Abstract
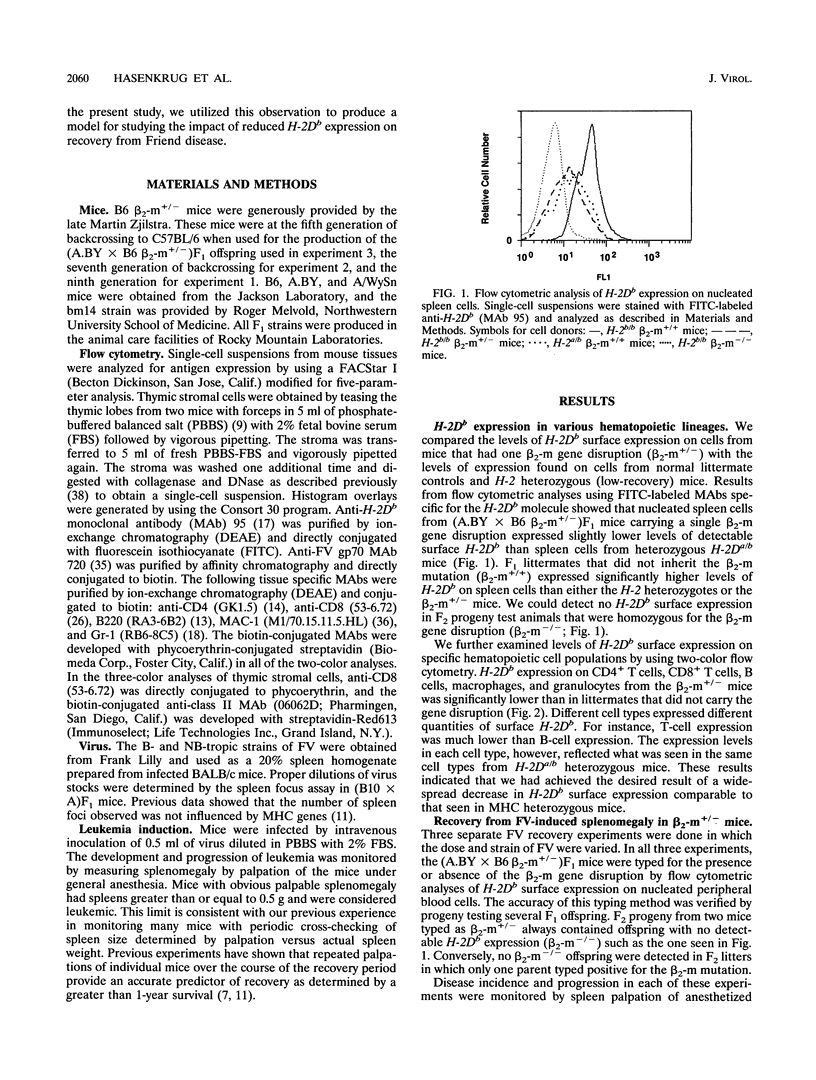
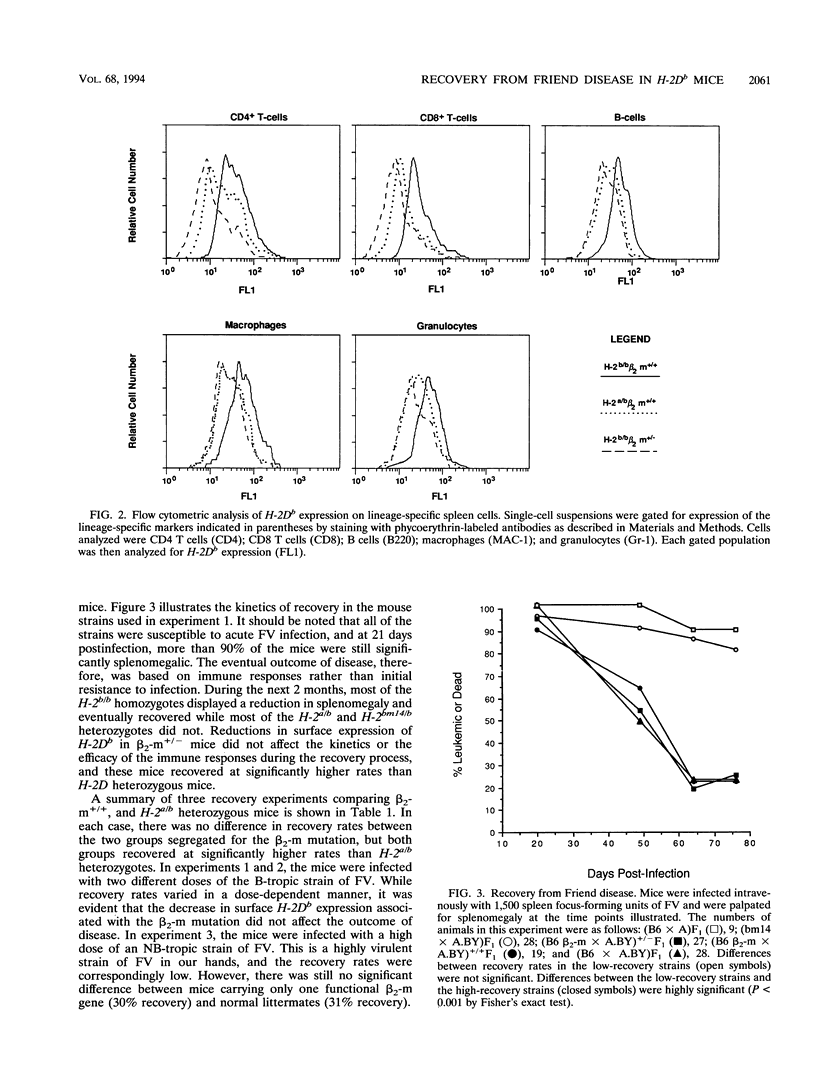
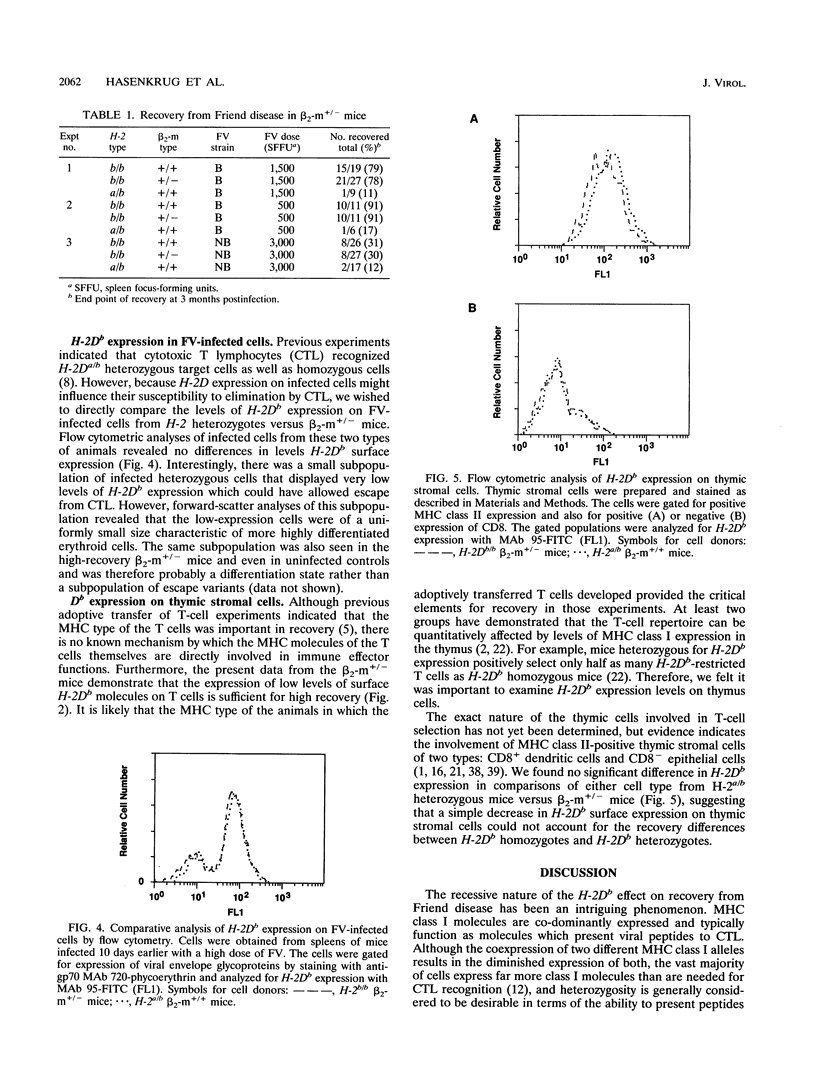
Mice homozygous for the b allele of the MHC gene, H-2D, have a high incidence of recovery from Friend virus infections, while mice heterozygous for the b allele at H-2D have a very low incidence of recovery. Previous experiments indicated that the low recovery rates associated with heterozygosity at H-2D might be related to a gene dosage effect requiring the expression of two H-2Db alleles for high recovery. We investigated the effects of reduced H-2Db expression on recovery from Friend disease by using H-2b homozygous mice carrying a single beta 2-microglobulin gene disruption. These mice had reductions in cell surface H-2Db expression comparable to those of H-2Da/b heterozygotes. Numerous cell types with various levels of H-2Db expression were examined, and in each case, the expression levels in the beta 2-microglobulin mutants closely reflected those observed in the H-2Da/b heterozygotes. We found, however, that reduced expression did not affect recovery from Friend disease, indicating that heterozygous levels of H-2Db expression are sufficient for the high-recovery phenotype previously associated only with H-2Db homozygotes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson G., Jenkinson E. J., Moore N. C., Owen J. J. MHC class II-positive epithelium and mesenchyme cells are both required for T-cell development in the thymus. Nature. 1993 Mar 4;362(6415):70–73. doi: 10.1038/362070a0. [DOI] [PubMed] [Google Scholar]
- Berg L. J., Frank G. D., Davis M. M. The effects of MHC gene dosage and allelic variation on T cell receptor selection. Cell. 1990 Mar 23;60(6):1043–1053. doi: 10.1016/0092-8674(90)90352-f. [DOI] [PubMed] [Google Scholar]
- Bix M., Raulet D. Functionally conformed free class I heavy chains exist on the surface of beta 2 microglobulin negative cells. J Exp Med. 1992 Sep 1;176(3):829–834. doi: 10.1084/jem.176.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt W. J., Chesebro B. H-2D (Rfv-1) gene influence on recovery from Friend virus leukemia is mediated by nonleukemic cells of the spleen and bone marrow. J Exp Med. 1980 Dec 1;152(6):1795–1804. doi: 10.1084/jem.152.6.