Abstract
Solar UV irradiation is the causal factor for the increasing incidence of human skin carcinomas. The activation of the transcription factor activator protein-1 (AP-1) has been shown to be responsible for the tumor promoter action of UV light in mammalian cells. We demonstrate that proteinase inhibitor I (Inh I) and II (Inh II) from potato tubers, when applied to mouse epidermal JB6 cells, block UV-induced AP-1 activation. The inhibition appears to be specific for UV-induced signal transduction for AP-1 activation, because these inhibitors did not block UV-induced p53 activation nor did they exhibit any significant influence on epidermal growth factor-induced AP-1 transactivation. Furthermore, the inhibition of UV-induced AP-1 activity occurs through a pathway that is independent of extracellular signal-regulated kinases and c-Jun N-terminal kinases as well as P38 kinases. Considering the important role of AP-1 in tumor promotion, it is possible that blocking UV-induced AP-1 activity by Inh I or Inh II may be functionally linked to irradiation-induced cell transformation.
Proteinase inhibitors I (Inh I) and II (Inh II) from potatoes are two well characterized inhibitors of chymotrypsin and trypsin (1, 2). Both inhibitors are heat-stable, Inh I having one disulfide bond and Inh II having six (1, 2). Both inhibitors are induced to accumulate in potato and tomato leaves in response to wounding and UV irradiation (3, 4), and have been shown to be involved with the induced defense response of plants against herbivores and pathogens (3). These inhibitors, as well as other plant proteinase inhibitors, have an inhibitory effect on x-irradiation-induced mammalian cell transformation (5), although the mechanism underlying their anticarcinogenic activity is not known. Because activator protein-1 (AP-1) is one of the most important transcription factors in the UV response in mammalian cells (6–8), we investigated the effects of Inh I and Inh II on UV-induced AP-1 transactivation. We report the both Inh I and Inh II block UV-induced AP-1 activity and that the induction is independent of extracellular signal-regulated kinases (Erks) and c-Jun N-terminal kinases (JNKs), as well as p38 kinase.
MATERIALS AND METHODS
Plasmids and Reagents.
CMV-neu marker vector plasmid was constructed as reported (9); P53 luciferase reporter plasmid was the same as reported (10); fetal bovine serum (FBS), Lipofectamine, MEM, and G418 were from GIBCO/BRL; epidermal growth factor (EGF) was from Collaborative Research; luciferase substrate was from Promega; the proteinase inhibitors I and II were isolated from potato tubers as described (1, 2). Inh I contains a reactive site that powerfully inhibits chymotrypsin, whereas Inh II is a “double-headed” inhibitor and strongly inhibits both trypsin and chymotrypsin; lima bean inhibitor (LBI) and soybean trypsin inhibitor (SBI) were purchased from Sigma.
Generation of P53 Luciferase Reporter Stable Transfectant.
JB6 cells, Cl 41, were cultured in six-well plates until they reached 85–90% confluence. Six micrograms of P53 luciferase reporter plasmid (PG13-Luc) and 0.3 μg of cytomegalovirus-nue marker vector and 15 μl of Lipofectamine reagent were used to transfect each well in the absence of serum. After 10–12 h, the medium was replaced with 5% FBS MEM. Approximately 30–36 h after the beginning of the transfection, the cells were digested with 0.033% trypsin and the cell suspensions were transferred to 75-ml culture flasks and cultured for 24–28 days with G418 selection (300 μg/ml). Stable transfectants were screened by assay of the luciferase activity. The stable transfectant, C1 41 P53, was cultured in G418-free MEM for at least two passages before each experiment.
Cell Culture.
JB6 P+ mouse epidermal cell line, C1 41, and its stable transfectants, P+1-1 or C1 41 P53 were cultured in monolayers at 37°C, 5% CO2 using MEM containing 5% fetal calf serum, 2 mM l-glutamine, and 25 μg of gentamicin per ml.
Assay for AP-1 Activity and P53 Activity.
Confluent monolayers of P+1-1 or C1 41 P53 cells were trypsinized and 8 × 103 viable cells suspended in 100 μl 5% FBS MEM medium were added into each well of a 96-well plate. Plates were incubated at 37°C in a humidified atmosphere of 5% CO2. Twelve- to twenty-four hours later, cells were starved by culturing them in 0.1% FBS MEM for 12 h. The cells were or were not treated with Inh I or Inh II for 30 min, and then were exposed to UVB (4 kJ/m2 with filter) or UVC (60 J/m2) for AP-1 or P53 induction for 24 hr. The cells were extracted with lysis buffer and luciferase activity was measured using a luminometer (Monolight 2010). The results are expressed as relative AP-1 activity or relative P53 activity.
