Abstract
Infusion of 192 IgG-saporin (SAP) into the medial septum (MS) of rats selectively destroys cholinergic neurons projecting to the hippocampus and impairs acquisition of a delayed matching to position (DMP) T-maze task. The present study evaluated whether introduction of a mild aversive stimulus 30 min prior to training would attenuate the deficit in DMP acquisition caused by the SAP lesions. Male Sprague–Dawley rats received medial septal infusions of either artificial cerebrospinal fluid or SAP (0.22 μg in 1.0 μl). Fourteen days later, all animals were trained to perform the DMP task. Half of the SAP-treated animals and controls received an intraperitoneal injection of saline each day, 30 min prior to training. Results show that intraperitoneal saline attenuated the impairment in DMP acquisition in SAP lesioned rats. These results suggest that a mild aversive stimulus can attenuate cognitive deficits caused by medial septal cholinergic lesions.
Keywords: Memory, 192 IgG, saporin, Arousal, Basal ganglia, Hippocampus, Acetylcholine
1. Introduction
The rat basal forebrain, including the medial septum (MS), nucleus basalis magnocellularis (NBM) and vertical limb of the diagonal band of Broca (VDM), contains cholinergic neurons that provide the major source of cholinergic innervation to the amygdala, cerebral cortex, and hippocampus [7,38]. Based on experiments utilizing muscarinic antagonists and/or non-selective physical or chemical lesions, cholinergic neurons projecting to the cerebral cortex and hippocampus have been identified as playing an important role in learning, memory, and attentional processes [2,3,15,19]. Moreover, in humans the loss of basal forebrain cholinergic neurons has been associated with Alzheimer’s disease [1]. An understanding of the role of the basal forebrain in learning and memory has accelerated since the development of agents such as 192 IgG-saporin (SAP), that selectively lesion p75NTR-containing cholinergic neurons [9,46,48]. However, there is a lack of consistency in the literature that utilize SAP regarding the importance of MS cholinergic neurons in spatial working memory.
Previously we showed that SAP can be used to produce selective cholinergic lesions in the MS, resulting in a marked decrease in markers of hippocampal cholinergic function, as well as significant impairment in the acquisition of a delayed matching-to-position (DMP) T-maze task [22,23,28]. Other investigations, though, have reported only limited effects of MS SAP lesions in other spatial tasks. For example, Dornan et al. [16] reported that a selective reduction of cholinergic transmission in the basal forebrain was by itself insufficient to account for the functional impairments in spatial learning of rats using a Morris water maze (MWM) paradigm. Baxter et al. [4,5], also utilizing a MWM task, reported similar findings.
These conflicting results may be the consequence of the environment and stress associated with the particular task. For example, Sandi et al., found that lowering water temperature from 25 to 19 °C resulted in both increased post training corticosterone levels and enhanced acquisition and retention in the MWM [42]. Moreover, emotional and stressful experiences, via the activation of specific hormonal and brain systems, alter learning and memory processes [32,36,40]. Stress, depending on intensity and duration, can either facilitate or impair cognitive functions, particularly spatial learning and memory performance [35]. Short periods of a mild stressor can enhance acquisition of certain spatial learning tasks [12,49], while longer periods of stress have impaired performance in a variety of spatial tasks [8,33]. Therefore, we hypothesize that the effects of basal fore-brain cholinergic lesions on acquisition of a spatial task may have different outcomes as a consequence of the differing levels of stress inherent to the tasks.
A number of studies have shown that intraperitoneal (IP) injection of saline acts as a mild stressor, resulting in modest elevations in plasma corticosterone [6,41]. In the present study, we evaluated whether daily introduction of this mild aversive stimulus prior to training would alter the effect of septal cholinergic lesions on acquisition of a DMP task. Specifically, we predicted that introduction of the stressor would reduce the impairment produced by septal cholinergic lesions, consistent with the relative lack of effect of SAP lesions on acquisition of the more stressful MWM task previously described.