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt W. J., Chesebro B. H-2D control of recovery from Friend virus leukemia: H-2D region influences the kinetics of the T lymphocyte response to Friend virus. J Exp Med. 1983 Jun 1;157(6):1736–1745. doi: 10.1084/jem.157.6.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Bloom M., Wehrly K., Nishio J. Persistence of infectious Friend virus in spleens of mice after spontaneous recovery from virus-induced erythroleukemia. J Virol. 1979 Dec;32(3):832–837. doi: 10.1128/jvi.32.3.832-837.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Miyazawa M., Britt W. J. Host genetic control of spontaneous and induced immunity to Friend murine retrovirus infection. Annu Rev Immunol. 1990;8:477–499. doi: 10.1146/annurev.iy.08.040190.002401. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K. Rfv-1 and Rfv-2, two H-2-associated genes that influence recovery from Friend leukemia virus-induced splenomegaly. J Immunol. 1978 Apr;120(4):1081–1085. [PubMed] [Google Scholar]
- Chesebro B., Wehrly K., Stimpfling J. Host genetic control of recovery from Friend leukemia virus-induced splenomegaly: mapping of a gene within the major histocompatability complex. J Exp Med. 1974 Dec 1;140(6):1457–1467. doi: 10.1084/jem.140.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K. Studies on the role of the host immune response in recovery from Friend virus leukemia. II. Cell-mediated immunity. J Exp Med. 1976 Jan 1;143(1):85–99. doi: 10.1084/jem.143.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christinck E. R., Luscher M. A., Barber B. H., Williams D. B. Peptide binding to class I MHC on living cells and quantitation of complexes required for CTL lysis. Nature. 1991 Jul 4;352(6330):67–70. doi: 10.1038/352067a0. [DOI] [PubMed] [Google Scholar]
- Coffman R. L., Weissman I. L. B220: a B cell-specific member of th T200 glycoprotein family. Nature. 1981 Feb 19;289(5799):681–683. doi: 10.1038/289681a0. [DOI] [PubMed] [Google Scholar]
