Abstract
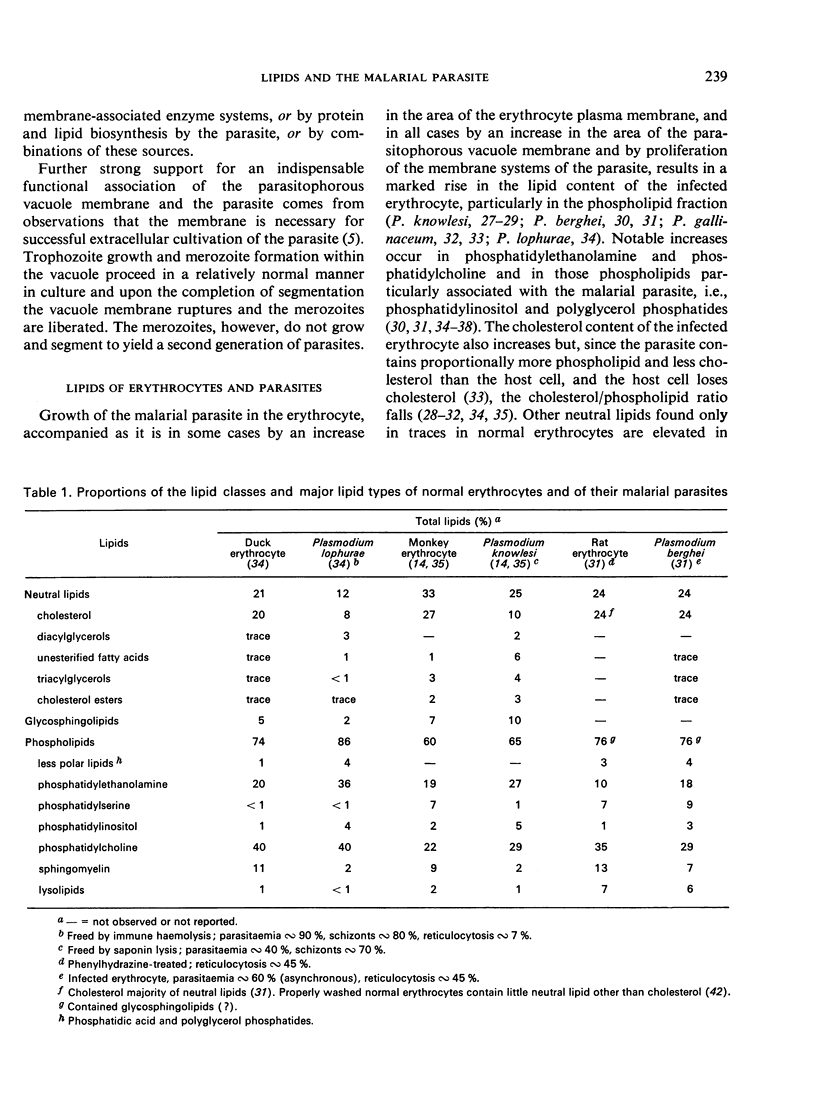
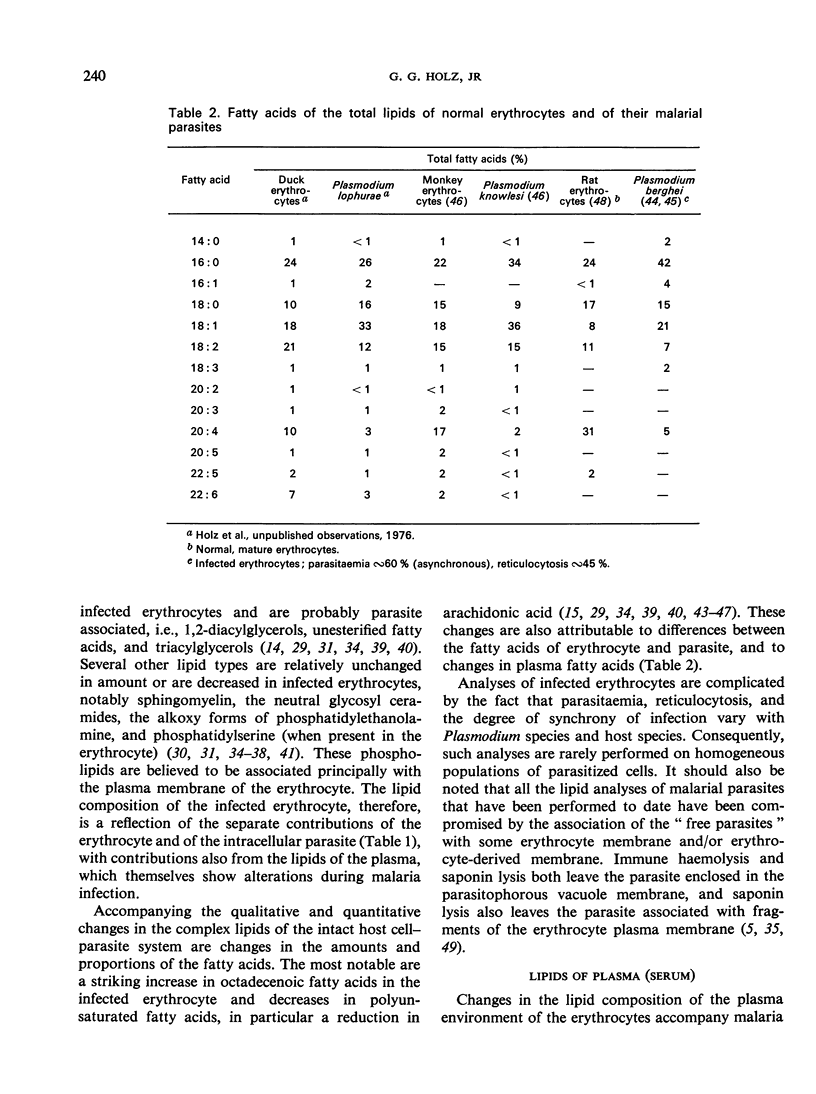
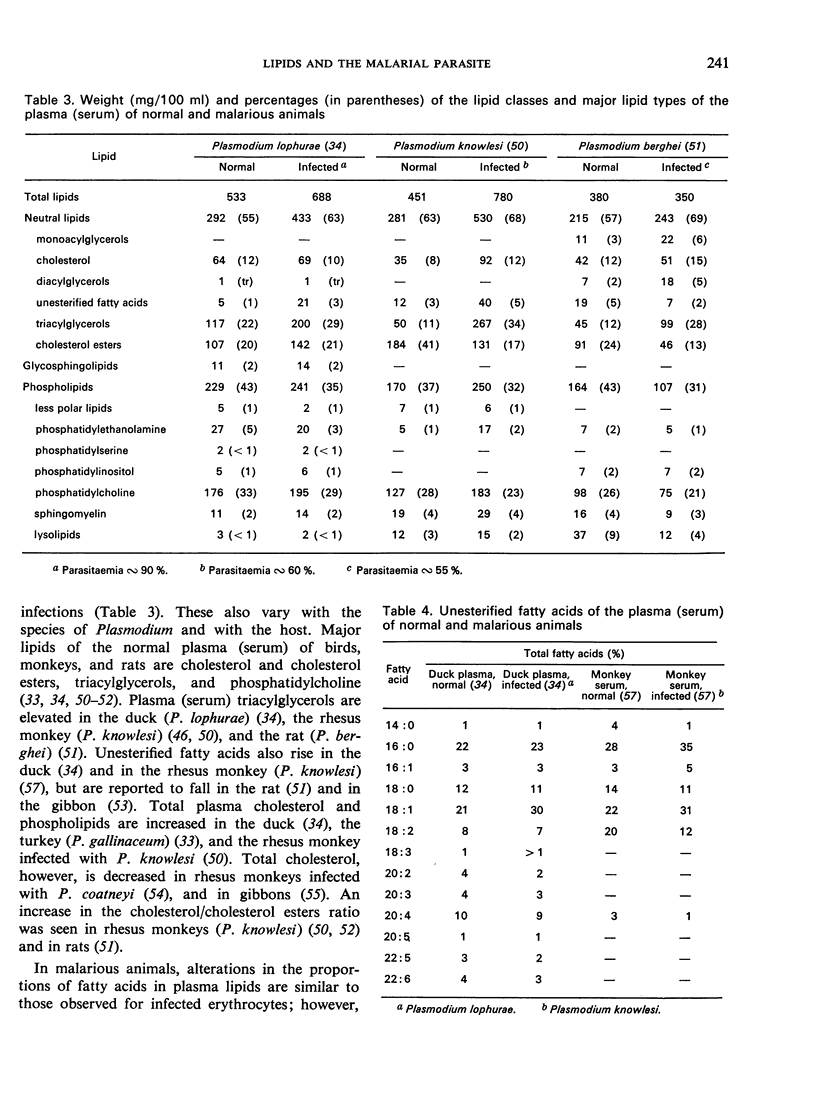
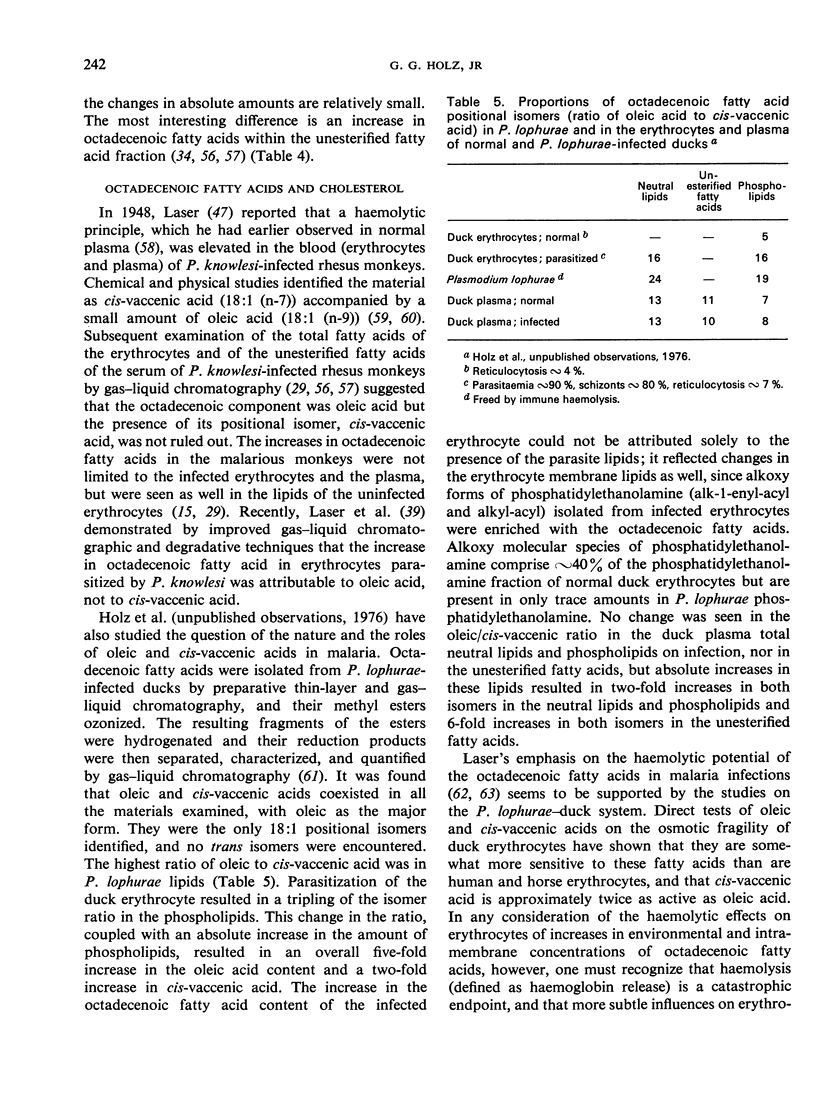
Merozoite endocytosis initiates Plasmodium development in a vacuole bounded by an erythrocyte-derived membrane, whose asymmetrical distribution of lipids and proteins is reversed in its orientation with respect to the parasite plasma membrane. Reorientation may accompany the proliferation of the membrane associated with the parasite's growth and phagocytic and pinocytic feeding. Increases in the membrane surface area of the parasite, and in some cases of the erythrocyte, parallel parasite growth and segmentation. Augmentation of all the membrane systems of the infected erythrocyte causes the lipid content to rise rapidly, but the parasite lipid composition differs from that of the erythrocyte in many respects: it is higher in diacyl phosphatidylethanolamine, phosphatidylinositol, polyglycerol phosphatides, diacylglycerols, unesterified fatty acids, triacylglycerols, and hexadecanoic and octadecenoic fatty acids and lower in sphingomyelin, phosphatidylserine, alkoxy phosphatidylethanolamine, cholesterol, and polyunsaturated fatty acids. Active lipid metabolism accompanies the membrane proliferation associated with feeding, growth, and reproduction. Plasmodium is incapable of de novo biosynthesis of fatty acids and cholesterol; however, it can fabricate its glycerides and phosphoglycerides with host-supplied fatty acids, nitrogenous bases, alcohols, ATP, and