Abstract
BACKGROUND
Problems with the voluntary control of behavior, such as those leading to increased antisaccade errors, are accepted as evidence of prefrontal dysfunction in schizophrenia. We previously reported that speeded prosaccade responses, i.e. shorter response latencies for automatic shifts of attention to visual targets, were associated with higher antisaccade error rates in schizophrenia. This suggests that dysregulation of automatic attentional processes may contribute to disturbances in prefrontally-mediated control of voluntary behavior.
METHODS
Twenty-four antipsychotic-naïve schizophrenia patients and 30 healthy individuals completed three tasks: a no-gap prosaccade task in which subjects shifted gaze toward a peripheral target that appeared coincident with the disappearance of a central fixation target, and separate prosaccade and antisaccade tasks in which a temporal gap or overlap of the central target offset and peripheral target onset occurred. Sixteen patients were retested after 6-weeks of antipsychotic treatment.
RESULTS
Patients’ prosaccade latencies in the no-gap task were speeded compared to healthy individuals. While patients were not atypical in the degree to which response latencies were speeded or slowed by the gap and overlap manipulations, those patients with diminished attentional engagement on the prosaccade task (i.e., reduced overlap effect) had significantly elevated antisaccade error rates. This effect persisted in patients evaluated after antipsychotic treatment.
CONCLUSIONS
This study provides evidence that a reduced ability to engage attention may render patients more distracted by sensory inputs, thereby further compromising impaired executive control during antisaccade tasks. Thus, alterations in attentional and executive control functions can synergistically disrupt voluntary behavioral responses in schizophrenia.
INTRODUCTION
Prefrontal cortical systems dysfunction is believed to underlie many cognitive abnormalities in schizophrenia including the regulation of attention and behavior. Cognitive neuroscience provides a useful framework for understanding mechanisms that support automatic and voluntary attention (1) and how disturbances in these processes may contribute to problems in the executive control of behavior (2). The linkage between oculomotor and attentional brain systems (3,4) make eye movement paradigms particularly relevant for investigating mechanisms of attentional and inhibitory control deficits in schizophrenia (5).
Prosaccade paradigms measure saccadic eye movements influenced by automatic shifts of visual attention. In these tasks, subjects must disengage attention from a central location and re-direct their gaze to a peripheral target when it appears. In contrast, antisaccade tasks require greater executive control of visual attention shifts because subjects must inhibit the automatic response to look toward the target and instead generate an eye movement to a mirrored location. These operations depend upon voluntary executive control processes regulated by prefrontal cortex (6,7,8,9).
Multiple studies have reported elevated antisaccade error rates in schizophrenia patients(10,11,12) which are believed to represent disturbances in the prefrontally-mediated ability to voluntarily suppress prepotent responses (7,13). Patients followed longitudinally show a persistent elevation in antisaccade error rates over time, suggesting an enduring deficit of prefrontal functioning (14,15,16).
We previously reported that antipsychotic-naïve first-episode schizophrenia patients responded more quickly than healthy individuals to visual targets in a prosaccade task, suggesting an abnormality in automatic attentional regulation of sensorimotor systems by neocortical regions (17). Moreover, this speeded responding was associated with increased errors on an antisaccade task (15). These findings suggest that abnormalities in the regulation of automatic orienting and the executive control of attention may both contribute to deficits on antisaccade tasks in schizophrenia.
One approach for examining automatic attentional control is through the manipulation of visual fixation offset and peripheral target onset temporal asynchronies (Figure 1). Introduction of a temporal gap between the offset of the fixation target and peripheral cue onset reduces saccade latencies – the so called gap effect (18,19). The offset of the central cue reduces activity in the visual fixation system, releasing the visual attention and saccade systems to more quickly respond to new stimuli (20). In contrast, overlap effects occur when the central target persists after the appearance of the peripheral cue which prolongs activity in visual fixation systems and, in turn, increases response latencies. Gap and overlap effects thus reflect the balance between attentional systems that respond automatically to sensory input and fixation systems that maintain attention on objects of interest. Prior studies have reported that schizophrenia patients show the expected change in prosaccade latencies in gap and overlap conditions, however, findings are mixed as whether the degree of change under these manipulations is atypical in schizophrenia (21,22,23). It is not known how the degree of release or maintenance of visual attention under these conditions is associated with antisaccade errors in schizophrenia.
Figure 1.
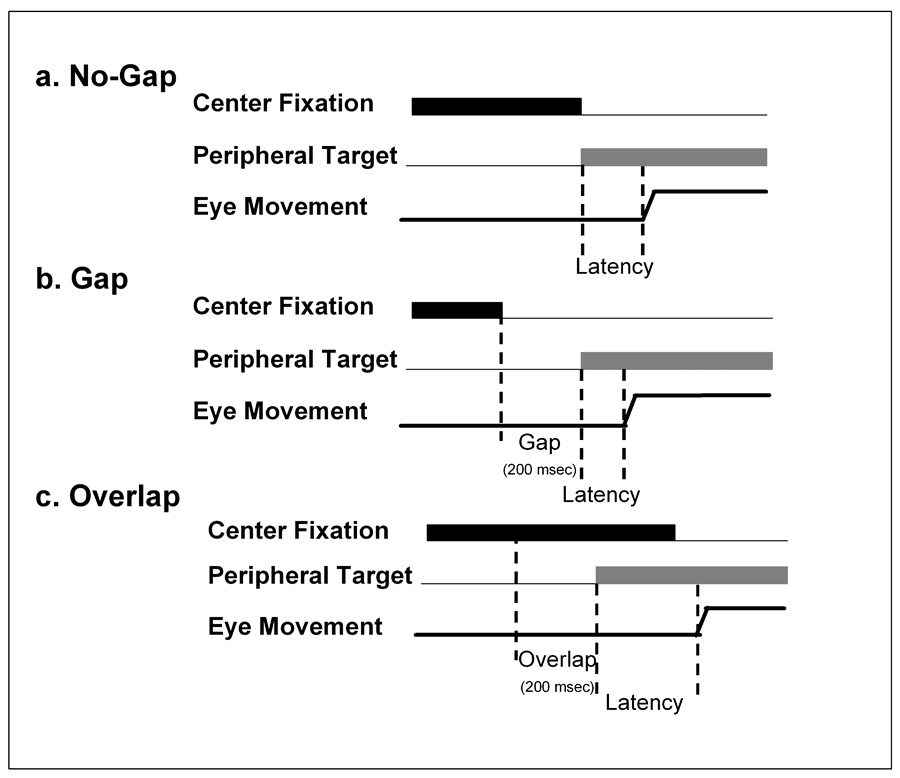
Manipulation of Fixation at the Central Cue. A. In no-gap trials extinction of the central fixation target occurred simultaneous to the appearance of the peripheral target. B. In gap trials, the central fixation target was extinguished 200 msec prior to the appearance of the peripheral target. Response latencies under gap conditions are speeded relative to those in no-gap or overlap conditions due to a reduction of activity in the visual fixation system that frees the visual attention and saccade systems to more quickly respond to visual inputs. C. In overlap trials the central fixation target remains illuminated for 200 msec after the appearance of the peripheral target, thus maintaining attentional engagement at the central location and prolonging response latencies.