- Doig D., Chesebro B. Anti-Friend virus antibody is associated with recovery from viremia and loss of viral leukemia cell-surface antigens in leukemic mice. Identification of Rfv-3 as a gene locus influencing antibody production. J Exp Med. 1979 Jul 1;150(1):10–19. doi: 10.1084/jem.150.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild P. J., Austyn J. M. Thymic dendritic cells: phenotype and function. Int Rev Immunol. 1990;6(2-3):187–196. doi: 10.3109/08830189009056629. [DOI] [PubMed] [Google Scholar]
- Hasenkrug K. J., Cory J. M., Stimpfling J. H. Monoclonal antibodies defining mouse tissue antigens encoded by the H-2 region. Immunogenetics. 1987;25(2):136–139. doi: 10.1007/BF00364282. [DOI] [PubMed] [Google Scholar]
- Hestdal K., Ruscetti F. W., Ihle J. N., Jacobsen S. E., Dubois C. M., Kopp W. C., Longo D. L., Keller J. R. Characterization and regulation of RB6-8C5 antigen expression on murine bone marrow cells. J Immunol. 1991 Jul 1;147(1):22–28. [PubMed] [Google Scholar]
- Hoatlin M. E., Kozak S. L., Lilly F., Chakraborti A., Kozak C. A., Kabat D. Activation of erythropoietin receptors by Friend viral gp55 and by erythropoietin and down-modulation by the murine Fv-2r resistance gene. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9985–9989. doi: 10.1073/pnas.87.24.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochman J. H., Shimizu Y., DeMars R., Edidin M. Specific associations of fluorescent beta-2-microglobulin with cell surfaces. The affinity of different H-2 and HLA antigens for beta-2-microglobulin. J Immunol. 1988 Apr 1;140(7):2322–2329. [PubMed] [Google Scholar]
- Hugo P., Kappler J. W., Godfrey D. I., Marrack P. C. A cell line that can induce thymocyte positive selection. Nature. 1992 Dec 17;360(6405):679–682. doi: 10.1038/360679a0. [DOI] [PubMed] [Google Scholar]
- Kisielow P., Teh H. S., Blüthmann H., von Boehmer H. Positive selection of antigen-specific T cells in thymus by restricting MHC molecules. Nature. 1988 Oct 20;335(6192):730–733. doi: 10.1038/335730a0. [DOI] [PubMed] [Google Scholar]
- Koller B. H., Marrack P., Kappler J. W., Smithies O. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science. 1990 Jun 8;248(4960):1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- Lavigueur A., Bernstein A. p53 transgenic mice: accelerated erythroleukemia induction by Friend virus. Oncogene. 1991 Dec;6(12):2197–2201. [PubMed] [Google Scholar]
