Abstract
Local nucleosome-nucleosome interactions in cis drive chromatin folding, whereas interactions in trans lead to fiber-fiber oligomerization. Here we show that peptides derived from the histone H4 tail and Kaposi's sarcoma herpesvirus LANA protein can replace the endogenous H4 tail, resulting in array folding and oligomerization. Neutralization of a LANA binding site on the histone surface enhanced rather than abolished nucleosome-nucleosome interactions. We maintain that the contoured nucleosome surface is centrally involved in regulating chromatin condensation.
We have previously shown that a peptide spanning the N-terminal 23 amino acids of the viral latency-associated nuclear antigen (LANA) protein (LANA1–23) occupies the same negatively charged and contoured region on the nucleosomal surface as the H4 tail from a symmetry-related particle in the crystal lattice1. This same region has been implicated as an H4 tail binding site that mediates nucleosome-nucleosome interactions during chromatin folding and self-association2. We hypothesized that if this charged nucleosome surface region were involved in H4 tail–dependent interactions, exogenous LANA1–23 would compete with the H4 tail and bind to the nucleosome surface, thereby preventing H4-mediated chromatin condensation.
To test this hypothesis, we used a previously established model system reconstituted from recombinant histones (containing either full-length or tailless H4 (gH4) and full-length H3, H2A and H2B) and a 12-mer repeat of a 208-bp DNA fragment derived from the 5S rRNA gene3 to yield wild-type and gH4 nucleosomal arrays (Supplementary Methods). All arrays were carefully matched to contain 11 ± 2 octamers per 208–12-mer DNA fragment (Supplementary Fig. 1a online). We assayed self-association, which is thought to mimic fiber-fiber interactions in cellular chromatin4,5, by measuring the abundance of monomeric arrays as a function of MgCl2 concentration3,6. The Mg50 value is defined as the concentration of MgCl2 at which 50% of nucleosomal arrays are oligomerized and pellet upon centrifugation. We assayed chromatin fiber folding using sedimentation velocity to monitor progression from a ∼27S 11-nm fiber to the 40S intermediate and ∼55S ‘30-nm’ folded states under MgCl2 conditions where the arrays were monomeric3,6.
We incubated wild-type nucleosomal arrays with LANA1–23 at various molar ratios and measured the arrays' ability to self-associate as a function of MgCl2 concentration. A representative experiment is shown (Fig. 1a). The curves were cooperative (Fig. 1a) and fully reversible upon removal of Mg2+ from solution (data not shown). Mg50 values as a function of increasing amounts of LANA1–23 are shown in Figure 1b. Contrary to our hypothesis, LANA1–23 lowered the Mg50 needed for fiber-fiber self-association (Fig. 1a,b). This demonstrates that the binding of LANA1–23 to the nucleosomal surface as an untethered peptide promotes self-association. This effect was saturable because the addition of excess LANA1–23 at >2.5 equivalents of LANA1–23 per nucleosomal binding site did not lower Mg50 further. The effect was also specific because a mutant of LANA1–23 (LRS) that has the same charge but is unable to bind to the nucleosomal surface1 did not induce self-association (Fig. 1b). A glutathione S-transferase–LANA1–23 fusion (GST-LANA1–23) bound to the H2A-H2B dimer with an apparent Kd of 4.5 nM (Supplementary Fig. 2a online), whereas GST-LRS did not bind (Supplementary Table 1 online). These data suggest that LANA1–23 does not act as a polycationic salt but instead promotes compaction through the specific interactions observed by crystallography1.
Figure 1.

LANA1–23 promotes chromatin self-association. (a) Self-association of wild-type nucleosomal arrays (WT-NA) is plotted as a function of the MgCl2 concentration (shown in red). The same experiment done in the presence of a fivefold excess of LANA1–23 or a triple alanine substitution of LANA1–23 substituted at positions 8LRS10 (LRS) that abrogates binding to nucleosomes1 is shown in blue and green, respectively. Each nucleosome has two charged surfaces and thus two binding sites for LANA1–23. (b) Mg50 values (obtained from experiments such as those shown in a) are plotted as a function of the molar ratio of nucleosomes in the array to LANA1–23, assuming a saturation of 12. (c) Self-association of gh4 arrays (gh4-NA) is plotted as a function of the MgCl2 concentration (shown in orange). Blue and green curves show gh4-NA in the presence of a fivefold molar excess of LANA1–23 or LRS. Wild-type arrays are shown in red. (d) Mg50 values are plotted as a function of the molar ratio of gh4 nucleosome to LANA1–23. Error bars in all panels represent s.d. from three separate experiments.
