Abstract
Pituitary Fshb concentrations increase markedly and selectively beginning on Postnatal Day 20 in the male rat. To evaluate the factors potentially responsible for this rise in FSH, we adjusted the time of weaning, which is generally also on Day 20. Male rat pups were provided nutrients by suckling only and were weaned to laboratory chow earlier (Day 17) or later (Day 23) than normally performed in animal facilities (Day 20). Between ages 17 and 29 days, significant increases were seen in serum LH (1.4-fold) and FSH (2.4-fold) levels; pituitary expression of Lhb (5.4-fold), Fshb (21.3-fold), and inhibin beta B (Inhbb, 2.26-fold) mRNAs; and testicular expression of Inhbb (10-fold) mRNA. Concurrently, significant decreases occurred in serum inhibin B levels (1.8-fold); pituitary adenylate cyclase-activating polypeptide (Adcyap1, 4.2-fold), total follistatin (Fst, 3.5-fold), and Fst isoform 288 (5.6-fold) mRNAs; and testicular expression of inhibin beta A (8.2-fold) mRNA. Early weaning significantly increased serum FSH but not LH and increased pituitary expression of Fshb and GnRH receptor (Gnrhr) mRNAs but not Lhb. Early weaning also significantly decreased serum inhibin B but increased testicular expression of the Inhbb subunit. Early weaning also caused pituitary expression of Fst and Adcyap1 to decline earlier than in the control group. Immediately after weaning, growth accelerated substantially, and the time of weaning produced significant and differential effects on circulating leptin levels that were not related to indices of FSH production. From these observations, we propose the novel hypothesis that the increase in growth rate subsequent to weaning signals circulating inhibin B levels to fall and pituitary Adcyap1 and consequently Fst expression to decrease, and that these events together facilitate the rise in Fshb and Gnrhr expression by increasing pituitary activin signaling.
Keywords: follicle-stimulating hormone, gonadotropins, inhibin, luteinizing hormone, male reproduction, male sexual function, pituitary
INTRODUCTION
Previous investigations in the male rat have demonstrated that circulating levels of FSH at birth are equivalent to levels in adult animals [1–3]. However, FSH concentrations decrease between the ages of 4 and 23 days and, beginning around Day 23, rise dramatically to peak levels at approximately 35 days that significantly exceed adult concentrations. On the other hand, there is very little change in LH throughout sexual maturation. Recent work in our laboratory and by others [4, 5] demonstrated that Fshb subunit mRNA expression parallels the developmental pattern of circulating FSH with a significant rise between ages 20 and 30 days. Because of the presumed importance of these events to testicular development and fertility, it is essential to elucidate the factors that are responsible for the selective regulation of FSH.
It has become increasingly clear that the primary mechanism by which FSH is differentially regulated from LH is through the activin signaling pathway. Activin, a member of the TGFβ superfamily of proteins, selectively stimulates Fshb synthesis with no direct effect on Lhb expression [6]. Activin also stimulates transcription of the gonadotropin-releasing hormone receptor (Gnrhr), however, and thereby indirectly increases LH by increasing GnRH responsiveness [7]. Conversely, Fshb expression levels are decreased when activin signaling is repressed [6, 8, 9]. The structurally similar dimeric protein inhibin is produced primarily within the gonads and, as part of a negative feedback control mechanism, competes with activin for binding to its receptor but fails to initiate activin signaling. A second regulator of activin is follistatin (FST), an activin-binding protein that is produced within the anterior pituitary and most peripheral tissues and neutralizes the actions of activin [10]. While there appears to be little change in pituitary activin, Fst expression in the pituitary is robustly regulated by GnRH, activin, testosterone, and pituitary adenylate cyclase-activating polypeptide (ADCYAP1) [11–13].
The single most dramatic event that occurs between the ages of 20 and 30 days in the life of the laboratory rat is weaning away from the nutritional environment of a lactating female. In the wild, rats will gradually wean themselves from nursing based on the availability of alternative food sources and the ability of the mother's milk to meet their nutritional needs. In many rodent research facilities, weaning occurs through separate caging, usually around Day 20, immediately prior to the time that FSH levels rise. Therefore, we developed the hypothesis that weaning and the ensuing acceleration in growth rate provide the physiological signal for the cessation of some factor(s) that repress maturation of the gonadotropin system.
In our experiments, we manipulated the timing of weaning of male rat pups and investigated the effects of early and late weaning on pituitary expression and serum concentrations of the gonadotropins. We also examined pituitary expression of Fst, inhibin beta B (Inhbb, also known as activin beta B), pituitary Adcyap1 (also known as PACAP), and serum inhibin B levels, and testicular expression of inhibin alpha subunit (Inha), inhibin beta A (Inhba, also known as activin beta A), and Inhbb mRNAs in order to examine the potential role of these candidate regulators of FSH during this time period. We assessed pituitary expression of Gnrhr as its expression is also stimulated by activin signaling [7]. Finally, we recorded body weights and measured the serum concentrations of leptin in our weaning paradigm since leptin is a marker of adipose tissue mass and has been suggested to play a permissive role in pubertal maturation in mammals [14].
MATERIALS AND METHODS
Animals
All experiments were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals according to a protocol approved by the Animal Care and Use Committee of the University of Louisville. Lactating Sprague-Dawley females and six male rat pups randomly assigned to the dam by the animal provider (Charles Rivers Laboratories) were housed together with free access to water. Once the pups were 10 days old, the lactating female was removed from the cage for 1 h twice daily and fed laboratory rat chow to ensure that suckling was the only source of nutrients to the pups. At the various times of weaning, pups were placed into cages without a lactating female, and one half of the cage was placed on a heating pad for 2 days to ensure adequate warmth. After weaning, water and laboratory rat chow were available ad libitum. Control rats were weaned from the lactating female at 20 days of age, and experimental pups were weaned on either Day 17 (early weaning) or Day 23 (late weaning). Body weight was determined daily starting at Postnatal (PN) Day 13. Pups from each group were killed on PN Days 17, 19, 21, 23, 25, and 29 (six per age). The pituitary gland from each animal was collected for the measurement of Lhb and Fshb subunits by Northern hybridization and mRNA levels of Gnrhr, Adcyap1, total Fst, and Fst isoform 288 by quantitative RT-PCR (qRT-PCR). To evaluate the peripheral effects of weaning, we processed the testes for qRT-PCR analysis of Inhbb, Inhba, and Inha subunit mRNA expression. Trunk blood was collected for analysis of circulating levels of LH, FSH, inhibin B, and leptin.
Serum Hormone Determinations
Enzyme immunoassays developed by Amersham Pharmacia Biotech were used to measure FSH and LH protein in serum. The within-assay coefficient of variation in the standard curves was 11.4% for FSH and 7.6% for LH. The range of the standards was 6.25 to 400 ng/ml for FSH and 0.41 to 100 ng/ml for LH.
Serum leptin levels were determined by a rat leptin ELISA kit (Crystal Chem, Inc., Downers Grove, IL). The within-assay coefficient of variation was 5.4%. The interassay coefficient of variation was 6.9%. The range of the standard curve was 200 to 12,800 pg/ml. Serum inhibin B levels were determined with an ultrasensitive ELISA kit (Serotec, Washington, DC). The range of the standards was 15.6 to 1000 pg/ml. The intraassay coefficient of variation was 4.7%.