The present study used three paradigms to examine the relationship of automatic attention and executive control on antisaccade task performance in schizophrenia: 1) no-gap prosaccade trials; 2) interspersed gap and overlap prosaccade trials; and 3) interspersed gap and overlap antisaccade trials. We had three aims. First, we sought to replicate in an independent sample the prior finding of speeded prosaccade latencies among untreated schizophrenia patients in a no-gap prosaccade task. Second, we aimed to determine whether any group difference in prosaccade latencies was greater in gap conditions or reduced in overlap conditions. And third, we sought to evaluate how individual differences in the extent to which visual attention was automatically released or maintained by central fixation targets was related to antisaccade error rates. This last aim sought to clarify whether our prior finding of an association of speeded orienting responses on a prosaccade task and elevated antisaccade error rates among schizophrenia patients (15) was due to a speeded release from fixation in the absence of target (increased gap effect), a less robust maintenance of visual fixation (reduced overlap effect), or a combination thereof. A subset of patients was retested after 6-weeks of treatment with antipsychotic medication to examine changes in performance after treatment and clinical stabilization.
METHODS AND MATERIALS
Subjects
Twenty-four antipsychotic-naïve individuals (16 males, 8 females) with a DSM-IV diagnosis of schizophrenia (n=22) or schizoaffective disorder (n=2) according to structured clinical interviews (SCID; (24)) and additional clinical data reviewed at consensus diagnosis meetings participated. Thirty healthy individuals (14 males, 16 females) recruited from the community matched patients on age, sex, and IQ (Table 1). Patients and healthy individuals were completely independent from those reported in prior studies (15,17). Patients first experienced psychotic symptoms 15 months on average prior to entering the study. Healthy individuals did not meet current or past criteria for any Axis I disorder according to SCID interviews, nor did they report psychotic or mood disorders among first-degree relatives. All subjects met the following criteria: (1) no systemic or neurologic disease; (2) no past head trauma with loss of consciousness; (3) no lifetime history of substance dependence and no substance abuse for at least 3 months prior to participation; (4) no benzodiazepines (for at least 5 half-lives) prior to testing; and (5) no coffee, tea or cigarettes from 1 hour prior to testing. All subjects provided verbal and written informed consent. This study was approved by the University of Pittsburgh Institutional Review Board.
Table 1.
Demographic comparison of schizophrenia patients and healthy individuals and clinical characteristics of the entire patient sample at baseline (n=24) and the subset of patients with baseline and follow-up assessments (n=16).
| Healthy Individuals (n=30) | Patients (n=24/16) | pa | ||||
|---|---|---|---|---|---|---|
| Demographics | (n=24) | (n=16) | ||||
| Age (years) | 22.2(4.9) | 25.8(8.3) | 23.1(4.6) | .06 | ||
| IQ | 97.2(8.2) | 93.7(11.5) | 91.6(8.1) | .20 | ||
| Male/ Female | 14/16 | 8/16 | 4/12 | .14 | ||
| Baseline (n=24) | Baseline (n=16) | 6-week follow-up (n=16) | pb | |||
| Clinical Ratings | ||||||
| BPRS | 49.6(6.6) | 49.1(5.0) | 37.9 (9.5) | .001 | ||
| SANS | 12.6(2.9) | 12.7(2.9) | 12.1(2.9) | .11 | ||
| SAPS | 8.6(2.9) | 8.9(2.7) | 6.3(4.1) | .03 | ||
| HAM-D | 18.4(7.5) | 18.3(7.6) | 14.4(8.2) | .05 | ||
| Daily Medication Dose (mg) | ||||||
| Risperidone mg/day (n=13) | 0 | 0 | 2.6(1.4) | |||
| Olanzapine mg/day (n=3) | 0 | 0 | 10.0(0.0) | |||
Note: Mean (SD) reported for age, and IQ (Ammon’s Quick Test (Ammons and Ammons,1962)), with pa values reflecting significance level for independent sample t-tests or X2 (for sex) comparing complete patient group to healthy individuals at baseline; Mean (SD) reported for clinical ratings; BPRS= Brief Psychiatric Rating Scale; SANS= Schedule for the Assessment of Negative Symptoms; SAPS= Schedule for the Assessment of Positive Symptoms; HAM-D= Hamilton Depression Inventory (24-item). pb comparisons by paired sample t-tests for change in patients with both baseline and follow-up testings.
Procedures
Patients’ baseline eye movement studies were completed prior to any lifetime antipsychotic treatment. Symptom ratings were completed by clinicians who remained blind to performance on eye movement tasks using the Brief Psychiatric Rating Scale (BPRS; (25)), the Schedules for the Assessment of Positive (SAPS; (26)) and Negative (SANS; (27)) Symptoms, and the 24-item Hamilton Depression Rating Scale (HAM-D-24; (28)) (Table 1). Sixteen patients were available for re-testing after an average of 6-weeks of treatment with antipsychotic medications [13 treated with risperidone (2.6 ± 1.4 mg/ day); 3 treated with olanzapine (all 10.0 mg/ day)] (Table 1). These patients did not differ on demographic or baseline clinical or eye movement performance from those available only at baseline.
Oculomotor Tasks
Subjects were tested in a darkened black room. Stimuli subtending 1 degree of visual angle were presented in the horizontal plane at eye level on a 20 inch monitor (Silicon Graphics, Model GDM-20E21) located 27 inches from the subject. A chin rest with forehead and occipital restraints minimized head movement. An examiner in an adjacent room provided instructions via intercom. Eye movements were monitored using infrared reflection sensors mounted on spectacle frames (Applied Science Laboratories, Inc., Model 210). Blinks were identified using electrodes placed above and below the left eye. Data were digitized on-line at 500 Hz and stored for off-line analysis. Tasks were administered to all subjects in the order described below.
