Abstract
Purpose
As deficits in memory and cognition are commonly observed in survivors of traumatic brain injury (TBI), causing reduced quality of life for the patient, a major goal in experimental TBI research is to identify and evaluate cognitive dysfunction. The present study assessed the applicability of the serial Morris water maze (MWM) test to determine cognitive function following experimental TBI in the same group of rats which is particularly important for long-term studies and increasingly valuable for the evaluation of novel treatment strategies.
Methods
Male Sprague-Dawley rats (n = 27) were anesthetized and subjected to either sham injury (n = 9) or lateral fluid percussion (FP) brain injury of moderate severity (n = 18). At 4 weeks post-injury, animals were trained in a water maze over 3 days (acquisition/learning phase) to find a submerged platform. At 8 weeks post-injury the hidden platform was then moved to the opposite quadrant, and animals were trained to find the new position of the platform over 3 days. Forty-eight hours later, animals were tested for memory retention in a probe trial in which the platform was not present.
Results
Brain-injured animals had significant learning impairment (p < 0.0001), shifted-learning impairment (p < 0.001) and memory retention deficits (p < 0.01) in comparison to their sham-injured counterparts over the 8 week testing period. Swim speed and distance were not significantly altered by brain injury at any time point.
Conclusion
The validation of this testing paradigm using a clinically relevant experimental brain injury model is an important addition to behavioral outcome testing.
Keywords: Head injury, water maze, shift learning, reversal learning, multiple time points
1. Introduction
Following severe traumatic brain injury (TBI), the most common cognitive impairment seen clinically is memory dysfunction, including the ability to form and retain new memories [17]. Post-traumatic amnesia following TBI in humans is a selective memory deficit which may be anterograde (impaired memory after the TBI) or retrograde (impaired memory of events that happened prior to the TBI). The Morris water maze (MWM) has been frequently used as a tool to evaluate the effects of brain injury on either memory (retrograde amnesia) or learning (anterograde amnesia) in experimental models of brain injury at single points in time as it has conceptual equivalence ([16]; for reviews see [10,11,24]). Animals are usually trained to either the memory or the learning paradigm, and not tested across multiple time points. However, several authors have identified the need for pre-clinical studies with long-term evaluation of cognitive function at multiple time points to improve translation of experimental findings to the clinical situation [4,24]. The validation of such a test in brain-injured animals would allow for longer evaluation of cognitive functioning in these animals as well as reducing the number of animals required for long-term studies using multiple time points and repetitive testing of cognitive evaluation.
The shift learning and reversal learning tests have previously been used in the MWM to assess cognitive ability and were adapted for use in this study. The shift learning paradigm has been utilized in both atropine-treated [8] and hippocampal-lesioned rats [9], showing impaired ability to utilize more efficient learning strategies when faced with a new learning task in the MWM. In this paradigm, the animal is first trained to learn the position of a hidden submerged platform, superficially similar to the learning task paradigm currently used in various studies following experimental TBI [5,20,21]. In the shift learning paradigm, however, the platform is of varying size, thigmotaxis (swimming exclusively in the periphery of the pool characterized by “hugging the wall”) is prevented in initial trials, and in the last trial, the platform is shifted diagonally across from the old location in a probe trial to determine the animal's ability to effectively switch search strategies [8,9].
The MWM “reversal test” has been successfully used in rats to evaluate the impact of various factors on memory and learning [2,6–8,18]. The reversal test involves several steps: the first is an acquisition phase in the MWM similar to the first phase of shift learning. The acquisition phase may be followed by a memory probe test with the platform removed, to assess place location ability in the rat [2,6,7,18]. The memory test currently used in experimental TBI research involves training prior to the injury, with a probe trial at 24−48 hours post-injury, used as an isolated test for cognitive function [5,11]. The last phase of this test is “reversal” learning, where the position of the hidden platform is moved diagonally from its former position, and the rat learns the new position over a period of several days [2, 6,18]. A memory probe may again follow the reversal learning phase, as an additional testing option [2,6,7,18]. The interval between the initial phase/probe and reversal phase has varied widely between these studies, however despite differences; the ability to detect cognitive disturbances has been reported with both short and long intervals between learning phases [7,18]. An important component of reversal learning is that both control and experimental groups meet set criteria for learning before further testing (either probe or reversal learning) is initiated. In the present report, we evaluated whether serial testing in the MWM with an adapted version of two established paradigms (shift and reversal) could be of use for evaluating cognitive deficits in traumatically brain-injured rats.
