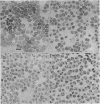
Abstract
Separation and concentration of parasitized erythrocytes from infected blood was achieved by centrifugation of a sample placed in a layer on top of a cushion of a Ficoll solution with a critical density. Pure suspensions of parasitized erythrocytes were obtained from Plasmodium berghei infected rodent blood, whereas results with P. vivax infected monkey (Aotus trivirgatus) blood were partially successful. Titration experiments revealed that the parasitized erythrocytes obtained by Ficoll fractionation were infective.
Full text
PDF

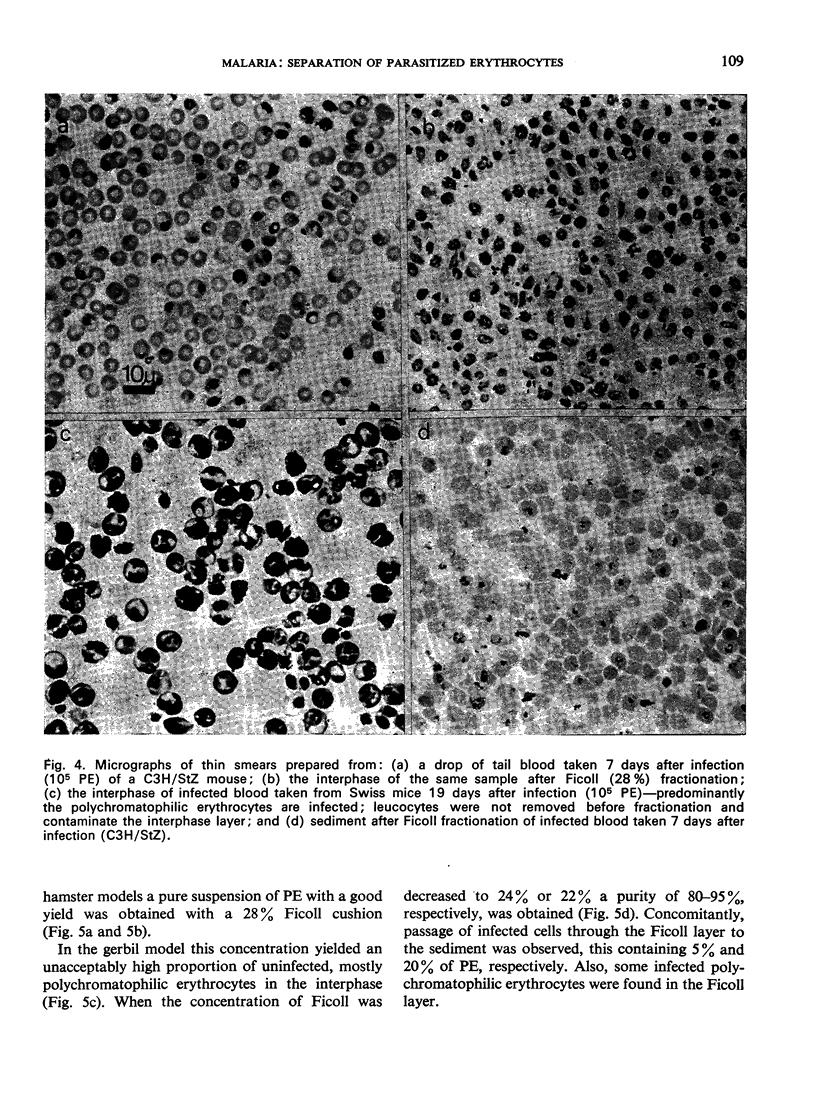





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COHEN S., McGREGOR I. A., CARRINGTON S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961 Nov 25;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- Cohen S., Butcher G. A., Crandall R. B. Action of malarial antibody in vitro. Nature. 1969 Jul 26;223(5204):368–371. doi: 10.1038/223368a0. [DOI] [PubMed] [Google Scholar]
- FULTON J. D., GRANT P. T. The sulphur requirements of the erythrocytic from of Plasmodium knowlesi. Biochem J. 1956 Jun;63(2):274–282. doi: 10.1042/bj0630274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loos J. A., Roos D. Ficoll-isopaque gradients for the determination of density distributions of human blood lymphocytes and other reticulo-endothelial cells. Exp Cell Res. 1974 Jun;86(2):333–341. doi: 10.1016/0014-4827(74)90721-6. [DOI] [PubMed] [Google Scholar]
- Miller L. H., Chien S. Density distribution of red cells infected by Plasmodium knowlesi and plasmodium coatneyi. Exp Parasitol. 1971 Jun;29(3):451–456. doi: 10.1016/0014-4894(71)90054-3. [DOI] [PubMed] [Google Scholar]
- Noble P. B., Cutts J. H., Carroll K. K. Ficoll flotation for the separation of blood leukocyte types. Blood. 1968 Jan;31(1):66–73. [PubMed] [Google Scholar]
- Rowley P. T., Siddiqui W. A., Geiman Q. M. Separation of malarial parasites according to age by density gradient centrifugation. J Lab Clin Med. 1967 Dec;70(6):933–937. [PubMed] [Google Scholar]
- Siddiqui W. A., Schnell J. V., Geiman Q. M. In vitro cultivation of Plasmodium falciparum. Am J Trop Med Hyg. 1970 Jul;19(4):586–591. doi: 10.4269/ajtmh.1970.19.586. [DOI] [PubMed] [Google Scholar]
- TRAGER W. Folinic acid and non-dialyzable materials in the nutrition of malaria parasites. J Exp Med. 1958 Nov 1;108(5):753–772. doi: 10.1084/jem.108.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Weiss M. L. Plasmodium berghei: adaptation of a mouse-adapted strain to the Mongolian jird (Meriones unguiculatus); infectivity and immunogenicity. Exp Parasitol. 1976 Aug;40(1):103–111. doi: 10.1016/0014-4894(76)90071-0. [DOI] [PubMed] [Google Scholar]