas-induced apoptosis, reaper-induced apoptosis is mediated by caspases, as evidenced by the fact that specific caspase inhibitors prevent reaper-induced cell death (10, 11).
No mammalian homologues for reaper have yet been identified. However, close inspection of the amino acid sequence of FasC and reaper indicates that the death domains of the Fas and TNF type I receptors share a limited homology with reaper (13), suggesting that Fas and reaper induce apoptosis by similar mechanisms. In this report, we used an inducible promoter to express FasC and reaper in Drosophila cell lines. The induced expression of either reaper or FasC in Drosophila cell lines caused cell death, which could be blocked by inhibitors specific for caspases. Studies using specific caspase inhibitors indicated that Fas predominantly activated caspase 1 (ICE)-like protease, whereas reaper activated caspase 3 (CPP32)-like proteases, suggesting that Fas and reaper use different signal transduction pathways to cause apoptosis. The caspase-like proteases activated by Fas and reaper were partially purified and biochemically characterized. These analyses indicated that at least three different caspase-like proteases, which are differentially activated by Fas and reaper, occur in Drosophila cells.
MATERIALS AND METHODS
Materials and Expression Vectors.
Acetyl-Tyr-Val-Ala-Asp aldehyde (Ac-YVAD-CHO), acetyl-Asp-Glu-Val-Asp aldehyde (Ac-DEVD-CHO), acetyl-Tyr-Val-Ala-Asp chloromethyl ketone (Ac-YVAD-CMK), and benzyloxycarbonyl-Leu-Leu-Leu aldehyde (Z-LLL-CHO) were purchased from the Peptide Institute (Osaka, Japan). Nα-Tosyl-Phe chloromethyl ketone (TPCK) was obtained from Calbiochem. MCA-YVADAPK(DNP) and MCA-DEVDAPK(DNP), carrying the specific peptide between the highly fluorescent (7-methoxycoumarin-4-yl)acetyl group (MCA) and its quenching 2,4-dinitrophenyl (DNP) group, were custom-synthesized at the Peptide Institute.
The DNA fragment that encodes a chimeric protein consisting of two tandem FK506-binding protein (FKBP) and the human Fas cytoplasmic region (amino acids 175–319) (14) was provided by M. Gilman (ARIAD Gene Therapeutics, Cambridge, MA). A 1.5-kb cowpox virus DNA fragment encoding crmA (15) was provided by D. J. Pickup (Duke University Medical Center, Durham, NC). The 198-bp coding sequence of reaper (9) was amplified by PCR from a Drosophila melanogaster cDNA library (CLONTECH), using oligonucleotides (5′-ACTGGATCCCAATGGCAGTGGCATTCTA-3′ and 5′-AAAGGATCCTCATTGCGATGGCTTGC-3′), and inserted into pBluescript (Stratagene) to generate pBS-rpr. The 900-bp coding sequence of p35 (16) was amplified by PCR from Autographa californica nuclear polyhedrosis virus, using oligonucleotides (5′-CCCGAATTCATGTGTGTAATTTTTCCG-3′ and 5′-CCCGGATCCTTATTTAATTGTGTTTAA-3′). The authenticity of the cDNAs was confirmed by DNA sequencing. To express FasC, reaper, crmA, and p35 in Drosophila cells, the respective DNA fragments were placed under the control of the Drosophila metallothionein promoter of pRmHa3 (17), and the resultant plasmid DNAs were designated pMT-FasC, pMT-rpr, pMT-crmA, and pMT-p35, respectively.
Drosophila Cell Lines, Transformation, and Cell Death Assay.
The Drosophila cell lines, Schneider’s Drosophila line 2 (ATCC CRL 1963) and GM2 cells (18), both established from Drosophila embryos, were maintained at 25°C in Schneider’s Drosophila medium (GIBCO/BRL) supplemented with 15% fetal bovine serum (HyClone). As selection markers, the G418- and hygromycin-resistance genes were transferred to the Drosophila expression vector pAct5CO (19), which carries the Drosophila actin 5C promoter, and were designated pACTneo and pACThyg, respectively. Transfection of Drosophila cells was performed by electroporation. In brief, 5 × 106 cells in 0.8 ml of K-PBS buffer (30.8 mM NaCl/120.7 mM KCl/8.1 mM Na2HPO4/1.46 mM KH2PO4) containing 50 μg of ScaI-digested pMT-FasC, pMT-rpr, pMT-p35, or pMT-crmA and 5 μg of XhoI-digested pACTneo or pACThyg were exposed to a 230-V pulse with a capacitance of 960 μF using a Gene Pulsar (Bio-Rad). Cells were cultured in 16 ml of growth medium containing 1 mg/ml G418 or 0.6 mg/ml hygromycin for 10 days, and individual clones were isolated by limiting dilution.
