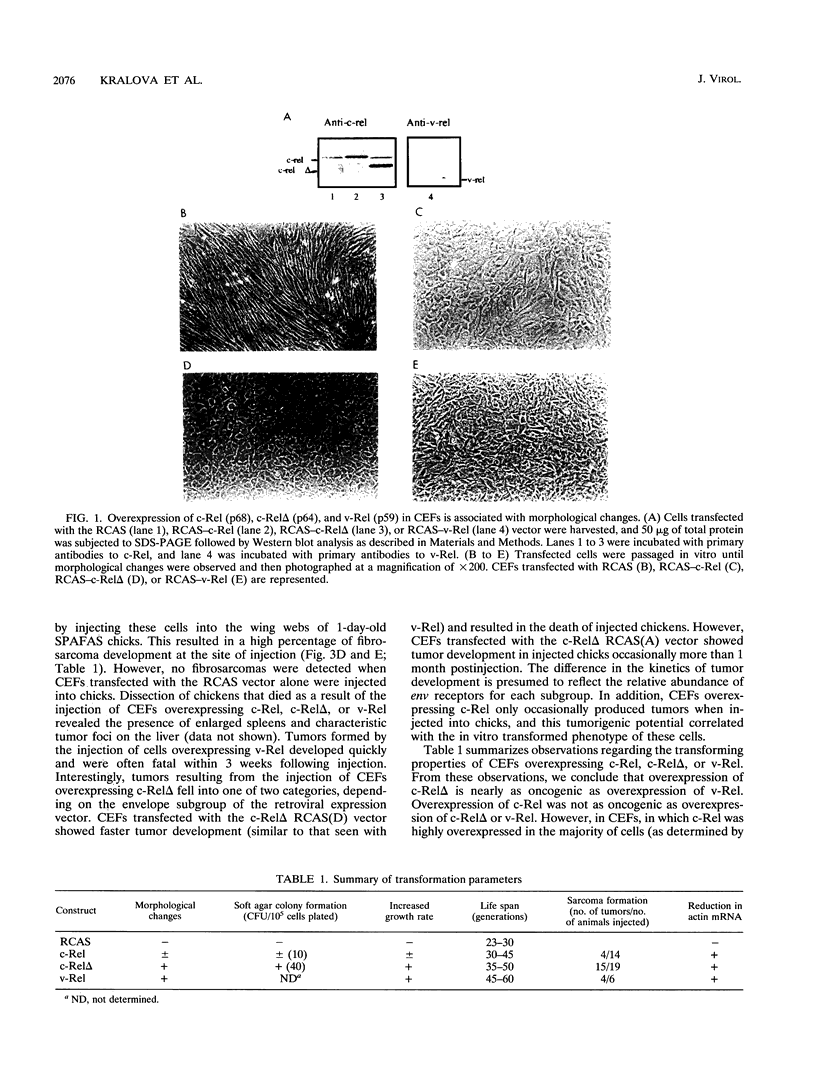
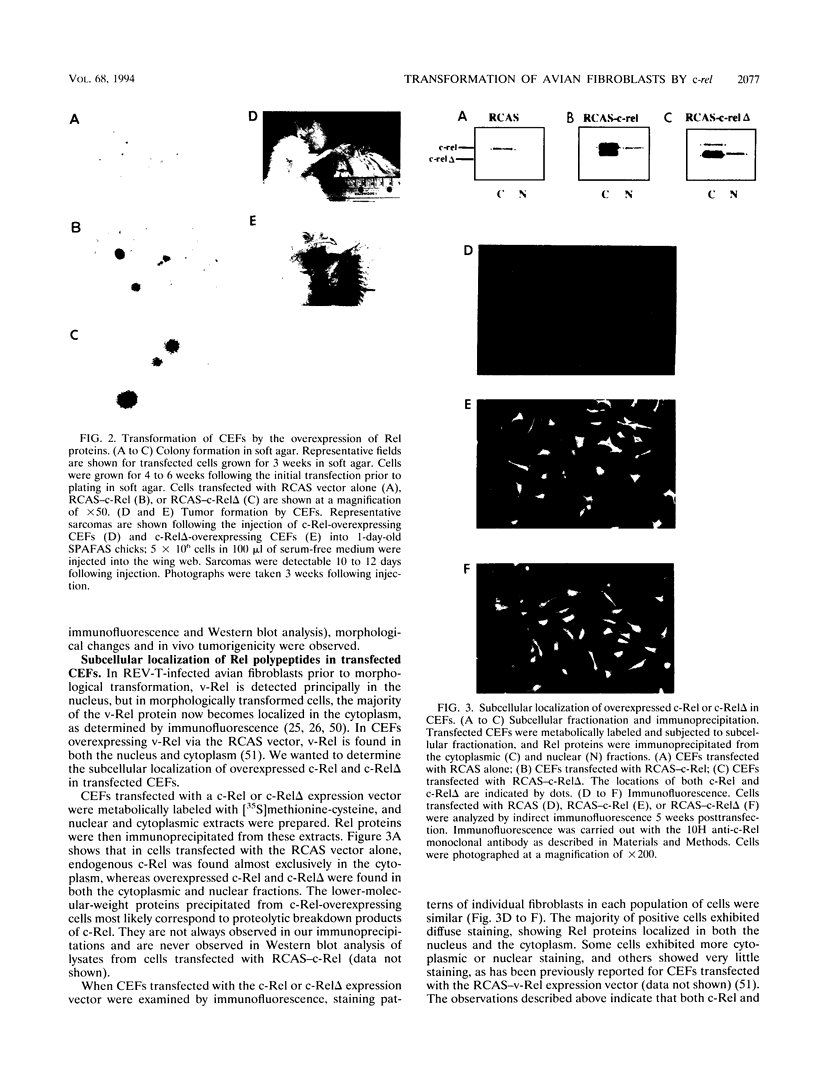
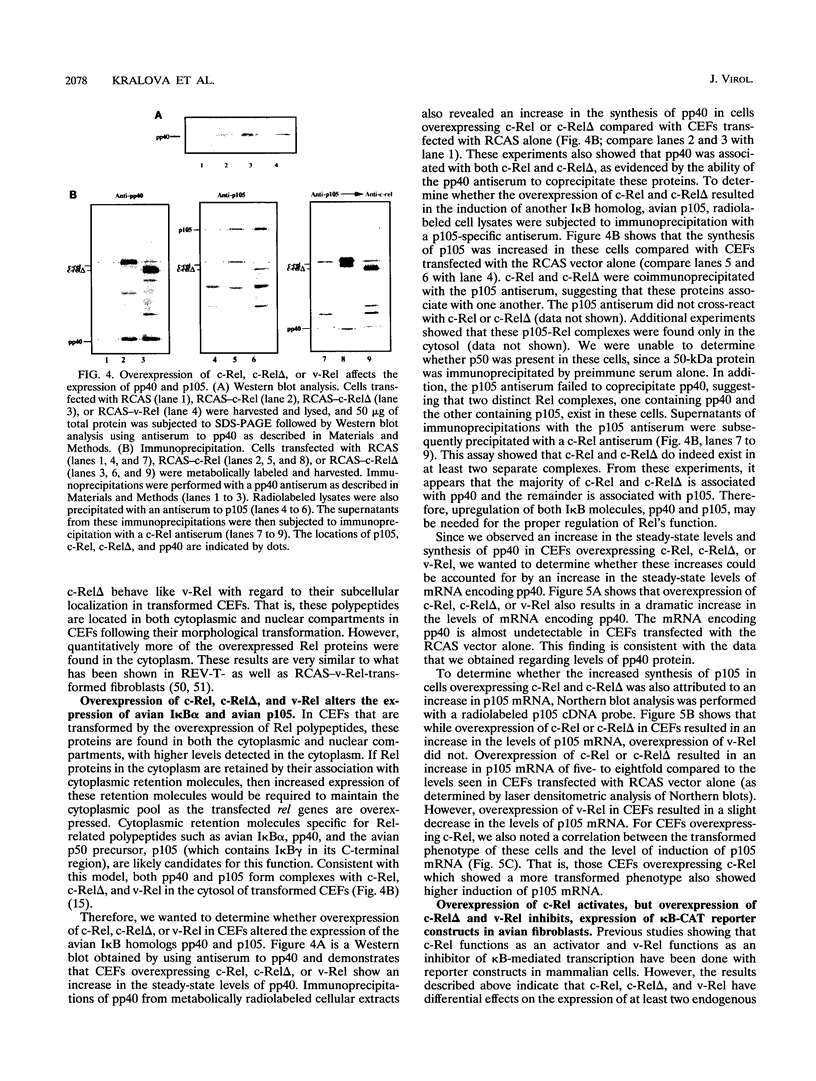
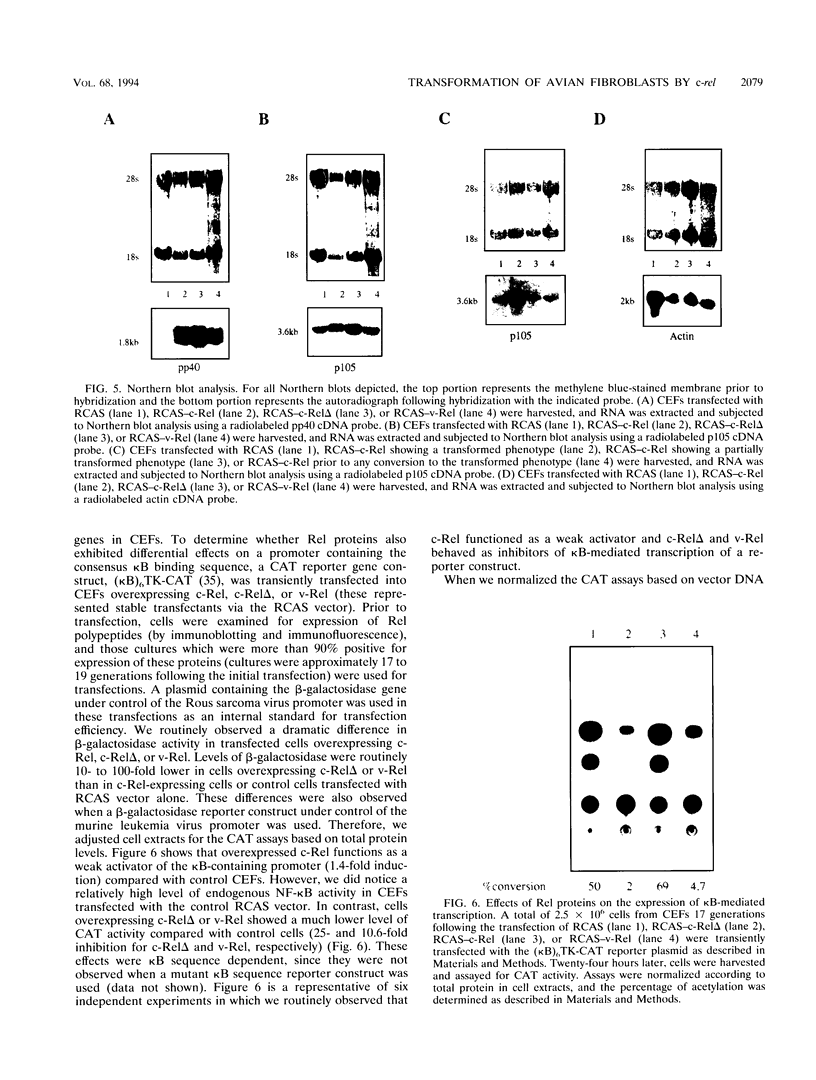
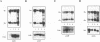Abstract
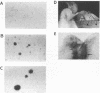
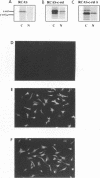
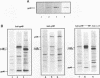
The v-rel oncogene was derived from the c-rel proto-oncogene, which encodes a transcriptional activator. Expression of v-rel transforms avian hematopoietic cells and fibroblasts. Here we report that overexpression (via a replication-competent retroviral vector) of full-length c-Rel as well as a 40-amino-acid, carboxy-terminal deletion construct of c-Rel (c-Rel delta) resulted in the morphological transformation of chicken embryo fibroblasts (CEFs). Subcellular localization of Rel polypeptides in these transformed cells as determined by immunofluorescence and immunoprecipitation revealed their presence in both the nucleus and the cytoplasm, with the majority of Rel polypeptides showing cytoplasmic localization. Cytoplasmic localization could be due to interaction with I kappa B molecules, and in fact, the overexpression of c-Rel or the C-terminal deletion construct of c-Rel resulted in an increase in the levels of mRNA encoding the avian I kappa B protein pp40 and the avian homolog of the NF-kappa B protein, p105. However, expression of v-Rel resulted in the induction of pp40 mRNA only. While c-Rel was a weak activator of kappa B-mediated transcription of a reporter construct in transformed CEFs, v-Rel and c-Rel delta were transcriptional repressors. However, in spite of these differences, all of these proteins resulted in the transformation of CEFs.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A. The inducible transcription activator NF-kappa B: regulation by distinct protein subunits. Biochim Biophys Acta. 1991 Apr 16;1072(1):63–80. doi: 10.1016/0304-419x(91)90007-8. [DOI] [PubMed] [Google Scholar]
- Ballard D. W., Walker W. H., Doerre S., Sista P., Molitor J. A., Dixon E. P., Peffer N. J., Hannink M., Greene W. C. The v-rel oncogene encodes a kappa B enhancer binding protein that inhibits NF-kappa B function. Cell. 1990 Nov 16;63(4):803–814. doi: 10.1016/0092-8674(90)90146-6. [DOI] [PubMed] [Google Scholar]
- Beg A. A., Ruben S. M., Scheinman R. I., Haskill S., Rosen C. A., Baldwin A. S., Jr I kappa B interacts with the nuclear localization sequences of the subunits of NF-kappa B: a mechanism for cytoplasmic retention. Genes Dev. 1992 Oct;6(10):1899–1913. doi: 10.1101/gad.6.10.1899. [DOI] [PubMed] [Google Scholar]