- Lawlor D. A., Zemmour J., Ennis P. D., Parham P. Evolution of class-I MHC genes and proteins: from natural selection to thymic selection. Annu Rev Immunol. 1990;8:23–63. doi: 10.1146/annurev.iy.08.040190.000323. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Li J. P., D'Andrea A. D., Lodish H. F., Baltimore D. Activation of cell growth by binding of Friend spleen focus-forming virus gp55 glycoprotein to the erythropoietin receptor. Nature. 1990 Feb 22;343(6260):762–764. doi: 10.1038/343762a0. [DOI] [PubMed] [Google Scholar]
- Miyazawa M., Nishio J., Wehrly K., Jay G., Melvold R. W., Chesebro B. Detailed mapping of the Rfv-1 gene that influences spontaneous recovery from Friend retrovirus-induced leukaemia. Eur J Immunogenet. 1992 Jun;19(3):159–164. doi: 10.1111/j.1744-313x.1992.tb00054.x. [DOI] [PubMed] [Google Scholar]
- Moreau-Gachelin F., Tavitian A., Tambourin P. Spi-1 is a putative oncogene in virally induced murine erythroleukaemias. Nature. 1988 Jan 21;331(6153):277–280. doi: 10.1038/331277a0. [DOI] [PubMed] [Google Scholar]
- Morrison R. P., Earl P. L., Nishio J., Lodmell D. L., Moss B., Chesebro B. Different H-2 subregions influence immunization against retrovirus and immunosuppression. Nature. 1987 Oct 22;329(6141):729–732. doi: 10.1038/329729a0. [DOI] [PubMed] [Google Scholar]
- Paul R., Schuetze S., Kozak S. L., Kozak C. A., Kabat D. The Sfpi-1 proviral integration site of Friend erythroleukemia encodes the ets-related transcription factor Pu.1. J Virol. 1991 Jan;65(1):464–467. doi: 10.1128/jvi.65.1.464-467.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploegh H. L., Cannon L. E., Strominger J. L. Cell-free translation of the mRNAs for the heavy and light chains of HLA-A and HLA-B antigens. Proc Natl Acad Sci U S A. 1979 May;76(5):2273–2277. doi: 10.1073/pnas.76.5.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polsky D., Lilly F. Suppression of H-2b-associated resistance to Friend erythroleukemia virus by a class I gene from the H-2d major histocompatibility complex haplotype. