Abstract
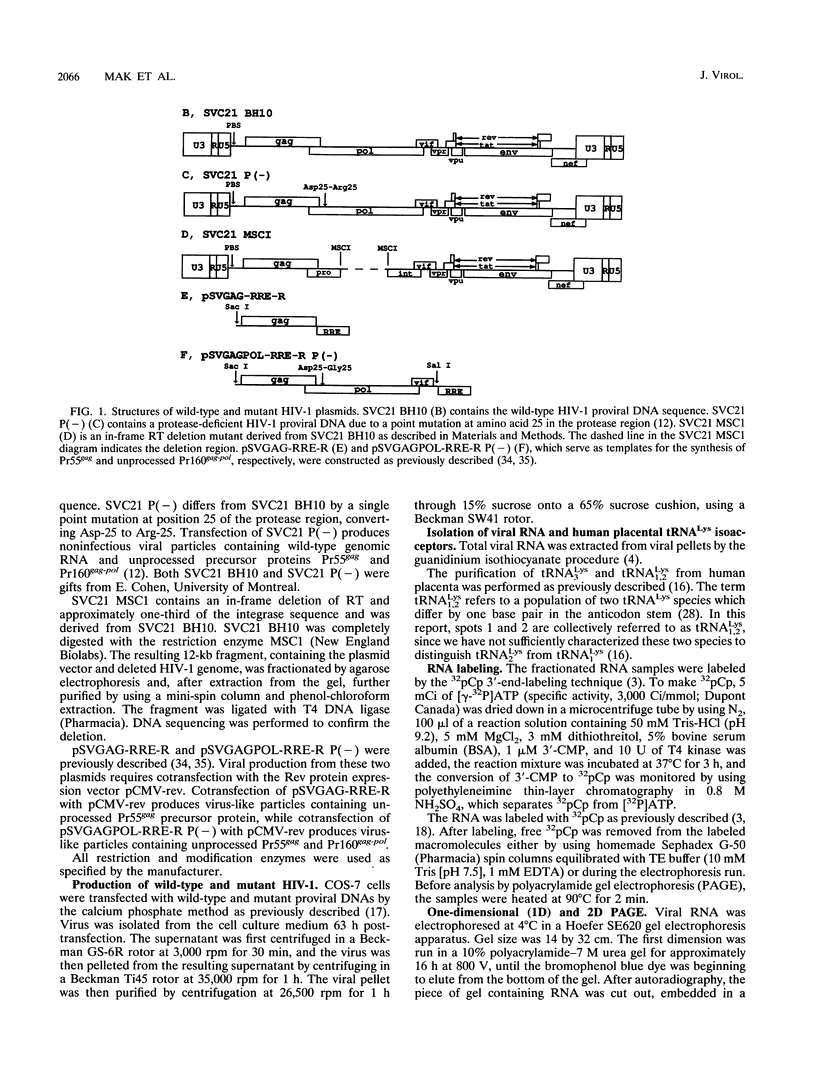
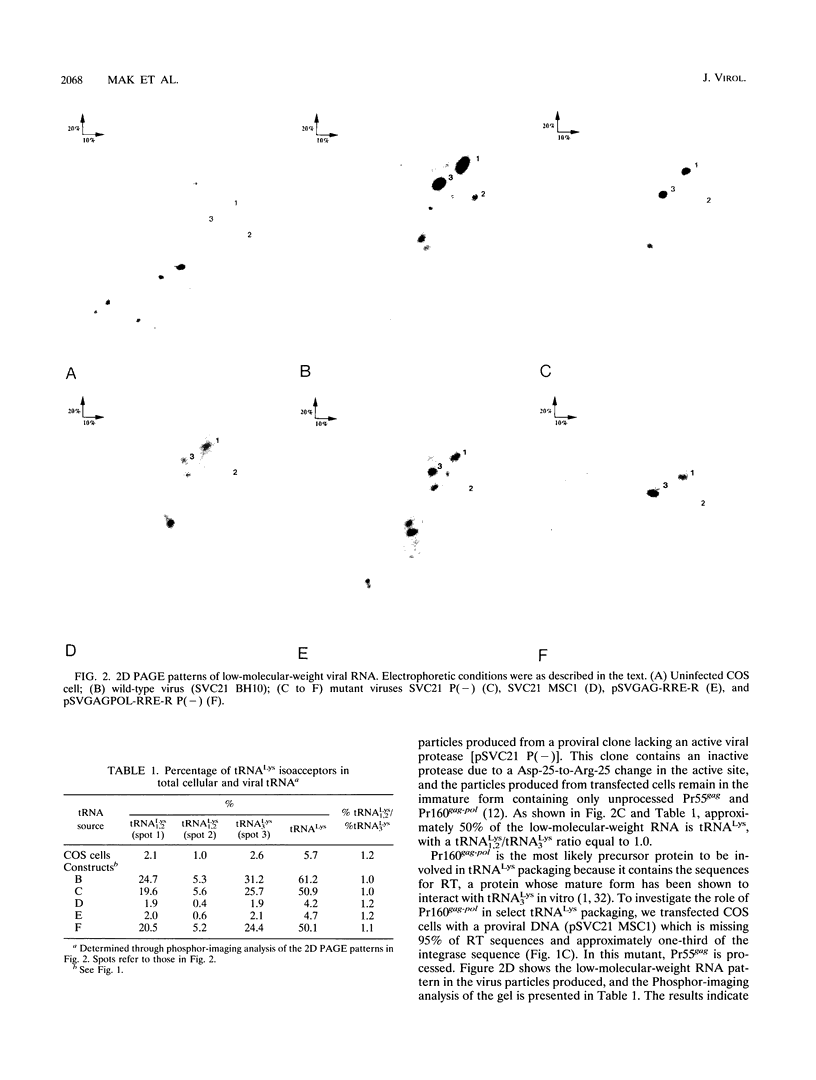
COS-7 cells transfected with human immunodeficiency virus type 1 (HIV-1) proviral DNA produce virus in which three tRNA species are most abundant in the viral tRNA population. These tRNAs have been identified through RNA sequencing techniques as tRNA(3Lys) the primer tRNA in HIV-1, and members of the tRNA(1,2Lys) isoacceptor family. These RNAs represent 60% of the low-molecular-weight RNA isolated from virus particles, while they represent only 6% of the low-molecular-weight RNA isolated from the COS cell cytoplasm. Thus, tRNA(Lys) is selectively incorporated into HIV-1 particles. We have measured the ratio of tRNA(3Lys) molecules to copies of genomic RNA in viral RNA samples and have calculated that HIV-1 contains approximately eight molecules of tRNA(3Lys) per two copies of genomic RNA. We have also obtained evidence that the Pr160gag-pol precursor is involved in primer tRNA(3Lys) incorporation into virus. First, selective tRNA(Lys) incorporation and wild-type amounts of tRNA(3Lys) were maintained in a protease-negative virus unable to process Pr55gag and Pr160gag-pol precursors, indicating that precursor processing was not required for primer tRNA incorporation. Second, viral particles containing only unprocessed Pr55gag protein did not selectively incorporate tRNA(Lys), while virions containing both unprocessed Pr55gag and Pr160gag-pol proteins demonstrated select tRNA(3Lys) packaging. Third, studies with a proviral mutant containing a deletion of most of the reverse transcriptase sequences and approximately one-third of the integrase sequence in the Pr160gag-pol precursor resulted in the loss of selective tRNA incorporation and an eightfold decrease in the amount of tRNA(3Lys) per two copies of genomic RNA. We have also confirmed herein finding of a previous study which indicated that the primer binding site is not required for the selective incorporation of tRNA(Lys).