coenzyme A, and can generate the glyceryl moiety during glycolysis. Cholesterol is obtained from the host but nothing is known of sphingolipid origins. Lipid metabolism of the parasite may be associated with alterations in the amounts of octadecenoic fatty acids and cholesterol in the erythrocyte plasma membrane, which in turn are responsible for changes in permeability and fragility.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aikawa M., Cook R. T. Plasmodium: electron microscopy of antigen preparations. Exp Parasitol. 1972 Feb;31(1):67–74. doi: 10.1016/0014-4894(72)90048-3. [DOI] [PubMed] [Google Scholar]
- Beach D. H., Sherman I. W., Holz G. G., Jr Lipids of Plasmodium lophurae, and of erythrocytes and plasma of normal and P. lophurae-infected Pekin ducklings. J Parasitol. 1977 Feb;63(1):62–75. [PubMed] [Google Scholar]
- Beckwith R., Schenkel R. H., Silverman P. H. Qualitative analysis of phospholipids isolated from nonviable Plasmodium antigen. Exp Parasitol. 1975 Apr;37(2):164–172. doi: 10.1016/0014-4894(75)90067-3. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S., Raff M. C. Mammalian plasma membranes. Nature. 1975 Nov 6;258(5530):43–49. doi: 10.1038/258043a0. [DOI] [PubMed] [Google Scholar]
- Brohn F. H., Trager W. Coenzyme A requirement of malaria parasites: enzymes of coenzyme A biosynthesis in normal duck erythrocytes and erythrocytes infected with Plasmodium lophurae. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2456–2458. doi: 10.1073/pnas.72.6.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenedella R. J., Jarrell J. J., Saxe L. H. Lipid synthesis in vivo from 1-14C-oleic acid and 6-3H-glucose by intraerythrocytic Plasmodium berghei. Mil Med. 1969 Sep;134(10):1045–1055. [PubMed] [Google Scholar]
