Abstract
We have demonstrated that soft substrate induced apoptosis in polarized cells, but not in transformed cells by disturbance of Ca2+ homeostasis. This study aims to further investigate the regulatory mechanisms underlying the disruption of Ca2+-signaling integrity in soft substrate–induced epithelial apoptosis. Soft substrate up-regulated the store-operated Ca2+ (SOC) entry across the plasma membrane of normal cervical epithelial cells, which resulted in increased cytosolic Ca2+ levels. Concomitantly, soft substrate induced the aggregation and translocation of stromal interacting molecule 1 (STIM1) toward the cell periphery to colocalize with Orai1, an essential pore subunit of SOC channel, detected by fluorescence resonance energy transfer approach and confocal image analyses. The disturbed Ca2+ homeostasis resulted in the activation of μ-calpain, which cleaved α-spectrin, induced actin disorganization, and caused apoptosis. In contrast, soft substrate did not disturb Ca2+ homeostasis or induce apoptosis in cervical cancer cells. Chelating extracellular Ca2+ by EGTA and down-regulated SOC entry by small interfering RNA targeting STIM1 or inhibitors targeting Ca2+-binding site of calpain significantly inhibited soft substrate–induced activation of μ-calpain and epithelial cell apoptosis. Thus, soft substrate up-regulates the interaction of STIM1 with SOC channels, which results in the activation of μ-calpain and subsequently induces normal epithelial cell apoptosis.
INTRODUCTION
Physical forces between cellular adhesion sites and substrate may play an important role in the regulation of cellular function, as evidenced by the responses of cell morphology, locomotion, growth, and gene expression to mechanical forces such as fluid shear stress or substrate stretching (Pelham and Wang, 1997; Wang et al., 2000; Benjamin and Hillen, 2003; Discher et al., 2005; Vogel and Sheetz, 2006). Consequently, cells possess rather sophisticated mechanosensory devices, which can detect physical forces and respond to them (Beningo et al., 2002). The rigidity of substrate also influences cell contractility, cell movement, Rho activity, focal adhesion assembly, and differentiation of myotubes (Lo et al., 2000; Wang et al., 2003; Hung and Ingber, 2005; Paszek et al., 2005; Engler et al., 2004). However, the mechanisms by which cells precisely sense mechanical stimuli and how these factors alter cellular structure and function are far less explored than those triggered by chemical ligands.
Microenvironments between cell–substrate adhesions appear important in controlling cell proliferation (Chen et al., 1997; Chicurel et al., 1998) or even critical in stem cell lineage specification (Engler et al., 2004, 2006). Although a wide variation in substrate rigidities for differentiated cells is known to influence focal-adhesion structure and cytoskeleton (Cukierman et al., 2001; Bershadsky et al., 2003; Discher et al., 2005), microenvironments between cell–substrate adhesion can be difficult to adequately characterize or control. It has not been possible to specifically identify the role of physical parameters because of the use of substrates that differ in both chemical and physical properties. Collagen gel may exert both biochemical and biophysical impact on cells, but little is known about its biophysical impact on cell biology. The biophysical effects of collagen gel on cells could be due to its physical property, i.e., very low rigidity. To address this problem, we have developed the collagen-coated substrates, which, through minor changes in the concentration of cross-linkers, allowed the regulation of flexibility over a wide range without altering their chemical properties (Wang et al., 2003, 2005). We demonstrated that different rigidities of adhesive collagen substrate affected cell growth, morphology, activation of protein kinases and stability of focal adhesion complex proteins (Wang et al., 2003). In addition, soft substrate of collagen gel induced apoptosis in polarized cells, but not in transformed cells or fibroblasts (Wang et al., 2007). We also showed that soft substrate induced apoptosis in renal epithelial LLC-PK1 cells but not in cervical cancer HeLa cells by the disturbance of Ca2+ homeostasis (Chiu et al., 2007).