2. Materials and methods
2.1. Intraseptal infusions
All experiments followed NIH guidelines for the care and use of laboratory animals and were approved by the Duquesne University Institutional Animal Care and Use Committee. Thirty-two male Sprague–Dawley rats (275–300 g) were obtained from Hilltop Lab Animals (Scottdale, PA). Animals were housed individually in a temperature and humidity controlled facility on a 12:12 h light: dark cycle and had unrestricted access to food and water. For intraseptal infusion of SAP, animals were anesthetized with pentobarbital (50 mg/kg; IP) and placed into a stereotaxic frame. An incision was made exposing the dorsal aspect of the skull and a hole drilled over the MS (+0.2 bregma, 0.0 lateral). A stainless steel cannula was lowed 5.6 mm from dura into the MS. Animals were infused with either 1 μl of vehicle (artificial cerebrospinal fluid; CMA Inc., North Chelms-ford, MA) or SAP (0.22 μg in 1 μl of ACSF; Chemicon, Temecula, CA) over 5 min at 0.2 μl/min. The dose of SAP was selected based on previous studies using the same lot number (#24–87) that demonstrated a substantial loss of cholinergic neurons in the MS with little non-selective damage to GABAergic neurons [28]. Following the infusion, the cannula remained in place for 5 min to allow diffusion of the solution into the tissue. The incision was closed and animals were allowed to recover for 2 weeks before behavioral testing.
2.2. Behavioral testing
Fourteen days following surgery, control (CON) and SAP-treated (SAP) animals were each randomly assigned to aversive (A) and non-aversive (NA) treatment groups. SAP-A and CON-A groups were administered a single IP injection of sterile saline 30 min before each training session. The procedure included restraint (approximately 10 s) and needle stick (3 s; 26 gauge) through the abdomen into the peritoneum with the injection of sterile saline (1.0 ml/kg).
Animals were trained on the DMP task as recently described [20,22,28]. Animals were food deprived to 85% of their normal body weight and then adapted to the T-maze by placing them into the maze with sweetened reward pellets (45 mg Noyes pellets; Research Diets; New Brunswick, NJ) once per day for 5 days. Animals were then trained to run to the ends of each goal arm by using a series of six forced choices per day for 3 days. To avoid the introduction of side bias, right and left goal arms were alternated in a random and balanced fashion. Animals then began DMP acquisition training. Each animal received eight trial pairs per day. The first trial consisted of a forced choice in which one of the goal arm doors was closed, forcing the animal to enter the open goal arm to receive a reward (two pellets). The animal was immediately returned to the approach alley for the second trial during which both goal arms were opened. Returning to the same goal arm as the first trial resulted in a reward (four pellets). Entering the opposing arm resulted in no reward and confinement in the goal arm for 15 s. Animals were run in groups of three or four. After each trial pair, animals were returned to their cages for 5–10 min, while training proceeded on the remaining animals of the group. Animals continued to receive eight trial pairs per day until a criterion of 15 correct choices out of 16 consecutive trial pairs was met.
2.3. Choline acetyltransferase activity
At the completion of behavioral training, animals were killed and ChAT activity in the hippocampus and frontal cortex was measured as previously described [21]. Briefly, animals were anesthetized with pentobarbital (100 mg/kg; IP), the brains removed, and tissue from the hippocampus and frontal cortex dissected, frozen, and processed. Tissues were not pooled. Frozen tissues were thawed and dissociated by sonication in a medium containing EDTA (10 mM) and Triton X-100 (0.5%) and diluted to a concentration of 10 mg tissue/ml. An aliquot of each sample was used for the determination of total protein [10]. Three 5 μl aliquots of each sample were incubated for 30 min at 37 °C in a medium containing 3[H] acetyl-CoA (50,000–60,000 d.p.m./tube, final concentration 0.25 mM acetyl-CoA; Sigma Inc., St. Louis, MO), choline chloride (10.0 mM), physostigmine sulfate (0.2 mM), NaCl (300 mM), sodium phosphate buffer (pH 7.4, 50 mM), and EDTA (10 mM). The reaction was terminated with 4 ml sodium phosphate buffer (10 mM) followed by the addition of 1.6 ml of ace-tonitrile containing 5 mg/ml tetrephenylboron. The amount of 3[H] acetylcholine produced was determined by adding 8 ml of EconoFluor scintillation cocktail (Packard Instruments, Meriden, CT) and counting total cpm in the organic phase using an LKB beta-counter. Background was determined using identical tubes to which no sample was added. For each sample, the three reaction tubes containing sample were averaged and the difference between total cpm and background cpm was used to estimate the total amount of ACh produced per sample. ChAT activity was then calculated for each sample as pmol ACh manufactured/h/μg protein.