- Dawson R. M. The exchange of phospholipids between cell membranes. Subcell Biochem. 1973 Jan;2(1):69–89. [PubMed] [Google Scholar]
- Dunn M. J. Alterations of red blood cell sodium transport during malarial infection. J Clin Invest. 1969 Apr;48(4):674–684. doi: 10.1172/JCI106025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak J. A., Miller L. H., Whitehouse W. C., Shiroishi T. Invasion of erythrocytes by malaria merozoites. Science. 1975 Feb 28;187(4178):748–750. doi: 10.1126/science.803712. [DOI] [PubMed] [Google Scholar]
- Emmelot P., Van Hoeven R. P. Phospholipid unsaturation and plasma membrane organization. Chem Phys Lipids. 1975 May;14(3):236–246. doi: 10.1016/0009-3084(75)90005-5. [DOI] [PubMed] [Google Scholar]
- GODDARD E. D., ALEXANDER A. E. The haemolytic acid present in horse brain; examination by the insoluble monolayer technique. Biochem J. 1950 Sep;47(3):331–334. doi: 10.1042/bj0470331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellerman J. L., Schlenk H. Preparation of fatty acids labeled with C-14 from Ochromonas danica. J Protozool. 1965 May;12(2):178–189. doi: 10.1111/j.1550-7408.1965.tb01834.x. [DOI] [PubMed] [Google Scholar]
- Ginn F. L., Hochstein P., Trump B. F. Membrane alterations in hemolysis: Internalization of plasmalemma induced by primaquine. Science. 1969 May 16;164(3881):843–845. doi: 10.1126/science.164.3881.843. [DOI] [PubMed] [Google Scholar]