Full text
PDF

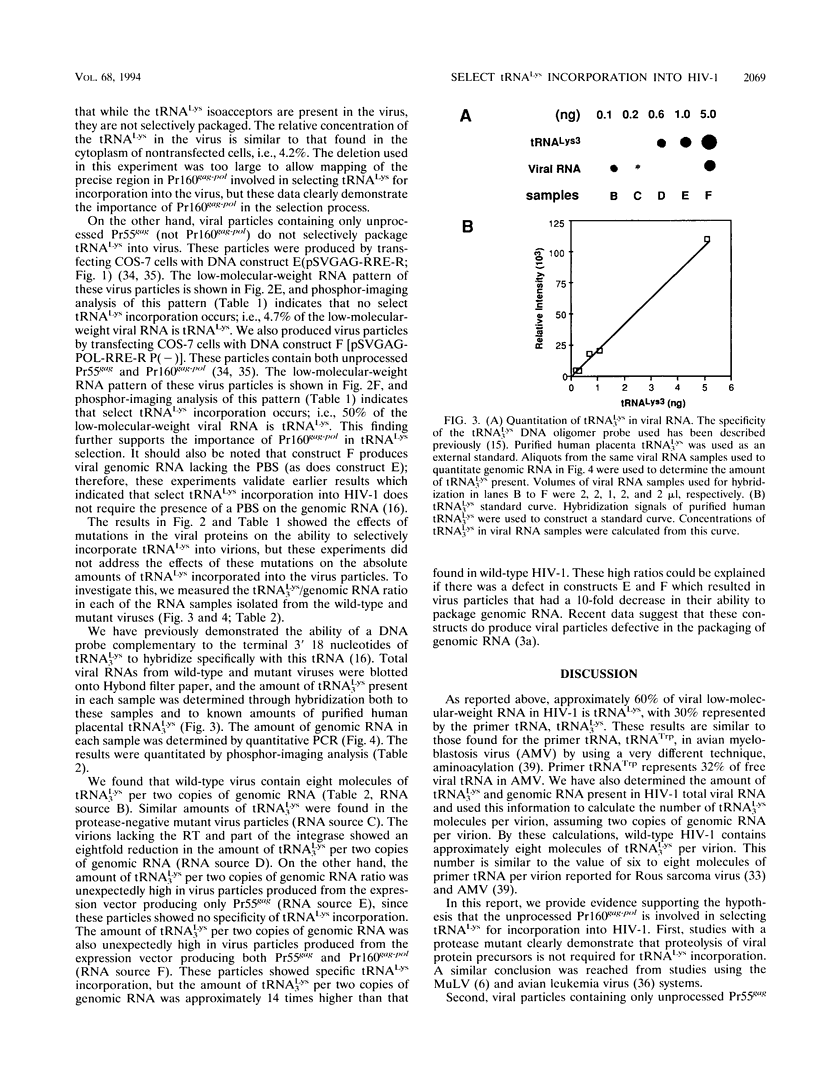
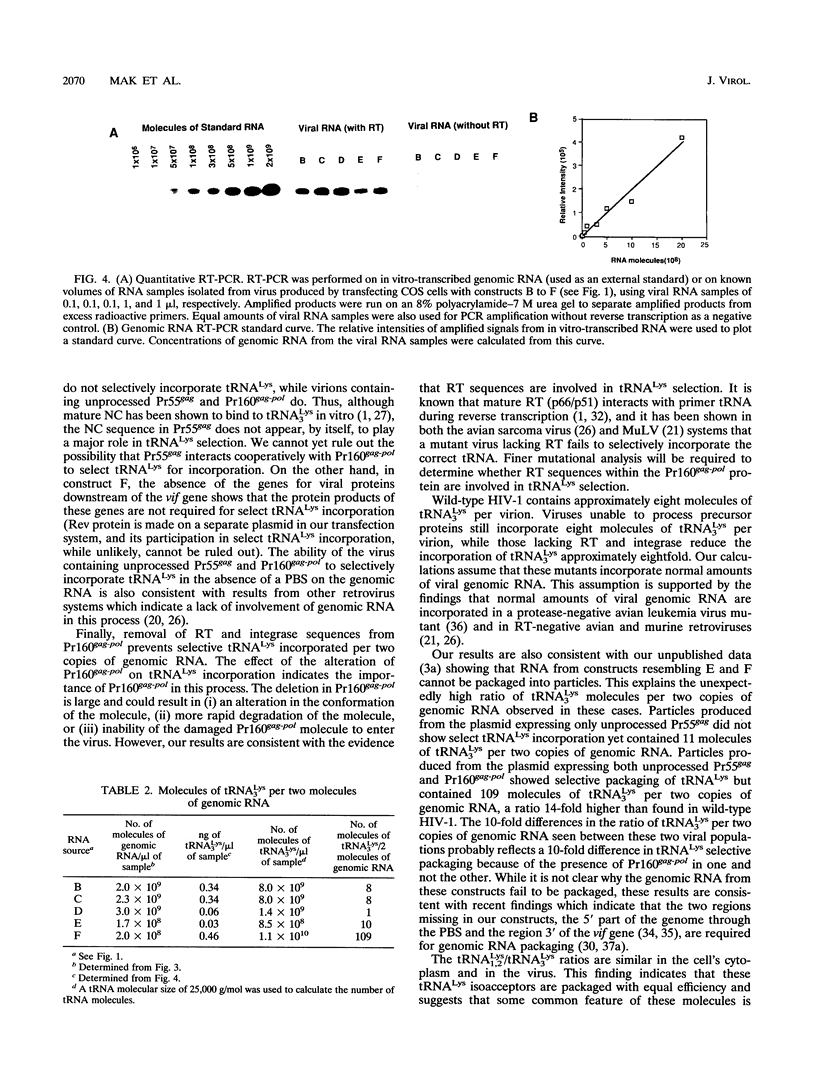


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barat C., Lullien V., Schatz O., Keith G., Nugeyre M. T., Grüninger-Leitch F., Barré-Sinoussi F., LeGrice S. F., Darlix J. L. HIV-1 reverse transcriptase specifically interacts with the anticodon domain of its cognate primer tRNA. EMBO J. 1989 Nov;8(11):3279–3285. doi: 10.1002/j.1460-2075.1989.tb08488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier B., Tarrago-Litvak L., Sallafranque-Andreola M. L., Robert D., Tharaud D., Fournier M., Barr P. J., Litvak S., Sarih-Cottin L. Inhibition of the p66/p51 form of human immunodeficiency virus reverse transcriptase by tRNA(Lys). Nucleic Acids Res. 1990 Feb 11;18(3):429–436. doi: 10.1093/nar/18.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce A. G., Uhlenbeck O. C. Reactions at the termini of tRNA with T4 RNA ligase. Nucleic Acids Res. 1978 Oct;5(10):3665–3677. doi: 10.1093/nar/5.10.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Colicelli J., Goff S. P. Isolation of a recombinant murine leukemia virus utilizing a new primer tRNA. J Virol. 1986 Jan;57(1):37–45. doi: 10.1128/jvi.57.1.37-45.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford S., Goff S. P. A deletion mutation in the 5' part of the pol gene of Moloney murine leukemia virus blocks proteolytic processing of the gag and pol polyproteins. J Virol. 1985 Mar;53(3):899–907. doi: 10.1128/jvi.53.3.899-907.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg J. E., Sawyer R. C., Taylor J. M., Faras A. J., Levinson W. E., Goodman H. M., Bishop J. M. Transcription of DNA from the 70S RNA of Rous sarcoma virus. I. Identification of a specific 4S RNA which serves as primer. J Virol. 1974 May;13(5):1126–1133. doi: 10.1128/jvi.13.5.1126-1133.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H. Physical properties of Rous Sarcoma Virus RNA. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1511–1518. doi: 10.1073/pnas.60.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson E., Erikson R. L. Association of 4S ribonucleic acid with oncornavirus ribonucleic acids. J Virol. 1971 Aug;8(2):254–256. doi: 10.1128/jvi.8.2.254-256.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faras A. J., Dibble N. A. RNA-directed DNA synthesis by the DNA polymerase of Rous sarcoma virus: structural and functional identification of 4S primer RNA in uninfected cells. Proc Natl Acad Sci U S A. 1975 Mar;72(3):859–863. doi: 10.1073/pnas.72.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faras A. J., Garapin A. C., Levinson W. E., Bishop J. M., Goodman H. M. Characterization of the low-molecular-weight RNAs associated with the 70S RNA of Rous sarcoma virus. J Virol. 