No-gap Prosaccade Task
Subjects fixated a central target for a variable period (2000 – 3000 msec) after which the central target extinguished simultaneously with a peripheral target unpredictably appearing at 7.5 or 15.0 degrees to the right or left of center (see Figure 1a). Subjects were instructed to look to the peripheral target as soon as it appeared. The peripheral target remained illuminated for 1500 msec after which it was extinguished and the central target reappeared indicating the start of the next trial. Thirty-two trials were administered. Saccade latency was measured for each trial.
Gap-Overlap Prosaccade Task
This task was similar to the no-gap prosaccade task except that the central target offset and peripheral target onset were not contemporaneous. In gap trials, the central fixation target was extinguished 200 msec before the peripheral target appeared (see Figure 1b). In overlap trials, the central fixation remained illuminated for 200 msec after the appearance of the peripheral target (see Figure 1c). Thirty-two trials of each trial type were pseudorandomly interleaved throughout this task. Saccade latency was measured for each trial.
A subject’s average saccade latency in no-gap trials was subtracted from the average latency in gap trials to index the degree to which attentional fixation was released in the gap condition. More negative differences on no-gap vs. gap trials reflect the facilitating effects of releasing attention from central fixation prior to target presentation. Differences between the overlap and no-gap trials were also calculated, with more positive differences on no-gap vs. overlap trials reflecting the effects of attentional maintenance at central fixation in delaying automatic shifts of attention and gaze.
Gap-Overlap Antisaccade Task
This task was similar to the gap-overlap prosaccade task except that rather than directing gaze to the appearance of the peripheral target, subjects were instructed to not to look to the peripheral target and instead immediately look to the mirror location in the opposite hemifield. The percentage of trials with antisaccade errors (looking toward rather than away from targets) and the latency of correct antisaccade responses were measured.
Analysis of Oculomotor Recordings
Eye movement recordings were analyzed using software developed in our laboratory. An algorithm identified the beginning of a saccade when eye acceleration rose above 1000 deg/sec2 and continuing until 25% of peak deceleration. A technician blind to subject characteristics visually reviewed recordings from each trial to verify saccades were measured correctly by the algorithm.
Statistical Analyses
Data for individual subjects were pooled across direction and amplitude of peripheral target displacement because there were no significant main effects or interactions of these factors with group. An arcsine transform was performed on the antisaccade error rate data to render them more appropriate for parametric analyses. Results from analyses of transformed and untransformed error rate data were similar. Comparisons between patients and healthy individuals on the no-gap prosaccade task were compared using analysis of variance (ANOVA). Repeated measures ANOVA was used to compare group performances on gap and overlap trials separately on gap-overlap prosaccade and antisaccade tasks, and to examine performance differences over time in the subgroup of patients who were retested after treatment. Correlation analyses were used to examine the association between prosaccade latencies and antisaccade errors.
RESULTS
Prosaccade Latencies
In the no-gap prosaccade task, patients’ latencies were faster than those of healthy individuals (Fgroup (1,53)=6.00, p=.02; Figure 2). For the gap-overlap prosaccade task, analyses revealed shorter latencies in the gap than overlap condition (Ftrial (1,52)=382.13, p<.001; Figure 2). However, there was no overall group difference (Fgroup(1,52)=0.10, p=.76) or differential effect of gap vs. overlap trials between groups (Fgroup × trial (1,52)=0.02, p=.89).
Figure 2.
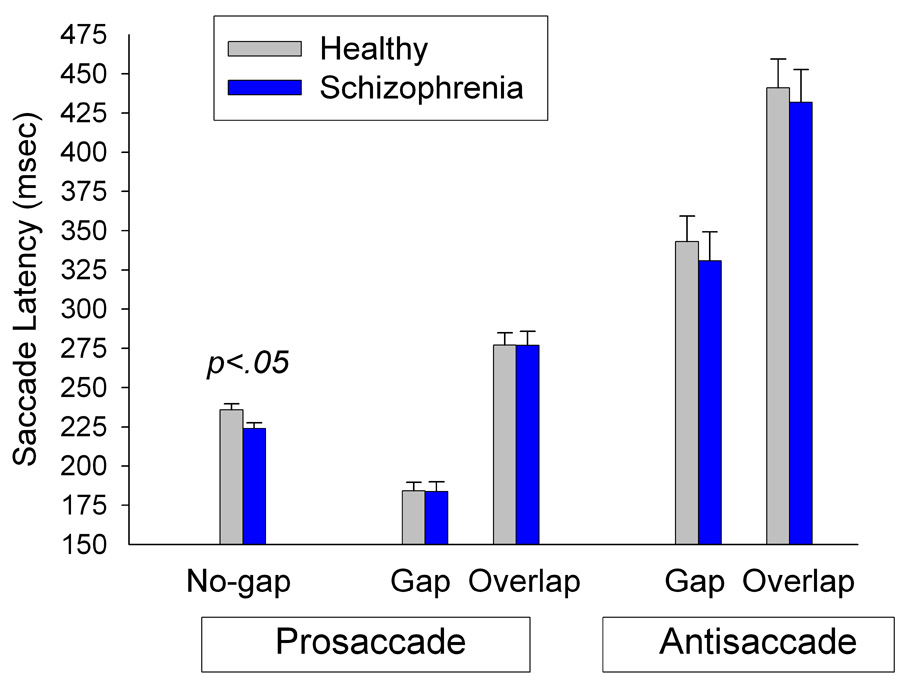
Mean (SE) of latencies in the no-gap prosaccade and gap-overlap prosaccade and antisaccade tasks. Patients had speeded response latencies in the no-gap condition compared to healthy individuals (p=.02). In the gap-overlap prosaccade task, both patients and healthy individuals showed similarly fast latencies on gap trials and similarly prolonged latencies on the overlap trials. Similarly, in the antisaccade task both groups showed comparably fast latencies on gap trials and prolonged latencies on overlap trials.
Figure 3 shows distributions of trial-wise data for the no-gap, gap and overlap prosaccade latencies for each group. The latency distribution of gap trials (diamonds) falls mostly leftward, followed by the distributions for the no-gap (squares) and then overlap (triangles) trials. The group difference in the no-gap condition is illustrated by the leftward shift in the patients’ distribution relative to that of the healthy individuals.