- Boehmelt G., Walker A., Kabrun N., Mellitzer G., Beug H., Zenke M., Enrietto P. J. Hormone-regulated v-rel estrogen receptor fusion protein: reversible induction of cell transformation and cellular gene expression. EMBO J. 1992 Dec;11(12):4641–4652. doi: 10.1002/j.1460-2075.1992.tb05566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose H. R., Jr The Rel family: models for transcriptional regulation and oncogenic transformation. Biochim Biophys Acta. 1992 Sep 14;1114(1):1–17. doi: 10.1016/0304-419x(92)90002-g. [DOI] [PubMed] [Google Scholar]
- Bours V., Franzoso G., Azarenko V., Park S., Kanno T., Brown K., Siebenlist U. The oncoprotein Bcl-3 directly transactivates through kappa B motifs via association with DNA-binding p50B homodimers. Cell. 1993 Mar 12;72(5):729–739. doi: 10.1016/0092-8674(93)90401-b. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bull P., Morley K. L., Hoekstra M. F., Hunter T., Verma I. M. The mouse c-rel protein has an N-terminal regulatory domain and a C-terminal transcriptional transactivation domain. Mol Cell Biol. 1990 Oct;10(10):5473–5485. doi: 10.1128/mcb.10.10.5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capobianco A. J., Chang D., Mosialos G., Gilmore T. D. p105, the NF-kappa B p50 precursor protein, is one of the cellular proteins complexed with the v-Rel oncoprotein in transformed chicken spleen cells. J Virol. 1992 Jun;66(6):3758–3767. doi: 10.1128/jvi.66.6.3758-3767.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capobianco A. J., Gilmore T. D. A conditional mutant of vRel containing sequences from the human estrogen receptor. Virology. 1993 Mar;193(1):160–170. doi: 10.1006/viro.1993.1112. [DOI] [PubMed] [Google Scholar]
- Castellazzi M., Dangy J. P., Mechta F., Hirai S., Yaniv M., Samarut J., Lassailly A., Brun G. Overexpression of avian or mouse c-jun in primary chick embryo fibroblasts confers a partially transformed phenotype. Oncogene. 1990 Oct;5(10):1541–1547. [PubMed] [Google Scholar]
- Chen I. S., Mak T. W., O'Rear J. J., Temin H. M. Characterization of reticuloendotheliosis virus strain T DNA and isolation of a novel variant of reticuloendotheliosis virus strain T by molecular cloning. J Virol. 1981 Dec;40(3):800–811. doi: 10.1128/jvi.40.3.800-811.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N., Bargmann W., Lim M. Y., Bose H., Jr Avian reticuloendotheliosis virus-transformed lymphoid cells contain multiple pp59v-rel complexes. J Virol. 1990 Feb;64(2):584–591. doi: 10.1128/jvi.64.2.584-591.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N., Ghosh S., Simmons D. L., Tempst P., Liou H. C., Baltimore D., Bose H. R., Jr Rel-associated pp40: an inhibitor of the rel family of transcription factors. Science. 1991 Sep 13;253(5025):1268–1271. doi: 10.1126/science.1891714. [DOI] [PubMed] [Google Scholar]