2.4. Statistical analysis
Days-to-criterion was analyzed using one-way ANOVA with Newman–Keuls post-hoc test for individual comparisons between treatment groups. To compare treatment effects during different stages of acquisition, performance data were blocked into training periods of 5 days each (a total of six periods). Once an animal achieved criterion, a performance value of 93.8% was assigned for the remaining days for the purposes of statistical analysis. Performance during acquisition testing was analyzed using two-way ANOVA with repeated measures on ‘Block’, and one-way ANOVA for treatment effects within training periods. Differences in ChAT activity in the hippocampi and frontal cortex of SAP treated and control animals were compared using one-way ANOVA (GraphPad Prism 3.0).
3. Results
3.1. Effects on ChAT activity
Of the original 32 animals, two animals (1 CON-NA, 1 SAP-NA) failed to run the maze or displayed side bias and were excluded from the study. For the remaining animals (seven CON-NA, eight CON-A, seven SAP-NA, eight SAP-A), the results of the ANOVA revealed a significant effect of SAP lesion on hippocampal ChAT activity [F(3,26) = 107.7, p < 0.0001]. There was a 70.5% decrease in ChAT activity in the hippocampi of SAP-NA versus CON-NA groups (7.85 ± 1.37 versus 26.61 ± 0.77 pmol ACh/h/μg protein; p < 0.001), and a 74.9% decrease in ChAT activity in the hippocampi of SAP-A versus CON-A (6.85 ± 1.10 versus 27.29 ± 1.05 pmol ACh/h/μg protein; p < 0.001). There was no significant difference in hippocampal ChAT activity between the SAP-A versus SAP-NA groups p > 0.05). For tissues collected from the frontal cortex, ANOVA revealed no significant differences in ChAT activity between the treatment groups [F(3,26) = 1.64, p = 0.21].
3.2. Effects on DMP acquisition
Treatment significantly affected the number of days that animals required to reach criterion [F(3,26) = 6.95, p = 0.0014] (Fig. 1). Specifically, SAP-NA animals required more days to reach criterion than corresponding CON-NA controls (21.7 ± 1.6 days versus 15.9 ± 0.5 days, p < 0.05). In contrast, SAP-treated animals that also received the mild aversive stimulus did not require significantly more days to reach criterion than corresponding CON-A animals (16.8 ± 1.3 versus 13.4 ± 1.4 days for CON-A animals, p > 0.05). Also, SAP-A treated animals took significantly fewer days to reach criterion than SAP-NA animals (p < 0.05), however, there was no significant difference in days-to-criterion between the CON-A and the CON-NA treated controls (p > 0.05). Note that by day 20 of training, all control animals had reached criterion, seven of eight SAP-A animals had reached criterion, and only three of seven SAP-NA animals had reached criterion. By day 30 of training, only a single animal in the SAP-A group had not reached criterion.
Fig. 1.
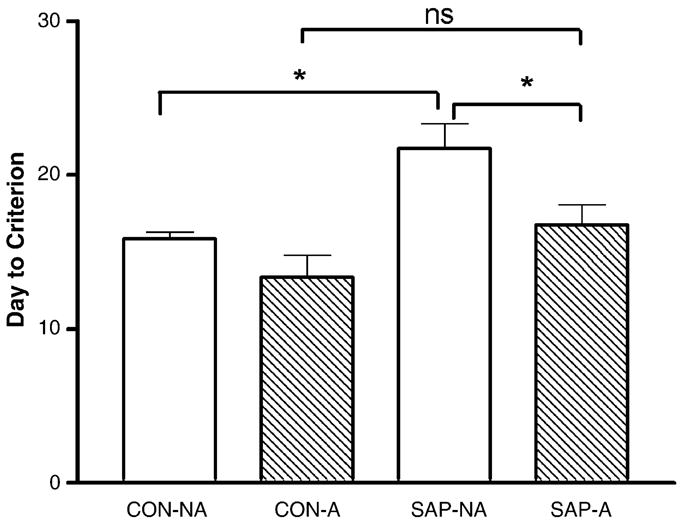
Graph summarizing the average number of days required to reach criterion for each treatment group. Bars represent the mean ± S.E.M. Note that the SAP-NA group required significantly more days to reach criterion than the corresponding CON-NA controls. In contrast, the SAP-A group did not require significantly more time to reach criterion than the corresponding CON-A controls. *p < 0.05; n.s. = no significance.