When compared to wild-type arrays, tailless H4 arrays require more MgCl2 for self-association3,6. LANA1–23 retained the ability to promote self-association of nucleosomal arrays lacking the endogenous H4 tail (Fig. 1c,d). In the presence of LANA (1:5), the Mg50 for gH4-arrays dropped to 2.5 mM, the same value observed for wild-type arrays in the absence of LANA. However, the Mg50 did not reach levels as low as those observed for wild-type arrays in the presence of LANA and the covalently attached H4 tail. Thus, we believe that LANA1–23 can mimic some, but not all, actions of the H4 tail. This, in turn, implies that there are more binding sites on the nucleosome for the H4 tail than for LANA. Consistent with this interpretation, the interaction of the GST–H4 tail peptide with the H2A-H2B dimer was indicative of the presence of multiple low-affinity sites of interaction (>45 μM) in addition to a relatively high-affinity interaction (∼11 nM, Supplementary Fig. 2b and Supplementary Table 1). The H4 tail showed a similar binding behavior with the (H3-H4)2 tetramer (Supplementary Table 1), whereas LANA1–23 did not interact with the (H3-H4)2 tetramer1. Additionally, GST–H4 tail (as shown previously7), but not GST-LANA1–23, bound to free DNA (Supplementary Fig. 2c). Thus, we believe that the H4 tail binds to nucleosomal arrays through both protein-protein and protein-DNA interactions.
How does LANA affect self-association in the presence and absence of covalently attached H4 N-terminal domains? One possibility is that LANA binding to the nucleosomal surface alters a ‘repulsive’ domain on the nucleosomal surface that may prevent the close association of nucleosomes (Fig. 2a,b). We hypothesized that nucleosome-nucleosome interactions are mediated by a collection of opposing and attractive domains on the nucleosome surface. This yields a carefully balanced condensation equilibrium that can easily be regulated in either direction by environmental conditions and chromatin-binding proteins. To test this hypothesis, we neutralized six of the seven acidic surface residues that contribute to the LANA binding region. All of these residues are either near crystal contacts or are directly involved in making hydrogen bonds with a symmetry-related particle. Mutant H2A molecules, together with the other three wild-type core histones, were assembled into nucleosome arrays that had partially neutralized histone surfaces (nhs arrays). Nucleosomes and nucleosomal arrays reconstituted with this histone mutant were indistinguishable from those reconstituted with wild-type H2A (Supplementary Fig. 1a,b). Nhs arrays required less MgCl2 than wild-type arrays did for self-association (Fig. 2c), indicating that the native charged and/or stereo-chemical character of this region indeed counteracts the ability of wild-type arrays to self-associate. The failure of the nhs arrays to completely recapitulate the Mg50 of a saturated LANA wild-type array (the Mg50 values observed were ∼1.8 versus ∼1.1–1.2 mM) reflects the fact that the local nucleosomal surface is affected differently by mutagenesis of acidic residues and by the binding of LANA peptide. The addition of LANA1–23 or LRS had little or no effect (Fig. 2c), most likely because neither peptide was able to bind to the mutated histone surface with high affinity (Supplementary Table 1). The observation that nhs H2A-H2B dimers have some residual binding to LANA1–23 (Supplementary Table 1) suggests that low-affinity binding sites on the H2A-H2B dimer in addition to the charged surface region may contribute to the effect observed with LANA1–23.
Figure 2.