- Glomset J. A. The plasma lecithins:cholesterol acyltransferase reaction. J Lipid Res. 1968 Mar;9(2):155–167. [PubMed] [Google Scholar]
- HALLINAN T., EDEN E., NORTH R. The structure and composition of rat reticulocytes. I. The ultrastructure of reticulocytes. Blood. 1962 Nov;20:547–556. [PubMed] [Google Scholar]
- Haynes J. D., Diggs C. L., Hines F. A., Desjardins R. E. Culture of human malaria parasites Plasmodium falciparum. Nature. 1976 Oct 28;263(5580):767–769. doi: 10.1038/263767a0. [DOI] [PubMed] [Google Scholar]
- Kimelberg H. K., Papahadjopoulos D. Effects of phospholipid acyl chain fluidity, phase transitions, and cholesterol on (Na+ + K+)-stimulated adenosine triphosphatase. J Biol Chem. 1974 Feb 25;249(4):1071–1080. [PubMed] [Google Scholar]
- Kury P. G., McConnell M. Regulation of Membrane Flexibility in Human Erythrocytes. Biochemistry. 1975 Jul;14(13):2798–2803. doi: 10.1021/bi00684a002. [DOI] [PubMed] [Google Scholar]
- Ladda R., Aikawa M., Sprinz H. Penetration of erythrocytes by merozoites of mammalian and avian malarial parasites. J Parasitol. 1969 Jun;55(3):633–644. [PubMed] [Google Scholar]
- Langreth S. G., Trager W. Fine structure of the malaria parasite Plasmodium lophurae developing extracellularly in vitro. J Protozool. 1973 Nov;20(5):606–613. doi: 10.1111/j.1550-7408.1973.tb03584.x. [DOI] [PubMed] [Google Scholar]
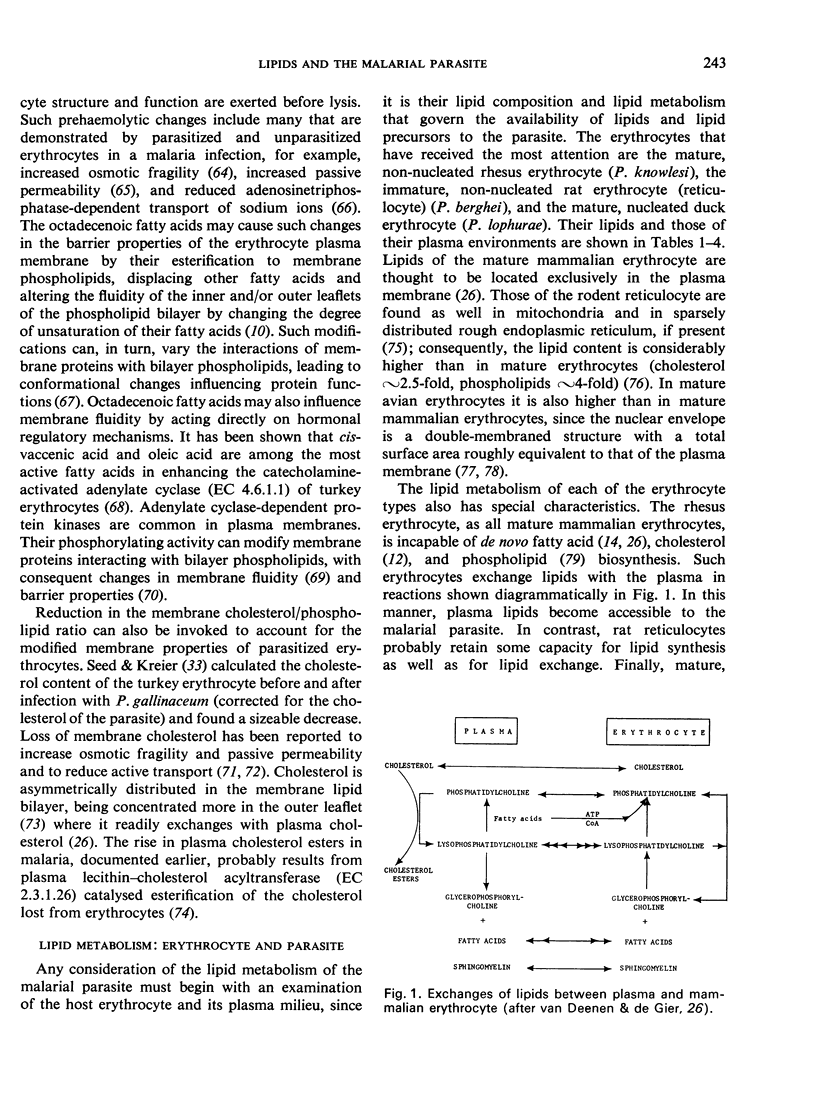