Analysis of the learning curves by two-way ANOVA demonstrated a significant effect of SAP treatment [F(3,156] = 103.0, p < 0.0001], a significant effect of ‘block’ [F(5,156) = 11.48, p < 0.0001), and a significant treatment × block interaction [F(15,156) = 1.811, p = 0.037; Fig. 2). A separate analysis of blocks 3 and 4 revealed significant differences between treatment groups [F(3,32) = 3.739, p = 0.021] for block 3; and [F(3,32) = 8.572, p = 0.0003] for block 4. Post-hoc analyses revealed a significant decrease in performance for SAP-NA-compared to CON-NA-treated animals during blocks 3 and 4. Additionally, in block 4 the SAP-A group performed significantly better than the SAP-NA group (p < 0.05). Differences between the CON-A and SAP-A treated groups were not statistically significant during any block.
Fig. 2.
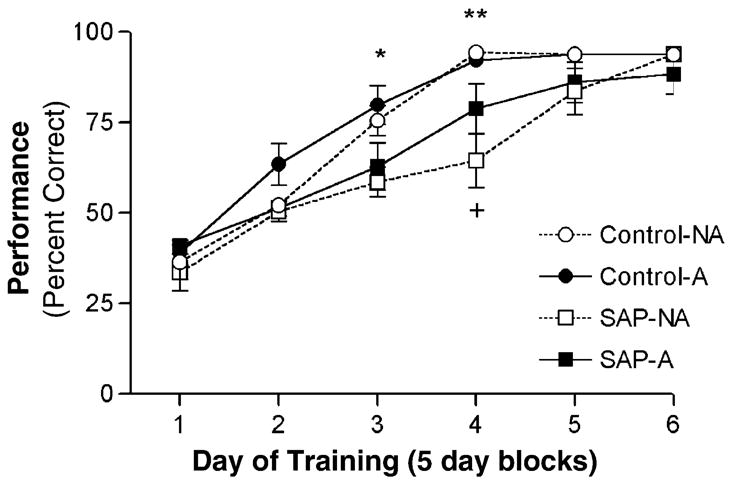
Learning curves showing acquisition of the DMP task across six 5-day blocks of training. Values represent the mean percentage correct choices for each treatment group during each period of training. Once an animal achieved criterion, a performance value of 93.8% was assigned for the remaining days for the purposes of statistical analysis. By day 20 of training, all control animals had reached criterion, seven of eight SAP-A animals had reached criterion, and only three of seven SAP-NA animals had reached criterion. By day 30 of training, only a single animal in the SAP-A group had not reached criterion. Note that all groups showed improved performance over time; however during blocks 3 and 4 the performance of the SAP-NA-treated animals was significantly worse than the corresponding CON-NA-treated controls (*p < 0.05; **p < 0.001). Also, during block 4, the performance of the SAP-A-treated animals was significantly better than the SAP-NA-treated animals (+p < 0.05). Differences in acquisition between the CON-A and SAP-A treatment groups were not statistically significant during any period.
4. Discussion
4.1. Effects on DMP acquisition
The data show that SAP lesions significantly impaired acquisition of the DMP task relative to controls. As predicted, this effect was significantly reduced by application of a mild aversive stimulus each day prior to training. This demonstrates that a mild aversive stimulus can attenuate or mask a deficit resulting from lesion of MS cholinergic neurons on acquisition of a spatial task. This is consistent with studies of working memory which used paradigms with an aversive component and that found that selective cholinergic lesions of the MS did not produce acquisition deficits in spatial working memory [4,5,16]. Notably, application of the aversive stimulus had no significant effect on DMP acquisition in the non-lesioned controls. This suggests that the aversive stimulus may only affect performance in the presence of the lesion; however, the possibility of a ceiling effect on performance in the non-lesioned animals cannot be excluded.
One might expect an aversive stimulus to have the greatest effect early during training, when the stimulus is relatively novel and a lesser effect as the animal becomes habituated to the stimulus later in training. The effect of the aversive stimulus in the SAP-A group, though, became apparent relatively late in training (days 16–20; block 4). It is possible that acquisition of the DMP task in this study may involve adopting different strategies over the course of training and the effect of the aversive stimulus in SAP lesioned animals may be associated with a strategy shift that occurs relatively late during acquisition of the task. An ongoing study in our laboratory is focused on this possibility.