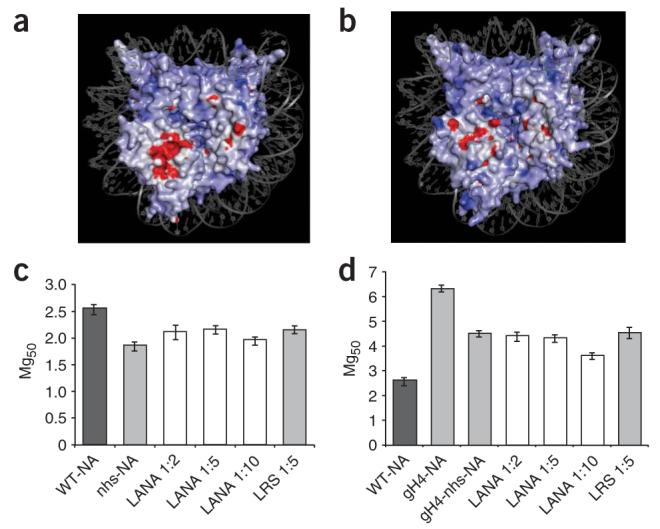
A negatively charged region on the surface of the nucleosome acts as a repulsive domain. (a) Charged surfaces for PDB entry 1ZLA (red, negatively charged; blue, positively charged) were calculated through Delphi/GRASP15 and the figure was rendered in PyMol16. (b) Upon interaction with LANA1–23, the surface contours and charge of the nucleosomal region are altered. (c,d) Mg50 values are plotted as a function of molar ratios of LANA1–23 to nhs nucleosomes in the nhs nucleosome array (nhs-NA; c) or to gh4-nhs nucleosomes in the gh4-nhs nucleosome array (gh4-nhs-NA) (d). Error bars in all panels represent s.d. from three separate experiments.
As suggested by the experiments described above, the endogenous H4 tail promotes self-association by binding to regions on the nucleosome that are at least partially distinct from that bound by LANA1–23. If this is the case, mutagenesis of the LANA binding region should not completely compensate for the deletion of the H4 tail. We prepared nucleosomal arrays with histone octamers that had neither the H4 tail nor the charged region (gH4-nhs arrays). Mg50 values for such arrays fell between those of wild-type and gH4 arrays (Fig. 2d), indicating that the H4 tail is at most only partially involved in neutralizing the region defined by crystallographic analysis8. Again, addition of LANA1–23 had no effect.
We next wanted to test whether a synthetic H4 tail peptide could promote self-association when added exogenously—an experiment analogous to those described above with LANA1–23. As for LANA1–23, the addition of H4 tail peptide to wild-type nucleosomal arrays lowered the Mg50 value (Fig. 3a). However, this effect was nonsaturable, as molar ratios as high as 10- to 16-fold continued to lower Mg50 (Fig. 3a and data not shown). This is consistent with our observation that the interaction of H4 with H2A-H2B had a nonsaturable component (Supplementary Fig. 2b). We next tested whether the exogenous H4 tail peptide could replace the missing H4 tail in trans in gH4 arrays (Fig. 3b) and in arrays carrying both neutralizing surface mutations and tail deletions (gH4-nhs arrays). We used arrays with a mutated charged region as controls (nhs arrays, Fig. 3c,d). In all cases, addition of the H4 tail further promoted self-association and addition of increasing amounts of H4 tail peptide further lowered the Mg50 values, with a linear dependency. This is consistent with our finding that the H4 tail could still bind to nhs histone dimers, whereas LANA could not (Supplementary Fig. 2d and Supplementary Table 1), and suggests that the H4 tail is capable of binding to multiple binding sites on nucleosomal surface during self-association.
Figure 3.