RNA Extraction and Northern Analysis
RNA was extracted with the Perfect RNA Total RNA Isolation Kit (5 Prime-3 Prime, Boulder, CO). We determined the concentration of total RNA by reading the optical density at 260 nm. We determined sample purity by calculating the ratio of sample absorbance at 260:280 nm; samples were rejected if it was below 1.8. For Northern analysis, aliquots of pituitary RNA samples (2.5 μg) were subjected to electrophoresis on 1.2% agaroseformaldehyde gels. RNAs were transferred to Nytran membranes (Schleicher & Schuell, Keene, NH) and cross-linked to the membranes by baking for 2 h at 80° to 90°C followed by irradiation for 2 min with UV light. Purified cDNAs for rat Fshb (Dr. Richard Maurer) and Lhb (Dr. James Roberts) were labeled by the random primer method with [α-32P]dCTP (3000 Ci/mmol; New England Nuclear Research Products) to a specific activity of 6 × 108 to 8 × 108 dpm/μg as described elsewhere [15]. Labeled probes were added to the hybridization solutions at a concentration of approximately 5 ng/ml for 48 to 72 h. Membranes were washed, autoradiographed, and analyzed using a BioRad GS-700 imaging densitometer. Membranes were rehybridized without stripping for normalization to cyclophilin cDNA.
RT-PCR for Pituitary and Testis mRNAs
Construction of cRNA standard curves. For qRT-PCR, a standard curve of known amounts of target mRNA was prepared by the method of Fronhoffs et al. [16]. Briefly, target-specific primers (Table 1) were synthesized with and without a T7 promoter sequence (5′-GGATCCTAATACGACTCACTATAGGGAGG-3′) at the 5′ end of the forward primer and with an oligo(dT)15 at the 5′ end of the reverse primer. PCR was performed to produce cDNA containing the T7 promoter sequence. The PCR product (1 μg) was then used as template in an in vitro transcription reaction (MAXIscript T7 kit; Ambion, Austin, TX). The subsequent cRNA was quantified with a spectrophotometer and serial diluted to make standards containing 1011 to 104 molecules of cRNA. These standards were processed in parallel with experimental RNA samples for qRT-PCR with the primers lacking the T7 and dT sequences.
TABLE 1.
Primers utilized for real-time PCR analyses.
| Gene | Forward | Reverse | GenBank accession no. |
|---|---|---|---|
| Gapdh | CTGGAAGATGGTGATGGGTT | ATGATTCTACCCACGGCAAG | M17701 |
| Inha | CTGAACCAGAGGAGGAGGAT | CCAGATGACAGCACCAGAAG | NM_012590 |
| Inhbb | TGAGATCATCAGCTTTGCAG | CTGCACCACAAATAGGTTCT | XM_344130 |
| Inhbba | GCAGACCTCGGAGATCATCA | GGAAGATGTGCCAAGTGCTC | NM_017128 |
| Total Fst | GCTCCACTTGTGTGGTGGA | CCGCACTGGATGTCTTCACA | NM_012561 |
| Fst isoform 288 | GAGGCCCAAAAGACAAAACA | ATGGGGGAATACAGGGAGAG | AY280523 |
| Gnrhr | TCACACGAGTCCTTCATCA | GCCACTGTCATCTTTAGAGTTC | NM_031038 |
| Adcyap1 | CCTACCGCAAAGTCTTGGAC | TTGACAGCCATTTGTTTTCG | NM_016989 |
Quantitative RT-PCR. RNA (2 μg) isolated from pituitary and testis samples was reverse transcribed in parallel with cRNA standards using an oligo(dT)12−24 as the primer. Reverse-transcribed cRNA standards and samples were amplified in parallel by PCR on a Stratagene Mx4000 multiplex qPCR system (Stratagene, La Jolla, CA) using the Brilliant SYBR Green qPCR master mix (Stratagene) and the primers designed for use in the cRNA standard preparation but without the T7 and oligo(dT) sequences. Accumulation of PCR product was monitored in real time (Mx4000), and the crossover threshold (Ct) was determined with the Mx4000 software. For each set of primers, a no-template control and a no–reverse amplification control were included. Postamplification dissociation curves were performed to verify the presence of a single amplification product in the absence of DNA contamination. Concentrations of mRNAs were determined by means of the change in crossover time method with normalization to Gapdh mRNA and interpolation using a standard curve of known starting mRNA concentrations. Briefly, the Ct cycle defined as the fractional cycle at which the fluorescence signal becomes significantly different from the baseline signal, was determined by the fit-point method provided by the Mx4000 software. The Mx4000 software system generated the cRNA standard curve by plotting the Cts against the logarithm of the calculated initial copy numbers. The sample copy numbers were calculated from their Cts based on the cRNA standard curve.
Statistical Analysis
It was necessary to perform multiple serum protein and tissue RNA assays to analyze the large number of samples in these investigations. We controlled for interassay variations by analyzing a pooled sample (serum or tissue RNA) from PN Day 17 control rats in each assay. In addition, samples from each of the groups at each time point were included in every assay.
Data for each assay were normalized to the Day 17 control values and analyzed by two-way ANOVA with age and weaning group as the independent variables. This analysis determined whether there was a statistically significant overall effect of age or weaning group on the end points of interest. To determine whether weaning affected the timing of the end points, however, it was necessary to perform one-way ANOVA within each group and between groups at each time point in order to detect the age at which significant changes occurred.
RESULTS
Effects of Weaning Manipulations on Serum Gonadotropins
Serum FSH and LH concentrations increased significantly (P < 0.0001, two-way ANOVA) between PN Day 17 and PN Day 29 in each of the weaning groups (Fig. 1). Two-way ANOVA revealed a significant (P < 0.01) effect of weaning on serum FSH concentrations. Male rats in the early weaning group had higher levels of FSH (P = 0.01, post-hoc, Fisher PLSD) than the control weaning group, whereas the values in control and late weaning groups were not significantly different from each other. Altering the day of weaning had no significant effect on the concentration of LH. Thus, the experimental model modified the developmental rise in FSH secretion and allowed us to probe potential mechanisms for this change.
FIG. 1.
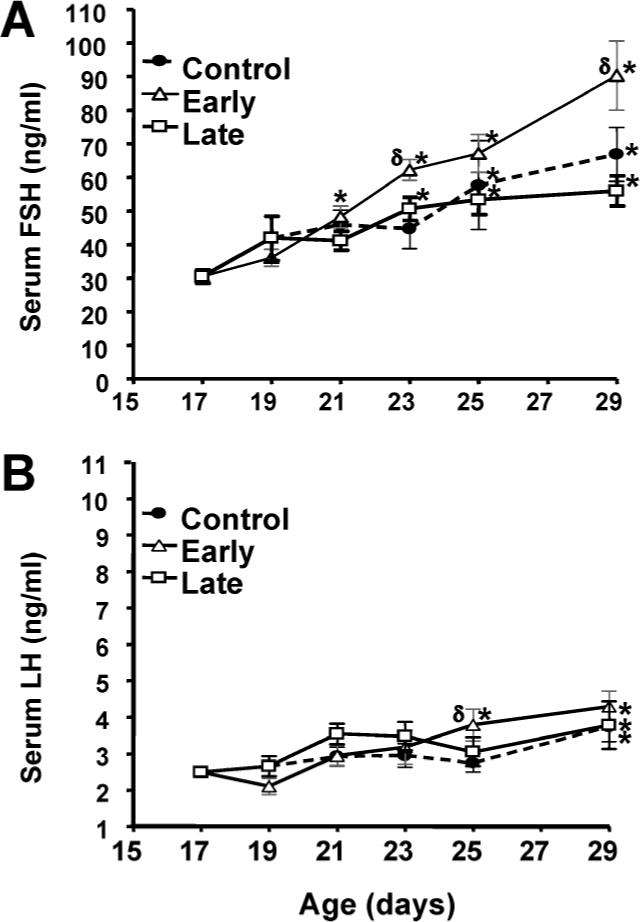
Effects of weaning manipulations on serum FSH and LH concentrations. Serum FSH (A) and LH (B) concentrations were determined at the indicated ages by ELISA. Each value represents the mean ± SEM of 6 animals. *, significantly different (ANOVA, P < 0.05) from Day 17 values within groups; δ, significantly greater (ANOVA, P < 0.05) than control values on the same day.