4.2. Associations with ChAT activity
The analysis of ChAT activity shows that the SAP-lesion paradigm significantly reduced ChAT activity in the hippocampus, but not in the frontal cortex. This is consistent with studies showing substantial loss of cholinergic neurons in the medial septum, but not in the nucleus basalis magnocellularis, with this paradigm [22,28]. Previous studies have also shown that the dose of SAP used in this study is selective for the cholinergic neurons in that there is little effect on GABAergic parvalbumin-containing neurons located in the same regions of the medial septum [28]. We conclude, therefore, that the learning deficit observed in the SAP-NA treated group is the consequence of a septal-hippocampal cholinergic lesion. Notably, there was no significant difference in hippocampal ChAT activity between the SAP-A and the SAP-NA treated groups, suggesting that the differences seen in T-maze acquisition between the two groups were not due to a bias resulting from a greater loss of septo-hippocampal cholinergic projections in one group compared to the other.
4.3. Potential mechanisms and implications
The sensitivity of any task to lesions of a single pathway depends on whether the lesion involves the principal pathway activated during the task, as opposed to several pathways that are activated. The hippocampus receives inputs from a variety of cortical and subcortical structures, including inputs from entorhinal cortex, amygdala, medial septum, thalamus, and monoaminergic cell groups in the midbrain and hindbrain. Mild stress has been shown to enhance acquisition of certain spatial learning tasks via hippocampal activation utilizing neuronal pathways that do not involve the MS [12,50]. Therefore, a task with a mildly aversive component may possibly preserve acquisition of a spatial task in animals with a septal-hippocampal cholinergic lesion via pathways that are independent of the septal-hippocampal tract. The result would be a loss of sensitivity with tests for impairments in learning/memory resulting from a septal-hippocampal lesion that involved a mildly aversive stimulus, e.g. the MWM.
One must consider the likelihood that there is no such thing as a pure hippocampal task. Most spatial tasks can be solved using a variety of strategies [17], each of which may rely to varying degrees on different neural substrates. During stress, it is well established that epinephrine released from the adrenal cortex can result in the activation of adrenergic receptors that facilitate memory function [26,37,43]. Adrenergic receptors found on vagal afferents project to the nucleus of the solitary tract and can subsequently activate neurons that project to the amygdala [11]. The amygdala has long been associated with the acquisition of memory related to aversive stimuli [11,13,25,31,34] and the modulation of memory processes involving the hippocampus [14,27,44]. Consequently, it is possible that in addition to enhancing the activity of non-cholinergic inputs to the hippocampus, administration of a mild aversive stimulus also activates parallel memory systems [29,47] that enhance performance, perhaps by strengthening the use of strategies less dependent on hippocampal cholinergic inputs. This is consistent with, and may help to explain, the reported discrepancies between the effects of septal cholinergic lesions on acquisition of MWM versus land-based (e.g., T-maze) tasks. While both types of tasks can be used to evaluate spatial learning and memory, the levels of stress are higher in a water maze task due to the aversive environment of the pool [18,24]. Therefore, performance of the DMP T-maze task may be more sensitive to the SAP lesion because it is less aversive. This is consistent with the current study which shows that administration of a mild stressor eliminates the sensitivity of the DMP task to the cholinergic lesions, producing results more like those reported with the MWM.
There have been reports where SAP lesion does induce a deficit in MWM performance [30]. However, the methodology utilized intraventricular injection of SAP, resulting not only in a broader cholinergic lesion of brain structures than intra-parenchymal injection in the MS, but the loss of Purkinje cells of the cerebellum as well [45]. Other studies, after injecting SAP into the MS and NBM failed to detect a deficit in performance in the radial arm maze, a non-aversive task [39]. However, this result may have been a consequence of the particular environment in which the animals were tested or specific aspects of the testing paradigm.
In summary, the findings demonstrate that cholinergic inputs to the hippocampus can play an important role in the acquisition of a spatial task under low-stress conditions, but that the impact of the cholinergic lesions is attenuated in the presence of a mild aversive stimulus. In addition, the data suggest caution when interpreting negative findings based on a single behavioral task, or a set of tasks that emulate a single environmental condition.
Acknowledgments
The authors wish to acknowledge the excellent technical assistance of Douglas Nelson and Christine Close. This research was supported by NIH grants RO1-AG16261 (DAJ) and RO1-AG021471 (RBG).