Erks and P38 Kinase Phosphorylation Assay.
Immunoblot assays for phosphorylation of Erks and P38 kinase were carried out as described by New England Biolabs using phosphospecific antibodies against phosphorylated sites of Erks and P38 kinase, respectively.
JNK Activity Assay.
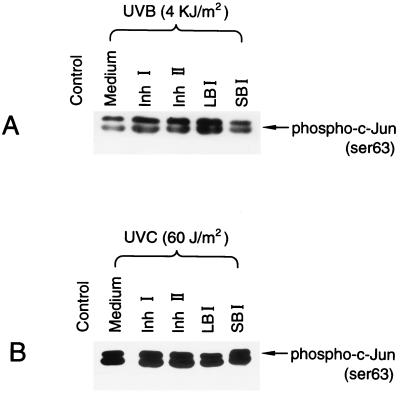
JNK activity was assayed as described in the protocol of New England Biolabs. In brief, JB6 C1 41 cells were starved for 48 hr in 0.1% FBS MEM at 37°C, 5% CO2 incubator. The cells were first treated with different concentrations of Inh I and Inh II for 30 min and then exposed to UVB (4 kJ/m2 with filter) or UVC (60 J/m2) for another 20 min. The cells were washed once with ice-cold PBS and lysed in 300 μl of lysis buffer per sample (20 mM Tris, pH 7.4/150 mM NaCl, 1 mM EDTA/1 mM EGTA/1% Triton/2.5 mM sodium pyrophosphate/1 mM β-glycerol phosphate/1 mM Na3VO4/1 mg/ml leupeptin). The lysates were sonicated and centrifuged, and the supernatant incubated with 2 μg of N-terminal c-Jun (1–89) fusion protein bound to glutathione Sepharose beads overnight at 4°C. The beads were washed twice with 500 ml of lysis buffer with phenylmethylsulfonyl fluoride and twice with 500 μl of kinase buffer (25 mM Tris, pH 7.5/5 mM β-glycerol phosphate/2 mM DTT/0.1 mM Na3VO4/10 mM MgCl2). The kinase reactions were carried out in the presence of 100 μm ATP at 30°C for 30 min. c-Jun phosphorylation was selectively measured by Western blot analysis using a chemiluminescent detection system and specific c-Jun antibodies against phosphorylation of c-Jun at Ser-63.
RESULTS AND DISCUSSION
Blocking of UV-Induced AP-1 Activity by Inh I and Inh II.
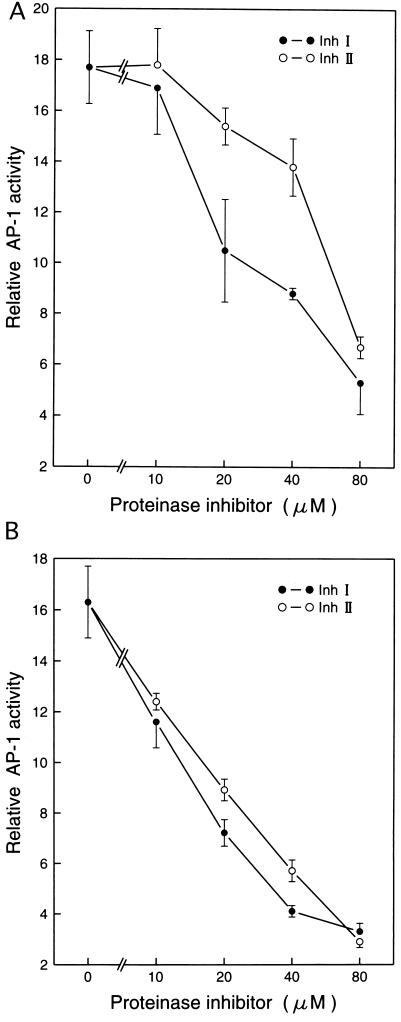
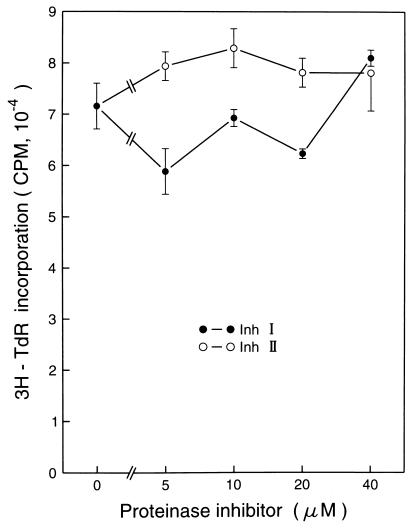
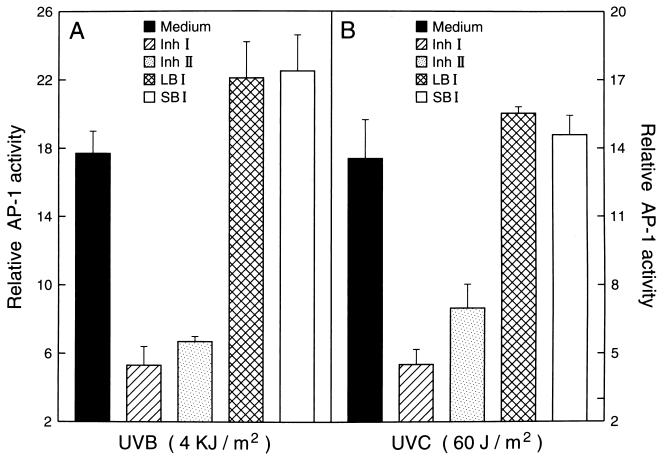
Inh I and Inh II have been shown to be effective reagents in arresting x-irradiation-induced cell transformation in mammalian cells (5), but no proteinase inhibitor proteins have been assayed for their effects in UVB or UVC induction of cellular events leading to carcinogenesis. To explore the possibility that proteinase inhibitors might also inhibit events leading to UV-induced carcinogenesis, we used P+1-1 cells, which are a well-characterized mouse epidermal JB6 stable transfectant with the AP-1 luciferase reporter [73/63 collagenase promoter driving the luciferase gene (6, 7)]. The JB6 cell line is an established mouse epidermal cell line that has been used extensively as