Here we further investigate the regulatory signals underlying the disturbance of Ca2+ homeostasis in soft substrate–induced epithelial apoptosis. We used normal cervical epithelial cells and cervical cancer cells as a pair to study the cell type–specific responses to different substrate rigidities. The data indicate that soft substrate induces the translocation of stromal interaction molecule 1 (STIM1), a putative endoplasmic reticulum (ER) Ca2+ sensor, toward the plasma membrane to interact with Orai1, an essential pore subunit of store-operated Ca2+ (SOC) channels. The enhancing STIM1 translocation correlates with the up-regulation of Ca2+ entry in soft substrate, which disrupts the integrity of Ca2+-signaling complexes and subsequently induces normal epithelial cell apoptosis.
MATERIALS AND METHODS
Preparation of Collagen Gel and Quantification of Substrate Rigidity
Type I collagen and 0.3% collagen solution were prepared as described previously (Jiang et al., 2000). To overlay the culture dishes with collagen gel, 1.5 ml chilled 0.3% collagen solution was dispensed in a 6-cm culture dish and allowed for gelation at 37°C. In contrast, to prepare the collagen gel–coated dish (control group), the chilled collagen solution was added into culture dishes to cover the surface. The culture dishes were then tilted, and the excess amount of collagen gel was immediately aspirated. The collagen gel–coated dishes were air dried and washed twice by culture medium before use. The detailed protocol of the quantification of collagen–gel rigidity was previously described (Wu et al., 2005).
Calpain Inhibitors
The selective inhibitor for μ- and m-calpain directed to Ca2+-binding sites, 3-(4-iodophenyl)-2-mercapto-(Z)-2-propenoic acid (PD150606), the 20-fold selective inhibitor for μ-calpain directed to Ca2+-binding sites, 3-(5-fluoro-3-indolyl)-2-mercapto-(Z)-2-propenoic acid (PD151746), and the negative control for calpain inhibitors, 2-mercapto-3-phenylpropanonic acid (PD145305), were obtained from Calbiochem (EMD Biosciences, San Diego, CA).
Cell Culture
Cultures of normal human cervical epithelial cells and cervical cancer cell lines (SiHa and CaSki) were prepared as described previously (Chou et al., 1995). For experiments, cells were seeded on different rigidities of substrate at a density of 3 × 105 cells/60-mm dish.
Scanning Electron Microscopy
Cells and collagen substrate were rinsed twice with phosphate-buffered saline (PBS) and then fixed with 2% buffered glutaraldehyde for 1 h. Samples were then rinsed twice with PBS to remove glutaraldehyde and dehydrated by incubation with gradient alcohol from 50 to 95% for 10 min under each concentration. Absolute alcohol was finally used for the complete dehydration. Samples were processed critical point dried in liquid CO2 solution, coated with a thin layer of gold particle film and visualized under Hitachi scanning electron microscope (SEM; S5200, Tokyo, Japan).
Analysis of Apoptosis
Two methods were used to assess apoptosis in different culture conditions: 1) annexin V staining. By conjugating fluorescein isothiocyanate (FITC) to annexin V, which preferentially binds to negatively charged phosphatidylserine, it is possible to identify and quantitate apoptotic cells on a single-cell basis by flow cytometry. Cells cultured on collagen gel–coated dishes or on collagen gel for 4 h were washed twice and then stained with annexin-V-FITC (Roche, Indianapolis, IN). Samples were then analyzed by flow cytometer (FACSCalibur system, Becton Dickinson, San Jose, CA). 2) Propidium iodide (PI) staining. This is to stain DNA and look for the subdiploid population of cells from a cell cycle profile. Cells cultured on collagen gel–coated dishes or on collagen gel for 24, 48, and 72 h were washed and then fixed in 70% alcohol. After fixation, cells were treated with RNase (100 mg ml−1) and stained with 40 mg ml−1 PI (Sigma, St. Louis, MO). The PI-stained cells were analyzed by flow cytometry. The apoptotic ratio was analyzed from hypodiploid DNA peak of apoptotic cells by Cell Quest software (Becton Dickinson).
Western Blot
Cell lysates were harvested in modified radioimmune precipitation assay buffer (RIPA; 150 mM NaCl, 1 mM EGTA, 50 mM Tris, pH 7.4, 10% glycerol, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, and Complete™) at distinct times. The lysates were analyzed by Western blot using antibodies against STIM1 (BD Biosciences, San Jose, CA), μ-calpain, m-calpain (Sigma), α-spectrin, talin, FAK (Chemicon, Temecula, CA), and β-actin (Sigma).