To examine the ability of Fas and reaper to kill the cells, the transformants (1 × 105 cells in 100 μl wells of a 96-well microtiter plate) were cultured in medium containing 0.5 mM CuSO4 for various periods of time, and their growth was examined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (20). In brief, 20 μl of MTT (2.5 μg/ml) dissolved in PBS was added to each well and incubated for 5 hr at 25°C. The precipitates were dissolved by adding 100 μl of acid isopropyl alcohol (0.04 M HCl in isopropyl alcohol), and the absorbance was measured using a MicroELISA reader with a test wavelength of 540 nm and a reference wavelength of 690 nm. In some cases, the apoptotic cells were stained by a modified TUNEL (terminal deoxynucleotidyltransferase-mediated UTP end-labeling) procedure using an ApopTag-fluorescein in situ apoptosis detection kit (Oncor) and analyzed by flow cytometry as described (21).
Northern Blotting.
Schneider cell transformants of Fas or reaper were treated with 0.5 mM CuSO4, and poly(A)+ RNA was prepared by using the Quick Prep micro mRNA preparation Kit (Pharmacia). Poly(A)+ RNA (0.5 μg) was fractionated by electrophoresis on a 1.5% agarose gel in the presence of 2.2 M formaldehyde and transferred to a nitrocellulose filter. The filter was hybridized with 32P-labeled probes under high-stringency conditions. The probe DNA fragments for Fas and reaper were obtained from pMT-FasC and pBS-rpr, respectively. The 461-bp coding sequence of the Drosophila ribosomal protein 49 gene (rp49) (22) was amplified by PCR from the cDNA library of D. melanogaster, using the oligonucleotides (5′-ATGACCATCCGCCCAGCA-3′ and 5′-TTACCTCGTTCTTCTTGA-3′) and inserted into pBluescript, and its insert was used as a probe.
Assay for Caspase Activity.
The cell lysates were prepared essentially as described (23). In brief, 1 × 108 cells were washed with PBS, suspended in 1 ml of 0.1 M Hepes–KOH buffer (pH 7.5) containing 10% sucrose, 0.1% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid (CHAPS), 2 mM dithiothreitol, 0.1 mg/ml ovalbumin, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM pepstatin, and 1 μg/ml leupeptin (ICE buffer), and lysed by freezing and thawing twice. The cell lysates were spun at 30,000 × g for 10 min to remove cell debris and nuclei, then spun again at 100,000 × g for 2 hr to obtain the cytosolic fraction (S-100).
The caspase 1- and caspase 3-like activities were measured with specific fluorescent substrates as described (24). Proteins were incubated at 30°C for 60 min in a final volume of 100 μl of ICE buffer containing the fluorescent substrate (1 μM) in the presence or absence of the specific inhibitor (10 μM) for caspase 1 (Ac-YVAD-CMK) or caspase 3 (Ac-DEVD-CHO). After incubation, the fluorescence of the cleaved substrates was determined in a spectrofluorometer set at an excitation wavelength of 325 nm and an emission wavelength of 392 nm. The specific caspase 1- and caspase 3-like activities were determined by subtracting the value obtained in the presence of the inhibitor from the value obtained in the absence of inhibitors.
DEAE-Sephadex Column Chromatography.
Schneider cell transformants of FasC and reaper were cultured for 12 hr in medium containing 0.5 mM CuSO4. The cell lysates (cytosolic fraction) were prepared as described above. Twenty-five milligrams of protein was applied to a DEAE-Sephadex column (0.8 cm × 4 cm) that was equilibrated with 0.1 M Hepes–KOH buffer (pH 7.5) containing 10% sucrose, 0.1% CHAPS, 2 mM dithiothreitol, and 1 mM PMSF (buffer A). After the column had been washed with buffer A, proteins were eluted with a linear NaCl gradient from 0 to 0.5 M in buffer A, in a total volume of 20 ml. The flow rate was 10 ml/hr, and 0.5-ml fractions were collected. The protein concentration was monitored by measuring absorbance at 280 nm, and the caspase activity was measured in 20-μl aliquots, as described above.
RESULTS
Overexpression of Fas Induces Cell Death in Drosophila Cells.