an in vitro model for studying tumor promotion (6, 7, 9–11). Pretreatment of P+1-1 cells with either Inh I or Inh II markedly blocked both UVB- or UVC-induced AP-1 transactivation in a dose-dependent manner (Fig. 1A). These inhibitory effects were not due to cytotoxic effects because 3H-thymidine incorporation in the inhibitor-treated cells was not affected at the doses used in the present study (Fig. 2). To determine whether the blocking activity by Inh I and Inh II in UV-induced AP-1 activity is proteinase inhibitor specific, we also investigated the effects of LBI and SBI on UV-induced AP-1 activation. We found that pretreatment of cells with LBI or SBI had no significant inhibitory effect on UV-induced AP-1 activity (Fig. 3). Therefore, among these four proteinase inhibitors, the inhibition of UV-induced AP-1 activity appears to be specific for the potato proteinase Inh I and Inh II. This specificity is interesting because SBI is a potent trypsin inhibitor, with weak antichymoptrypsin activity, whereas both LBI and Inh II inhibit both trypsin and chymotrypsin and Inh I strongly inhibits chymotrypsin, but is a weak trypsin inhibitor. However, both Inh I and II are potent inhibitors of elastase. Although the specificity of Inh I and II in inhibiting AP-1 activity is not known, the data in Fig. 3 imply that Inh I and Inh II can regulate UV-induced signal transduction leading to the activation of the AP-1. We reported previously that induced AP-1 activity is required for tumor promoter-induced transformation (11). Therefore, the inhibitory effects of Inh I and Inh II proteins on UV-induced AP-1 activity may be related to both tumor-promoter-induced cell transformation as well as x-irradiation-induced cell transformation (5).
Figure 1.
Inhibition of UV-induced AP-1 activity by proteinase inhibitors I and II (Inh I and Inh II) in JB6 cells. JB6 AP-1 reporter stable transfected P+1-1 cells suspended in 5% FBS MEM were added to each well of 96-well plates. After overnight culture at 37°C, the cells were starved by replacing the medium with 0.1% FBS MEM for 12–24 h. Then, the cells were first treated with increasing concentrations of Inh I or Inh II for 30 min and exposed to either (A) UVB (4 kJ/m2 with filter), or (B) UVC (60 J/m2). After 24 h culture in 37°C, 5% CO2 incubator, the AP-1 transcriptional activity was measured using a luciferase activity assay. The results are presented as relative AP-1 activity.
Figure 2.