Immunofluorescence Imaging
Cells were rinsed twice with PBS, fixed with 3.7% buffered paraformaldehyde, and permeabilized with 0.5% Triton X-100 for 15 min. To detect nucleus and actin filaments, cells were stained with Hoechst 33258 and phalloidin conjugated with Alexa 594 (Molecular Probes, Eugene, OR) for 1 h at room temperature. To detect nucleus and STIM1, cell were stained with mouse anti-STIM1 antibody (BD Biosciences) for 1 h and then stained with Hoechst 33258 and anti-mouse IgG conjugated with Alexa 488 (Molecular Probes) for 1 h at room temperature. The fluorophore was excited by laser at 405, 488, or 543 nm and detected by a scanning confocal microscope (FV-1000, Olympus, Tokyo, Japan). Cells transfected with DNA plasmids of enhanced green fluorescent protein (EGFP)-STIM1 and monomeric red fluorescent protein (mOrange)-Orai1 were direct activated by laser at 488 or 543 nm for EGFP and mOrange, respectively.
Single-Cell Intracellular Ca2+ Measurement
Intracellular Ca2+ ([Ca2+]i) was measured at 37°C with the Fura-2 fluorescence ratio method on a single-cell fluorimeter as previously described (Ross and Cahalan, 1995; Shen et al., 2003). In brief, cells attached on a coverslip were loaded with 2 μM Fura-2/acetoxymethyl ester (Fura-2/AM) in DMEM culture medium at 37°C for 30 min. After loading, cells were washed three times with PBS. Coverslips were then placed on the stage of an Olympus IX71 inverted microscope equipped with a xenon illumination system and an IMAGO CCD camera (Till Photonics, Grafelfing, Germany). The excitation wavelength was alternated between 340 nm (I340) and 380 nm (I380) using the Polychrome IV monochromator (Till Photonics). The fluorescence intensity was monitored at 510 nm, stored digitally, and analyzed by the program of TILLvisION 4.0 (Till Photonics). [Ca2+]i was calculated as previously described (Grynkiewicz et al., 1985). In some experiments, cytosolic Ca2+ levels were estimated by confocal images (Olympus FV-1000) for cells loaded with 2 μM Fluo-4/AM (Molecular Probes).
Measurement of μ-Calpain Activity
The μ-calpain activity was assessed by generation of the fluorescent product from hydrolysis of an artificial μ-calpain fluorescent substrate t-butoxycarbonyl-Leu-Met-chloromethylaminocoumarin (t-Boc-LM-CMAC; Molecular Probes; Xu and Deng, 2004). Cells were pretreated with 10 μM t-Boc-LM-CMAC for 30 min and then cultured on collagen gel or gel-coated dishes for 1, 4, and 6 h. Intracellular fluorescence was sequentially imaged under a confocal imaging system (Olympus FV-1000) and mean fluorescence intensity (excitation by 405-nm LD laser) of individual cells was quantitatively analyzed.
Small Interfering RNA
For siRNA knockdown of μ-calpain or STIM1, normal cervical epithelial cells were transfected with 50, 100, and 200 nM of either targeting small interfering RNA (siRNA) or a control nontargeting siRNA (all purchased from Ambion, Austin, TX) using Oligofectamine (Invitrogen, San Diego, CA) for 24, 48, and 72 h. Preliminary results indicated that a siRNA concentration of 100 nM for 48 h exerted the maximum inhibition of mRNA and protein expression for μ-calpain or STIM1 without affecting the cell viability. The siRNA targets human large subunits of μ-calpain (sense siRNA strand: 5′GCUAGUGUUCGUGCACUCUtt3′; antisense siRNA strand: 5′AGAGUGCACGAACACUAGCtt3′; Honda et al., 2004). The sequences of siRNA targeting STIM1 are as follows: sense: 5′AAGGCUCUGGAUACAGUGCUCtt3′; antisense: 5′GAGCACUGUAUCCAGAGCCUUtt 3′; Roos et al., 2005).