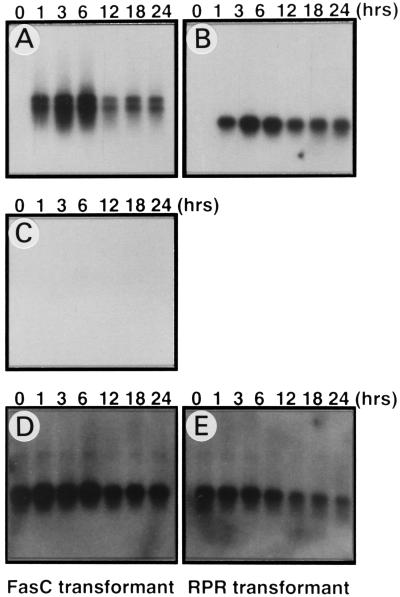
The Fas cytoplasmic region must be oligomerized to induce apoptosis (25), and the Fas cytoplasmic region has a tendency to self-aggregate (26). We used a chimeric protein consisting of the cytoplasmic region of human Fas attached to two copies of FKBP that can be induced to aggregate by FK1012 (14). The chimeric cDNA was placed under the control of a Drosophila metallothionein promoter (17), and stable transformant cell lines of Schneider’s Drosophila line 2 (S2) and GM2 cells that harbor the Fas expression plasmid (MT-FasC) were established. A similar expression plasmid (MT-rpr) for Drosophila reaper gene was also constructed and was stably introduced into GM2 and S2 cells. When the transformants were grown in medium without CuSO4, no detectable expression of Fas or reaper mRNA was detected by Northern hybridization (Fig. 1 A and B). However, the treatment of the transformed cell lines with 0.5 mM CuS04 quickly induced mRNA for Fas or reaper, which peaked 6 hr after treatment with CuSO4. Aberrant development or γ-ray irradiation activates reaper gene expression in Drosophila and causes cell death (27). However, the CuSO4 treatment of the parental Schneider cells or their transformants harboring the Fas expression plasmid did not induce reaper gene expression (Fig. 1C).
Figure 1.
Induced expression of FasC and reaper mRNAs in Drosophila cell transformants. Schneider cells transformed with either pMT-FasC (A, C, and D) or pMT-rpr (B and E) were treated with 0.5 mM CuSO4 for the indicated times. Poly(A)+ RNA (0.5 μg per lane) was resolved by electrophoresis on 1.5% agarose gels containing 2.2 M formaldehyde and was analyzed by Northern hybridization using the cDNA for the human Fas cytoplasmic region (A), reaper (B, C), and rp49 (D, E) as probes.
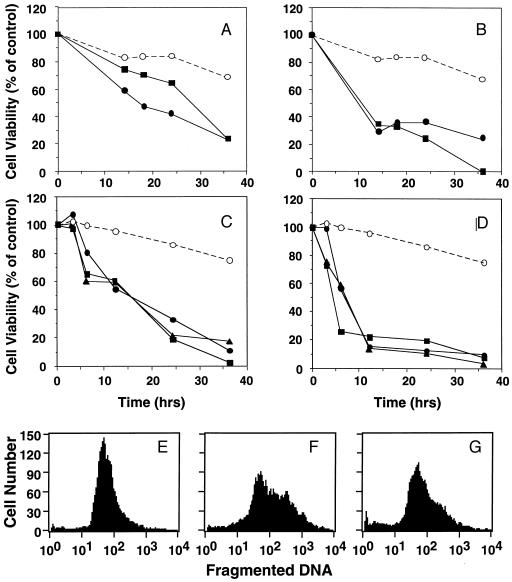
We then examined whether the induced expression of FasC or of reaper could kill the cells. As shown in Fig. 2, 0.5 mM CuSO4 was slightly toxic to the parental S2 or GM2 cells, and about 30% of the cells gradually died during the 36-hr period. On the other hand, as previously reported (10, 11), the cells were efficiently killed by the induced expression of reaper by CuSO4 treatment (Fig. 2 B and D). The induced expression of FasC in GM2 or S2 cells also caused cell death, although this process was significantly slower than that observed with the induced expression of reaper (Fig. 2 A and B). The cell death of Drosophila cells induced by reaper and Fas was accompanied by DNA fragmentation. That is, when the dying S2 cells were analyzed by the TUNEL procedure, which detects fragmented DNA, about 50% reaper transformants or about 30% Fas transformants were strongly positive 18 hr after treatment with CuSO4 (Fig. 2 E–G).
Figure 2.
Cell death of Drosophila cell transformants expressing either FasC or reaper. (A–D) Time course of cell death. Samples (1 × 105) of the parental Drosophila cells (○) or their transformants (two or three clones) harboring the expression plasmid for either human FasC or Drosophila reaper (filled symbols) were treated with 0.5 mM CuSO4, and the cell viability was determined by MTT assay. The cell viability is expressed as percentage of the control cells that were cultured for the same periods without CuSO4. (A) GM2 cells and their transformants harboring the expression plasmid for human FasC. (B) GM2 cells and their transformants harboring the expression plasmid for reaper. (C) Schneider S2 cells and their transformants for human Fas. (D) Schneider S2 cells and their reaper transformants. (E–G) TUNEL staining of dying Schneider cells. The parental S2 cells (E) and their transformants harboring pMT-rpr (F) or pMT-FasC (G) were treated for 18 hr with 0.5 mM CuSO4, stained with ApopTag, and analyzed by flow cytometry in a FACScan (Becton Dickinson).