The effects of Inh I or Inh II on JB6 cell proliferation. JB6 P+1-1 cells (5 × 103) were seeded in 96-well plates in the presence of different concentrations of Inh I or Inh II. After 36 h culturing in a 37°C incubator, [3H]thymidine (0.5 μCi per well; 1 Ci = 37 GBq) was added to each well. The cells were harvested 12 h later and incorporation of [3H]thymidine was detected with a liquid scintillation counter. The results are presented as cpm. Bar = the mean and SD of assays from triplicates.
Figure 3.
Comparisons of the effects of Inh I and Inh II with those of LBI and SBI on the UVB-induction of the AP-1 transcriptional activity. JB6 P+1-1 cells stably transfected with the AP-1-luciferase reporter plasmid, suspended in 5% FBS MEM, were added to each well of 96-well plates. After overnight culture at 37°C, the cells were starved by replacing the medium with 0.1% FBS MEM for 12–24 h. Then, the cells were first treated with 80 μM Inh I, Inh II, LBI, or SBI for 30 min and sequentially were exposed to (A) UVB (4 kJ/m2) or (B) UVC (60 J/m2). After 24 h culture in 37°C, 5% CO2 incubator, the AP-1 activity was measured with a luciferase activity assay. The results are presented as relative AP-1 activity.
Inh I and Inh II Specifically Block AP-1 Activity Induced by UV Irradiation, but Not Activity Induced by EGF.
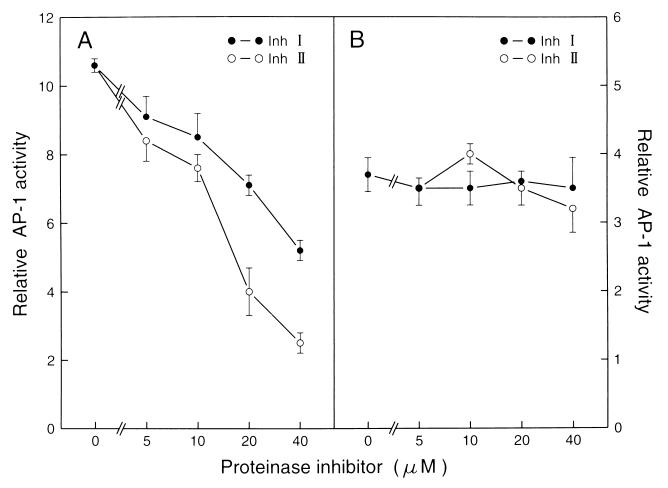
To determine whether the proteinase inhibitors also inhibit the signal transduction pathway for EGF induction of AP-l, we examined the effects of both Inh I and Inh II on EGF-induced AP-1 activity. As shown in Fig. 4, no inhibition of EGF-induced AP-1 activity was observed (Fig. 4). Additionally, no inhibition of the 12-O-tetradecanoylphobol-13-acetate-induced AP-1 activity was found (data not shown). These findings suggest that blocking AP-1 activity by Inh I or Inh II is specific for UV-induced signal transduction pathway leading to AP-1 activation, and provide direct evidence that the activation of the AP-1 by UV irradiation differs from those induced by EGF or 12-O-tetradecanoylphorbol 13-acetate. This is consistent with our previous finding that the UV-induced AP-1 activation pathway does not occur through EGF receptors (6, 7).
Figure 4.
Inhibition of UV-induced, but not EGF-induced AP-1 activity by Inh I or Inh II. P+1-1 cells suspended in 5% FBS MEM were added to each well of 96-well plates. The cells were cultured and starved as described in Materials and Methods. Then, the cells were pretreated with different concentrations of Inh I or Inh II for 30 min, then the cells were exposed to UVB (4 kJ/m2 with filter) or EGF (20 ng/ml). Twenty-four hours later, the AP-1 activity was determined as in Fig. l. The results are presented as relative AP-1 activity.
Blocking the Activity of Inh I or Inh II in the UV-Induced Signal Transduction Pathway Is Transcription Factor-Specific.