1973 Aug;12(2):334–342. doi: 10.1128/jvi.12.2.334-342.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göttlinger H. G., Sodroski J. G., Haseltine W. A. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5781–5785. doi: 10.1073/pnas.86.15.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada F., Peters G. G., Dahlberg J. E. The primer tRNA for Moloney murine leukemia virus DNA synthesis. Nucleotide sequence and aminoacylation of tRNAPro. J Biol Chem. 1979 Nov 10;254(21):10979–10985. [PubMed] [Google Scholar]
- Harada F., Sawyer R. C., Dahlberg J. E. A primer ribonucleic acid for initiation of in vitro Rous sarcarcoma virus deoxyribonucleic acid synthesis. J Biol Chem. 1975 May 10;250(9):3487–3497. [PubMed] [Google Scholar]
- Hershey J. W. Translational control in mammalian cells. Annu Rev Biochem. 1991;60:717–755. doi: 10.1146/annurev.bi.60.070191.003441. [DOI] [PubMed] [Google Scholar]
- Jiang M., Mak J., Ladha A., Cohen E., Klein M., Rovinski B., Kleiman L. Identification of tRNAs incorporated into wild-type and mutant human immunodeficiency virus type 1. J Virol. 1993 Jun;67(6):3246–3253. doi: 10.1128/jvi.67.6.3246-3253.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M., Mak J., Wainberg M. A., Parniak M. A., Cohen E., Kleiman L. Variable tRNA content in HIV-1IIIB. Biochem Biophys Res Commun. 1992 Jun 30;185(3):1005–1015. doi: 10.1016/0006-291x(92)91727-8. [DOI] [PubMed] [Google Scholar]
- Jiang M., Parniak M. A., Kleiman L. Isolation and fractionation of retroviral tRNAs. J Virol Methods. 1992 Aug;38(2):205–213. doi: 10.1016/0166-0934(92)90111-p. [DOI] [PubMed] [Google Scholar]
- Kohlstaedt L. A., Steitz T. A. Reverse transcriptase of human immunodeficiency virus can use either human tRNA(3Lys) or Escherichia coli tRNA(2Gln) as a primer in an in vitro primer-utilization assay. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9652–9656. doi: 10.1073/pnas.89.20.9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J. G., Seidman J. G. Effect of polymerase mutations on packaging of primer tRNAPro during murine leukemia virus assembly. J Virol. 1981 Apr;38(1):403–408. doi: 10.1128/jvi.38.1.403-408.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J. G., Seidman J. G. Selective packaging of host tRNA's by murine leukemia virus particles does not require genomic RNA. J Virol. 1979 Jan;29(1):328–335. doi: 10.1128/jvi.29.1.328-335.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund A. H., Duch M., Lovmand J., Jørgensen P., Pedersen F. S. Mutated primer binding sites interacting with different tRNAs allow efficient murine leukemia virus replication. J Virol. 1993 Dec;67(12):7125–7130. doi: 10.1128/jvi.67.12.7125-7130.