Involvement of Caspases in Cell Death of Drosophila Cells.
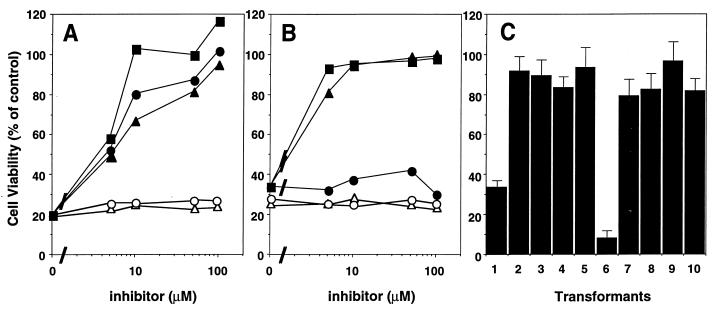
The Fas-induced apoptosis in mammalian cells is mediated by caspases (1), and competitive inhibitors of caspases prevent the process (24). To examine whether caspases are also involved in Fas- and reaper-induced cell death in Drosophila cells, the cell death process was assayed in the presence of caspase inhibitors. Ac-YVAD-CHO and Ac-DEVD-CHO bind to the active sites of caspase 1- and caspase 3-like proteases, respectively, and specifically inhibit their activities (28). Ac-YVAD-CMK covalently binds to the active site of caspase and has a broad specificity. None of the inhibitors at 500 μM had any effect on the growth of the parental Schneider cells (data not shown), although this concentration was sufficient to completely block the death process induced by FasC for all of the inhibitors (Fig. 3A). Further examination showed that the inhibition of the cell death process by these reagents was dose-dependent, with a concentration of 100 μM being sufficient for each inhibitor to completely prevent cell death. On the other hand, Z-LLL-CHO (an inhibitor of proteasome) or TPCK (an inhibitor of chymotrypsin) did not inhibit the cell death caused by Fas or reaper (Fig. 3). These results indicate that both caspase 1- and caspase 3-like proteases are involved in the Fas-induced cell death of Schneider cells. On the other hand, Ac-DEVD-CHO and Ac-YVAD-CMK prevented the reaper-induced cell death, but Ac-YVAD-CHO had little effect on this process, suggesting that a caspase 3-like protease(s) plays a major role in reaper-induced cell death in Schneider cells (Fig. 3B).
Figure 3.
Effect of caspase inhibitors on FasC- and reaper-induced cell death in Schneider cells. (A and B) Effect of the peptide inhibitors. Samples (1 × 105) of Drosophila Schneider cell transformants harboring the expression plasmid for FasC (A) or reaper (B) were treated at 25°C for 24 hr with 0.5 mM CuSO4 in the presence of the indicated concentrations of Ac-DEVD-CHO (▪), Ac-YVAD-CHO (•), Ac-YVAD-CMK (▴), Z-LLL-CHO (○), or TPCK (▵). The cell viability was determined by MTT assay, and values are expressed as percentages of the control cells, which were cultured without CuSO4. (C) Effect of crmA and p35. The transformants harboring the expression plasmids for FasC alone (lane 1), FasC and crmA (lanes 2 and 3), FasC and p35 (lanes 4 and 5), reaper alone (lane 6), reaper and crmA (lanes 7 and 8), or reaper and p35 (lanes 9 and 10), were treated at 25°C for 18 hr with 0.5 mM CuSO4. The cell viability was determined by MTT assay.
The product of crmA, a cytokine-response modifier gene carried by cowpox virus, and p35 protein, encoded by baculovirus, inhibit some caspases and block Fas-induced apoptosis (29–31). To confirm the involvement of caspases in Fas-induced apoptosis of Drosophila cells, the genes for crmA or p35 were placed under the promoter of metallothionein and introduced into S2 cell transformants harboring FasC or reaper. As shown in Fig. 3C, the expression of p35 or crmA blocked the cell death of Scheider cells induced by overexpression of FasC or reaper. These results suggested that human Fas killed Drosophila cells by activating caspases
Activation of Caspases in Drosophila Cells.