Fluorescence Resonance Energy Transfer Measurements
Fluorescence resonance energy transfer (FRET) approach was used to 1) visualize and quantify the interaction between STIM1 and Orai1 and 2) estimate cytosolic Ca2+ levels by fluorescent indicator protein cameleon (Nagai et al., 2004). EGFP and mOrange were conjugated with STIM1 and Orai1, respectively (kindly provided by Liangyi Chen, Chinese Academy of Science, China) and were used as a FRET pair. We initially cloned the cells with stable expression of STIM1–EGFP. These cells were transiently transfected with Orai1-mOrange by using Lipofectamine 2000 (Invitrogen). Live-cell imaging was acquired on a confocal imaging system (Olympus FV-1000). To avoid the bias incurred by different Orai1 protein expressions, cells with similar mOrange fluorescent intensity (3000 ± 100 arbitrary units in the fluorescent scale ranged from 0 to 4095) were selected for FRET measurement. An optional 488 nm laser was used to directly excite EGFP without exciting mOrange, which was ideal for EGFP and mOrange FRET imaging. On the other hand, emission spectra of EGFP and mOrange were 500–535 and 560–650 nm, respectively. Intermolecular FRET between EGFP–STIM1 and mOrange– Orai1 measured by the analysis program in FV-1000 allows ratio calculation and ratio image acquisition after subtracted background fluorescent intensity. The ratio of mOrange to EGFP emissive fluorescence intensity was presented as the pseudocolor image. Cameleon is a chimeric protein composed of a short-wavelength variant of GFP, calmodulin (CaM), a glycylglycine linker, the CaM-binding peptide of myosin light-chain kinase (M13), and a long-wavelength variant of GFP. Ca2+ binding to CaM initiates an intramolecular interaction between CaM and M13, which changes the chimeric protein from an extended to a more compact conformation, thereby increasing the efficiency of FRET from the shorter- to the longer-wavelength variant of GFP. Yellow cameleons (YCs) have cyan and yellow fluorescent proteins (CFP and YFP) as the FRET donor and acceptor, respectively. We selected the cells stable expression of cameleon plasmid (kindly provided by Takeharu Nagai, Hokkaido University, Japan) and the live-cell imaging was acquired by a confocal imaging system (Olympus FV-1000). An optional 440 nm LD laser was used to directly excite ECFP without exciting EYFP, which was ideal for EYFP/ECFP ratio image. On the other hand, emission spectra of ECFP and EYFP were 470–500 and 535–565 nm, respectively. Intramolecular FRET between ECFP and EYFP measured by the analysis program in FV-1000 allows ratio calculation and ratio image acquisition after subtracted background fluorescent intensity. The ratio of EYFP to ECFP emissive fluorescence intensity was presented as the pseudocolor image.
Colocalization and Statistical Analyses
A pixel-by-pixel colocalization analysis, using FV-1000 software, was used to assess levels of STIM1 colocalization with Orai1 in confocal images. All values were reported as means ± SEM. Student's pair or unpaired t test was used for statistical analyses. Differences between values were considered significant when p < 0.05.
RESULTS
Soft Substrate Regulates Growth and Apoptosis of Normal Cervical Epithelial Cells But Not Cervical Cancer Cells
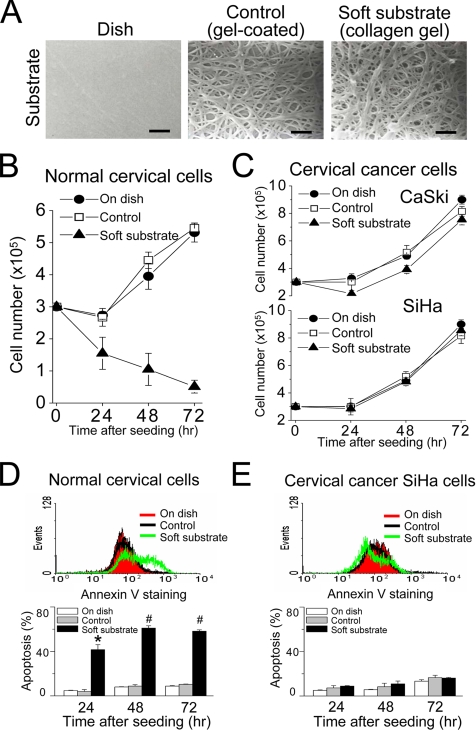
To study the effect of substrate rigidity on the cellular function, we developed a cell culture system, in which culture dishes were coated with a very thin layer of collagen gel or were overlaid with collagen gel (Figure 1A). Detected by the dynamic mechanical analyzer, the substrate rigidity of culture dish coated with a very thin layer of collagen gel is more than 1 giga pascal, which was similar to that of culture dish without any coated substances and was referred as the control condition. In contrast, overlaying the culture dish with collagen gel remarkably decreased the substrate rigidity to 30–100 pascals and was therefore referred as soft substrate. The SEM examination indicates a similar density of collagen fibril cross-link in gel-coated dish and gel-overlaid dish (Figure 1A). Therefore, this culture system is able to differentiate the biophysical effects of collagen gel from its biochemical impacts on the cellular function.