References
- 1.Araujo DM, Lapchak PA, Robitaille Y, Gauthier S, Quirion R. Differential alteration of various cholinergic markers in cortical and sub-cortical regions of the human brain in Alzheimer’s disease. J Neurochem. 1988;50:1914–1923. doi: 10.1111/j.1471-4159.1988.tb02497.x. [DOI] [PubMed] [Google Scholar]
- 2.Bartus RT, Dean RL, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–417. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 3.Bartus RT, Flicker C, Dean RL, Pontecorvo M, Figueiredo JC, Fisher SK. Selective memory loss following nucleus basalis lesions. doi: 10.1016/0091-3057(85)90139-x. [DOI] [PubMed] [Google Scholar]
- 4.Baxter MG, Bucci DJ, Gorman LK, Wiley RG, Gallagher M. Selective immunotoxic lesions of basal forebrain cholinergic cells: effects on learning and memory in rats. Behav Neurosci. 1995;109:714–722. doi: 10.1037//0735-7044.109.4.714. [DOI] [PubMed] [Google Scholar]
- 5.Baxter MG, Bucci DJ, Sobel TJ, Williams MJ, Gorman LK, Gallagher M. Intact spatial learning following lesions of basal forebrain cholinergic neurons. Neuroreport. 1996;7:1417–1420. doi: 10.1097/00001756-199605310-00019. [DOI] [PubMed] [Google Scholar]
- 6.Belz EE, Kennell JS, Czambel RK, Rubin RT, Rhodes ME. Environmental enrichment lowers stress-responsive hormones in singly housed male and female rats. Pharmacol Biochem Behav. 2003;76:481–486. doi: 10.1016/j.pbb.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Bigl V, Woolf NJ, Butcher LL. Cholinergic projections from the basal forebrain to frontal, parietal, temporal, occipital, and cingulate cortices: a combined flourescent tracer and acetylcholinesterase analysis. Brain Res Bull. 1982;8:727–749. doi: 10.1016/0361-9230(82)90101-0. [DOI] [PubMed] [Google Scholar]
- 8.Bodnoff SR, Humphreys AG, Leftman JC, Diamond DM, Rose GM, Meaney MJ. Enduring effects of chronic corticosterone treatment on spatial learning, synaptic plasticity, and hippocampal neuropathology in young and middle-aged rats. J Neurosci. 1995;15:61–69. doi: 10.1523/JNEUROSCI.15-01-00061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Book AA, Wiley RG, Schweitzer JB. Specificity of 192-IgG-saporin for NGF receptor-positive cholinergic basal forebrain neurons in the rat. Brain Res. 1992;590:350–355. doi: 10.1016/0006-8993(92)91121-t. [DOI] [PubMed] [Google Scholar]
- 10.Bradford M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principal of protein dye binding. Anal Biochem. 1976;72:248–253. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 11.Calderazzo L, Cavalheiro EA, Macchi G, Molinari M, Bentivoglio M. Branched connections to the septum and to the entorhinal cortex from the hippocampus, amygdala and diencephalon in the rat. Brain Res Bull. 1996;40:245–251. doi: 10.1016/0361-9230(96)00033-0. [DOI] [PubMed] [Google Scholar]
- 12.Clayton EC, Williams CL. Adrenergic activation of the nucleus tractus solitarius potentiates amygdala norepinephrine release and enhances retention performance in emotionally arousing and spatial memory tasks. Behav Brain Res. 2000;112:151–158. doi: 10.1016/s0166-4328(00)00178-9. [DOI] [PubMed] [Google Scholar]
- 13.Davis M. The role of the amygdala in fear and anxiety. Ann Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 14.Decker MW, Curzon P, Brioni JD. Influence of separate and combined septal and amygdala lesions on memory, acoustic startle, anxiety, and locomotor activity in rats. Neurobiol Learn Mem. 1995;64:156–168. doi: 10.1006/nlme.1995.1055. [DOI] [PubMed] [Google Scholar]
- 15.DeKosky ST, Scheff SW, Markesbery WR. Laminar organization of cholinergic circuits in human frontal cortex in Alzheimer’s disease and aging. Neurology. 1985;35:1425–1431. doi: 10.1212/wnl.35.10.1425. [DOI] [PubMed] [Google Scholar]