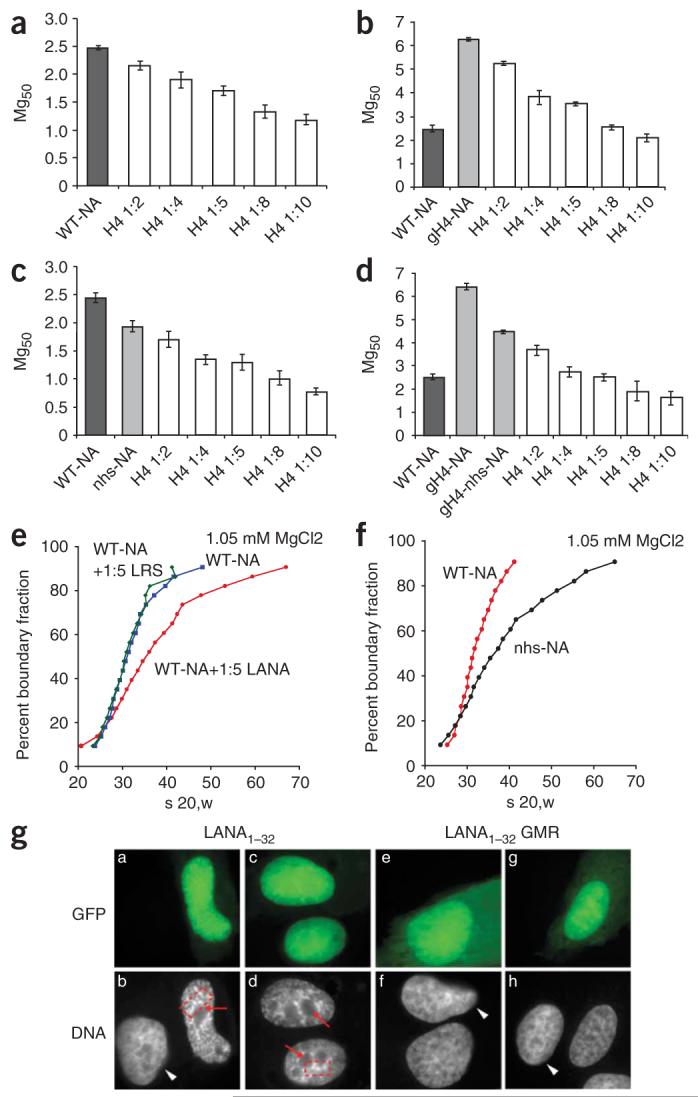
The H4 tail and LANA peptide act in trans to promote self-association and folding. (a–d) Mg50 values are plotted as a function of the molar ratios of H4 tail peptide added to wild-type nucleosomes (a) or as a function of the molar ratio of H4 to gh4 nucleosomes (b), nhs nucleosomes (c) or gh4-nhs nucleosomes (d). WT-NA, wild-type nucleosome array. Error bars represent s.d. from three separate experiments. (e) Folding of wild-type arrays at 1.05 mM MgCl2 in the presence of a fivefold excess of LANA over either nucleosome (red), LRS (green) or buffer (blue). s 20, w indicates sedimentation coefficient in water at 20 °C. (f) Folding of wild-type arrays (red) and nhs arrays (black) at 1.05 mM MgCl2. (g) GFP fusion with LANA1–32 (a–d within panel g) or GFP-LANA1–32 with three alanine substitutions at positions 4GMR6 (e–h within panel g) were expressed in U2OS cells. DNA was visualized by fluorescent microscopy after Hoechst 33258 staining. Regions of Hoechst exclusion (red arrows) and focus formation (red boxes) are indicated. White arrowheads indicate nuclei of untransfected cells. Details are found in the Supplementary Methods online. A quantification of these data is shown in Supplementary Figure 3.
We used sedimentation velocity to study the MgCl2-dependent transition from the 27S 11-nm fiber to the 40S intermediate folded state and the 55S 30-nm structure4. We incubated wild-type arrays with LANA1–23, LRS or buffer in the presence of 1.05 mM MgCl2. Under these conditions, no self-association occurred and folding was limited to the 30S-to-40S transition in the absence of LANA1–23. A substantial proportion of the arrays sedimented between 40S and 55S upon addition of a fivefold excess of LANA1–23, confirming that LANA1–23 promotes formation of a compact 30-nm conformation (Fig. 3e). As with self-association, the effect is specific, because LRS had no discernible effect. Mutagenesis of the histone surface (nhs arrays) also promoted folding of a portion of arrays into a 55S species (Fig. 3f). As with self-association, the addition of LANA1–23 had no effect as a result of its inability to interact with these mutant nucleosomes (data not shown). Thus, altering the charged surface region either by interaction with LANA1–23 or by mutation of selected charged residues in H2A has a profound effect on chromatin fiber folding.
We next wished to determine whether N-terminal LANA peptide affects the structure of chromatin in vivo. LANA residues 1–32, which include a nuclear localization signal at positions 24–30, were fused to green fluorescent protein (GFP) and expressed in U2OS cells. Our results (Fig. 3g; Supplementary Fig. 3 online) demonstrated that LANA peptide acted in vivo to alter nuclear architecture and influence chromatin condensation, consistent with our in vitro findings. Notably, the changes in nuclear architecture observed here are similar to those described for heterochromatin formation induced by cellular senescence9.