- Diehl J. A., McKinsey T. A., Hannink M. Differential pp40I kappa B-beta inhibition of DNA binding by rel proteins. Mol Cell Biol. 1993 Mar;13(3):1769–1778. doi: 10.1128/mcb.13.3.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckett C. S., Perkins N. D., Kowalik T. F., Schmid R. M., Huang E. S., Baldwin A. S., Jr, Nabel G. J. Dimerization of NF-KB2 with RelA(p65) regulates DNA binding, transcriptional activation, and inhibition by an I kappa B-alpha (MAD-3). Mol Cell Biol. 1993 Mar;13(3):1315–1322. doi: 10.1128/mcb.13.3.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. B., Gottlieb P. D., Bose H. R., Jr Identification of a rel-related protein in the nucleus during the S phase of the cell cycle. Mol Cell Biol. 1993 Oct;13(10):6147–6156. doi: 10.1128/mcb.13.10.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganchi P. A., Sun S. C., Greene W. C., Ballard D. W. I kappa B/MAD-3 masks the nuclear localization signal of NF-kappa B p65 and requires the transactivation domain to inhibit NF-kappa B p65 DNA binding. Mol Biol Cell. 1992 Dec;3(12):1339–1352. doi: 10.1091/mbc.3.12.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., Gifford A. M., Riviere L. R., Tempst P., Nolan G. P., Baltimore D. Cloning of the p50 DNA binding subunit of NF-kappa B: homology to rel and dorsal. Cell. 1990 Sep 7;62(5):1019–1029. doi: 10.1016/0092-8674(90)90276-k. [DOI] [PubMed] [Google Scholar]
- Gilmore T. D. Malignant transformation by mutant Rel proteins. Trends Genet. 1991 Oct;7(10):318–322. doi: 10.1016/0168-9525(91)90421-l. [DOI] [PubMed] [Google Scholar]
- Gilmore T. D. NF-kappa B, KBF1, dorsal, and related matters. Cell. 1990 Sep 7;62(5):841–843. doi: 10.1016/0092-8674(90)90257-f. [DOI] [PubMed] [Google Scholar]
- Gilmore T. D., Temin H. M. Different localization of the product of the v-rel oncogene in chicken fibroblasts and spleen cells correlates with transformation by REV-T. Cell. 1986 Mar 14;44(5):791–800. doi: 10.1016/0092-8674(86)90845-7. [DOI] [PubMed] [Google Scholar]
- Gilmore T. D., Temin H. M. v-rel oncoproteins in the nucleus and in the cytoplasm transform chicken spleen cells. J Virol. 1988 Mar;62(3):703–714. doi: 10.1128/jvi.62.3.703-714.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hannink M., Temin H. M. Transactivation of gene expression by nuclear and cytoplasmic rel proteins. Mol Cell Biol. 1989 Oct;9(10):4323–4336. doi: 10.1128/mcb.9.10.4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen S. K., Nerlov C., Zabel U., Verde P., Johnsen M., Baeuerle P. A., Blasi F. A novel complex between the p65 subunit of NF-kappa B and c-Rel binds to a DNA element involved in the phorbol ester induction of the human urokinase gene. EMBO J. 1992 Jan;11(1):205–213. doi: 10.1002/j.1460-2075.1992.tb05043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskill S., Beg A. A., Tompkins S. M., Morris J. S., Yurochko A. D., Sampson-Johannes A., Mondal K., Ralph P., Baldwin A. S., Jr Characterization of an immediate-early gene induced in adherent monocytes that encodes I kappa B-like activity. Cell. 1991 Jun 28;65(7):1281–1289. doi: 10.1016/0092-8674(91)90022-q. [DOI] [PubMed] [Google Scholar]