2. Materials and methods
All procedures used in the experiments were approved by the University of Pennsylvania's Animal Use and Care Committee and conducted in accordance with National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80−23, 1996). Rats (n = 27) were maintained in the colony under standard laboratory conditions (lights on 0600/off 1800) housed in pairs and had access to food and water ad libitum. Following anesthesia with sodium pento-barbital (65 mg/kg intraperitoneally, i.p.), adult male Sprague-Dawley rats (350−400 g, Harlan, Indianapolis, In, n = 18) underwent craniectomy and were subjected to lateral FP brain injury of moderate severity (2.4−2.7 atm) as previously described in detail [15]. Normothermia was maintained throughout all procedures and for 120 minutes following injury. Surviving animals (n = 15) were returned to home cages following recovery from anesthesia. Sham-injured rats (n = 9) were anesthetized and surgically prepared in the same manner but did not receive lateral FP brain injury. Animal numbers were determined a priori based upon previous experience with the MWM and mindful of animal welfare concerns frequently encountered with studies involving long-term behavioral evaluation in brain-injured animals.
An experienced investigator, blinded to injury status of each animal, carried out the behavioral testing of the animals at the same time each day. The maze consists of a circular pool (1.83 m diameter, 0.6 m depth) with black interior filled with water maintained at a temperature of 22−24°C. A clear Plexiglas escape platform (10 cm diameter) was placed 1 cm below the surface of the water in the learning phases,rendering the platform invisible to the animal. The testing room had several prominent black and white cues placed visibly on the surrounding walls which remained consistent throughout the testing period. A computerized tracking system (Accutrak®, San Diego, CA) was used to track and record animal movement and swimming pattern in the maze for all tests. Average swim speed (swim distance/time in maze) was calculated for each trial.
Four weeks following experimental TBI, surviving animals underwent three days of testing, starting on post-injury day 28. Testing consisted of 2 blocks each day of 4 trials (8 total trials per day) over a 3-day interval during which external visual cues were used to find the submerged platform. Learning trials began by placing animals into the maze and releasing them at one of four sites separated by 90° around the periphery of the maze. Animals were allowed up to 60 seconds per trial to locate the hidden platform, after which time they were guided to the platform. Rats were allowed to remain on the platform for 15 seconds and were returned to a warm, opaque drying cage between trials. Training blocks were kept constant time-wise for each animal and the initial release site was consistent for each training block. The animal's latency to reach the platform was recorded for each of the 24 trials and the average for each day was calculated as a measure of post-injury learning.
Eight weeks post-injury, the platform position was moved from its previous location (NE quadrant) to a new location (SW quadrant), and animals were reintroduced to the same 1.83-meter maze. Learning trials were conducted in the same manner over days 56−58 post-injury, as animals were released at one of four sites separated by 90° around the periphery of the maze and latency was recorded for up to 60 seconds for each of the 24 trials. Forty-eight hours following the last learning trial (day 60), animals were placed in the maze for two probe trials (60 s each) where the platform was removed. The time spent in each zone and the latency to pass through the target zone was recorded and was used to generate the memory score as previously described [23]. The zones are mathematically weighted based on the proximity to the platform. The memory score for each animal is determined by multiplying the time spent in each zone by the weighted factor and summing the product [23]. Currently most preclinical studies evaluate short term cognitive outcomes; however, the need to evaluate long-term outcome led us to choose the time points for analysis in this study (4 and 8 weeks post-injury) in an attempt to make future studies more clinically-relevant.