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick W. C. Mechanism and regulation of eukaryotic protein synthesis. Microbiol Rev. 1992 Jun;56(2):291–315. doi: 10.1128/mr.56.2.291-315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G. G., Hu J. Reverse transcriptase as the major determinant for selective packaging of tRNA's into Avian sarcoma virus particles. J Virol. 1980 Dec;36(3):692–700. doi: 10.1128/jvi.36.3.692-700.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G., Glover C. tRNA's and priming of RNA-directed DNA synthesis in mouse mammary tumor virus. J Virol. 1980 Jul;35(1):31–40. doi: 10.1128/jvi.35.1.31-40.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G., Harada F., Dahlberg J. E., Panet A., Haseltine W. A., Baltimore D. Low-molecular-weight RNAs of Moloney murine leukemia virus: identification of the primer for RNA-directed DNA synthesis. J Virol. 1977 Mar;21(3):1031–1041. doi: 10.1128/jvi.21.3.1031-1041.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prats A. C., Sarih L., Gabus C., Litvak S., Keith G., Darlix J. L. Small finger protein of avian and murine retroviruses has nucleic acid annealing activity and positions the replication primer tRNA onto genomic RNA. EMBO J. 1988 Jun;7(6):1777–1783. doi: 10.1002/j.1460-2075.1988.tb03008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raba M., Limburg K., Burghagen M., Katze J. R., Simsek M., Heckman J. E., Rajbhandary U. L., Gross H. J. Nucleotide sequence of three isoaccepting lysine tRNAs from rabbit liver and SV40-transformed mouse fibroblasts. Eur J Biochem. 1979 Jun;97(1):305–318. doi: 10.1111/j.1432-1033.1979.tb13115.x. [DOI] [PubMed] [Google Scholar]
- Ratner L., Haseltine W., Patarca R., Livak K. J., Starcich B., Josephs S. F., Doran E. R., Rafalski J. A., Whitehorn E. A., Baumeister K. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985 Jan 24;313(6000):277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- Richardson J. H., Child L. A., Lever A. M. Packaging of human immunodeficiency virus type 1 RNA requires cis-acting sequences outside the 5' leader region. J Virol. 1993 Jul;67(7):3997–4005. doi: 10.1128/jvi.67.7.3997-4005.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal L. J., Zamecnik P. C. Amino-acid acceptor activity of the "70S-associated" 4S RNA from avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1184–1185. doi: 10.1073/pnas.70.4.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarih-Cottin L., Bordier B., Musier-Forsyth K., Andreola M. L., Barr P. J., Litvak S. Preferential interaction of human immunodeficiency virus reverse transcriptase with two regions of primer tRNA(Lys) as evidenced by footprinting studies and inhibition with synthetic oligoribonucleotides. J Mol Biol. 1992 Jul 5;226(1):1–6. doi: 10.1016/0022-2836(92)90117-3. [DOI] [PubMed] [Google Scholar]