- 16.Dornan WA, McCampbell AR, Tinkler GP, Hickman LJ, Bannon AW, Decker MW, Gunther KL. Comparison of site-specific injections into the basal forebrain on water maze and radial arm performance in the male rat after immunolesioning with 192-IgG-saporin. Behav Brain Res. 1996;82:93–101. doi: 10.1016/s0166-4328(97)81112-6. [DOI] [PubMed] [Google Scholar]
- 17.Dudchenko PA. How do animals actually solve the T-maze. Behav Neurosci. 2001;115:850–860. [PubMed] [Google Scholar]
- 18.Dudchenko PA, Goodridge JP, Seiterle DA, Taube JS. Effects of repeated disorientation on the acquisition of spatial tasks in rats: dissociation between appetitive radial arm maze and aversive water maze. J Exp Psychol: Anim Behav Process. 1997;23:194–210. doi: 10.1037//0097-7403.23.2.194. [DOI] [PubMed] [Google Scholar]
- 19.Everitt BJ, Robins TW. Central cholinergic systems and cognition. Ann Rev Psychol. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- 20.Gibbs RB. Estrogen replacement enhances acquisition of a spatial memory task and reduces deficits associated with hippocampal muscarinic receptor inhibition. Horm Behav. 1999;36:222–233. doi: 10.1006/hbeh.1999.1541. [DOI] [PubMed] [Google Scholar]
- 21.Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol Aging. 2000;21:107–116. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- 22.Gibbs RB. Basal forebrain cholinergic neurons are necessary for estrogen to enhance acquisition of a delayed matching to position T-maze task. Horm Behav. 2002;42:245–257. doi: 10.1006/hbeh.2002.1825. [DOI] [PubMed] [Google Scholar]
- 23.Gibbs RB, Gabor R. Estrogen and cognition: applying preclinical findings to clinical perspectives. J Neurosci Res. 2003;74:637–643. doi: 10.1002/jnr.10811. [DOI] [PubMed] [Google Scholar]
- 24.Hodges H. Maze procedures: the radial-arm and water maze compared. Cognit Brain Res. 1996;3:167–181. doi: 10.1016/0926-6410(96)00004-3. [DOI] [PubMed] [Google Scholar]
- 25.Hyman BT, Van Hoesen GW, Damasio AR. Memory-related neural systems in Alzheimer’s disease: an anatomic study. Neurology. 1990;40:1721–1730. doi: 10.1212/wnl.40.11.1721. [DOI] [PubMed] [Google Scholar]
- 26.Izquierdo I, Diaz RD. Influences on memory of posttraining or pretest injections of ACTH, vasopressin, epinephrine, or b-endorphin and their interaction with naloxone. Psychoneuroendocrinology. 1985;10:165–172. doi: 10.1016/0306-4530(85)90054-x. [DOI] [PubMed] [Google Scholar]
- 27.Izquierdo I, Medina JH, Bianchin M, Walz R, Zanatta MS, Da Silva RC, Bueno e Silva M, Ruschel AC, Paczko N. Memory processing by the limbic system: role of specific neurotransmitter systems. Behav Brain Res. 1993;58:91–98. doi: 10.1016/0166-4328(93)90093-6. [DOI] [PubMed] [Google Scholar]
- 28.Johnson DA, Zambon NJ, Gibbs RB. Selective lesion of cholinergic neurons in the medial septum by 192 IgG-saporin impairs learning in a delayed matching to position T-maze paradigm. Brain Res. 2002;943:132–141. doi: 10.1016/s0006-8993(02)02623-9. [DOI] [PubMed] [Google Scholar]
- 29.Kesner RP, Rogers J. An analysis of independence and interactions of brain substrates that subserve multiple attributes, memory systems, and underlying processes. Neurobiol Learn Mem. 2004;82:199–215. doi: 10.1016/j.nlm.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Leanza G, Nilsson OG, Wiley RG, Bjorklund A. Selective lesioning of the basal forebrain cholinergic system by intraventricular 192 IgG-saporin: behavioral, biochemical, and stereological studies in the rat. Eur J Neurosci. 1995;7:329–343. doi: 10.1111/j.1460-9568.1995.tb01068.x. [DOI] [PubMed] [Google Scholar]
- 31.LeDoux JE. Emotion: clues from the brain. Ann Rev Psychol. 1995;46:209–235. doi: 10.1146/annurev.ps.46.020195.001233. [DOI] [PubMed] [Google Scholar]
- 32.Levy A, Dachir S, Arbel I, Kadar T. Aging, stress, and cognitive function. Ann NY Acad Sci. 1994;717:79–88. doi: 10.1111/j.1749-6632.1994.tb12075.x. [DOI] [PubMed] [Google Scholar]