- Hatada E. N., Nieters A., Wulczyn F. G., Naumann M., Meyer R., Nucifora G., McKeithan T. W., Scheidereit C. The ankyrin repeat domains of the NF-kappa B precursor p105 and the protooncogene bcl-3 act as specific inhibitors of NF-kappa B DNA binding. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2489–2493. doi: 10.1073/pnas.89.6.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel T., Zabel U., van Zee K., Müller J. M., Fanning E., Baeuerle P. A. Intramolecular masking of the nuclear location signal and dimerization domain in the precursor for the p50 NF-kappa B subunit. Cell. 1992 Mar 20;68(6):1121–1133. doi: 10.1016/0092-8674(92)90083-o. [DOI] [PubMed] [Google Scholar]
- Herrin D. L., Schmidt G. W. Rapid, reversible staining of northern blots prior to hybridization. Biotechniques. 1988 Mar;6(3):196-7, 199-200. [PubMed] [Google Scholar]
- Hughes S. H., Greenhouse J. J., Petropoulos C. J., Sutrave P. Adaptor plasmids simplify the insertion of foreign DNA into helper-independent retroviral vectors. J Virol. 1987 Oct;61(10):3004–3012. doi: 10.1128/jvi.61.10.3004-3012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue J., Kerr L. D., Ransone L. J., Bengal E., Hunter T., Verma I. M. c-rel activates but v-rel suppresses transcription from kappa B sites. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3715–3719. doi: 10.1073/pnas.88.9.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue J., Kerr L. D., Rashid D., Davis N., Bose H. R., Jr, Verma I. M. Direct association of pp40/I kappa B beta with rel/NF-kappa B transcription factors: role of ankyrin repeats in the inhibition of DNA binding activity. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4333–4337. doi: 10.1073/pnas.89.10.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip Y. T., Kraut R., Levine M., Rushlow C. A. The dorsal morphogen is a sequence-specific DNA-binding protein that interacts with a long-range repression element in Drosophila. Cell. 1991 Jan 25;64(2):439–446. doi: 10.1016/0092-8674(91)90651-e. [DOI] [PubMed] [Google Scholar]
- Kamens J., Richardson P., Mosialos G., Brent R., Gilmore T. Oncogenic transformation by vrel requires an amino-terminal activation domain. Mol Cell Biol. 1990 Jun;10(6):2840–2847. doi: 10.1128/mcb.10.6.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S. M., Tran A. C., Grilli M., Lenardo M. J. NF-kappa B subunit regulation in nontransformed CD4+ T lymphocytes. Science. 1992 Jun 5;256(5062):1452–1456. doi: 10.1126/science.1604322. [DOI] [PubMed] [Google Scholar]