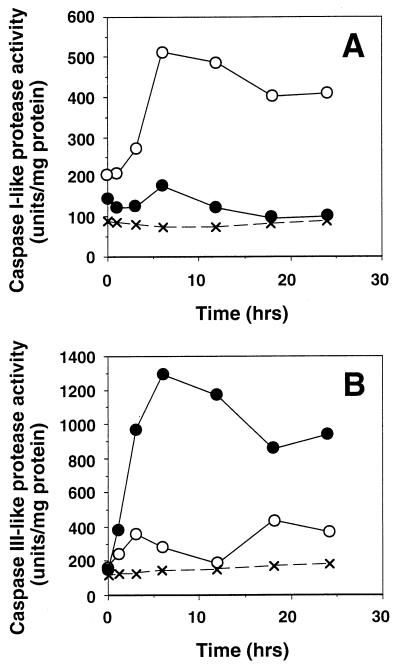
We then monitored the caspase activities in the Schneider cells undergoing apoptosis. We used two different fluorescent substrates, MCA-YVADAPK(DNP) and MCA-DEVDAPK(DNP), which are specific substrates for caspase 1- and caspase 3-like proteases, respectively (24). The Fas and reaper transformants of Schneider cells were cultured for various periods of time in the presence of 0.5 mM CuSO4, after which the caspase-like activities in the cytosolic extracts were determined by using the two fluorescent substrates. Low levels of caspase 1- and caspase 3-like activities were detected in the extracts from uninduced cell lines. The induced expression of Fas caused a gradual increase in the caspase 1-like activity, which peaked approximately 8 hr after induction, and remained relatively high throughout the incubation period (Fig. 4A). Caspase 3-like protease activity was also detected in the cells expressing FasC, and this activity appeared biphasically, with the first peak at 3 hr after induction, and the second peak at a later stage of cell death (Fig. 4B). The expression of reaper also induced the caspase 1-like activity, but this activity was only one-fifth of that detected in the extracts from the Fas-activated cells (Fig. 4A). In contrast, the overexpression of reaper induced caspase 3-like proteases much more strongly than did FasC. As shown in Fig. 4B, the caspase 3-like activity peaked approximately 6 hr after induction, and its high activity level remained throughout the rest of the incubation period. These results suggest that Fas and reaper activate caspases in different manners.
Figure 4.
Activation of caspase 1-like and caspase 3-like proteases by Fas or reaper expression. Schneider cells (×) and their transformants harboring the expression plasmid for either FasC (○) or reaper (•) were treated with CuSO4 for the indicated periods of time. Cytosolic extracts were prepared from these cells, and caspase 1 and caspase 3 protease activities were determined using the fluorescent substrate MCA-YVADAPK(DNP) (A) or MCA-DEVDAPK(DNP) (B).
Caspases Activated by Fas and Reaper.
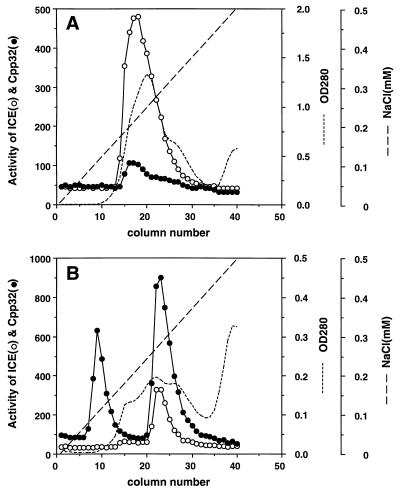
To biochemically characterize the caspases activated by Fas and reaper, Schneider cells harboring the Fas or reaper expression vector were treated for 12 hr with CuSO4, then the cell extracts were prepared and fractionated by DEAE-Sephadex column chromatography. As shown in Fig. 5A, chromatography of the extracts from the Fas-activated cells gave rise to a single peak of protease activity (ICE-Fas), which preferentially cleaved MCA-YVADAPK(DNP). On the other hand, when the extracts from the reaper-activated cells were fractionated, two peaks of protease activity were observed. The first peak (ICE-RPR-A) cleaved MCA-DEVDAPK(DNP) but not MCA-YVADAPK(DNP). On the other hand, the second peak (ICE-RPR-B) preferentially cleaved MCA-DEVDAPK(DNP) but also showed a weak MCA-YVADAPK(DNP)-cleaving activity. These results confirm that Fas and reaper activate distinct caspase-like proteases in Drosophila cells.
Figure 5.
Fractionation of caspases activated by FasC or reaper by DEAE-column chromatography. Schneider’s cell transformants for human FasC (A) or reaper (B) were treated with 0.5 mM CuSO4 for 12 hr. Cytosolic extracts (S100) were prepared from these cells. Samples of extract containing approximately 25 mg of protein from the FasC and reaper transformants were fractionated by DEAE-column chromatography with a linear gradient of NaCl from 0 to 0.5 M. Caspase 1-like (○) and caspase 3-like (•) activities in each fraction were determined by using specific fluorescent substrates. The short-dashed lines indicate the absorbance at 280 nm. The fractions containing the peaks of caspase activity from FasC transformants (fractions 15–21) and from reaper transformants (fractions 8–11 and 21–26) were pooled. These samples were designated as ICE-FasC, ICE-RPR-A, and ICE-RPR-B, respectively, and used for subsequent experiments described in Fig. 6.