Effects of Weaning Manipulations on Gonadotropin Subunit Gene Expression
As demonstrated in Figure 2, a significant effect of age on pituitary expression of Lhb and Fshb was observed in each experimental group (P < 0.0001, two-way ANOVA). In control rats, there was a 5-fold increase in Lhb and a 21-fold increase in Fshb expression between Days 17 and 29. Manipulation of the day of weaning had a slight but nonsignificant effect on pituitary expression of Lhb. Conversely, altering the day of weaning significantly (P = 0.0008, two-way ANOVA) changed pituitary expression of Fshb mRNA. Early weaning induced a significantly (P = 0.03, Fisher PLSD) greater rise in Fshb mRNA expression compared to male pups in the control group. In the early weaning group, the level of Fshb mRNA on Day 21 was equivalent to the value in the control group on Day 29. Late weaning resulted in a modest, nonsignificant reduction in pituitary Fshb mRNA levels compared to control rats.
FIG. 2.
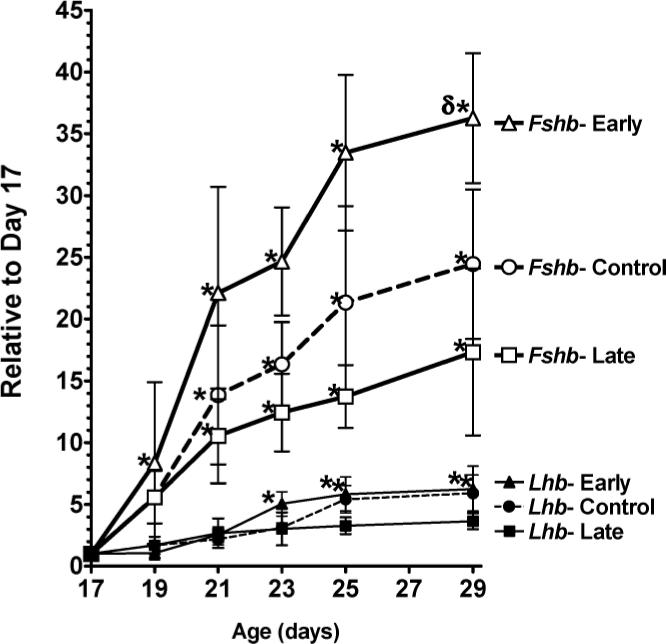
Effects of weaning manipulations on pituitary Fshb and Lhb mRNA concentrations. Lhb and Fshb mRNA levels were determined by Northern hybridization with normalization to cyclophilin mRNA levels. Data are expressed as mRNA expression levels relative to Day 17 values. Each value represents the mean ± SEM of 6 animals. *, significantly different (P < 0.05) from Day 17 values within groups; δ, significantly greater (P < 0.05) than control values on the same day.
Effects of Weaning Manipulations on Pituitary Gnrhr Expression
Analysis of the relative expression levels of pituitary Gnrhr mRNA revealed a significant effect of age and day of weaning (P < 0.001, two-way ANOVA). Pituitary Gnrhr mRNA levels increased significantly between Days 17 and 29 in controls, with an accelerated increase in the early weaning group (Fig. 3). Subsequent to weaning, the levels of pituitary Gnrhr mRNA were significantly higher (P = 0.01, Fisher PLSD) in the early weaning group compared to the control group. Pituitary Gnrhr mRNA levels were moderately but not significantly lower in the late weaned animals until Day 29, at which time there was a significant difference between the late and control groups. At the beginning of the experiment on Day 17, the pituitary Gnrhr mRNA concentration was 5.25 pg per microgram of total pituitary RNA.
FIG. 3.
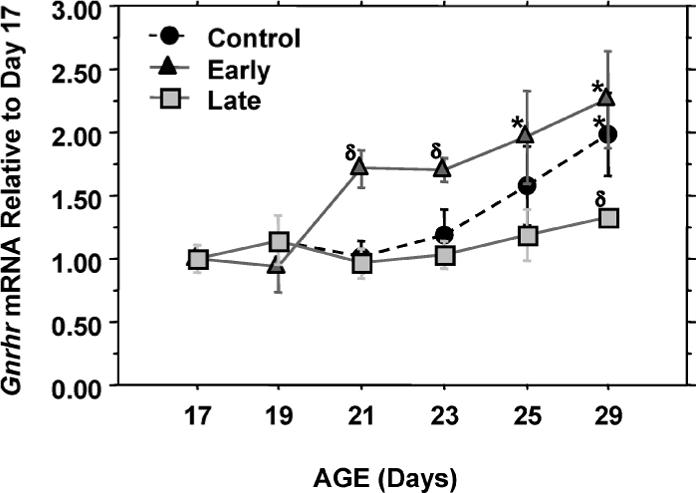
Effects of weaning manipulations on pituitary expression of Gnrhr. Gnrhr mRNA levels were determined by RT-PCR. Data are expressed as mRNA expression levels relative to Day 17 values. Each value represents the mean ± SEM of 6 animals. *, significantly different (P < 0.05) from Day 17 values within groups; δ, significantly different (P < 0.05) from control values on the same day.
Effects of Weaning Manipulations on Pituitary Fst Expression
The mRNA species encoding Fst isoform 288 has a 10-fold greater FSH-suppressing activity than the more abundant Fst-315 isoform [17]. We showed previously that the rise in Fshb and Gnrhr mRNA levels in the male rat pituitary between Days 20 and 30 occurs together with a decline in the level of pituitary Fst isoform 288 expression [5]. Because early weaning increased both Fshb and Gnrhr expression, Fst isoform 288 as well as total pituitary Fst mRNA levels were evaluated. Analysis of the relative expression levels of total pituitary Fst mRNAs revealed a significant effect of age (P < 0.001; Fig. 4A). Pituitary Fst mRNA levels declined significantly between Days 17 and 29, and the decline was accelerated in the early weaning group. Analysis of the individual weaning groups by one-way ANOVA revealed a significant decline in Fst expression by Day 23 in the control group and by Days 19 and 25 in the early and late weaning groups, respectively. A similar pattern to total Fst was observed for Fst isoform 288 mRNA expression except that, in the control group, pituitary expression of Fst isoform 288 was significantly lower by Day 21 rather than Day 23 (Fig. 4B). Therefore, we conclude that early weaning advances the decline in pituitary expression of Fst. Animals on Day 17 had pituitary mRNA concentrations of 1.2× 106 molecules of total Fst and 3.7 × 105 molecules of Fst isoform 288 mRNA per microgram of total pituitary RNA. Fst isoform 288 accounted for 20% to 25% of total pituitary Fst mRNAs during the various stages of development studied.