Figure 1.
Soft substrate regulates growth of normal cervical epithelial cells but not cervical cancer cells through apoptosis. (A) Cell culture system with collagen substrates of different elastic modulus. The culture dish was coated with a very thin layer of collagen gel (referred as “control group”) or overlaid with collagen gel (referred as “soft substrate”). Scale bars, 1 μm. (B and C) Different growth responses to soft substrate between normal cervical epithelial cells and cervical cancer cells (SiHa and CaSki). Each point represents mean ± SEM (n = 6). (D and E) Soft substrate induces apoptosis in normal cervical epithelial cells but not in cervical cancer SiHa cells. Top panels, annexin V staining of normal cervical epithelial cells and cervical cancer SiHa cells cultured on dish (On dish), collagen gel–coated dish (Control), or on collagen gel (On gel) for 4 h. X-axis indicates annexin V-FITC fluorescent intensity and Y-axis is the cell number. Bottom panels, apoptotic analysis was assessed by flow cytometry (FACScan) of propidium iodide (PI)-stained normal cervical epithelial cells and cervical cancer SiHa cells that were cultured on various conditions for 24, 48, and 72 h. Apoptosis indicates the cell population in sub-G1 phase of cell cycle. Each column represents mean ± SEM (n = 6). * p < 0.001; # p < 0.0001, compared with control group.
We cultured normal cervical epithelial cells and two cervical cancer cell lines on different substrate rigidities. Culture on soft substrate inhibited the proliferation of normal cervical epithelial cells (Figure 1B), whereas that of cervical cancer cells was not affected by the substrate rigidity (Figure 1C). The cell population with positive annexin V staining, an early apoptotic marker, happened as early as 4 h after normal cervical epithelial cells cultured on soft substrate (top panel, Figure 1D). Analyzed by PI staining, a high sub-G1 population (i.e., apoptotic cells) was increased in a time-dependent manner for normal cervical epithelial cells cultured on soft substrate (bottom panel, Figure 1D). In contrast, there were no such phenomena for normal cervical epithelial cells cultured on the control condition or cervical cancer SiHa cells cultured on different substrate rigidities (Figure 1, B–E). This indicates that soft substrate regulates the growth of normal cervical epithelial cells through apoptotic pathways.