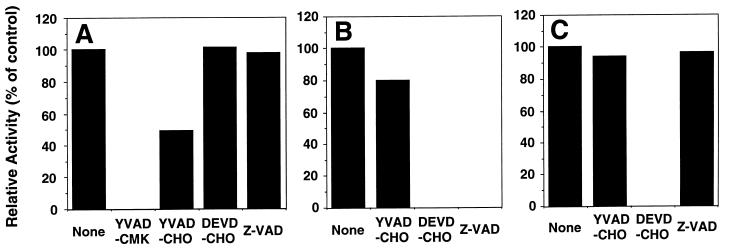
To further characterize the substrate specificities of the caspase-like proteases activated by Fas and reaper, the effects of various caspase inhibitors on these enzyme activities were examined. As shown in Fig. 6, the caspase 1-like protease activity of ICE-Fas was inhibited completely by 10 μM Ac-YVAD-CMK and partially by 10 μM Ac-YVAD-CHO. Even 1 μM Ac-YVAD-CMK was potent to inhibit the ICE-Fas (data not shown). In contrast, neither Ac-DEVD-CHO nor Z-VAD-fluoromethyl ketone (FMK) inhibited ICE-Fas at the same concentration. On the other hand, the caspase 3-like protease activity of ICE-RPR-A was completely inhibited by 10 μM either Ac-DEVD-CHO or Z-VAD-FMK, but Ac-YVAD-CHO had only a marginal effect on this protease activity. The caspase 3-like activity of ICE-RPR-B was completely inhibited by Ac-DEVD-CHO but by neither Ac-YVAD-CHO nor Z-VAD-FMK. These results suggested that ICE-Fas, ICE-RPR-A, and ICE-RPR-B are caspase-like proteases that have distinct substrate specificities.
Figure 6.
Three distinct caspases in Drosophila that are activated by FasC and reaper. ICE-FasC (A), ICE-RPR-A (B), and ICE-RPR-B (C) (25 μl) were preincubated at 4°C for 30 min in the absence or presence of 10 μM Ac-YVAD-CMK, Ac-YVAD-CHO, Ac-DEVD-CHO, or Z-VAD-fluoromethyl ketone. Caspase 1-like and caspase 3-like activities were then determined by using specific fluorescent substrates.
DISCUSSION
The Fas cytoplasmic region should be trimerized to transduce the apoptotic signal, specifically to recruit the signaling molecule to the Fas receptor (25, 32). We showed here that the induced expression of monomeric Fas cytoplasmic region caused cell death in Drosophila cells. When FK1012, which is known to cross-link FKBP (33), was added to the Fas transformants, the cell death kinetics were similar to those observed in the absence of FK1012 (data not shown). The death domain of the Fas cytoplasmic region has a tendency to aggregate (26) and its overexpression causes apoptosis in mammalian cells (34). Therefore, it is possible that the overexpressed FasC in Drosophila cells also underwent self-aggregation and killed the cells.
The cell death kinetics in Drosophila cell lines induced by mammalian Fas is significantly slower than that observed with reaper. When Drosophila cells are induced to die by γ-ray irradiation or by aberrant development, the cells express reaper, which appears to mediate the cell death process (27). On the other hand, no reaper mRNA was detected when the Schneider cells underwent the Fas-induced apoptosis (Fig. 1), suggesting that FasC induces cell death in Drosophila cells independently from reaper. In the mammalian system, the adaptor molecule called FADD/MORT1 links the Fas death domain to a caspase (caspase 8), thereby activating it (1). It will be interesting to study whether the caspases activated by Fas and reaper in Drosophila cells directly bind to reaper or Fas death domains, or whether additional adaptor molecules are required to link them.
In mouse WR19L cells, the Fas engagement activates a caspase 1-like protease, and the activation of a caspase 3-like protease follows (24). Like the apoptosis in mammalian cells, the Fas-induced apoptosis of Drosophila cells was inhibited by caspase inhibitors, crmA and p35. Furthermore, Ac-YVAD-CHO, an inhibitor of caspase 1, as well as Ac-DEVD-CHO, an inhibitor of caspase 3, prevented the Fas-induced apoptosis, indicating that both caspase-1 and -3-like proteases are involved in Fas-induced apoptosis in Drosophila cells. Accordingly, we could detect protease activities in Drosophila cells undergoing apoptosis by Fas activation. The major protease activated by Fas recognized the YVAD tetrapeptide but not the DEVD tetrapeptide, and it could be inhibited by Ac-YVAD-CHO but not by Ac-DEVD-CHO, suggesting that this protease has enzymatic properties similar to the mammalian caspase 1. However, it will be necessary to purify and molecularly clone the cDNA coding for this protease to determine whether or not it is a member of the Drosophila caspase family. Furthermore, the fact that in addition to Ac-YVAD-CHO, Ac-DEVD-CHO inhibited the Fas-induced cell death suggests that another protease, possibly a caspase 3-like protease, is involved in the Fas-induced apoptosis in Drosophila cells. As found in the mammalian system (24), the signaling pathway for the Fas-induced apoptosis in Drosophila cells is likely to be composed of a protease cascade. In any case, the apparent conservation of the signaling pathway for Fas-induced apoptosis in Drosophila cells suggests that it might be possible to study the signal-transduction system for Fas-induced apoptosis by using genetics in Drosophila. Moreover, the conservation of the Fas signaling pathway in Drosophila cells suggests that the apoptosis or cell death in invertebrates is controlled by a death factor and receptor system in addition to suicide genes such as reaper, hid, and grim (12, 35, 36).