FIG. 4.
Effects of weaning on expression levels of pituitary-derived FSH regulating factors. Levels of pituitary Fst (A), Fst isoform 288 (labeled Fst-288)(B), Adcyap1 (C), and Inhbb (D) mRNAs were determined by RT-PCR. Data are expressed as mRNA expression levels relative to Day 17 values. Each value represents the mean ± SEM of 6 animals. *, significantly different (P < 0.05) from Day 17 values within groups; δ, significantly different (P < 0.05) from control values on the same day.
Effects of Weaning Manipulations on Pituitary Adcyap1 Expression
Insofar as ADCYAP1 stimulates Fst expression in vitro [15, 18], the decline in pituitary Fst between ages 17 and 29 days could result from a decrease in pituitary Adcyap1 expression. To evaluate whether ADCYAP1 plays an autocrine/paracrine role in the developmental rise in FSH, we measured pituitary Adcyap1 mRNA levels in this model of FSH regulation by early and late weaning. Analysis of the relative expression levels of pituitary Adcyap1 mRNA by two-way ANOVA revealed a significant effect of age (P = 0.008) but no difference in overall levels associated with the time of weaning (Fig. 4C). Analysis of the individual weaning groups by one-way ANOVA revealed a significant decline in Adcyap1 expression by Day 21 in the control and late weaning groups, whereas the value on Day 19 was significantly less than the Day 17 value in the early group. Therefore, we conclude that early weaning may advance the decline in pituitary expression of Adcyap1. Furthermore, the timing of the significant changes in pituitary Adcyap1 mRNA was exactly reciprocal to that of the changes observed in Fshb mRNA expression in each group and paralleled the changes observed in Fst isoform 288 mRNA expression levels in the control and early weaning groups. The pituitary Adcyap1 mRNA concentration of Day 17 animals was 3.85 × 105 molecules of Adcyap1 mRNA per microgram of total pituitary RNA.
Effects of Weaning Manipulations on Pituitary Inhbb Expression
Previous studies have shown that Inhbb is the predominant isoform of activin expressed within the pituitary gland [19]. To evaluate a possible role of activin expression in this model of selective regulation of FSH, we measured pituitary Inhbb mRNA by quantitative RT-PCR. Two-way ANOVA revealed a significant effect of age (P = 0.0001) but no effect of time of weaning (Fig. 4D). Moreover, the temporal pattern of expression, with higher levels at weaning and on Day 29 and lower levels on Days 23 and 25, differed from the pattern of Fshb and Gnrhr mRNA expression. On Day 17, the pituitary Inhbb mRNA concentration was 3.1 × 104 molecules per microgram of total pituitary RNA.
Effects of Weaning Manipulations on Serum Inhibin B
Because inhibin B levels were reported to decline in male rats between ages 20 to 30 days [20], we sought to determine whether circulating inhibin B, like Fshb, is influenced by weaning. Analysis of serum inhibin B concentrations by two-way ANOVA revealed a significant effect of age (P < 0.001) and day of weaning (P < 0.001). Inhibin B concentrations declined significantly in the control group between Days 17 and 29, with an accelerated decline in the early weaning group. Moreover, the decline in inhibin B levels was delayed in the late weaning group (Fig. 5A).
FIG. 5.
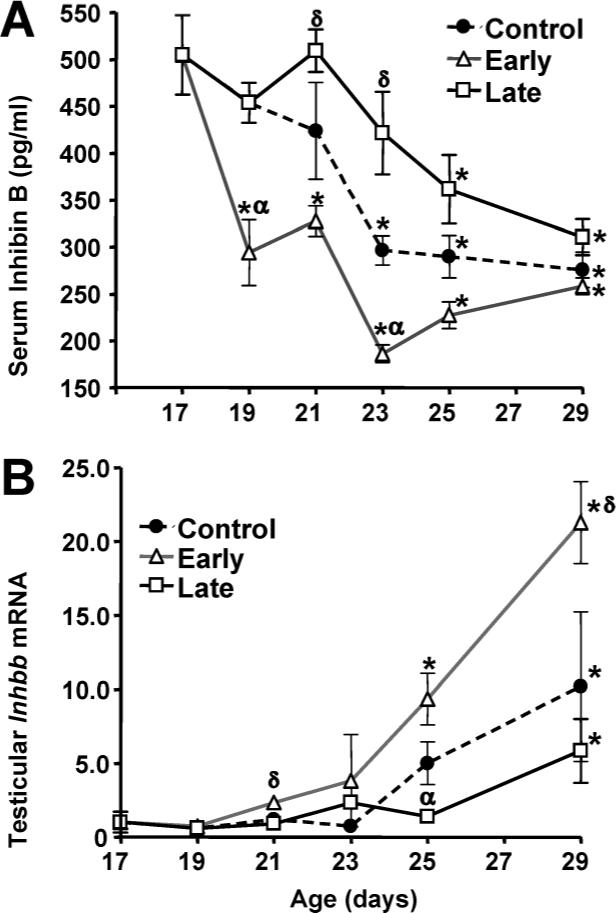
Effects of weaning manipulations on serum inhibin B and testicular Inhbb mRNA levels. Serum inhibin B concentrations (A) were measured by ELISA. Each value represents the mean ± SEM of 6 animals. Levels of testicular Inhbb mRNA (B) were determined by RT-PCR. Data are expressed as mRNA expression levels relative to Day 17 values. Each value represents the mean ± SEM of 6 animals. *, significantly different (P < 0.05) from Day 17 values within groups; δ, significantly greater (P < 0.05) than control values on the same day; α, significantly less (P < 0.05) than control values on the same day.
Effects of Weaning Manipulations on Testicular Activin/Inhibin Subunit Expression
Inhibin subunit mRNA levels in testis were measured to determine whether the decline in circulating inhibin B during sexual maturation and weaning was explained by reduced inhibin production. Testicular levels of Inhbb mRNA (Fig. 5B) were significantly affected by age (P < 0.001) and by day of weaning (P < 0.001). Unlike the fall in serum inhibin B levels, however, Inhbb mRNA levels in testis increased significantly between Days 17 and 29, with an accelerated increase in male rats in the early weaning group. Levels of testicular Inhbb mRNAs were significantly higher (P = 0.04, Fisher PLSD) in the early weaning group compared to the control group. Testicular Inhbb mRNA levels were moderately but not significantly lower in the late weaned rats compared to control animals. Two-way ANOVA also revealed a significant effect of age (P < 0.01) on the expression levels of Inha and Inhba mRNAs but no effect of the time of weaning (Fig. 6). Inhba expression levels fell dramatically between Days 19 and 29, while Inha expression levels rose following weaning and then declined. On Day 17, animals had testicular mRNA concentrations of 4.3 × 106 molecules of Inhbb, 2.4 × 108 molecules Inhba, and 2.9 × 108 molecules Inha mRNA per microgram of total testicular RNA. However, by Day 29, the concentration of Inhbb mRNA was sixfold higher than that of Inhba mRNA.
FIG. 6.

Effects of weaning on Inhba and Inha levels in testis. Levels of testicular Inhba (A) and Inha (B) mRNAs were determined by RT-PCR. Data are expressed as mRNA expression levels relative to Day 17 values. Each value represents the mean ± SEM of 6 animals. *, significantly different (P < 0.05) from Day 17 values within groups; δ, significantly different (P < 0.05) from control values on the same day.