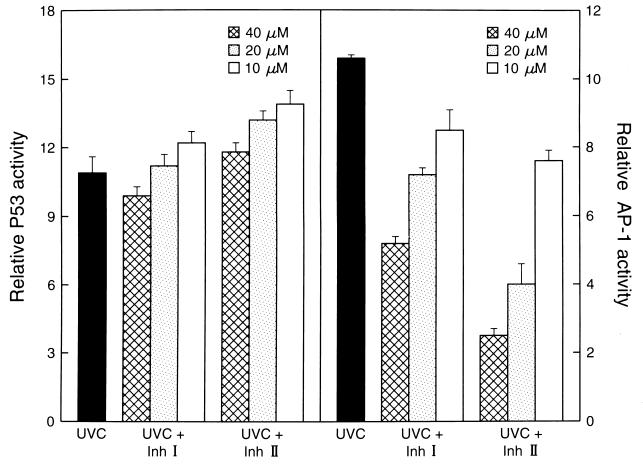
It was proposed that UV-induced AP-1 activation originates at the plasma membrane and the signal is subsequently transferred to the nucleus via several signaling cascades involving Ras/Raf and the mitogen-activated protein kinase family (8, 12). In contrast, UV-induced P53 activation results from UV-induced DNA damage (8, 12, 16). To further examine the nature of the signal transduction pathway that is inhibited by Inh I and Inh II in response to UV irradiation, we compared the effects of Inh I and Inh II on the UVC-induced AP-1 activation and P53 activation. We found that Inh I and Inh II did not inhibit P53 activation (P > 0.05) by UVC under conditions in which the UV-induced AP-1 activity was significantly blocked by Inh I or Inh II (Fig. 5). Interestingly, at low concentrations, Inh I or Inh II slightly enhanced p53 activity (Fig. 5). These data support a mechanism of inhibition by Inh I and Inh II in which the UV-induced signaling is signal transduction pathway and transcription factor-specific.
Figure 5.
Inh I and Inh II do not block UV-induced P53 activity. Cl 41 P53 or P+1-1 cells suspended in 5% FBS MEM were added to each well of 96-well plates. The cells were cultured and starved as described in Materials and Methods. Then, the cells were pretreated with Inh I or Inh II at the concentration indicated. Then the cells were exposed to UVC (60 J/m2). Twenty-four hours later, the AP-1-dependent transcriptional activity, or P53-dependent transcriptional activity, was determined as in Fig. l. The results were presented as relative AP-1 activity or relative P53 activity.
Inhibition of UV-Induced AP-1 Activity by Inh I and Inh II Is Through Erks, JNKs-, or P38 Kinase-Independent Path-ways.
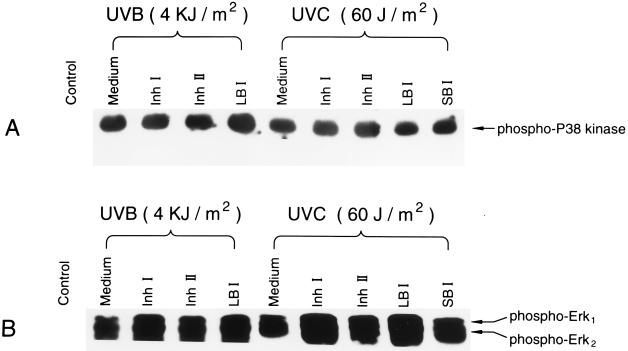
In most studies to date, the signaling leading to AP-1 activation is through three mitogen-activated protein kinase pathways, including Erks, JNKs, and P38 kinase (13–22). To further analyze the mechanism of the inhibitory effect of Inh I or Inh II on UV-induced AP-1 activation, we investigated the influence of Inh I and Inh II on UV-induced activations of JNKs, Erks, and P38 kinase. UV-induced activation of JNKs (Fig. 6) and the phosphorylation of Erks and P38 kinase (Fig. 7) were not inhibited by Inh I or Inh II at the same dose range that blocked UV-induced AP-1 activity. However, Inh I and Inh II, as well as the LBI and the SBI, all appeared to enhance the UV-induced phosphorylation of Erks (Fig. 7B). These data, together with the data from Figs. 1, 2, 3, 4, 5, indicate that both Inh I and Inh II inhibit UV-induced AP-1 activation through a pathway that is independent of Erks, JNKs, and P38 kinase.
Figure 6.
Lack of effect of proteinase inhibitor proteins on UV-induced JNK activity. JB6 C1 41 cells were cultured in monolayers in 100-mM diameter dishes until 90% confluent. The cells were starved by changing the medium with 0.1% FBS MEM medium for 24–48 h. The cells were treated with 80 μM of each of the proteinase inhibitors, Inh I, Inh II, LBI, or SBI for 30 min, and then exposed to (A) UVB (4 kJ/m2 with filter) or (B) UVC (60 J/m2) and cultured for another 20 min. The cells were harvested and JNK activity was measured as described in Materials and Methods.
Figure 7.