Homology screening of various cDNA libraries and the human genome database have identified at least 10 members in the human caspase family (37), all of which cause apoptosis when they are overexpressed in mammalian cells. Biochemical analysis of the respective recombinant caspases has indicated that each caspase recognizes specific target sequences, with some overlapping specificity (38). However, it has not been elucidated how and when each caspase member is activated during apoptosis. Here, we have shown that there are at least three caspase-like proteases in Drosophila cells. One, caspase 1-like protease, is activated during Fas-induced apoptosis, while two others, both caspase 3-like proteases, are activated by reaper. Recently, a Drosophila caspase protease (Drosophila caspase 1, DCP-1) was molecularly cloned (39). Since the recombinant DCP-1 recognizes the DEVD but not the YVAD sequence, DCP-1 probably corresponds to one of the caspase 3-like proteases activated by reaper. The identification of at least three caspases in Drosophila cells so far suggests that there are probably even more caspase-like proteases in Drosophila, which can be distinctly activated by different stimuli.
Acknowledgments
We thank Dr. A. Fujisawa-Sehara (Department of Molecular Genetics, National Institute of Neuroscience, Tokyo, Japan) for Schneider cells and pRmHa-3 and pAct5CO vectors, and Ms. S. Kumagai and Ms. H. Fujiwara for secretarial assistance. This work was supported in part by Grants-in-Aid from the Ministry of Education, Science and Culture of Japan.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: TNF, tumor necrosis factor; ICE, interleukin 1β-converting enzyme; FasC, Fas cytoplasmic region; peptide-CHO, peptide analogue with a C-terminal aldehyde group; peptide-CMK, peptide analogue with a C-terminal chloromethyl ketone; TPCK, Nα-tosyl-Phe chloromethyl ketone; MCA, (7-methoxycoumarin-4-yl)acetyl; DNP, 2,4-dinitrophenyl; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; CHAPS, 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid; PMSF, phenylmethylsulfonyl fluoride; FKBP, FK506-binding protein.
References
- 1.Nagata S. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 2.Itoh N, Nagata S. J Biol Chem. 1993;268:10932–10937. [PubMed] [Google Scholar]
- 3.Tartaglia L A, Ayres T M, Wong G H W, Goeddel D V. Cell. 1993;74:845–853. doi: 10.1016/0092-8674(93)90464-2. [DOI] [PubMed] [Google Scholar]
- 4.Fraser A, Evan G. Cell. 1996;85:781–784. doi: 10.1016/s0092-8674(00)81005-3. [DOI] [PubMed] [Google Scholar]
- 5.Chinnaiyan A M, O’Rourke K, Tewari M, Dixit V M. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 6.Muzio M, Chinnaiyan A M, Kischkel F C, O’Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz J D, Zhang M, Gentz R, Mann M, Krammer P H, Peter M E, Dixit V M. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 7.Boldin M P, Varfolomeev E E, Pancer Z, Mett I L, Camonis J H, Wallach D. J Biol Chem. 1995;270:7795–7798. doi: 10.1074/jbc.270.14.7795. [DOI] [PubMed] [Google Scholar]
- 8.Boldin M P, Goncharov T M, Goltsev Y V, Wallach D. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 9.White K, Grether M, Abrams J, Young L, Farrell K, Steller H. Science. 1994;264:677–683. doi: 10.1126/science.8171319. [DOI] [PubMed] [Google Scholar]
- 10.White K, Tahaoglu E, Steller H. Science. 1996;271:805–807. doi: 10.1126/science.271.5250.805. [DOI] [PubMed] [Google Scholar]
- 11.Pronk G, Ramer K, Amiri P, Williams L. Science. 1996;271:808–810. doi: 10.1126/science.271.5250.808. [DOI] [PubMed] [Google Scholar]
- 12.Steller H. Science. 1995;267:1445–1449. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- 13.Golstein P, Marguet D, Depraetere V. Cell. 1995;81:185–186. doi: 10.1016/0092-8674(95)90327-5. [DOI] [PubMed] [Google Scholar]