Effects of Weaning Manipulations on Growth and Serum Leptin Concentrations
Body weight increased in each group of rat pups immediately following weaning (Fig. 7A), and the rate of increase was linear and comparable for each group. Consequently, the early weaned rats were heavier throughout the course of the experiment, whereas the control and late weaned pups were similar in weight between PN Days 13 and 29.
FIG. 7.
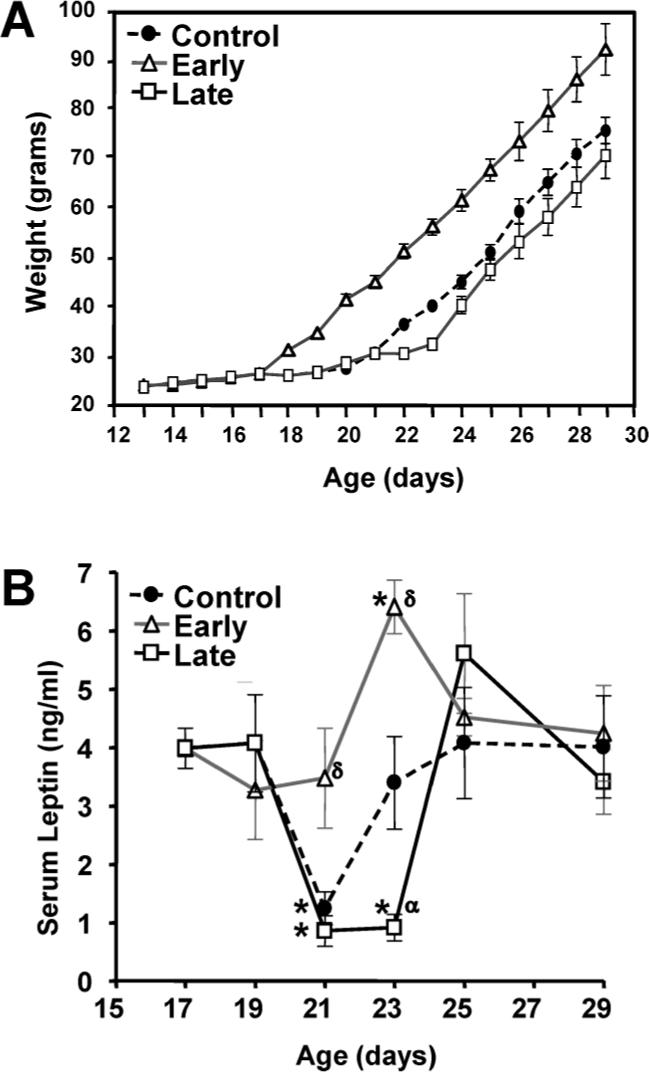
Effects of weaning manipulations on body weight and serum concentrations of leptin. Body weights (A) of pups in the control, early weaning, and late weaning groups were recorded beginning on Day 13. There were no apparent differences between groups in the rate of growth before or after weaning. Values represent the mean ± SEM of 12 to 18 animals at each time point. Serum leptin concentrations (B) were measured by specific ELISA. Each value represents the mean ± SEM of 6 animals. *, significantly different (P < 0.05) from Day 17 values within groups; δ, significantly greater (P < 0.05) than control values on the same day; α, significantly less (P < 0.05) than control values on the same day.
There was a significant effect of age (P = 0.0002) and day of weaning (P = 0.012) on serum leptin concentrations. The results in Figure 7B reveal that serum leptin levels in control rats declined on Day 21 subsequent to weaning on Day 20 but returned to Day 19 values on Day 23. A decrease in leptin was also observed on Day 21 in the late weaning group; however, the subsequent rise was delayed until Day 25, 2 days after weaning on Day 23. Serum leptin levels in the early weaning group declined slightly but not significantly on Day 19 following weaning on Day 17 and increased on Day 23, as in controls. Thus, early weaning prevented the decline in serum leptin concentrations that occurred in the final days of suckling in control rats. On the other hand, late weaning prolonged the period of decreased leptin concentrations. Finally, the pattern of circulating leptin concentrations was unrelated to FSH, which rose continuously from Day 17 to Day 29 (Fig. 1).
DISCUSSION
The data in this report imply that the increase in growth that follows weaning provides a developmental cue for Fshb gene expression to increase from Day 17 to Day 29 in male rats. Furthermore, the mechanism for repressed FSH during infancy appears to involve inhibition of pituitary activin signaling by inhibin and FST since, subsequent to weaning, there were reciprocal changes in Fshb mRNA and plasma inhibin B and pituitary Fst levels.
Specific two-site immunoassays have shown that inhibin B is the predominant circulating isoform in males [21, 22]. Our results confirm those of Sharpe et al. [20], who reported that circulating inhibin B levels decrease from Day 20 to Day 30 in male Sprague-Dawley rats, suggesting that postweaning somatic growth may regulate this decline. A shift in the day of weaning by 3 days produced a marked advance or delay in the timing of the decline in circulating inhibin B concentrations. This decline permits enhanced activin signaling due to less competition by inhibin for the activin receptor. The end result, as demonstrated in this investigation, is a significant increase in expression of activin-responsive mRNAs, including Fshb and Gnrhr. Another study [23] did not observe a decrease in circulating concentrations of inhibin B until after Day 40, well after the peak in circulating FSH; the reason for these dissimilar results is uncertain. However, those results suggest that a decline in inhibin is not solely responsible for increased Fshb expression.
Inhibin B is primarily produced in and released from the testes [21, 23]. Inhibin B is a dimer of the Inha and inhibin beta B subunit proteins. In this investigation, we observed a rise in the concentration of testicular Inha subunit mRNA following weaning that was presumably a consequence of the rise in FSH [21] followed by a decrease by Day 29, which may have been partly due to germ cell expansion diluting the Inha from Sertoli cells. When adjusted for total RNA content, our results are similar to those of Klaij et al. [24], revealing an increase in total testicular Inha mRNA levels between the ages of 23 and 25 days. However, there was no effect of weaning on testicular Inha expression levels. By contrast, the concentration of testicular Inhbb mRNA increased substantially from Day 17 to Day 29, perhaps because this gene is expressed in germ cells as well as Sertoli cells [23].
Thus, the developmental decrease in circulating inhibin B concentrations is unrelated to changes in testicular Inhbb or Inha gene expression. There are several potential explanations for decreased circulating inhibin levels. First, it is possible that weaning influences the posttranscriptional processing of the inhibin subunit mRNAs. Second, the rat blood-testis barrier becomes functional at this time [25] and may limit the release of inhibin into the circulation. Third, the metabolic clearance of inhibin appears to be greater in adult than in immature male rats [26], and the change in diet with weaning and the associated increase in somatic growth may affect peptide clearance rates.
We also observed a significant increase in pituitary Inhbb expression from Day 17 to Day 29. Activin stimulates and inhibin decreases expression of the activin/inhibin subunit genes [27]. Therefore, decreased circulating inhibin B concentrations during development might allow for increased activin signaling to increase pituitary activin expression and thereby stimulate FSH.