Effects of pretreatment of JB6 cells with proteinase inhibitors on UV-induced phosphorylation of P38 kinase and Erks. The cells were cultured in monolayers of six-well plates and starved by replacing the medium with 0.1% FBS MEM for 48 h. Four hours before the cells were pretreated with Inh I, Inh II, LBI, or SBI, the medium was changed to serum-free MEM. The cells were then treated with 80 μM of the different inhibitors for 30 min and subsequently exposed to UV and cultured as described in Fig. 6. The cells were extracted and phosphorylated forms of P38 kinase (A) or Erk (B) were determined using phosphospecific antibodies as described by New England Biolabs.
Exposure of mammalian cells to UV irradiation leads to activation of transcription factor AP-1 (7, 23–25). It is known that the UV signal(s) leading to the activation of AP-1 in mammalian cells involves three distinct but related pathways and culminates in the selective activation of the three different sets of mitogen-activated protein kinases, including Erks, JNKs, and P38 kinase (17–22). In the series of experiments reported here, we found that UV-induced activations of Erks, JNKs, as well as P38 kinase, were not inhibited by Inh I and Inh II at a dose range that blocked AP-1 activation and, in contrast to the inhibition of UV activation of AP-l, the activation of Erks was enhanced. Because the EGF-induced AP-1 activity is not suppressed by Inh I and Inh II (Fig. 4), the transcription machinery for AP-1 activation by EGF is also likely different than the pathway for activation by UV irradiation. We suggest that blocking UV-induced AP-1 activity by Inh I and Inh II is through a fourth pathway, which is independent of Erks, JNKs, and P38 kinases, or through the inhibition of downstream Erks, JNKs, or P38 kinases. This suggestion is supported by the previous finding that there are at least four distinct mitogen-activated protein kinase signaling pathways in budding yeast (26), in which the mitogen-activated protein kinase signaling pathways exhibit a strong evolutionary conservation with those of mammalian cells (26–28). The results that Inh I and Inh II block UV-induced AP-l activity, but not EGF-induced AP-1 activity, support our previous finding that EGF receptors are not involved in UV-induced AP-1 activation (6, 7).
Proteinase inhibitors from plants have been known for some time to have anticarcinogenic properties (29) and potato Inh I had been shown to inhibit carcinogen-induced transformation of C3H/10T½ cells by x-irradiation (30). Inh I and Inh II were also found to be the most potent among several inhibitors of the formation of H2O2 in polymorphonuclear leukocytes, activated by the tumor promoter 12-O-tetradecanoylphorbol 13-acetate (31). The mechanism(s) for the effects of protease inhibitors on these processes are not known, but a protease was partially purified from C3H/10T½ cells whose activity increased in the cells 5–10-fold in response to 12-O-tetradecanoylphorbol 13-acetate. The protease is a candidate target for the protease inhibitors, although no functional role for a protease has yet been established as a component of the events leading to cancer in response to carcinogenic agents.
UV irradiation acts as both tumor initiator and promoter in animal models (32–34). It is generally accepted that the initiating effect of UV light occurs through absorbing energy by the DNA of irradiated cells and results in formation of pyrimidine dimers, whereas the activation of membrane-regulated signal transduction pathways, leading to activation of AP-1, is responsible for the tumor promotion action of UV light (9, 10, 35–39). Considering the important role of AP-1 activation in chemical- and radiation-carcinogenesis (40–46), we believe that specific inhibition of radiation-induced AP-1 activity by Inh I and Inh II may be related to their inhibition of radiation-induced cell transformation.
In summary, we have demonstrated that Inh I and Inh II specifically block UV-induced AP-1 activity through the pathways that are independent of Erks, JNKs, and P38 kinase, and we suggest that the UV-induced AP-1 activation by Inh I and Inh II may result from specific interactions with a component(s) of the signaling mechanism that mediates UV irradiation-induced cell transformation.
Acknowledgments
We thank Dr. Harald H. O. Schmid for critically reading the manuscript. This work was supported by the Hormel Foundation National Institutes of Health Grant CA 74916-01 (to Z.D.), and a Grant-in-Aid of the Graduate School, University of Minnesota; and by the College of Agriculture and Home Economics, Washington State University, Project 1791 (to C.A.R.).