Figure 3.
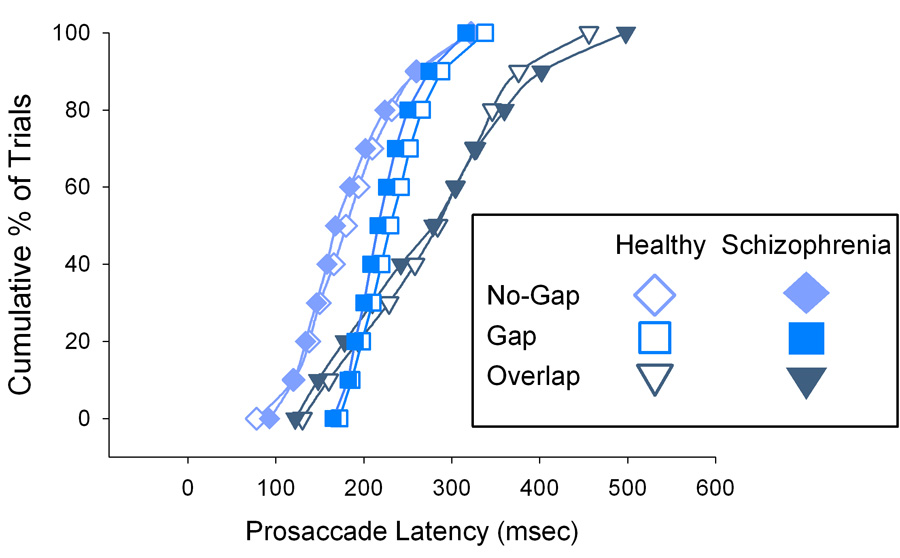
Cumulative percentages of trial-wise prosaccade latencies in gap, no-gap and overlap conditions for patients (filled symbols) and healthy subjects. As expected, saccade latencies in the gap condition (diamonds) are furthest to the left reflecting the greatest percentage of trials with faster latencies, followed by the distributions from no-gap (squares) and overlap (triangles) trials. Interestingly, approximately 20% of overlap trials (which were interleaved with the gap trials in the task) have latencies faster than those from no-gap trials. This effect was similar for patients and healthy individuals. Note the leftward shift in the patients’ curve in the no-gap condition (squares) reflects the shortening of saccade latency relative to healthy individuals.
Patients and healthy individuals did not differ in the extent of change in latency from the no-gap vs. gap (Fgroup(1, 53)=1.89, p=.18) or no-gap vs. overlap (Fgroup(1,53)=1.00, p=.33) conditions. Thus, the degree to which attention at center fixation was released by the central target offset or maintained by the central target persistence was comparable in both groups.
Antisaccade Latencies
Patients and healthy individuals did not differ in the speed with which they initiated correct responses in the antisaccade task (Fgroup(1, 52)=.13, p=.72; Figure 2). As with the prosaccade task, latencies were faster on gap than overlap trials (Ftrial(1, 52)=246.26, p<.001). The difference in response latency between gap and overlap trials was comparable in both groups (Fgroup × trial(1, 52)=.09, p=.76).
Antisaccade Errors
Patients’ antisaccade error rates were significantly greater than those of healthy individuals (Fgroup(1,52)=15.32, p<.001). There was no significant difference between gap and overlap conditions on error rates (Ftrial(1,52)=1.74, p= .19) nor was there a significant interaction between trial condition and group (Fgroup × trial(1,52)=1.23, p=.27). The proportion of trials with antisaccade errors for gap and overlap trials are presented for patients and healthy individuals in Figure 4.
Figure 4.
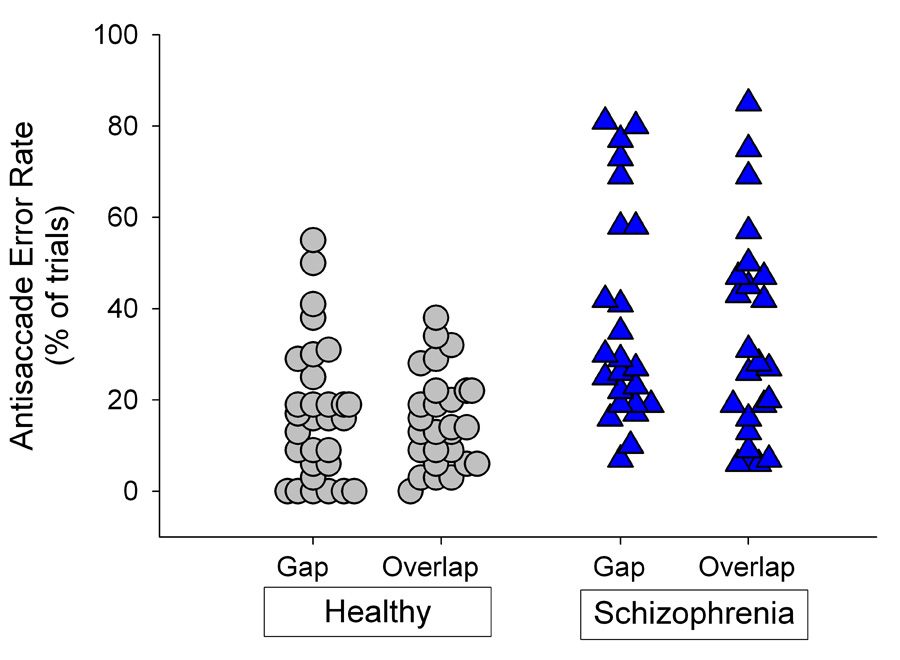
Individual error rates on the antisaccade task. Patients had increased antisaccade error rates compared to healthy individuals regardless of trial type.
Prosaccade Latencies and Antisaccade Errors
To examine whether the degree of release (on gap trials) or maintenance (on overlap trials) of attention at central fixation on the prosaccade task was related to errors on the antisaccade task, the change in latency between no-gap vs. gap and between no-gap vs. overlap prosaccade conditions were correlated with antisaccade error rates (collapsed across both antisaccade gap and overlap trials). As shown in the left side of Figure 5, there was no significant association between the no-gap vs. gap change with antisaccade errors for the patient (r=−.16, p=.50) or healthy group (r=−.19, p=.31). In contrast, the change in latency from no-gap vs. overlap conditions was strongly associated with antisaccade errors among schizophrenia patients (r=−.60, p=.002) but not healthy individuals (r=−.19, p=.32) (Figure 5 right side). Follow-up analyses revealed that healthy individuals’ prosaccade response latencies in no-gap and gap-overlap trials were moderately associated with antisaccade error rates (rs <−37), suggesting a generalized effect of speeded responding regardless of the experimental release or maintenance of visual fixation. Among patients, only diminished response latencies under overlap conditions was associated with elevated antisaccade error rates(r−=.60). Thus, there is a selectivity of diminished maintenance of attention at central fixation with antisaccade errors in schizophrenia patients.