In this investigation, we confirmed our previous finding [5] that the expression level of the Fst isoform 288 mRNA isoform in the male rat pituitary declines significantly between Days 20 and 30. RT-PCR analysis also revealed a significant decline in total pituitary Fst mRNA not previously observed by semiquantitative PCR. Furthermore, early weaning accelerated the age at which Fst isoform 288 and total Fst mRNA levels were significantly lowered. Inhibin B is thought to suppress Fst expression by blocking activin signaling [28]; therefore, some other signal consequent to weaning must be responsible for the fall in pituitary Fst expression that occurs simultaneously with a fall in inhibin B.
ADCYAP1 (PACAP) administration causes a robust increase in Fst expression within primary cultures of rat pituitary cells [15, 18]. Moreover, we previously reported a significant decrease in Adcyap1 mRNA levels within the paraventricular nucleus of the hypothalamus between the ages of 20 and 30 days in male rats [5]. In this study, we observed a significant decrease in pituitary Adcyap1 mRNA levels between 20 and 30 days. Furthermore, the timing of decreased pituitary Adcyap1 expression was advanced in the subjects that were weaned early compared to the developmental pattern in the control group. Thus, these findings further strengthen the hypothesis that a developmental decrease in ADCYAP1 contributes to the increase in Fshb expression by reducing Fst expression, which increases activin signaling.
The reciprocal relationship between pituitary Adcyap1 and Fshb mRNA levels is an especially novel discovery. Until recently, ADCYAP1 has been considered a hypophysiotropic hormone as high concentrations of ADCYAP1 peptide and Adcyap1 mRNA are found within the hypothalamus [29]. In a previous report, we determined that hypothalamic expression of Adcyap1 is reciprocal to pituitary Fshb expression between ages 20 and 30 days in the male rat [5]. The results of this study suggest that an autocrine/paracrine mechanism links ADCYAP1 to Fshb expression in the male rat during peripubertal sexual maturation. ADCYAP1 has been demonstrated to have an autoregulatory effect to stimulate Adcyap1 mRNA accumulation in cultures of tilapia astrocytes [30]. Thus far, only cAMP signaling has been reported to regulate mammalian Adcyap1 expression [31]. Therefore, due to potent activation of cAMP signaling, changes in hypothalamic expression of Adcyap1 during development may influence pituitary expression of Adcyap1 and further strengthen its effects on gonadotrophs.
ADCYAP1 has been demonstrated to selectively inhibit Fshb mRNA expression, and this effect is thought to occur through its potent stimulatory action on Fst expression (e.g. [18]). Increased Fst expression in response to ADCYAP1 stimulation is thought to diminish activin signaling and thus selectively repress expression of Fshb. In this model of weaning-associated regulation of gonadotropin expression, the levels of pituitary Adcyap1 and Fst mRNAs declined substantially at the time when Fshb mRNA levels were increasing. Thus, this relationship appears to provide an additional mechanism by which LH and FSH are differentially regulated during development in the male rat.
While less pronounced than the rise in FSH, serum LH levels and pituitary expression of Lhb also increased significantly between Days 20 and 30. This rise in LH may partially reflect increased GnRH secretion [32, 33]; however, Gnrhr mRNA levels also increased following weaning, and increased Gnrhr will enhance gonadotroph responsiveness to GnRH to increase secretion of both gonadotropins. The rise in Gnrhr mRNA levels is likely to result from increased activin signaling due to suppressed inhibin B and Fst expression as well as to increased GnRH.
Repression of FSH production during early development is thought to prevent premature maturation of the testes. In previous investigations in which FSH was administered to immature male rats, there were multiple changes to testis morphology that persisted into adulthood [34, 35]. Administration of recombinant human FSH to immature rats accelerated testicular growth, including increases in seminiferous tubule volume and length with proportionate increases in Sertoli and germ cell numbers as well as increased numbers of spermatogonia and serum levels of testosterone. Human FSH also stimulated spermatogonia differentiation, which resulted in a fivefold increase in the number of spermatocytes. The male rats in the early weaning group in this investigation had significantly elevated circulating FSH levels compared to those in the control group. Further studies are required to determine the long-term effects of early and elevated serum FSH levels in our weaning paradigm.
Fst and Adcyap mRNAs as well as serum inhibin B levels were not significantly different between groups at the time when serum FSH and pituitary Fshb mRNAs differed maximally (Days 25 and 29). Increased Fshb expression in the early weaning rats at these later time points could be due to the earlier increases in activin signaling. Alternatively, ADCYAP1 and inhibin modulation of activin signaling may not be the sole mechanism by which FSH is regulated during development. Extending our studies with the weaning paradigm should readily determine whether the control and late weaning groups ultimately achieve similar levels of FSH.
In designing this study, we were concerned that the animals in the early weaning group would experience malnutrition due to premature removal of suckling nutrients and an inability to consume sufficient amounts of rat chow. However, animals in each weaning group displayed a marked increase in growth rate after being switched to laboratory chow as their exclusive dietary source. Thus, animals in each group had adequate nourishment after weaning, as reflected by a dramatic increase in growth.
Our data reveal a relationship between serum leptin concentrations and weaning. Leptin is produced within adipocytes and is secreted into the circulation to affect multiple tissues, including hypothalamic neurons important for reproductive function [36]. Leptin is thought to play a permissive role in sexual maturation, and animals deficient in leptin peptide or its receptors do not develop to reproductive maturity [37, 38]. In this investigation, animals in the control and late weaning groups displayed a significant, albeit transient, decrease in circulating concentrations of leptin toward the end of the suckling period. However, animals that were weaned early did not experience this decline. Several previous studies in which suckling pups were provided laboratory chow ad libitum also demonstrated a significant decline in serum concentrations of leptin at or near the time of weaning [39–41]. Circulating levels of leptin decrease with fasting in rodents [42] and are an indication of a change in metabolic status. Therefore, we hypothesize that a fall in leptin levels may provide a metabolic signal for rat pups to seek novel food sources; however, further studies are required to delineate the role of leptin in the cessation of suckling.
Other data from this investigation and previously published studies imply, however, that leptin plays an insignificant role in the delay in Fshb and Gnrhr maturation in control and late weaning groups. First, the decline in serum leptin that occurred at approximately Day 19 appears to be the normal pattern of circulating leptin in immature male rats [39–41]. Second, in a recent study that employed severe nutritional deprivation in preweanling male rats, a 38% decrease in body weight and a 70% decrease in serum leptin levels produced no significant change in serum gonadotropins at the time of weaning [43]. Finally, in this investigation, at the time when serum leptin levels were decreasing, pituitary expression of Fshb was rising, and serum leptin levels were comparable in each weaning group by Day 25.
In conclusion, the results of this investigation suggest that weaning and the ensuing acceleration in growth provide a signal for the rise in Fshb and Gnrhr expression that occurs in the male rat between Days 20 and 30. Weaning and somatic growth are associated with a decrease in circulating inhibin B and pituitary Fst mRNA levels and thereby enhanced activin signaling. The decline in serum inhibin B while testicular Inhbb expression levels rise suggests that weaning may accelerate inhibin clearance. In addition, the weaning-related decline in pituitary Adcyap1 expression is likely to contribute to decreased pituitary Fst expression. Finally, controlled alterations in weaning may represent a valuable new model to study sexual maturation in the male rat.
ACKNOWLEDGMENTS
The authors would like to acknowledge the expert technical assistance of Mr. Dushan Ghooray and Mr. Alan Icard.
Footnotes
Supported in part by NIH grants RR-P20 RR17702-SPID 0005 from the Institutional Development Award (IdeA) Program of the NCRR (J.P.M.) and R01-HD-036034 (S.J.W.), by the Walter F. and Avis Jacobs Foundation, and by the Commonwealth of Kentucky Research Challenge Fund.