- Laser H., Kemp P., Miller N., Lander D., Klein R. Malaria, quinine and red cell lysis. Parasitology. 1975 Oct;71(2):167–181. doi: 10.1017/s003118200004662x. [DOI] [PubMed] [Google Scholar]
- Laser H., Kemp P., Miller N., Lander D., Klein R. Malaria, quinine and red cell lysis. Parasitology. 1975 Oct;71(2):167–181. doi: 10.1017/s003118200004662x. [DOI] [PubMed] [Google Scholar]
- Lawrence C. W., Cenedella R. J. Lipid content of Plasmodium berghei-infected rat red blood cells. Exp Parasitol. 1969 Oct;26(2):181–186. doi: 10.1016/0014-4894(69)90110-6. [DOI] [PubMed] [Google Scholar]
- MORRISON D. B., JESKEY H. A. Alterations in some constituents of the monkey erythrocyte infected with Plasmodium knowlesi as related to pigment formation. J Natl Malar Soc. 1948 Dec;7(4):259–264. [PubMed] [Google Scholar]
- MORTON I. D., TODD A. R. The haemolytic acid present in horse brain; purification and identification as cis-octadec-11-enoic acid. Biochem J. 1950 Sep;47(3):327–330. doi: 10.1042/bj0470327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClean S., Kabat A., Sampugna J., Purdy W. C. Analysis of the effect of malaria on lipid composition of rhesus plasma. Anal Chim Acta. 1976 Oct;86(1):255–261. doi: 10.1016/s0003-2670(01)83042-7. [DOI] [PubMed] [Google Scholar]
- McClean S., Purdy W. C., Kabat A., Sampugna J., DeZeeuw R. Analysis of the phospholipid composition of Plasmodium knowlesi and rhesus erythrocyte membranes. Anal Chim Acta. 1976 Mar;82(1):175–185. doi: 10.1016/s0003-2670(01)82215-7. [DOI] [PubMed] [Google Scholar]