Soft Substrate–induced Apoptosis Results from μ-Calpain Activation
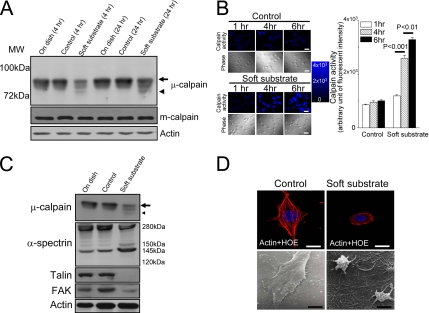
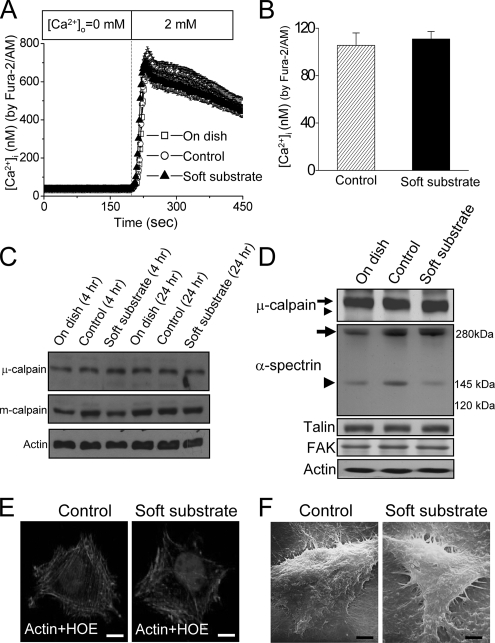
We dissected the signal pathways involved in soft substrate–induced apoptosis. As depicted in Figure 2A, a breakdown product of μ-calpain appeared when normal cervical epithelial cells cultured on soft substrate for 4 h. Concomitantly, full-length μ-calpain significantly decreased. Cleavage of μ-calpain into a near 72-kDa breakdown product became more obvious when normal cervical epithelial cells cultured on soft substrate for 24 h. These results imply the activation of μ-calpain. On the other hand, m-calpain was not cleaved when normal cervical epithelial cells cultured on soft substrate. To confirm that calpain was indeed activated in soft substrate, we monitored and measured the changes of intracellular calpain activity by using a fluorogenic membrane-permeable calpain substrate t-Boc-LM-CMAC. Cleavage of t-Boc-LM-CMAC by active calpain can induce the retention of CMAC portion of the molecule in cells, which results in increased fluorescence. The fluorescent intensity of CMAC portion kept constant when normal cervical epithelial cells grew on control culture condition (Figure 2B). On the other hand, the fluorescent intensity of CMAC portion was increased to 2.5- and 3.2-fold when normal cervical epithelial cells cultured on soft substrate for 4 and 6 h, respectively (Figure 2B). This confirms that soft substrate potently stimulates the calpain activity.
Figure 2.
Soft substrate induces μ-calpain activation and disrupts the cellular structure of normal cervical epithelial cells. (A) Normal cervical epithelial cells were cultured on various conditions. Cell lysates were harvested at the indicated time points. Arrow and arrowhead, the inactive and active catalytic μ-calpain subunit, respectively. (B) Left panel, representative pictures of time courses of CMAC fluorescent intensity in various culture conditions. Normal cervical epithelial cells were cultured on control condition or on soft substrate for 1, 4, and 6 h. Intracellular calpain activities were measured by using a fluorogenic membrane-permeable calpain substrate t-Boc-LM-CMAC. Cleavage of t-Boc-LM-CMAC by active calpain induced the retention of CMAC portion of the molecule in the cells and resulted in increased fluorescence. Scale bar, 30 μm. Right panel, quantification of the arbitrary unit of fluorescent intensity, which represents the calpain activity. Each column represents mean ± SEM from at least 150 different cells. (C) Soft substrate–induced cleavage of α-spectrin and degraded adhesion molecules. Normal cervical epithelial cells were cultured on various conditions for 6–8 h. Small arrow and small arrowhead, the inactive and active catalytic μ-calpain subunit, respectively. (D) Soft substrate–induced disorganization of actin filaments in normal cervical epithelial cells. Top panel, cells were cultured on control condition or on soft substrate for 8–10 h, and immunofluorescence study was performed to detect actin filaments and nucleus by immunoreaction with phalloidin-Alexa 594 and Hoechst 33258 (HOE), respectively. Scale bars, 10 μm. Bottom panel, morphology of normal cervical epithelial cells cultured on control condition or on soft substrate for 8–10 h, detected by scanning electron microscope. The SEM picture in the control culture condition shows 4–5 confluent cells, but the SEM picture in the soft substrate culture condition shows two round-up cells. Scale bars, 4 μm.
We also analyzed the calpain activation by monitoring the appearance of calpain-specific α-spectrin breakdown products. α-Spectrin is a cytoskeletal scaffold protein which plays an important role in the maintenance of actin architecture. Cleavage of α-spectrin into 150- and 145-kDa breakdown products is a characteristic of calpain activity (Wang, 2000). As shown in Figure 2C, the calpain-specific 150-/145-kDa breakdown products of α-spectrin appeared strongly when normal cervical epithelial cells cultured on soft substrate for 6–8 h. Concomitantly, full-length α-spectrin significantly decreased. No caspase-specific 120-kDa α-spectrin breakdown products were found. The downstream targets of μ-calpain on adhesion molecules such as talin and FAK were also degraded (Figure 2C). The soft substrate–induced cleavage of α-spectrin and the degradation of talin and FAK resulted in the disorganization of actin cytoskeleton and contributed to structural derangement in normal cervical epithelial cells cultured on soft substrate (Figure 2D, top panel). As a consequence, most normal cervical epithelial cells contracted when they grew on soft substrate for more than 8 h and became round-up, detected by the scanning electron microscope (Figure 2D, bottom panel).