Figure 5.
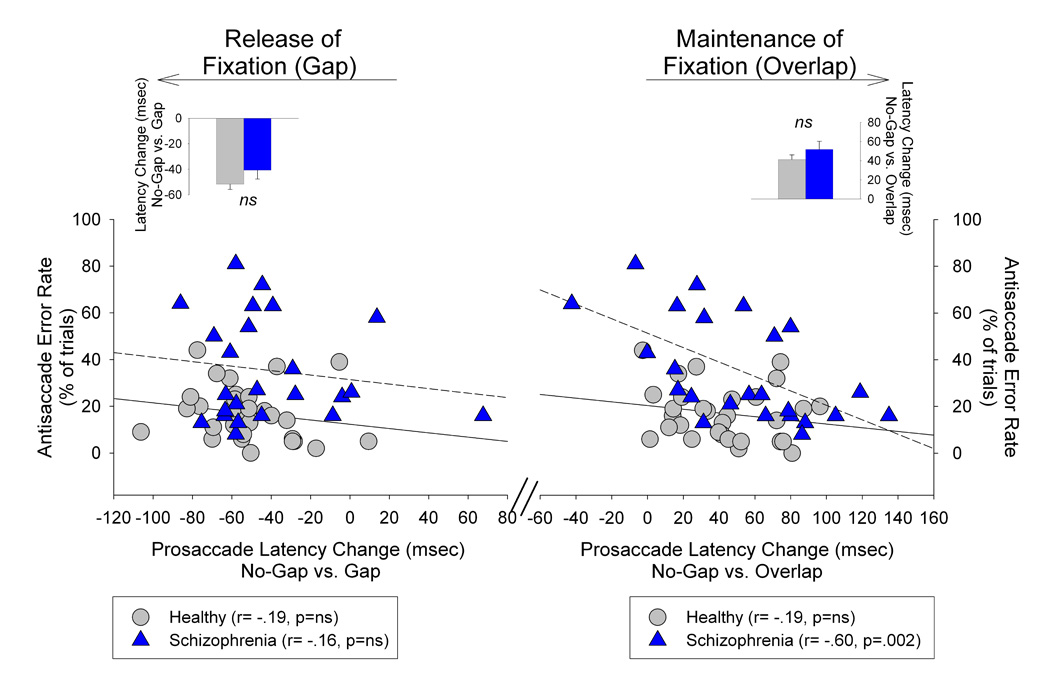
Relationship between degree of release (on gap trials) or maintenance (on overlap trials) of attention at central fixation and antisaccade error rates. Scatter plots reflect associations between antisaccade error rates and the extent which attention fixation was released in the prosaccade task by the gap manipulation (left side) or maintained by the overlap manipulation (right side). There was no association among either patients’ or healthy individuals’ antisaccade error rates and the degree to which their prosaccade latencies were shortened by the gap manipulation. Schizophrenia patients’ increased antisaccade error rates were highly associated with decreased change in latency in the overlap condition indicating a reduced maintenance of attention at central fixation was strongly related to response suppression failures among schizophrenia patients. No such relationship was observed among healthy individuals. Inserts are mean (SE) for the change in prosaccade latency from the gap vs. no-gap conditions (left side; decreased latency) and from the no-gap vs. overlap condition (right side, increased latency). Patients and healthy individuals showed comparable changes in saccade latency in the gap and overlap manipulations.
Change in Performance after Treatment in Schizophrenia Patients
No change in the no-gap prosaccade latency from baseline was observed among patients evaluated at the 6-week follow-up (Ftime(1,15)=.86, p=.37). In the gap-overlap prosaccade task, there were also no changes after treatment (Ftime (1,15)=2.70, p=.12) or interaction between time and trial (Ftime × trial(1,15)=.17, p=.69). Despite the lack significant change over time in the nogap and gap-overlap conditions, the reduction of saccade latencies observed in no-gap vs. gap was greater after treatment (Ftime(1,15)=6.02, p=.03). Follow-up analyses indicated that this effect resulted from a reduction over time in latency on gap trials (t(15)=2.15, p=.05). No change over time was observed in the difference of response latency in no-gap vs. overlap trials (Ftime(1,15)=2.75, p=.12).
On the antisaccade task, there was no significant change after treatment in the error rate (Ftime (1,15)=1.33, p=.27), nor was there any differential change in error rates across gap and overlap trials (Ftime × trial(1,15)=2.00, p=.18). A trend was observed for a slight increase in response latencies over time in overlap trials relative to gap trials (Ftime × trial(1,15)=3.71, p=.07). At follow-up, antisaccade error rates remained robustly associated with the change in response latency from the no-gap vs. overlap prosaccade conditions (r=−.61, p=.01). As at baseline testing, there was no association between antisaccade error rates and the change in latency in gap vs. no-gap conditions (r=−15, p=.58).
Associations among Clinical Ratings and Task Performance
There were no associations between clinical ratings and performance on the prosaccade or antisaccade tasks at baseline. There were also no associations among clinical ratings at follow-up, clinical change from baseline, or medication dose with task performances at followup or changes in task performances over time.
DISCUSSION
The established inability to suppress context-appropriate responses on antisaccade tasks in schizophrenia is widely considered to reflect prefrontal cortical dysfunction that impairs the voluntary inhibition of prepotent responses, akin to disinhibition of a visual grasp reflex. Using no-gap and gap-overlap paradigms to manipulate the balance between neurophysiological mechanisms supporting sustained visual attention and the automatic shift of attention to new visual stimuli, we report the novel finding that a reduced level of attentional focus on central cues is associated with increased rates of antisaccade errors in schizophrenia. Essentially, when patients less effectively fixate attention on central targets, they were more vulnerable to having their attention and gaze automatically drawn to peripheral visual stimuli. This finding is important because it suggests that a diminished ability to sustain focused attention may interact with executive control deficits to cause deficits in suppressing context-inappropriate responses to prepotent stimuli. Thus, antisaccade deficits may result not only from disturbances in the top-down control of behavioral responses by executive prefrontal systems, but also when patients less effectively engage visual attention.