Swim paths were analyzed by 2 independent investigators blinded to the treatment status of each animal. The tracking/swimming patterns of the two groups were also assessed to determine the ability to “shift” strategies within the maze. In assessing the individual swim paths, we employed the pattern categorization developed by Graziano and colleagues [13] which is a 7 category qualitative analysis of prototypical behavior in the MWM: thigmotaxis, circling, random searching, scanning, self-orienting, approaching target, and ability to directly locate the platform. The strategies are ordered on a continuum of difficulty and efficiency where thigmotaxis is considered the least efficient way of solving the task [9,13], while direct finding of the hidden platform is the most efficacious [13]. Slope of learning was determined by plotting the learning latencies and obtaining the slope of the linear regression. The “slope of learning” is a relatively new method for analysis of MWM data, but has been used in several labs to examine rate of learning (Dr. Alex Harper, personal communication, [14]). Its benefit is to distinguish fast learners from delayed learners which may be especially important in studies of neuroplasticity and repair.
Differences in swim speed, slope of learning, and mean MWM probe trial score were analyzed by independent t-tests. Repeated measures ANOVAs were used to analyze MWM learning data followed by Student Neuman-Keuls post-hoc tests where appropriate. Data are reported as mean +/− SEM.
3. Results and discussion
Lateral FP injury of moderate severity resulted in a 17% mortality in the immediate post-injury period due to prolonged apnea, which is consistent with our previously published results [1,22]. There were no significant differences in mean swim speed between sham-injured and brain-injured animals on any of the three tasks (data not shown). In the first phase (learning/acquisition), there was a progressively decreased latency to find the hidden platform over time, demonstrating learning ability at four-weeks post-injury, regardless of group (p < 0.0001, Fig. 1A). Brain-injured animals showed significantly impaired ability to learn the position of the submerged platform over time (p < 0.0001, Fig. 1A) when compared to sham-injured animals. When animals were reintroduced to the same maze at eight weeks post-injury with the platform location relocated, there was again a time effect demonstrating learning over time, regardless of group (p < 0.0001, Fig. 1B). Again, sham-injured animals were able to learn the new location of the platform significantly faster then their brain-injured counterparts (p < 0.001, Fig. 1B). The overall slope of learning of the two groups was not significantly different at the 8-week time point: −6.2 in brain-injured animals versus − 6.1 in sham-injured animals. This was in contrast to the much slower rate of learning of brain-injured animals at 4 weeks indicated by the slope of −2.5 compared to −5.9 in sham-injured animals. However, the mean latency per day averaged over 15 seconds lower in sham-injured animals than in brain-injured animals at 8 weeks post injury (p < 0.001). Brain-injured animals had significantly lower mean Memory Scores (122.1 ± 13.3) than their sham-injured counterparts (188.7 14.6; p < 0.01) during the probe trials at the 8 week time point.
Fig. 1.
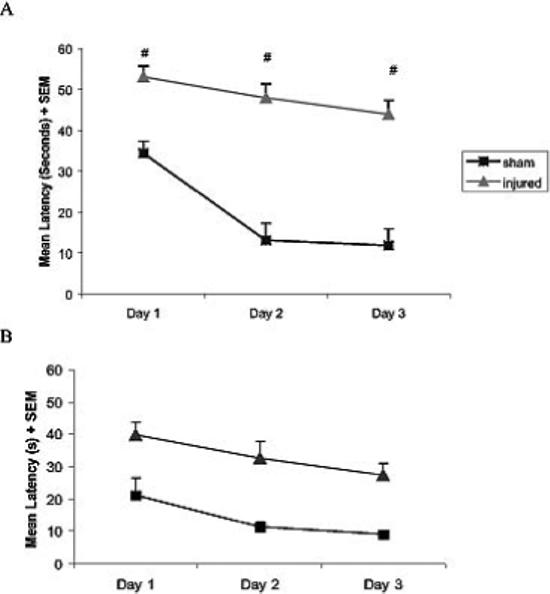
Acquisition and shift learning performance of rats in the Morris water maze (MWM). (A) Acquisition/Initial learning: Sham-injured and brain-injured rats were subjected to a visuospatial Morris water task at 4 weeks post-injury, showing the average of each training day (8 trials) per day. Brain-injury resulted in significant learning deficits over time in comparison to sham injury (# = p < 0.0001). (B) Platform relocation learning: The same rats were subjected to a relocated platform in the MWM at 8 weeks post-injury. Brain-injury significantly impaired the animals' ability to locate the hidden platform in comparison to sham-injury (p < 0.001). The mean latency to reach the hidden platform (+ SEM) of the training block at 4 and 8 weeks is shown.