- Sawyer R. C., Dahlberg J. E. Small RNAs of Rous sarcoma virus: characterization by two-dimensional polyacrylamide gel electrophoresis and fingerprint analysis. J Virol. 1973 Dec;12(6):1226–1237. doi: 10.1128/jvi.12.6.1226-1237.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. J., Cho M. I., Hammarskjöld M. L., Rekosh D. Human immunodeficiency virus type 1 Pr55gag and Pr160gag-pol expressed from a simian virus 40 late replacement vector are efficiently processed and assembled into viruslike particles. J Virol. 1990 Jun;64(6):2743–2750. doi: 10.1128/jvi.64.6.2743-2750.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. J., Srinivasakumar N., Hammarskjöld M. L., Rekosh D. Requirements for incorporation of Pr160gag-pol from human immunodeficiency virus type 1 into virus-like particles. J Virol. 1993 Apr;67(4):2266–2275. doi: 10.1128/jvi.67.4.2266-2275.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart L., Schatz G., Vogt V. M. Properties of avian retrovirus particles defective in viral protease. J Virol. 1990 Oct;64(10):5076–5092. doi: 10.1128/jvi.64.10.5076-5092.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M. An analysis of the role of tRNA species as primers for the transcription into DNA of RNA tumor virus genomes. Biochim Biophys Acta. 1977 Mar 21;473(1):57–71. doi: 10.1016/0304-419x(77)90007-5. [DOI] [PubMed] [Google Scholar]
- Waters L. C. Lysine tRNA is the predominant tRNA in murine mammary tumor virus. Biochem Biophys Res Commun. 1978 Apr 14;81(3):822–827. doi: 10.1016/0006-291x(78)91425-0. [DOI] [PubMed] [Google Scholar]
- Waters L. C., Mullin B. C., Ho T., Yang W. K. Ability of tryptophan tRNA to hybridize with 35S RNA of avian myeloblastosis virus and to prime reverse transcription in vitro. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2155–2159. doi: 10.1073/pnas.72.6.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters L. C., Mullin B. C. Transfer RNA into RNA tumor viruses. Prog Nucleic Acid Res Mol Biol. 1977;20:131–160. doi: 10.1016/s0079-6603(08)60471-7. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Baltimore D. Relationship of retrovirus polyprotein cleavages to virion maturation studied with temperature-sensitive murine leukemia virus mutants. J Virol. 1978 Jun;26(3):750–761. doi: 10.1128/jvi.26.3.750-761.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wöhrl B. M., Ehresmann B., Keith G., Le Grice S. F. Nuclease footprinting of human immunodeficiency virus reverse transcriptase/tRNA(Lys-3) complexes. J Biol Chem. 1993 Jun 25;268(18):13617–13624. [PubMed] [Google Scholar]