- 14.Spencer D, Belshaw P, Chen L, Ho S, Randazzo R, Crabtree G, Schreiber S. Curr Biol. 1996;6:839–847. doi: 10.1016/s0960-9822(02)00607-3. [DOI] [PubMed] [Google Scholar]
- 15.Pickup D J, Ink B S, Hu W, Ray C A, Joklik W K. Proc Natl Acad Sci USA. 1986;83:7698–7702. doi: 10.1073/pnas.83.20.7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clem R J, Fechheimer M, Miller L K. Science. 1991;254:1388–1390. doi: 10.1126/science.1962198. [DOI] [PubMed] [Google Scholar]
- 17.Bunch T, Grinblat Y, Goldstein L. Nucleic Acids Res. 1988;16:1043–1061. doi: 10.1093/nar/16.3.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mosna G, Dolfini S. Chromosoma. 1972;38:1–9. doi: 10.1007/BF00319954. [DOI] [PubMed] [Google Scholar]
- 19.Angelichio M L, Beck J A, Johansen H, Ivey-Hoyle M. Nucleic Acids Res. 1991;19:5037–5043. doi: 10.1093/nar/19.18.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mosmann T R, Fong T A T. J Immunol Methods. 1989;116:151–158. doi: 10.1016/0022-1759(89)90198-1. [DOI] [PubMed] [Google Scholar]
- 21.Suda T, Tanaka M, Miwa K, Nagata S. J Immunol. 1996;157:3918–3924. [PubMed] [Google Scholar]
- 22.O’Connell P, Rosbach M. Nucleic Acids Res. 1984;12:5495–5513. doi: 10.1093/nar/12.13.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Enari M, Hase A, Nagata S. EMBO J. 1995;14:5201–5208. doi: 10.1002/j.1460-2075.1995.tb00204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Enari M, Talanian R V, Wong W W, Nagata S. Nature (London) 1996;380:723–726. doi: 10.1038/380723a0. [DOI] [PubMed] [Google Scholar]
- 25.Kischkel F C, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer P H, Peter M E. EMBO J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang B, Eberstadt M, Olejniczak E T, Meadows R P, Fesik S W. Nature (London) 1996;384:638–641. doi: 10.1038/384638a0. [DOI] [PubMed] [Google Scholar]
- 27.Nordstrom W, Chen P, Steller H, Abrams J. Dev Biol. 1996;180:213–226. doi: 10.1006/dbio.1996.0296. [DOI] [PubMed] [Google Scholar]
- 28.Thornberry N A, Miller D, Nicholson D. Perspect Drug Discovery Des. 1995;2:389–399. [Google Scholar]
- 29.Enari M, Hug H, Nagata S. Nature (London) 1995;375:78–81. doi: 10.1038/375078a0. [DOI] [PubMed] [Google Scholar]
- 30.Tewari M, Dixit V M. J Biol Chem. 1995;270:3255–3260. doi: 10.1074/jbc.270.7.3255. [DOI] [PubMed] [Google Scholar]
- 31.Beidler D R, Tewari M, Friesen P D, Poirier G, Dixit V M. J Biol Chem. 1995;270:16526–16528. doi: 10.1074/jbc.270.28.16526. [DOI] [PubMed] [Google Scholar]
- 32.Dhein J, Daniel P T, Trauth B C, Oehm A, Möller P, Krammer P H. J Immunol. 1992;149:3166–3173. [PubMed] [Google Scholar]
- 33.Spencer D M, Wandless T J, Schreiber S L, Crabtree G R. Science. 1993;262:1019–1024. doi: 10.1126/science.7694365. [DOI] [PubMed] [Google Scholar]
- 34.Boldin M P, Mett I L, Varfolomeev E E, Chumakov I, Shemer-Avni Y, Camonis J H, Wallach D. J Biol Chem. 1995;270:387–391. doi: 10.1074/jbc.270.1.387. [DOI] [PubMed] [Google Scholar]
- 35.Grether M E, Abrams J M, Agapite J, White K, Steller H. Genes Dev. 1995;9:1694–1708. doi: 10.1101/gad.9.14.1694. [DOI] [PubMed] [Google Scholar]
- 36.Chen P, Nordstrom W, Gish B, Abrams J M. Genes Dev. 1996;10:1773–1782. doi: 10.1101/gad.10.14.1773. [DOI] [PubMed] [Google Scholar]
- 37.Alnemri E S, Livingston D J, Nicholson D W, Salvesen G, Thornberry N A, Wong W W, Yuan J. Cell. 1996;87:171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- 38.Talanian R V, Quinlan C, Trautz S, Hackett M C, Mankovich J A, Banach D, Ghayur T, Brady K D, Wong W W. J Biol Chem. 1997;272:9677–9682. doi: 10.1074/jbc.272.15.9677. [DOI] [PubMed] [Google Scholar]
- 39.Song Z, McCall K, Steller H. Science. 1997;275:536–540. doi: 10.1126/science.275.5299.536. [DOI] [PubMed] [Google Scholar]