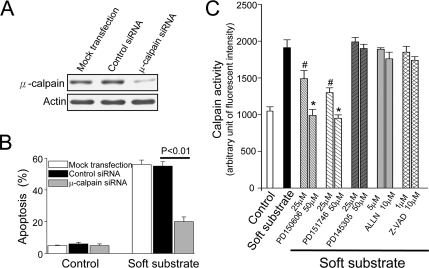
To provide the direct evidence of the involvement of μ-calpain in soft substrate–induced apoptosis, we used siRNA to knockdown μ-calpain and investigated this effect on soft substrate–induced apoptosis. Normal cervical epithelial cells were transfected with 100 nM siRNA specific for μ-calpain for 48 h. Then at 48 h after transfection, the cells were 1) harvested for analysis of protein expression by immunoblotting, 2) cultured on various conditions for 24 h, and 3) analyzed the apoptosis by PI staining. As shown in Figure 3A, endogenous μ-calpain protein levels were specifically reduced in the presence of a μ-calpain–specific double-stranded RNA oligomer. The control siRNA did not alter μ-calpain protein levels. Reduction of μ-calpain protein levels with siRNA significantly inhibited 60–70% apoptosis induced by soft substrate (Figure 3B), indicating that the activation of μ-calpain significantly contributes to soft substrate–induced epithelial apoptosis. We further studied the mechanisms of μ-calpain activation in soft substrate by utilizing inhibitors acting at different target sites of calapin. PD150606 and PD151746, two specific inhibitors targeting Ca2+-binding site of calpain (Fettucciari et al., 2006), inhibited soft substrate–induced calpain activation in a dose-dependent manner (Figure 3C). PD145305, a negative control for PD150606 and PD151746 (Fettucciari et al., 2006), did not show any effect on the activation of calpain. In contrast, ALLN, an inhibitor targeting catalytic binding site of calpain (Debiasi et al., 1999), showed no significant effect on soft substrate–induced calpain activity. The pan-caspase inhibitor, Z-VAD, had no effect on soft substrate–induced activation of calpain, either. This indicates that dysregulation of Ca2+ homeostasis likely plays a causal role to activate μ-calpain activity in soft substrate.
Figure 3.
The involvement of μ-calpain in soft substrate–induced apoptosis. siRNA was used to knockdown μ-calpain and investigated this effect on soft substrate–induced apoptosis. Normal cervical epithelial cells were transfected with 100 nM siRNA specific for μ-calpain for 48 h. Then at 48-h after transfection, the cells were harvested for analysis of protein expression by immunoblotting (A) or cultured on various conditions for 24 h and analyzed the apoptosis by PI staining (B). (C) Effects of various inhibitors on the soft substrate–induced activation of μ-calpain. PD150606 and PD151746, two specific inhibitors by blocking Ca2+-binding site of calpain; PD145305, a negative control for calpain inhibitors; ALLN, an inhibitor targeting catalytic binding site of calpain; Z-VAD, a pan-caspase inhibitor. Normal cervical epithelial cells were cultured on collagen gel–coated dish (control) or on collagen gel (soft substrate) for 4 h in the absence or presence of various inhibitors. Intracellular calpain activities were measured by using a fluorogenic membrane-permeable calpain substrate t-Boc-LM-CMAC. Each column represents mean ± SEM (n = 6).