- Kao K. R., Hopwood N. D. Expression of a mRNA related to c-rel and dorsal in early Xenopus laevis embryos. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2697–2701. doi: 10.1073/pnas.88.7.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr L. D., Duckett C. S., Wamsley P., Zhang Q., Chiao P., Nabel G., McKeithan T. W., Baeuerle P. A., Verma I. M. The proto-oncogene bcl-3 encodes an I kappa B protein. Genes Dev. 1992 Dec;6(12A):2352–2363. doi: 10.1101/gad.6.12a.2352. [DOI] [PubMed] [Google Scholar]
- Kerr L. D., Inoue J., Davis N., Link E., Baeuerle P. A., Bose H. R., Jr, Verma I. M. The rel-associated pp40 protein prevents DNA binding of Rel and NF-kappa B: relationship with I kappa B beta and regulation by phosphorylation. Genes Dev. 1991 Aug;5(8):1464–1476. doi: 10.1101/gad.5.8.1464. [DOI] [PubMed] [Google Scholar]
- Kieran M., Blank V., Logeat F., Vandekerckhove J., Lottspeich F., Le Bail O., Urban M. B., Kourilsky P., Baeuerle P. A., Israël A. The DNA binding subunit of NF-kappa B is identical to factor KBF1 and homologous to the rel oncogene product. Cell. 1990 Sep 7;62(5):1007–1018. doi: 10.1016/0092-8674(90)90275-j. [DOI] [PubMed] [Google Scholar]
- Kumar S., Rabson A. B., Gélinas C. The RxxRxRxxC motif conserved in all Rel/kappa B proteins is essential for the DNA-binding activity and redox regulation of the v-Rel oncoprotein. Mol Cell Biol. 1992 Jul;12(7):3094–3106. doi: 10.1128/mcb.12.7.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenardo M. J., Baltimore D. NF-kappa B: a pleiotropic mediator of inducible and tissue-specific gene control. Cell. 1989 Jul 28;58(2):227–229. doi: 10.1016/0092-8674(89)90833-7. [DOI] [PubMed] [Google Scholar]
- Lim M. Y., Davis N., Zhang J. Y., Bose H. R., Jr The v-rel oncogene product is complexed with cellular proteins including its proto-oncogene product and heat shock protein 70. Virology. 1990 Mar;175(1):149–160. doi: 10.1016/0042-6822(90)90195-w. [DOI] [PubMed] [Google Scholar]
- Liou H. C., Nolan G. P., Ghosh S., Fujita T., Baltimore D. The NF-kappa B p50 precursor, p105, contains an internal I kappa B-like inhibitor that preferentially inhibits p50. EMBO J. 1992 Aug;11(8):3003–3009. doi: 10.1002/j.1460-2075.1992.tb05370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell P. C., Kumar S., Rabson A. B., Gélinas C. Transcriptional activity of rel family proteins. Oncogene. 1992 Jan;7(1):163–170. [PubMed] [Google Scholar]