We determined whether rats were seeking the platform in the previous, original location, followed by a switch to a new search strategy for the new platform location, or if no long-term memory process was active. Representative learning patterns of a sham-injured animal and a brain-injured animal are shown in Fig. 2. These patterns visually demonstrate the search strategy of individual animals over four 60-second trials and were chosen as they reflect a typical swim pattern for each group. Sham-injured animals, upon discovering the platform was not in the original location on the first relocation trial, switched to a “scanning” strategy [13] in the pool (Fig. 2A). By the second entry in the first trial block of the paradigm, the swim pattern of the majority of sham-injured animals was that of “approaching target” – the animal adjusted its trajectory while approaching the platform (Fig. 2B).
Fig. 2.
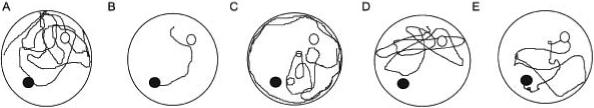
Search strategies used for new platform location in MWM. (A) Sham-injured animal at first shifted learning trial demonstrated a scanning strategy. (B) Sham-injured animal with approaching target swim pattern on second trial. (C) Brain-injured animal at first shift trial showed a circling pattern followed by thigmotaxis. (D) Brain-injured animal on second trial demonstrated random searching swim pattern in the MWM. (E) On third entry to the shift trial, the brain-injured animal was able to demonstrate a scanning strategy in order to locate the hidden platform. For each diagram: open circle represents old location of platform, dark circle represents new platform location for shift trial.
In contrast, brain-injured animals showed delayed ability to demonstrate these higher-level search strategies in the pool. In the initial trial, brain-injured animals first demonstrated “circling” activity, a swim pattern that moved away from the wall in a circular trajectory, with a switch to “thigmotaxic” behavior later in the trial (Fig. 2C). This ineffective, thigmotaxic pattern is typical of animals with impaired hippocampal function [8]. In the second trial, “random searching” behaviors occurred which were demonstrated by erratic trajectories with sudden changes in direction and velocity (Fig. 2D). It was only in the third trial that brain-injured animals were able to demonstrate “scanning” strategies to locate the platform (Fig. 2E), in contrast to sham-injured animals that were able to demonstrate these behaviors in the first trial.
The lack of pliancy in brain-injured animals to efficiently switch search strategies is also a hallmark of hippocampal damage [9]. Thus, differences in prototypical behavioral swim patterns were evident between the sham and brain-injured animals at 8 weeks post-injury and this information may also be useful in evaluating injury-related deficits through use of factor or discriminate analysis [3,13]. These patterns were generally consistent across animals within the trials. The brain-injured animals had difficulty integrating the relocation of the platform location to the task and continued to return to the old platform location. This finding is beneficial for the planning of long-term studies involving cognition in experimental brain injury in the future, particularly those involving neural repair or plasticity.