ABBREVIATIONS
- AP-1
activator protein-1
- EGF
epidermal growth factor
- Erk
extracellular signal-regulated protein kinase
- JNK
c-Jun N-terminal kinase
- Inh I and Inh II
proteinase inhibitors I and II
- LBI
lima bean inhibitor
- SBI
soybean trypsin inhibitor
- FBS
fetal bovine serum
References
- 1.Melville J C, Ryan C A. J Biol Chem. 1972;247:3445–3453. [PubMed] [Google Scholar]
- 2.Bryant J, Green T R, Gurusaddaiah T, Ryan C A. Biochemistry. 1976;15:3418–3424. doi: 10.1021/bi00661a004. [DOI] [PubMed] [Google Scholar]
- 3.Bergey D R, Howe G A, Ryan C A. Proc Natl Acad Sci USA. 1996;93:12053–12058. doi: 10.1073/pnas.93.22.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conconi A, Smerdon M J, Howe G A, Ryan C A. Nature (London) 1996;383:826–829. doi: 10.1038/383826a0. [DOI] [PubMed] [Google Scholar]
- 5.Billings P C, Morrow A R, Ryan C A, Kennedy A R. Carcinogenesis. 1989;10:687–691. doi: 10.1093/carcin/10.4.687. [DOI] [PubMed] [Google Scholar]
- 6.Huang C, Ma W-Y, Bowden G T, Dong Z. J Biol Chem. 1996;271:31262–32168. doi: 10.1074/jbc.271.49.31262. [DOI] [PubMed] [Google Scholar]
- 7.Huang C, Ma W-Y, Dong Z. Oncogene. 1997;14:1945–1954. doi: 10.1038/sj.onc.1201056. [DOI] [PubMed] [Google Scholar]
- 8.Devary Y, Gottlieb R A, Smeal T, Karin M. Cell. 1992;71:1081–1091. doi: 10.1016/s0092-8674(05)80058-3. [DOI] [PubMed] [Google Scholar]
- 9.Dong Z, Lavorsky V, Colburn N H. Carcinogenesis. 1995;16:749–756. doi: 10.1093/carcin/16.4.749. [DOI] [PubMed] [Google Scholar]
- 10.Sun Y, Dong Z, Nakamura K, Colburn N H. FASEB J. 1993;7:944–950. doi: 10.1096/fasebj.7.10.8344492. [DOI] [PubMed] [Google Scholar]
- 11.Dong Z, Birrer M J, Watts R G, Matrisian L M, Colburn N H. Proc Natl Acad Sci USA. 1994;91:609–613. doi: 10.1073/pnas.91.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radler-Pohl A, Sachsenmaier C, Gebel S, Auer H-P, Bruder J T, Rapp U, Angel P, Rahmsdorf H J, Herrlich P. EMBO J. 1993;12:1005–1012. doi: 10.1002/j.1460-2075.1993.tb05741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Derijard B, Hibi M, Wu I-H, Barret T, Su B, Deng T, Karin M, Davis R J. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 14.Devary Y, Rosette C, DiDonato J A, Karin M. Science. 1993;261:1442–1445. doi: 10.1126/science.8367725. [DOI] [PubMed] [Google Scholar]
- 15.Liu Z-G, Baskaran R, Lea-Chou E T, Wood L D, Chen Y, Karin M, Wang J Y J. Nature (London) 1996;384:273–276. doi: 10.1038/384273a0. [DOI] [PubMed] [Google Scholar]
- 16.Canman C E, Kastan M B. Nature (London) 1996;384:213–214. doi: 10.1038/384213a0. [DOI] [PubMed] [Google Scholar]
- 17.Janknecht R, Hunter T. EMBO J. 1997;16:1620–1627. doi: 10.1093/emboj/16.7.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitmarsh A J, Yang S-H, Su M S S, Sharrocks A D, Davis R J. Mol Cell Biol. 1997;17:2360–2371. doi: 10.1128/mcb.17.5.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitmarsh A J, Davis R J. J Mol Med. 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- 20.Price M A, Cruzalegui F H, Treisman R. EMBO J. 1996;15:6552–6563. [PMC free article] [PubMed] [Google Scholar]
- 21.Cavigelli M, Li W W, Lin A, Su B, Yoshioka K, Karin M. EMBO J. 1996;15:6269–6279. [PMC free article] [PubMed] [Google Scholar]