REFERENCES
- 1.Maeda KS, Ohkura S, Tsukamura H. Physiology of reproduction. In: Krinke GJ, editor. The Laboratory Rat. Academic Press; New York: 2000. pp. 145–176. [Google Scholar]
- 2.Ojeda SR, Andrews WW, Advis JP, White SS. Recent advances in the endocrinology of puberty. Endocr Rev. 1980;1:228–257. doi: 10.1210/edrv-1-3-228. [DOI] [PubMed] [Google Scholar]
- 3.Russell LD, Alger LE, Nequin LG. Hormonal control of pubertal spermatogenesis. Endocrinology. 1987;120:1615–1632. doi: 10.1210/endo-120-4-1615. [DOI] [PubMed] [Google Scholar]
- 4.Zapatero-Caballero H, Sanchez-Franco F, Guerra-Perez N, Fernandez-Mendez C, Fernandez-Vazquez G. Gonadotropin-releasing hormone receptor gene expression during pubertal development of male rats. Biol Reprod. 2003;68:1764–1770. doi: 10.1095/biolreprod.102.008821. [DOI] [PubMed] [Google Scholar]
- 5.Moore JP, Jr, Wilson L, Dalkin AC, Winters SJ. Differential expression of the pituitary gonadotropin subunit genes during male rat sexual maturation: reciprocal relationship between hypothalamic pituitary adenylate cyclase-activating polypeptide and follicle-stimulating hormone beta expression. Biol Reprod. 2003;69:234–241. doi: 10.1095/biolreprod.102.012757. [DOI] [PubMed] [Google Scholar]
- 6.Carroll RS, Corrigan AZ, Gharib SD, Vale W, Chin WW. Inhibin, activin, and follistatin: regulation of follicle-stimulating hormone messenger ribonucleic acid levels. Mol Endocrinol. 1989;3:1969–1976. doi: 10.1210/mend-3-12-1969. [DOI] [PubMed] [Google Scholar]
- 7.Braden TD, Conn PM. Activin-A stimulates the synthesis of gonadotropin-releasing hormone receptors. Endocrinology. 1992;130:2101–2105. doi: 10.1210/endo.130.4.1312442. [DOI] [PubMed] [Google Scholar]
- 8.Carroll RS, Kowash PM, Lofgren JA, Schwall RH, Chin WW. In vivo regulation of FSH synthesis by inhibin and activin. Endocrinology. 1991;129:3299–3304. doi: 10.1210/endo-129-6-3299. [DOI] [PubMed] [Google Scholar]
- 9.Besecke LM, Guendner MJ, Sluss PA, Polak AG, Woodruff TK, Jameson JL, Bauer-Dantoin AC, Weiss J. Pituitary follistatin regulates activin-mediated production of follicle-stimulating hormone during the rat estrous cycle. Endocrinology. 1997;138:2841–2848. doi: 10.1210/endo.138.7.5279. [DOI] [PubMed] [Google Scholar]
- 10.de Winter JP, ten Dijke P, de Vries CJ, van Achterberg TA, Sugino H, de Waele P, Huylebroeck D, Verschueren K, van den Eijnden-van Raaij AJ. Follistatins neutralize activin bioactivity by inhibition of activin binding to its type II receptors. Mol Cell Endocrinol. 1996;116:105–114. doi: 10.1016/0303-7207(95)03705-5. [DOI] [PubMed] [Google Scholar]
- 11.Burger LL, Haisenleder DJ, Wotton GM, Aylor KW, Dalkin AC, Marshall JC. The regulation of FSHbeta transcription by gonadal steroids: testosterone and estradiol modulation of the activin intracellular signaling pathway. Am J Physiol Endocrinol Metab. 2007;293:E277–E285. doi: 10.1152/ajpendo.00447.2006. [DOI] [PubMed] [Google Scholar]
- 12.Winters SJ, Ghooray D, Fujii Y, Moore JP, Jr, Nevitt JR, Kakar SS. Transcriptional regulation of follistatin expression by GnRH in mouse gonadotroph cell lines: evidence for a role for cAMP signaling. Mol Cell Endocrinol. 2007;271:45–54. doi: 10.1016/j.mce.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 13.DePaolo LV, Mercado M, Guo Y, Ling N. Increased follistatin (activin-binding protein) gene expression in rat anterior pituitary tissue after ovariectomy may be mediated by pituitary activin. Endocrinology. 1993;132:2221–2228. doi: 10.1210/endo.132.5.8477666. [DOI] [PubMed] [Google Scholar]
- 14.Cheung CC, Thornton JE, Kuijper JL, Weigle DS, Clifton DK, Steiner RA. Leptin is a metabolic gate for the onset of puberty in the female rat. Endocrinology. 1997;138:855–858. doi: 10.1210/endo.138.2.5054. [DOI] [PubMed] [Google Scholar]
- 15.Tsujii T, Ishizaka K, Winters SJ. Effects of pituitary adenylate cyclase-activating polypeptide on gonadotropin secretion and subunit messenger ribonucleic acids in perfused rat pituitary cells. Endocrinology. 1994;135:826–833. doi: 10.1210/endo.135.3.7915230. [DOI] [PubMed] [Google Scholar]
- 16.Fronhoffs S, Totzke G, Stier S, Wernert N, Rothe M, Bruning T, Koch B, Sachinidis A, Vetter H, Ko Y. A method for the rapid construction of cRNA standard curves in quantitative real-time reverse transcription polymerase chain reaction. Mol Cell Probes. 2002;16:99–110. doi: 10.1006/mcpr.2002.0405. [DOI] [PubMed] [Google Scholar]
- 17.Inouye S, Guo Y, DePaolo L, Shimonaka M, Ling N, Shimasaki S. Recombinant expression of human follistatin with 315 and 288 amino acids: chemical and biological comparison with native porcine follistatin. Endocrinology. 1991;129:815–822. doi: 10.1210/endo-129-2-815. [DOI] [PubMed] [Google Scholar]
- 18.Fujii Y, Okada Y, Moore JP, Jr, Dalkin AC, Winters SJ. Evidence that PACAP and GnRH down-regulate follicle-stimulating hormone-beta mRNA levels by stimulating follistatin gene expression: effects on folliculostellate cells, gonadotrophs and LbetaT2 gonadotroph cells. Mol Cell Endocrinol. 2002;192:55–64. doi: 10.1016/s0303-7207(02)00109-0. [DOI] [PubMed] [Google Scholar]
- 19.Bilezikjian LM, Vaughan JM, Vale WW. Characterization and the regulation of inhibin/activin subunit proteins of cultured rat anterior pituitary cells. Endocrinology. 1993;133:2545–2553. doi: 10.1210/endo.133.6.8243276. [DOI] [PubMed] [Google Scholar]
- 20.Sharpe RM, Turner KJ, McKinnell C, Groome NP, Atanassova N, Millar MR, Buchanan DL, Cooke PS. Inhibin B levels in plasma of the male rat from birth to adulthood: effect of experimental manipulation of Sertoli cell number. J Androl. 1999;20:94–101. [PubMed] [Google Scholar]
- 21.Woodruff TK, Besecke LM, Groome N, Draper LB, Schwartz NB, Weiss J. Inhibin A and inhibin B are inversely correlated to follicle-stimulating hormone, yet are discordant during the follicular phase of the rat estrous cycle, and inhibin A is expressed in a sexually dimorphic manner. Endocrinology. 1996;137:5463–5467. doi: 10.1210/endo.137.12.8940372. [DOI] [PubMed] [Google Scholar]