The present study demonstrates that serial use of the MWM may be adapted for use in the lateral FP brain injury model. Significant cognitive deficits were observed with the shift paradigm using a probe trial up to 8 weeks post-injury, even with a 4-week interval between initial testing and relocation of the platform. This paradigm can allow for serial cognitive testing of each animal. In addition, there is no plateau effect in learning seen in the sham-injured animals with the ordering of these tasks. From the end of the first task to the beginning of the second, the latencies increase since the animal is being introduced to a new paradigm, so concern about over training (practice) effects can be minimized using the shift paradigm. With the need for longer evaluation of therapeutic interventions, the ability to detect cognitive deficits using the MWM from 1-to 2-months post-injury in the same group of brain-injured animals was an important finding. Differences in individual animal swim patterns observed when the platform is relocated may also be a useful indicator of brain injury and recovery to evaluate in future studies. As cognitive deficits following TBI are one of the most debilitating sequelae observed in the clinical setting, pre-clinical measures of cognition might be the most critical outcome measure for studies of therapeutic efficacy. Serial testing in the MWM using the shift paradigm with probe is a valuable addition to the current battery of tests available for use in cognitive evaluation following traumatic brain injury in rats.
Acknowledgements
This paper was presented at the 39th Annual Communicating Nursing Research Conference/20th Annual WIN Assembly, sponsored by the Western Institute of Nursing. This work was supported, in part, by the National Institutes of Health (T32-NS043126, T32-NR007106, P50-NS08803, R01-NS40978) and a Merit Review Grant from the Veteran's Administration. Dr. Marklund was supported, in part, by a grant from the Swedish Brain Foundation.
References
- 1.Bareyre FM, Saatman KE, Helfaer MA, Sinson G, Weisser JD, Brown AL, McIntosh TK. Alterations in ionized and total blood magnesium after experimental traumatic brain injury: relationship to neurobehavioral outcome and neuroprotective efficacy of magnesium chloride. J. Neurochemistry. 1999;73:271–280. doi: 10.1046/j.1471-4159.1999.0730271.x. [DOI] [PubMed] [Google Scholar]
- 2.Blokland A, deVente J, Prickaerts J, Honig W, Markerink-van Ittersum M, Steinbusch H. Local inhibition of hippocampal nitric oxide synthase does not impair place learning in the Morris water escape task in rats. Eur. J of Neurosci. 1999;11:223–232. doi: 10.1046/j.1460-9568.1999.00431.x. [DOI] [PubMed] [Google Scholar]
- 3.Brody DL, Holtzman DM. Morris water maze search strategy analysis in PDAPP mice before and after experimental traumatic brain injury. Exp. Neurol. 2006;197:330–340. doi: 10.1016/j.expneurol.2005.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bullock MR, Lyeth BG, Muizelaar JP. Current status of neuroprotection trials for traumatic brain injury: lessons from animal models and clinical studies. Neurosurgery. 1999;45:207–217. doi: 10.1097/00006123-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Cheney JA, Weisser JD, Bareyre FM, Laurer HL, Saatman KE, Raghupathi R, Gribkoff V, Starrett JE, McIntosh TK. The maxi-K channel opener BMS-204352 attenuates regional cerebral edema and neurologic motor impairment after experimental brain injury. J Cereb. Blood Flow Metab. 2001;21:396–403. doi: 10.1097/00004647-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Cirulli F, Berry A, Alleva E. Intracerebroventricular administration of brain derived neurotrophic factor in adult rats affects analgesia and spontaneous behaviour but not memory retention in a Morris Water Maze task. Neuroscience Letters. 2000;287:207–210. doi: 10.1016/s0304-3940(00)01173-3. [DOI] [PubMed] [Google Scholar]
- 7.Cirulli F, Berry A, Chiarotti F, Alleva E. Intrahippocampal administration of BDNF in adult rats affects short-term behavioral plasticity in the Morris water maze and performance in the elevated plus-maze. Hippocampus. 2004;14:802–807. doi: 10.1002/hipo.10220. [DOI] [PubMed] [Google Scholar]