- 22.Raingeaud J, Gupta S, Rogers J S, Dickens M, Hans J, Ulevitch R J, Davis R J. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 23.Stein B, Reahmsdorf H J, Steffer A, Litfin M, Herrlich P. Mol Cell Biol. 1989;9:5169–5181. doi: 10.1128/mcb.9.11.5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Angel P. In: Induced Gene Expression. Baeuerle P A, editor. Boston: Birkhauser; 1995. pp. 63–92. [Google Scholar]
- 25.Engelberg D, Klein C, Martinetto H, Struhl K, Karin M. Cell. 1994;77:381–390. doi: 10.1016/0092-8674(94)90153-8. [DOI] [PubMed] [Google Scholar]
- 26.Levin D E, Errede B. Curr Opin Cell Biol. 1995;7:197–202. doi: 10.1016/0955-0674(95)80028-x. [DOI] [PubMed] [Google Scholar]
- 27.Davis R J. Trends Biochem Sci. 1994;19:470–473. doi: 10.1016/0968-0004(94)90132-5. [DOI] [PubMed] [Google Scholar]
- 28.Waskiewicz A J, Cooper J A. Curr Opin Cell Biol. 1995;7:798–805. doi: 10.1016/0955-0674(95)80063-8. [DOI] [PubMed] [Google Scholar]
- 29.Kennedy A R. In: Protease Inhibitors as Cancer Chemoproventative Agents. Troll W, Kennedy A R, editors. New York: Plenum; 1993. pp. 9–64. [Google Scholar]
- 30.Billings P C, St. Clair W, Ryan C A, Kennedy A R. Carcinogenesis. 1987;8:809–812. doi: 10.1093/carcin/8.6.809. [DOI] [PubMed] [Google Scholar]
- 31.Frenkel K, Chrzan K, Ryan C A, Wiesner R, Troll W. Carcinogenesis. 1987;8:1207–1212. doi: 10.1093/carcin/8.9.1207. [DOI] [PubMed] [Google Scholar]
- 32.Parrish J A. In: Biochemistry and Physiology of the Skin. Goldsmith L A, editor. Vol. 2. New York: Oxford Univ. Press; 1993. pp. 713–333. [Google Scholar]
- 33.Strickland P T. J Invest Dermatol. 1986;87:272–275. doi: 10.1111/1523-1747.ep12696669. [DOI] [PubMed] [Google Scholar]
- 34.Staberg B, Wolf H C, Klemp P, Poulsen T, Bradhagen H. J Invest Dermatol. 1983;81:517–519. doi: 10.1111/1523-1747.ep12522855. [DOI] [PubMed] [Google Scholar]
- 35.Matsui M S, Delco V A. In: Photocarcinogenesis by Ultraviolet A and B. Mukhtar H, editor. Boca Raton, FL: CRC; 1995. pp. 21–30. [Google Scholar]
- 36.Scotto J, Fears T R, Fraumeni J F. Incidence of Non-Melanoma Skin Cancers in the United States. Bethesda, MD: National Institutes of Health; 1983. , NIH Publ. No. 83–2433. [Google Scholar]
- 37.Danpure H J, Tyrrell R M. Photochem Photobiol. 1976;23:171. doi: 10.1111/j.1751-1097.1976.tb07238.x. [DOI] [PubMed] [Google Scholar]
- 38.Ronai Z, Weinstein I B. J Virol. 1988;62:1057–1060. doi: 10.1128/jvi.62.3.1057-1060.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buzzell R A. Otolaryngol Clin North Am. 1993;26:1–11. [PubMed] [Google Scholar]
- 40.Huang C, Ma W-Y, Dawson M I, Rincon M, Flavell R A, Dong Z. Proc Natl Acad Sci USA. 1997;94:5826–5830. doi: 10.1073/pnas.94.11.5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Angel P, Karin M. Biochim Biophys Acta. 1991;1072:129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- 42.Huang C, Ma W-Y, Dong Z. Int J Oncol. 1996;8:389–393. doi: 10.3892/ijo.8.2.389. [DOI] [PubMed] [Google Scholar]
- 43.Huang C, Ma W-Y, Dong Z. Mol Cell Biol. 1996;16:6427–6435. doi: 10.1128/mcb.16.11.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jonat C, Rahmsdorf H J, Park K K, Cato A C, Gebel S, Ponta H, Herrlich P. Cell. 1990;62:1189–1204. doi: 10.1016/0092-8674(90)90395-u. [DOI] [PubMed] [Google Scholar]
- 45.Dong Z, Huang C, Brown R E, Ma W-Y. J Biol Chem. 1997;272:9962–9970. doi: 10.1074/jbc.272.15.9962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holladay K, Fujiki H, Bowden G T. Mol Carcinog. 1992;6:16–24. doi: 10.1002/mc.2940050106. [DOI] [PubMed] [Google Scholar]