- 33.Luine V, Martinez C, Villeges M, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- 34.Maren S, Fanselow MS. The amygdala and fear conditioning: has the nut been cracked? Neuron. 1996;16:237–240. doi: 10.1016/s0896-6273(00)80041-0. [DOI] [PubMed] [Google Scholar]
- 35.Mcewen BS, Spoolsky RM. Stess and cognitive function. Curr Opin Neurobiol. 1995;5:205–216. doi: 10.1016/0959-4388(95)80028-x. [DOI] [PubMed] [Google Scholar]
- 36.Mcewen BS, De Kloet ER, Rostene W. Adrenal steroid receptors and action in the nervous system. Physiol Rev. 1986;66:1121–1188. doi: 10.1152/physrev.1986.66.4.1121. [DOI] [PubMed] [Google Scholar]
- 37.McGaugh JL. Memory—a century of consolidation. Science. 2000;287:28–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 38.Mesulam MM, Mufson EJ, Wagner BH, Levey AI. Central cholinergic pathways in the rat: an overveiw based on an alternative nomenclature. Neuroscience. 1983;10:11815–21201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- 39.Perry T, Hodges H, Gray JA. Behavioural, histological and immunocytochemical consequences following 192 IgG-saporin immunolesions of the basal forebrain cholinergic system. Brain Res Bull. 2001;54:29–48. doi: 10.1016/s0361-9230(00)00413-5. [DOI] [PubMed] [Google Scholar]
- 40.Reul JM, De Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- 41.Rhodes ME, Kennell JS, Belz EE, Czambel RK, Rubin RT. Rat estrous cycle influences the sexual diergism of HPA axis stimulation by nicotine. Brain Res Bull. 2004;64:205–213. doi: 10.1016/j.brainresbull.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 42.Sandi C, Loscertales M, Guaza C. Experience-dependent facilitating effect of corticosterone on spatial memory formation in the water maze. Eur J Neurosci. 1997;9:637–642. doi: 10.1111/j.1460-9568.1997.tb01412.x. [DOI] [PubMed] [Google Scholar]
- 43.Sternberg DB, Isaacs KR, Gold PE, McGaugh JL. Epinephrine facilitation of appetitive learning: attenuation with adrenergic receptor antagonists. Behav Neural Biol. 1985;44:447–453. doi: 10.1016/s0163-1047(85)90856-8. [DOI] [PubMed] [Google Scholar]
- 44.Von Cramon DY, Markowitsch HJ, Shuri U. The possible contributions of the septal region in memory. Neuropsychologia. 1993;31:1159–1180. doi: 10.1016/0028-3932(93)90065-8. [DOI] [PubMed] [Google Scholar]
- 45.Waite JJ, Chen AD, Wardlow ML, Wiley RG, Lappi DA, Thal LJ. 192 Immunoglobulin G-saporin produces graded behavioral and biochemical changes accompanying the loss of cholinergic neurons of the basal forebrain and cerebellar Purkinje cells. Neuroscience. 1995;65:463–476. doi: 10.1016/0306-4522(94)00479-o. [DOI] [PubMed] [Google Scholar]
- 46.Walsh U, Herzog CD, Gandhi C, Stackman RW, Wiley RG. Injection of 192 IgG-saporin into the medial septum produces cholinergic hypofunction and dose-dependent working memory deficits. Brain Res. 1996;726:69–79. [PubMed] [Google Scholar]
- 47.White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiol Learn Mem. 2002;77:125–184. doi: 10.1006/nlme.2001.4008. [DOI] [PubMed] [Google Scholar]
- 48.Wiley RG, Oeltmann TN, Lappi DA. Immunolesioning: selective destruction of neurons using immunotoxin to rat NGF receptor. Brain Res. 1999;847:284–298. doi: 10.1016/0006-8993(91)91199-b. [DOI] [PubMed] [Google Scholar]
- 49.Wrenn CC, Lappi DA, Wiley RG. Threshold relationship between lesion extent of the cholinergic basal forebrain in the rat and working memory impairment in the radial maze. Brain Res. 1999;847:284–298. doi: 10.1016/s0006-8993(99)02099-5. [DOI] [PubMed] [Google Scholar]
- 50.Zhao H, Bucci DJ, Weltzin M, Drew KL. Effects of aversive stimuli on learning and memory in Arctic ground squirrels. Behav Brain Res. 2004;151:219–224. doi: 10.1016/j.bbr.2003.08.017. [DOI] [PubMed] [Google Scholar]