- Mercurio F., DiDonato J. A., Rosette C., Karin M. p105 and p98 precursor proteins play an active role in NF-kappa B-mediated signal transduction. Genes Dev. 1993 Apr;7(4):705–718. doi: 10.1101/gad.7.4.705. [DOI] [PubMed] [Google Scholar]
- Moore B. E., Bose H. R., Jr Expression of the v-rel oncogene in reticuloendotheliosis virus-transformed fibroblasts. Virology. 1988 Feb;162(2):377–387. doi: 10.1016/0042-6822(88)90478-3. [DOI] [PubMed] [Google Scholar]
- Morrison L. E., Boehmelt G., Beug H., Enrietto P. J. Expression of v-rel in a replication competent virus: transformation and biochemical characterization. Oncogene. 1991 Sep;6(9):1657–1666. [PubMed] [Google Scholar]
- Morrison L. E., Kabrun N., Mudri S., Hayman M. J., Enrietto P. J. Viral rel and cellular rel associate with cellular proteins in transformed and normal cells. Oncogene. 1989 Jun;4(6):677–683. [PubMed] [Google Scholar]
- Mosialos G., Hamer P., Capobianco A. J., Laursen R. A., Gilmore T. D. A protein kinase-A recognition sequence is structurally linked to transformation by p59v-rel and cytoplasmic retention of p68c-rel. Mol Cell Biol. 1991 Dec;11(12):5867–5877. doi: 10.1128/mcb.11.12.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumann M., Wulczyn F. G., Scheidereit C. The NF-kappa B precursor p105 and the proto-oncogene product Bcl-3 are I kappa B molecules and control nuclear translocation of NF-kappa B. EMBO J. 1993 Jan;12(1):213–222. doi: 10.1002/j.1460-2075.1993.tb05647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M., Tsapos K., Scheppler J. A., Ross J., Franza B. R., Jr Identification of complex formation between two intracellular tyrosine kinase substrates: human c-Rel and the p105 precursor of p50 NF-kappa B. Oncogene. 1992 Nov;7(11):2095–2104. [PubMed] [Google Scholar]
- Nolan G. P., Ghosh S., Liou H. C., Tempst P., Baltimore D. DNA binding and I kappa B inhibition of the cloned p65 subunit of NF-kappa B, a rel-related polypeptide. Cell. 1991 Mar 8;64(5):961–969. doi: 10.1016/0092-8674(91)90320-x. [DOI] [PubMed] [Google Scholar]
- Rice N. R., MacKichan M. L., Israël A. The precursor of NF-kappa B p50 has I kappa B-like functions. Cell. 1992 Oct 16;71(2):243–253. doi: 10.1016/0092-8674(92)90353-e. [DOI] [PubMed] [Google Scholar]
- Richardson P. M., Gilmore T. D. vRel is an inactive member of the Rel family of transcriptional activating proteins. J Virol. 1991 Jun;65(6):3122–3130. doi: 10.1128/jvi.65.6.3122-3130.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer-Pokora B., Beug H., Claviez M., Winkhardt H. J., Friis R. R., Graf T. Transformation parameters in chicken fibroblasts transformed by AEV and MC29 avian leukemia viruses. Cell. 1978 Apr;13(4):751–760. doi: 10.1016/0092-8674(78)90225-8. [DOI] [PubMed] [Google Scholar]