- Neame K. D., Homewood C. A. Alterations in the permeability of mouse erythrocytes infected with the malaria parasite, Plasmodium berghei. Int J Parasitol. 1975 Oct;5(5):537–540. doi: 10.1016/0020-7519(75)90046-6. [DOI] [PubMed] [Google Scholar]
- Nelson G. J. Composition of neutral lipids from erythrocytes of common mammals. J Lipid Res. 1967 Jul;8(4):374–379. [PubMed] [Google Scholar]
- Orly J., Schramm M. Fatty acids as modulators of membrane functions: catecholamine-activated adenylate cyclase of the turkey erythrocyte. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3433–3437. doi: 10.1073/pnas.72.9.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poznansky M., Lange Y. Transbilayer movement of cholesterol in dipalmitoyllecithin-cholesterol vesicles. Nature. 1976 Feb 5;259(5542):420–421. doi: 10.1038/259420a0. [DOI] [PubMed] [Google Scholar]
- Roseman M., Litman B. J., Thompson T. E. Transbilayer exchange of phosphatidylethanolamine for phosphatidylcholine and N-acetimidoylphosphatidylethanolamine in single-walled bilayer vesicles. Biochemistry. 1975 Nov 4;14(22):4826–4830. doi: 10.1021/bi00693a008. [DOI] [PubMed] [Google Scholar]
- Rudolph S. A., Greengard P. Regulation of protein phosphorylation and membrane permeability by beta-adrenergic agents and cyclic adenosine 3':5'-monophosphate in the avian erythrocyte. J Biol Chem. 1974 Sep 10;249(17):5684–5687. [PubMed] [Google Scholar]
- Rudzinska M. A. The fine structure of malaria parasites. Int Rev Cytol. 1969;25:161–199. doi: 10.1016/s0074-7696(08)60203-x. [DOI] [PubMed] [Google Scholar]