Excitability of Exogenous Attention Systems in Schizophrenia
Patients’ response latencies in the no-gap prosaccade task were speeded, consistent with our prior observation in an independent sample of treatment-naïve first episode patients (17). This might result from decreased neocortical regulation of the superior colliculus (SC), which is the final common pathway of corticofugal inputs that maintain balance between inhibition and excitation in subcortical components of visual orienting systems. Excitatory input from frontoparietal attention systems to the fixation zone in the rostral pole of the SC, and indirect pathways via the dorsolateral prefrontal cortex (DLPFC) and frontal eye fields (FEF) to the caudate nucleus to the substantia nigra to SC, maintain gaze on targets of interest. Activity in the SC fixation zone provides inhibitory input to the saccade zone of the caudal SC, that itself receives excitatory input from neocortex to guide shifts of gaze to attended targets. This results in a dynamic balance between sustaining visual attention to targets of interest and shifting attention to new targets (29). Reduced tonic corticofugal activation of the fixation zone of the SC could result in diminished inhibition of saccade neurons in the caudal SC, thereby rendering that area more easily excited by visual inputs as reflected in speeded responses to novel targets. Consistent with this possibility, disturbances in this circuit have been reported in two independent fMRI studies that demonstrated reduced activation of cortical eye fields in a prosaccade task in antipsychotic-naïve first episode schizophrenia patients (30,31).
Other studies have demonstrated that patients with schizophrenia exhibit the expected shortening of prosaccade latencies in gap conditions relative to no-gap or overlap conditions (23,22). Our finding of speeded latencies among patients in the no-gap condition without similar findings in gap conditions may seem inconsistent and contrary to the explanation of reduced corticofugal regulation on automatic attention systems. However, in prior studies there was no overall mean difference in the latency of prosaccades in gap trials between healthy individuals and schizophrenia patients even though a greater proportion of fast latency (~ < 120 ms) “express saccades” did occur in patients (32,23,33). While we did not observe an increase of express saccades in patients (Figure 3), this may be consequent to interleaving gap and overlap trials rather than blocking trial conditions as in prior studies. Studies with healthy individuals have reported a decrease in express saccade frequency and a relative lengthening of saccade latency on gap trials when overlap trials are interleaved (34). This may also account for why we saw speeded responses only in the blocked no-gap task.
Decreased Attention Fixation and Inhibitory Deficit in Schizophrenia
Examining gap and overlap condition data in relation to a no-gap task permitted separate examination of the balance between systems that maintain visual attention and those that respond automatically to shift attention to new sensory input, and how this relate to errors on an antisaccade task. Patients with reduced attentional fixation effects in the prosaccade overlap condition (evidenced by a smaller difference between their no-gap and overlap prosaccade latencies) made more errors on the antisaccade task. In contrast, there was no association between patients’ response facilitation in prosaccade gap trials and antisaccade errors. The specificity of patients’ prosaccade latencies under overlap conditions and elevated antisaccade errors is very different from the pattern of associations observed in healthy individuals in which error rates were associated with response latency in general, but not differentially when fixation of attention was manipulated. These findings suggest that diminished fixation of attention, rather than an exaggerated release of attention (i.e., an excessive “go” response) in the presence of novel sensory input, lessens the capacity of prefrontal executive processes to suppress context inappropriate responses in schizophrenia.
To successfully execute an antisaccade, increased activity in the fixation zone of SC before peripheral stimulus presentation must inhibit activity of saccade generating neurons in caudal SC to suppress responses to visual input (35,36). Neurophysiological studies with behaving monkeys show that excitatory input from DLPFC supports preparatory activity in the fixation zone of SC as part of this process (37). Given multiple lines of work showing DLPFC disturbances in schizophrenia, reduced input from this region to pretectal systems is a likely cause of increased errors on the antisaccade task. Because inputs to maintain fixation during prosaccade tasks also comes from the FEF, reduced input from FEF to SC might also contribute deficits on the antisaccade task in schizophrenia.
In aggregate, our findings suggest that there are important functional synergies between sustained attention and executive processing deficits in schizophrenia. Deficits sustaining attention and inhibiting context-inappropriate responses are well established in schizophrenia (38,11). Both types of deficits are promising endophenotypes for the disorder insofar as they are evident at illness onset, persist over the course of the disorder, and are found among unaffected relatives (39,40,41,12). The replication of our finding of speeded saccades on a prosaccade task is consistent with an alteration of top down attentional control. However, group differences in gap and overlap effects with the prosaccade task were not significant. The data indicate that those patients at the lower end of the range of overlap effects had an especially pronounced difficulty performing an antisaccade task. Our findings thus suggest that in schizophrenia, lower sustained attention abilities can interact with executive function deficits to cause heightened distractibility and reduced volitional control of behavior. This possibility could be further explored in future studies using both oculomotor paradigms such as those used here and neuropsychological measures of sustained attention (e.g., CPT tasks) and executive abilities.
One question raised by the present study is whether reductions in sustained attention and executive control are independent with additive effects under certain circumstances or problems with a common origin. The DLPFC comprises part of the anterior attentional system that serves to focus attention (1), and dysfunction within that region has been demonstrated in functional neuroimaging studies of schizophrenia patients performing sustained attention tasks (42,43). Therefore, a functional disturbance of dorsolateral prefrontal cortex might alone cause both deficits in sustained attention and in the ability to voluntarily suppress prepotent behavioral responses. Further research is needed to examine whether common or separate pathophysiological mechanisms contribute to sustained attention and executive control deficits in schizophrenia patients.
Persistent of Impairments after Antipsychotic Treatment
Antisaccade error rates remained persistently elevated among the subset of patients who were retested after 6-weeks of antipsychotic treatment. Moreover, the robust association between reduced attentional fixation and increased antisaccade error rates was sustained in these patients. This suggests that the interaction of systems that maintain attention and support executive control of behavior for antisaccade task performance is independent of clinical state, and may not be affected by treatment with second-generation antipsychotics, at least early in the course of treatment. The lack of correlations of neurophysiological deficits with clinical measures, and the absence of change after acute treatment, are consistent the view that the deficits in sustained attention and executive control appear to be stable over time.