In conclusion, we have shown evidence that the histone octamer surface of the nucleosome has an important role in the regulation of chromatin higher-order structure(s). Our data indicate that the same surface of the nucleosome affects both folding and self-association processes. We postulate that the degree of chromatin compaction under any given set of conditions results from the net effects of opposing and attractive domains on the nucleosome surface. Its character may be individually and locally altered by unmodified histone tails, through post-translational modification of the tails10 and the structured region of the histones11, through the introduction of histone mutants12,13, by histone variants14 or through interactions with a multitude of chromatin architectural proteins. By extension, we propose that there are many different ways in which nucleosomal surfaces interact to promote chromatin condensation, resulting in numerous interchangeable types of higher-order structures of varying stability.
Supplementary Material
ACKNOWLEDGMENTS
We thank P.N. Dyer for help with the preparation of histones and DNA. Supported by US National Institutes of Health grants GM067777 to K.L., CA82036 to K.M.K. and GM45916 to J.C.H. K.L. is also supported by the Howard Hughes Medical Institute.
Footnotes
Note: Supplementary information is available on the Nature Structural & Molecular Biology website.
References
- 1.Barbera AJ, et al. Science. 2006;311:856–861. doi: 10.1126/science.1120541. [DOI] [PubMed] [Google Scholar]
- 2.Dorigo B, et al. Science. 2004;306:1571–1573. doi: 10.1126/science.1103124. [DOI] [PubMed] [Google Scholar]
- 3.Gordon F, Luger K, Hansen JC. J. Biol. Chem. 2005;280:33701–33706. doi: 10.1074/jbc.M507048200. [DOI] [PubMed] [Google Scholar]
- 4.Hansen JC. Annu. Rev. Biophys. Biomol. Struct. 2002;31:361–392. doi: 10.1146/annurev.biophys.31.101101.140858. [DOI] [PubMed] [Google Scholar]
- 5.Schwarz PM, Felthauser A, Fletcher TM, Hansen JC. Biochemistry. 1996;35:4009–4015. doi: 10.1021/bi9525684. [DOI] [PubMed] [Google Scholar]
- 6.Dorigo B, Schalch T, Bystricky K, Richmond TJ. J. Mol. Biol. 2003;327:85–96. doi: 10.1016/s0022-2836(03)00025-1. [DOI] [PubMed] [Google Scholar]
- 7.Cary PD, Crane-Robinson C, Bradbury EM, Dixon GH. Eur. J. Biochem. 1982;127:137–143. doi: 10.1111/j.1432-1033.1982.tb06847.x. [DOI] [PubMed] [Google Scholar]
- 8.Luger K, Maeder AW, Richmond RK, Sargent DF, Richmond TJ. Nature. 1997;389:251–259. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 9.Narita M, et al. Cell. 2003;113:703–716. doi: 10.1016/s0092-8674(03)00401-x. [DOI] [PubMed] [Google Scholar]
- 10.Shogren-Knaak M, et al. Science. 2006;311:844–847. doi: 10.1126/science.1124000. [DOI] [PubMed] [Google Scholar]
- 11.Freitas MA, Sklenar AR, Parthun MR. J. Cell. Biochem. 2004;92:691–700. doi: 10.1002/jcb.20106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park JH, Cosgrove MS, Youngman E, Wolberger C, Boeke JD. Nat. Genet. 2002;32:273–279. doi: 10.1038/ng982. [DOI] [PubMed] [Google Scholar]
- 13.Matsubara K, Sano N, Umehara T, Horikoshi M. Genes Cells. 2007;12:13–33. doi: 10.1111/j.1365-2443.2007.01031.x. [DOI] [PubMed] [Google Scholar]
- 14.Suto RK, Clarkson MJ, Tremethick DJ, Luger K. Nat. Struct. Biol. 2000;7:1121–1124. doi: 10.1038/81971. [DOI] [PubMed] [Google Scholar]
- 15.Nicholls A, Sharp KA, Honig B. Proteins. 1991;11:281–296. doi: 10.1002/prot.340110407. [DOI] [PubMed] [Google Scholar]
- 16.DeLano WL. The PyMOL User's Manual. DeLano Scientific; San Carlos, California, USA: 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