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9243–9247. doi: 10.1073/pnas.88.20.9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter T. A., Boyer C., Verhulst A. M., Golstein P., Rajan T. V. Expression of H-2Db on the cell surface in the absence of detectable beta 2 microglobulin. J Exp Med. 1984 Jul 1;160(1):317–322. doi: 10.1084/jem.160.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson M. N., Spangrude G. J., Hasenkrug K., Perry L., Nishio J., Wehrly K., Chesebro B. Role and specificity of T-cell subsets in spontaneous recovery from Friend virus-induced leukemia in mice. J Virol. 1992 Jun;66(6):3271–3277. doi: 10.1128/jvi.66.6.3271-3277.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T., Galfré G., Secher D. S., Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979 Apr;9(4):301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- Troxler D. H., Ruscetti S. K., Scolnick E. M. The molecular biology of Friend virus. Biochim Biophys Acta. 1980 Sep 22;605(3):305–324. doi: 10.1016/0304-419x(80)90014-1. [DOI] [PubMed] [Google Scholar]
- Vremec D., Zorbas M., Scollay R., Saunders D. J., Ardavin C. F., Wu L., Shortman K. The surface phenotype of dendritic cells purified from mouse thymus and spleen: investigation of the CD8 expression by a subpopulation of dendritic cells. J Exp Med. 1992 Jul 1;176(1):47–58. doi: 10.1084/jem.176.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukmanović S., Grandea A. G., 3rd, Faas S. J., Knowles B. B., Bevan M. J. Positive selection of T-lymphocytes induced by intrathymic injection of a thymic epithelial cell line. Nature. 1992 Oct 22;359(6397):729–732. doi: 10.1038/359729a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts S., Vogel J. M., Harriman W. D., Itoh T., Stauss H. J., Goodenow R. S. DNA sequence analysis of the C3H H-2Kk and H-2Dk loci. Evolutionary relationships to H-2 genes from four other mouse strains. J Immunol. 1987 Dec 1;139(11):3878–3885. [PubMed] [Google Scholar]
- Zijlstra M., Bix M., Simister N. E., Loring J. M., Raulet D. H., Jaenisch R. Beta 2-microglobulin deficient mice lack CD4-8+ cytolytic T cells. Nature. 1990 Apr 19;344(6268):742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]