We did not observe the slowing of patients’ speeded prosaccade responses in a no-gap task after antipsychotic treatment which in contrast to a prior report (17), perhaps due to lower medication doses in the current study. Interestingly, there was a small shortening of prosaccade latency in gap trials compared to no-gap latency and an opposite trend effect for slightly increased antisaccade latency in overlap trials. These findings raise the possibility that antipsychotic treatment may differentially impact the speed with which automatic and voluntary attentional shifts occur. In the case of schizophrenia this could be seen as advantageous as both the ability to shift and sustain attention may be enhanced. Additional data with larger samples are needed to better understand how these attentional processes are impacted by antipsychotic treatments.
By parsing different aspects of attentional control, the current study demonstrated that a hallmark impairment of executive control in schizophrenia, the failure to suppress contextinappropriate responses on an antisaccade task, may result in part from deficits in more basic processes of sustained attention. Thus, functional synergies between simple attentional and higher order processes may underlie deficits that patients exhibit on executive function tasks. Additional work with such paradigms that allow for the dissociation of generalized and specific deficits of cognitive processes is needed to further our understanding of the neural systems basis to complex behavioral deficits associated with schizophrenia.
GRANT SUPPORT AND OTHER ACKNOWLEDGEMENTS
This publication was supported by funds received from MH62134, MH01433, NIH/NCRR/GCRC Grant M01 RR00056, and NARSAD. We thank Drs. Cameron Carter, Gretchen Haas, and Debra Montrose, and the clinical core staff of the Center for the Neuroscience of Mental Disorders (MH45156 Director: David Lewis MD) for their assistance in diagnostic and psychopathological assessments.
Footnotes
None of the authors reported biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Posner MI. Cognitive Neuroscience of Attention. New York: Guilford Press; 2004. [Google Scholar]
- 2.Pierrot-Deseilligny C, Muri RM, Ploner CJ, Gaymard B, Demeret S, Rivaud-Pechoux S. Decisional role of the dorsolateral prefrontal cortex in ocular motor behaviour. Brain. 2003;126:1460–1473. doi: 10.1093/brain/awg148. [DOI] [PubMed] [Google Scholar]
- 3.Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC, Shulman GL. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- 4.Moore T, Fallah M. Control of eye movements and spatial attention. Proc.Natl.Acad.Sci.U.S.A. 2001 Jan 30;98:1273–1276. doi: 10.1073/pnas.021549498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sweeney JA, Luna B, Keedy SK, McDowell JE, Clementz BA. fMRI studies of eye movement control: Investigating the interaction of cognitive and sensorimotor brain systems. NeuroImage. 2007 doi: 10.1016/j.neuroimage.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Funahashi S, Chafee MV, Goldman-Rakic PS. Prefrontal neuronal activity in rhesus monkeys performing a delayed anti-saccade task. Nature. 1993;365:753–758. doi: 10.1038/365753a0. [DOI] [PubMed] [Google Scholar]
- 7.Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nat.Rev Neurosci. 2004;5:218–228. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- 8.Clementz BA, Brahmbhatt SB, McDowell JE, Brown R, Sweeney JA. When does the brain inform the eyes whether and where to move the eyes? an EEG study in humans. Cerebral Cortex. 2007 Feb 5; doi: 10.1093/cercor/bhl171. [DOI] [PubMed] [Google Scholar]
- 9.Sweeney JA, Mintun MA, Kwee S, Wiseman MB, Brown DL, Rosenberg DR, Carl JR. Positron emission tomography study of voluntary saccadic eye movements and spatial working memory. Journal of Neurophysiology. 1996;75:454–468. doi: 10.1152/jn.1996.75.1.454. [DOI] [PubMed] [Google Scholar]
- 10.Fukushima J, Morita N, Fukushima AK, Chiba T, Tanaka S, Yamashita I. Voluntary control of saccadic eye movements in patients with schizophrenic and affective disorders. Journal of Psychiatric Research. 1990;24:9–24. doi: 10.1016/0022-3956(90)90021-h. [DOI] [PubMed] [Google Scholar]
- 11.Hutton SB, Ettinger U. The antisaccade task as a research tool in psychopathology: a critical review. Psychophysiology. 2006;43:302–313. doi: 10.1111/j.1469-8986.2006.00403.x. [DOI] [PubMed] [Google Scholar]
- 12.Clementz BA, McDowell JE, Zisook S. Saccadic system functioning among schizophrenia patients and their first-degree biological relatives. Journal of Abnormal Psychology. 1994;103:277–287. [PubMed] [Google Scholar]
- 13.McDowell JE, Brown GG, Paulus M, Martinez A, Stewart SE, Dubowitz DJ, Braff DL. Neural correlates of refixation saccades and antisaccades in normal and schizophrenia subjects. Biol.Psychiatry. 2002 Feb 1;51:216–223. doi: 10.1016/s0006-3223(01)01204-5. [DOI] [PubMed] [Google Scholar]
- 14.Gooding DC, Mohapatra L, Shea HB. Temporal stability of saccadic task performance in schizophrenia and bipolar patients. Psychological Medicine. 2004;34:921–932. doi: 10.1017/s003329170300165x. [DOI] [PubMed] [Google Scholar]
- 15.Harris MS, Reilly JL, Keshavan MS, Sweeney JA. Longitudinal studies of antisaccades in antipsychotic-naive first-episode schizophrenia. Psychol.Med. 2006;36:485–494. doi: 10.1017/S0033291705006756. [DOI] [PubMed] [Google Scholar]
- 16.Hutton SB, Crawford TJ, Puri BK, Duncan LJ, Chapman M, Kennard C, Barnes TRE, Joyce EM. Smooth pursuit and saccadic abnormalities in first-episode schizophrenia. Psychological Medicine. 1998;28:685–692. doi: 10.1017/s0033291798006722. [DOI] [PubMed] [Google Scholar]
- 17.Reilly JL, Harris MS, Keshavan MS, Sweeney JA. Abnormalities in visually guided saccades suggest corticofugal dysregulation in never-treated schizophrenia. Biological Psychiatry. 2005 Jan 15;57:145–154. doi: 10.1016/j.biopsych.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 18.Saslow MG. Latency for saccadic eye movement. Journal of the Optical Society of America. 1967;57:1030–1033. doi: 10.1364/josa.57.001030. [DOI] [PubMed] [Google Scholar]