- Rushlow C. A., Han K., Manley J. L., Levine M. The graded distribution of the dorsal morphogen is initiated by selective nuclear transport in Drosophila. Cell. 1989 Dec 22;59(6):1165–1177. doi: 10.1016/0092-8674(89)90772-1. [DOI] [PubMed] [Google Scholar]
- Ryseck R. P., Bull P., Takamiya M., Bours V., Siebenlist U., Dobrzanski P., Bravo R. RelB, a new Rel family transcription activator that can interact with p50-NF-kappa B. Mol Cell Biol. 1992 Feb;12(2):674–684. doi: 10.1128/mcb.12.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S., Gilmore T. D. Transformation by the vRel oncoprotein requires sequences carboxy-terminal to the Rel homology domain. Oncogene. 1993 Aug;8(8):2245–2252. [PubMed] [Google Scholar]
- Schatzle J., Rathi A., Clarke M., Cardelli J. A. Developmental regulation of the alpha-mannosidase gene in Dictyostelium discoideum: control is at the level of transcription and is affected by cell density. Mol Cell Biol. 1991 Jun;11(6):3339–3347. doi: 10.1128/mcb.11.6.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid R. M., Perkins N. D., Duckett C. S., Andrews P. C., Nabel G. J. Cloning of an NF-kappa B subunit which stimulates HIV transcription in synergy with p65. Nature. 1991 Aug 22;352(6337):733–736. doi: 10.1038/352733a0. [DOI] [PubMed] [Google Scholar]
- Sif S., Capobianco A. J., Gilmore T. D. The v-Rel oncoprotein increases expression from Sp1 site-containing promoters in chicken embryo fibroblasts. Oncogene. 1993 Sep;8(9):2501–2509. [PubMed] [Google Scholar]
- Simek S., Rice N. R. p59v-rel, the transforming protein of reticuloendotheliosis virus, is complexed with at least four other proteins in transformed chicken lymphoid cells. J Virol. 1988 Dec;62(12):4730–4736. doi: 10.1128/jvi.62.12.4730-4736.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. M., Rice N. R., Hiebsch R. R., Bose H. R., Jr, Gilden R. V. Nucleotide sequence of v-rel: the oncogene of reticuloendotheliosis virus. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6229–6233. doi: 10.1073/pnas.80.20.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward R. Dorsal, an embryonic polarity gene in Drosophila, is homologous to the vertebrate proto-oncogene, c-rel. Science. 1987 Oct 30;238(4827):692–694. doi: 10.1126/science.3118464. [DOI] [PubMed] [Google Scholar]
- Steward R. Relocalization of the dorsal protein from the cytoplasm to the nucleus correlates with its function. Cell. 1989 Dec 22;59(6):1179–1188. doi: 10.1016/0092-8674(89)90773-3. [DOI] [PubMed] [Google Scholar]
- Sun S. C., Ganchi P. A., Ballard D. W., Greene W. C. NF-kappa B controls expression of inhibitor I kappa B alpha: evidence for an inducible autoregulatory pathway. Science. 1993 Mar 26;259(5103):1912–1915. doi: 10.1126/science.8096091. [DOI] [PubMed] [Google Scholar]