- Shattil S. J., Cooper R. A. Maturation of macroreticulocyte membranes in vivo. J Lab Clin Med. 1972 Feb;79(2):215–227. [PubMed] [Google Scholar]
- Siddiqui W. A., Schnell J. V., Geiman Q. M. Stearic acid as plasma replacement for intracellular in vitro culture of Plasmodium knowlesi. Science. 1967 Jun 23;156(3782):1623–1625. doi: 10.1126/science.156.3782.1623. [DOI] [PubMed] [Google Scholar]
- Solomonson L. P., Liepkalns V. A., Spector A. A. Changes in (Na+ + K+)-ATPase activity of Ehrlich ascites tumor cells produced by alteration of membrane fatty acid composition. Biochemistry. 1976 Feb 24;15(4):892–897. doi: 10.1021/bi00649a026. [DOI] [PubMed] [Google Scholar]
- Trager W., Brohn F. H. Coezyme A requirement of malaria parasites: effects of coenzyme A precursors on extracellular development in vitro of Plasmodium lophurae. Proc Natl Acad Sci U S A. 1975 May;72(5):1834–1837. doi: 10.1073/pnas.72.5.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Trager W. Malaria parasites (Plasmodium lophurae) developing extracellularly in vitro: incorporation of labeled precursors. J Protozool. 1971 Aug;18(3):392–399. doi: 10.1111/j.1550-7408.1971.tb03341.x. [DOI] [PubMed] [Google Scholar]
- Trager W. Some aspects of intracellular parasitism. Science. 1974 Jan 25;183(4122):269–273. doi: 10.1126/science.183.4122.269. [DOI] [PubMed] [Google Scholar]
- Walker B. L., Yurkowski M. Effect of cell age on erythrocyte fatty acid composition in rats on different dietary regimes. Biochem J. 1967 Apr;103(1):218–224. doi: 10.1042/bj1030218. [DOI] [PMC free article] [PubMed] [Google Scholar]