- 19.Fischer B, Weber H. Express saccades and visual attention. Behavioral and Brain Science. 1993;16:553–610. [Google Scholar]
- 20.Dorris MC, Munoz DP. A neural correlate for the gap effect on saccadic reaction times in monkey. Journal of Neurophysiology. 1995;73:2558–2562. doi: 10.1152/jn.1995.73.6.2558. [DOI] [PubMed] [Google Scholar]
- 21.Muller N, Riedel M, Eggert T, Straube A. Internally and externally guided voluntary saccades in unmedicated and medicated schizophrenic patients: Part II. Saccadic latency, gain, and fixation suppression errors. European Archives of Psychiatry and Clinical Neuroscience. 1999;249:7–14. doi: 10.1007/s004060050059. [DOI] [PubMed] [Google Scholar]
- 22.Smyrnis N, Malogiannis IA, Evdokimidis I, Stefanis NC, Theleritis C, Vaidakis A, Theodoropoulou S, Stefanis CN. Attentional facilitation of response is impaired for antisaccades but not for saccades in patients with schizophrenia: implications for cortical dysfunction. Exp Brain Res. 2004;159:47–54. doi: 10.1007/s00221-004-1931-0. [DOI] [PubMed] [Google Scholar]
- 23.Sereno AB, Holzman PS. Express saccades and smooth pursuit eye movement function in schizophrenic, affective disorder, and normal subjects. Journal of Cognitive Neuroscience. 1993;5:303–316. doi: 10.1162/jocn.1993.5.3.303. [DOI] [PubMed] [Google Scholar]
- 24.First M, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. Patient Edition (SCID-P) New York: New York State Psychiatric Institute; 1995. [Google Scholar]
- 25.Overall, JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports. 1962;10:799–812. [Google Scholar]
- 26.Andreasen NC. Scale for the Assessment of Positive Symptoms. Iowa City, IA: University of Iowa Press; 1984. [Google Scholar]
- 27.Andreasen NC. Scale for the Assessment of Negative Symptoms. Iowa City, IA: University of Iowa Press; 1984. [Google Scholar]
- 28.Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munoz DP, Fecteau JH. Vying for dominance: dynamic interactions control visual fixation and saccadic initiation in the superior colliculus. Progress in Brain Research. 2002;140:3–19. doi: 10.1016/S0079-6123(02)40039-8. [DOI] [PubMed] [Google Scholar]
- 30.Keedy SK, Ebens CL, Keshavan MS, Sweeney JA. Functional magnetic resonance imaging studies of eye movements in first episode schizophrenia: smooth pursuit, visually guided saccades and the oculomotor delayed response task. Psychiatry Res. 2006 Apr 30;146:199–211. doi: 10.1016/j.pscychresns.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Keedy SK, Harris MSH, Reilly JL, Marvin RW, Rosen C, Sweeney JA. Changes in the oculomotor system after antipsychotic treatment in first-episode schizophrenia: An fMRI study. Schizophrenia Bulletin. 2007;33:373. [Google Scholar]
- 32.Clementz BA. The ability to produce express saccades as a function of gap interval among schizophrenia patients. Experimental Brain Research. 1996;111:121–130. doi: 10.1007/BF00229561. [DOI] [PubMed] [Google Scholar]
- 33.Matsue Y, Osakabe K, Saito H, Goto Y, Ueno T, Matsuoka H, Chiba H, Fuse Y, Mitsumoto S. Smooth pursuit eye movements and express saccades in schizophrenic patients. Schizophrenia Research. 1994;12:121–130. doi: 10.1016/0920-9964(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 34.Weber H, Biscaldi M, Fischer B. Intertrial effects of randomization on saccadic reaction times in human observers. Vision Research. 1995;35:2615–2642. doi: 10.1016/0042-6989(95)00040-7. [DOI] [PubMed] [Google Scholar]
- 35.Everling S, Munoz DP. Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. Journal of Neuroscience. 2000;20:387–400. doi: 10.1523/JNEUROSCI.20-01-00387.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Everling S, Dorris MC, Klein RM, Munoz DP. Role of primate superior colliculus in preparation and execution of anti-saccades and pro-saccades. Journal of Neuroscience. 1999;19:2740–2754. doi: 10.1523/JNEUROSCI.19-07-02740.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnston K, Everling S. Monkey dorsolateral prefrontal cortex sends task-selective signals directly to the superior colliculus. J Neurosci. 2006 Nov 29;26:12471–12478. doi: 10.1523/JNEUROSCI.4101-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nuechterlein K. Vigilance in schizophrenia and related disorders. In: Steinhauer SR, Gruzelier JH, Zubin J, editors. Handbook of schizophrenia, neuropsychology, psychophysiology, and information processing. Amsterdam: Elsevier Science; 1991. pp. 397–433. [Google Scholar]
- 39.Cornblatt BA, Malhotra AK. Impaired attention as an endophenotype for molecular genetic studies of schizophrenia. Am.J Med Genet. 2001 Jan 8;105:11–15. [PubMed] [Google Scholar]
- 40.Wang Q, Chan R, Sun J, Yao J, Deng W, Sun X, Liu X, Sham PC, Ma X, Meng H, Murray RM, Collier DA, Li T. Reaction time of the Continuous Performance Test is an endophenotypic marker for schizophrenia: a study of first-episode neuroleptic-naive schizophrenia, their non-psychotic first-degree relatives and healthy population controls. Schizophrenia Research. 2007;89:293–298. doi: 10.1016/j.schres.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 41.Curtis CE, Calkins ME, Grove WM, Feil KJ, Iacono WG. Saccadic disinhibition in patients with acute and remitted schizophrenia and their first-degree biological relatives. American Journal of Psychiatry. 2001;158:100–106. doi: 10.1176/appi.ajp.158.1.100. [DOI] [PubMed] [Google Scholar]
- 42.Perlstein WM, Dixit NK, Carter CS, Noll DC, Cohen JD. Prefrontal cortex dysfunction mediates deficits in working memory and prepotent responding in schizophrenia. Biological Psychiatry. 2003 Jan 1;53:25–38. doi: 10.1016/s0006-3223(02)01675-x. [DOI] [PubMed] [Google Scholar]
- 43.Salgado-Pineda P, Junque C, Vendrell P, Baeza I, Bargallo N, Falcon C, Bernardo M. Decreased cerebral activation during CPT performance: structural and functional deficits in schizophrenic patients. Neuroimage. 2004;21:840–847. doi: 10.1016/j.neuroimage.2003.10.027. [DOI] [PubMed] [Google Scholar]