- Ten R. M., Paya C. V., Israël N., Le Bail O., Mattei M. G., Virelizier J. L., Kourilsky P., Israël A. The characterization of the promoter of the gene encoding the p50 subunit of NF-kappa B indicates that it participates in its own regulation. EMBO J. 1992 Jan;11(1):195–203. doi: 10.1002/j.1460-2075.1992.tb05042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thisse C., Perrin-Schmitt F., Stoetzel C., Thisse B. Sequence-specific transactivation of the Drosophila twist gene by the dorsal gene product. Cell. 1991 Jun 28;65(7):1191–1201. doi: 10.1016/0092-8674(91)90014-p. [DOI] [PubMed] [Google Scholar]
- Walker W. H., Stein B., Ganchi P. A., Hoffman J. A., Kaufman P. A., Ballard D. W., Hannink M., Greene W. C. The v-rel oncogene: insights into the mechanism of transcriptional activation, repression, and transformation. J Virol. 1992 Aug;66(8):5018–5029. doi: 10.1128/jvi.66.8.5018-5029.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walro D. S., Herzog N. K., Zhang J., Lim M. Y., Bose H. R., Jr The transforming protein of avian reticuloendotheliosis virus is a soluble cytoplasmic protein which is associated with a protein kinase activity. Virology. 1987 Oct;160(2):433–444. doi: 10.1016/0042-6822(87)90015-8. [DOI] [PubMed] [Google Scholar]
- Wilhelmsen K. C., Eggleton K., Temin H. M. Nucleic acid sequences of the oncogene v-rel in reticuloendotheliosis virus strain T and its cellular homolog, the proto-oncogene c-rel. J Virol. 1984 Oct;52(1):172–182. doi: 10.1128/jvi.52.1.172-182.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Zhou H., Duesberg P. Unmutated proto-src coding region is tumorigenic if expressed from the promoter of Rous sarcoma virus: implications for the gene-mutation hypothesis of cancer. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6393–6397. doi: 10.1073/pnas.89.14.6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel U., Henkel T., Silva M. S., Baeuerle P. A. Nuclear uptake control of NF-kappa B by MAD-3, an I kappa B protein present in the nucleus. EMBO J. 1993 Jan;12(1):201–211. doi: 10.1002/j.1460-2075.1993.tb05646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. Y., Olson W., Ewert D., Bargmann W., Bose H. R., Jr The v-rel oncogene of avian reticuloendotheliosis virus transforms immature and mature lymphoid cells of the B cell lineage in vitro. Virology. 1991 Aug;183(2):457–466. doi: 10.1016/0042-6822(91)90975-h. [DOI] [PubMed] [Google Scholar]
- de Martin R., Vanhove B., Cheng Q., Hofer E., Csizmadia V., Winkler H., Bach F. H. Cytokine-inducible expression in endothelial cells of an I kappa B alpha-like gene is regulated by NF kappa B. EMBO J. 1993 Jul;12(7):2773–2779. doi: 10.1002/j.1460-2075.1993.tb05938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]