- 8.Day LB, Schallert T. Anticholinergic effects on acquisition of place learning in the Morris water task: spatial mapping deficit or inability to inhibit nonplace strategies. Behavioral Neurosci. 1996;110:998–1005. doi: 10.1037//0735-7044.110.5.998. [DOI] [PubMed] [Google Scholar]
- 9.Day LB, Weisend M, Sutherland RJ, Schallert T. The hippocampus is not necessary for place response but may be necessary for pliancy. Behavioral Neurosci. 1999;113:914–924. doi: 10.1037//0735-7044.113.5.914. [DOI] [PubMed] [Google Scholar]
- 10.D'Hoog D, DeDeyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Research Reviews. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 11.Fujimoto ST, Longhi L, Saatman KE, McIntosh TK. Motor and cognitive function evaluation following experimental traumatic brain injury. Neurosci. Biobehav. Rev. 2004;28:365–378. doi: 10.1016/j.neubiorev.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of learning index for performance in the Morris water maze. Behavioral Neurosci. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- 13.Graziano A, Petrosini L, Bartoletti A. Automatic recognition of explorative strategies in the Morris water maze. J. Neuroscience Methods. 2003;130:33–44. doi: 10.1016/s0165-0270(03)00187-0. [DOI] [PubMed] [Google Scholar]
- 14.Giza CC, Griesbach CS, Hovda DA. Experience-dependent plasticity is disturbed following traumatic injury to the immature brain. Beh. Brain Res. 2005;157:11–22. doi: 10.1016/j.bbr.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 15.McIntosh TK, Vink R, Noble L, Yamakami I, Fernyak S, Soares H, Faden AL. Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience. 1989;28:233–244. doi: 10.1016/0306-4522(89)90247-9. [DOI] [PubMed] [Google Scholar]
- 16.Morris R. Development of a water-maze procedure for studying spatial learning in the rat. J. Neuroscience Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 17.National Institutes of Neurological Diseases and Stroke [November 2, 2004];Traumatic Brain Injury: Hope Through Research. (last updated Oct. 10, 2002.) http://www.ninds.nih.gov/health and medical/pubs/TBI.htm,
- 18.Pettenuzzo LF, Wyse ATS, Clovis MD, Dutra-Filho CS, Netto CA, Wajner M. Evaluation of the effect of chronic administration of drugs on rat behavior in the water maze task. Brain Research Protocols. 2003;12:109–115. doi: 10.1016/j.brainresprot.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Pierce JE, Smith DH, Eison MS, McIntosh TK. The nootropic compound BMY-21502 improves spatial learning ability in brain injured rats. Brain Research. 1993;624:199–208. doi: 10.1016/0006-8993(93)90078-2. [DOI] [PubMed] [Google Scholar]
- 20.Prins ML, Hovda DA. Mapping cerebral glucose metabolism during spatial learning: interactions of development and traumatic brain injury. J. Neurotrauma. 2001;18:31–46. doi: 10.1089/089771501750055758. [DOI] [PubMed] [Google Scholar]
- 21.Saatman KE, Murai H, Bartus RT, Smith DH, Hayward NJ, Perri BR, McIntosh TK. Calpain inhibitor AK295 attenuates motor and cognitive deficits following experimental brain injury in the rat. Proc Nat. Acad. Sci. USA. 1996;93:3428–3433. doi: 10.1073/pnas.93.8.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saatman KE, Bareyre FM, Grady MS, McIntosh TK. Acute cytoskeletal alterations and cell death induced by experimental brain injury are attenuated by magnesium treatment and exacerbated by magnesium deficiency. J. Neuropathol Exp. Neurol. 2001;60:183–194. doi: 10.1093/jnen/60.2.183. [DOI] [PubMed] [Google Scholar]
- 23.Smith D, Okiyama K, Thomas MJ, Claussen B, McIntosh TK. Evaluation of memory dysfunction following experimental brain injury using the MWM. J. Neurotrauma. 1991;8:259–269. doi: 10.1089/neu.1991.8.259. [DOI] [PubMed] [Google Scholar]
- 24.Thompson HJ, Lifshitz J, Marklund N, Grady MS, Graham DI, Hovda DA, McIntosh TK. Lateral fluid percussion: A 15 year review and evaluation. J. Neurotrauma. 2005;22:42–75. doi: 10.1089/neu.2005.22.42. [DOI] [PubMed] [Google Scholar]