- 22.Anawalt BD, Bebb RA, Matsumoto AM, Groome NP, Illingworth PJ, McNeilly AS, Bremner WJ. Serum inhibin B levels reflect Sertoli cell function in normal men and men with testicular dysfunction. J Clin Endocrinol Metab. 1996;81:3341–3345. doi: 10.1210/jcem.81.9.8784094. [DOI] [PubMed] [Google Scholar]
- 23.Buzzard JJ, Loveland KL, O'Bryan MK, O'Connor AE, Bakker M, Hayashi T, Wreford NG, Morrison JR, de Kretser DM. Changes in circulating and testicular levels of inhibin A and B and activin A during postnatal development in the rat. Endocrinology. 2004;145:3532–3541. doi: 10.1210/en.2003-1036. [DOI] [PubMed] [Google Scholar]
- 24.Klaij IA, Timmerman MA, Kramer P, Meijs-Roelofs HM, de Jong FH. Testicular and serum levels of inhibin and expression of inhibin subunit mRNAs are differentially affected by hemicastration in rats of various ages. J Endocrinol. 1994;141:143–151. doi: 10.1677/joe.0.1410143. [DOI] [PubMed] [Google Scholar]
- 25.Agarwal A, Hoffer AP. Ultrastructural studies on the development of the blood-epididymis barrier in immature rats. J Androl. 1989;10:425–431. doi: 10.1002/j.1939-4640.1989.tb00132.x. [DOI] [PubMed] [Google Scholar]
- 26.Winters SJ, Pohl CR, Adedoyin A, Marshall GR. Effects of continuous inhibin administration on gonadotropin secretion and subunit gene expression in immature and adult male rats. Biol Reprod. 1996;55:1377–1382. doi: 10.1095/biolreprod55.6.1377. [DOI] [PubMed] [Google Scholar]
- 27.Bilezikjian LM, Corrigan AZ, Blount AL, Vale WW. Pituitary follistatin and inhibin subunit messenger ribonucleic acid levels are differentially regulated by local and hormonal factors. Endocrinology. 1996;137:4277–4284. doi: 10.1210/endo.137.10.8828487. [DOI] [PubMed] [Google Scholar]
- 28.Phillips DJ. Regulation of activin's access to the cell: why is mother nature such a control freak? Bioessays. 2000;22:689–696. doi: 10.1002/1521-1878(200008)22:8<689::AID-BIES2>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 29.Mikkelsen JD, Hannibal J, Fahrenkrug J, Larsen PJ, Olcese JCM. Pituitary adenylate cyclase activating peptide-38 (PACAP-38), PACAP-27, and PACAP related peptide (PRP) in the rat median eminence and pituitary. J Neuroendocrinol. 1995;7:47–55. doi: 10.1111/j.1365-2826.1995.tb00666.x. [DOI] [PubMed] [Google Scholar]
- 30.Chi-Wei L, Chang SL, Weng CF. Pituitary adenylate cyclase-activating polypeptide (PACAP) regulates the expression of PACAP in cultured tilapia astrocytes. Exp Biol Med (Maywood) 2007;232:262–276. [PubMed] [Google Scholar]
- 31.Fukuchi M, Tabuchi A, Tsuda M. Activity-dependent transcriptional activation and mRNA stabilization for cumulative expression of pituitary adenylate cyclase-activating polypeptide mRNA controlled by calcium and cAMP signals in neurons. J Biol Chem. 2004;279:47856–47865. doi: 10.1074/jbc.M409090200. [DOI] [PubMed] [Google Scholar]
- 32.Dutlow CM, Rachman J, Jacobs TW, Millar RP. Prepubertal increases in gonadotropin-releasing hormone mRNA, gonadotropin-releasing hormone precursor, and subsequent maturation of precursor processing in male rats. J Clin Invest. 1992;90:2496–2501. doi: 10.1172/JCI116142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris GC, Levine JE. Pubertal acceleration of pulsatile gonadotropin-releasing hormone release in male rats as revealed by microdialysis. Endocrinology. 2003;144:163–171. doi: 10.1210/en.2002-220767. [DOI] [PubMed] [Google Scholar]
- 34.Meachem SJ, McLachlan RI, de Kretser DM, Robertson DM, Wreford NG. Neonatal exposure of rats to recombinant follicle stimulating hormone increases adult Sertoli and spermatogenic cell numbers. Biol Reprod. 1996;54:36–44. doi: 10.1095/biolreprod54.1.36. [DOI] [PubMed] [Google Scholar]
- 35.Kula K, Walczak-Jedrzejowska R, Slowikowska-Hilczer J, Oszukowska E. Estradiol enhances the stimulatory effect of FSH on testicular maturation and contributes to precocious initiation of spermatogenesis. Mol Cell Endocrinol. 2001;178:89–97. doi: 10.1016/s0303-7207(01)00415-4. [DOI] [PubMed] [Google Scholar]
- 36.Aubert ML, Pierroz DD, Gruaz NM, d'Alleves V, Vuagnat BA, Pralong FP, Blum WF, Sizonenko PC. Metabolic control of sexual function and growth: role of neuropeptide Y and leptin. Mol Cell Endocrinol. 1998;140:107–113. doi: 10.1016/s0303-7207(98)00058-6. [DOI] [PubMed] [Google Scholar]
- 37.Mounzih K, Lu R, Chehab FF. Leptin treatment rescues the sterility of genetically obese ob/ob males. Endocrinology. 1997;138:1190–1193. doi: 10.1210/endo.138.3.5024. [DOI] [PubMed] [Google Scholar]
- 38.Johnson LM, Sidman RL. A reproductive endocrine profile in the diabetes (db) mutant mouse. Biol Reprod. 1979;20:552–559. doi: 10.1095/biolreprod20.3.552. [DOI] [PubMed] [Google Scholar]
- 39.Watanabe H, Schioth HB. Postnatal profile of plasma leptin concentrations in male and female rats: relation with the maturation of the pituitary-gonadal axis. Regul Pept. 2002;105:23–28. doi: 10.1016/s0167-0115(01)00370-6. [DOI] [PubMed] [Google Scholar]
- 40.Smith JT, Waddell BJ. Developmental changes in plasma leptin and hypothalamic leptin receptor expression in the rat: peripubertal changes and the emergence of sex differences. J Endocrinol. 2003;176:313–319. doi: 10.1677/joe.0.1760313. [DOI] [PubMed] [Google Scholar]
- 41.Cheung CC, Thornton JE, Nurani SD, Clifton DK, Steiner RA. A reassessment of leptin's role in triggering the onset of puberty in the rat and mouse. Neuroendocrinology. 2001;74:12–21. doi: 10.1159/000054666. [DOI] [PubMed] [Google Scholar]
- 42.Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 43.Leonhardt M, Lesage J, Croix D, Dutriez-Casteloot I, Beauvillain JC, Dupouy JP. Effects of perinatal maternal food restriction on pituitary-gonadal axis and plasma leptin level in rat pup at birth and weaning and on timing of puberty. Biol Reprod. 2003;68:390–400. doi: 10.1095/biolreprod.102.003269. [DOI] [PubMed] [Google Scholar]