Soft Substrate Disturbs Ca2+ Homeostasis of Normal Cervical Cells
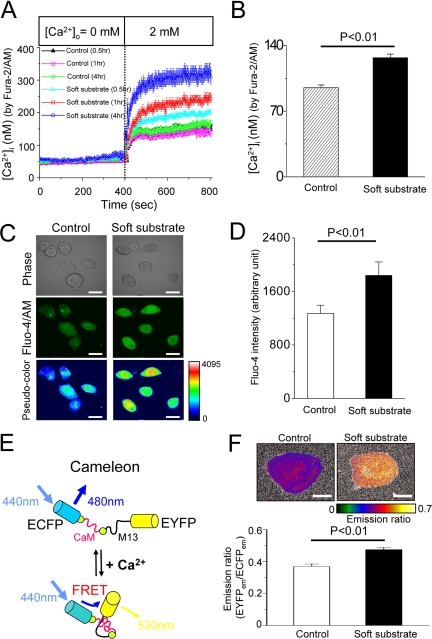
We then examined whether dysregulation of Ca2+ homeostasis happens and contributes to soft substrate–induced epithelial apoptosis. To study Ca2+ entry across the plasma membrane, normal cervical epithelial cells have been preincubated in Ca2+-free media plus 2 μM thapsigargin for 30 min to deplete the internal Ca2+ storage. [Ca2+]i rose as extracellular Ca2+ ([Ca2+]o) was replenished (Figure 4A). Normal cervical epithelial cells cultured on soft substrate had significantly higher levels of Ca2+ entry across the plasma membrane than cells cultured on control condition (Figure 4A). In addition, this up-regulated Ca2+ entry became more obviously with time. As a consequence of enhanced Ca2+ entry, the basal level of [Ca2+]i significantly raised from 95 ± 2 to 125 ± 3 nM (p < 0.01), when normal cervical epithelial cells cultured on soft substrate for 4 h (Figure 4B). To confirm that [Ca2+]i was indeed elevated in soft substrate, we used two other methods to estimate cytosolic Ca2+: 1) confocal image using Fluo-4/AM fluorescent dye and 2) FRET-based Ca2+ measurement by fluorescent indicator protein cameleon. As shown in Figure 4, C and D, cells seeding on soft substrate for 4 h increased the mean pixel intensity of Fluo-4 by 40%, compared with cells seeding on the control condition.
Figure 4.
Soft substrate disturbs Ca2+ homeostasis. (A) Soft substrate up-regulates Ca2+ influx in normal cervical epithelial cells. Cells were cultured on control condition or on soft substrate for 0.5, 1, and 4 h. To measure Ca2+ entry across the plasma membrane, normal cervical epithelial cells were preincubated in Ca2+-free media plus 2 μM thapsigargin (TG) for 30 min to deplete the internal Ca2+ storage. Each trace is mean ± SEM of at least 30 single cells. (B) Basal [Ca2+]i was calculated by Fura-2/AM measurement. Before [Ca2+]i measurement, normal cervical epithelial cells were cultured on the control condition or soft substrate for 4 h in the presence of 2 mM [Ca2+]o. Each column is mean ± SEM from at least 100 cells. (C) Cytosolic Ca2+ levels were estimated by Fluo-4/AM measurement. Representative pictures of phase contrast, green fluorescent intensity and pseudocolor Fluo-4 image in various culture conditions. Before measurement, normal cervical epithelial cells were cultured on the control condition or soft substrate for 4 h in the presence of 2 mM [Ca2+]o. Scale bar, 20 μm. (D) Quantification of Fluo-4 fluorescent intensity. Each column represents mean ± SEM from at least 100 different cells. (E) Model of FRET-based Ca2+ measurement by cameleon. M13, CaM-binding domain of myosin light-chain kinase. (F) Top panel, pseudocolor images indicating emission ratio of EYFP to ECFP for normal cervical epithelial cells seeding on soft substrate or control condition for 4 h in the presence of 2 mM [Ca2+]o. Scale bar, 10 μm. Bottom panel, quantification of emission ratio of EYFP to ECFP. Each column is mean ± SEM from at least 30 cells.
Figure 4E shows the model of FRET-based Ca2+ measurement by cameleon. When [Ca2+]i is elevated, Ca2+ binding to CaM causes intramolecular CaM binding to M13. This results in the conformation change of cameleon molecule, which would increase the emission ratio of EYFP to ECFP. The pseudocolor images of Ca2+ signal levels suggest that emission ratio of EYFP to ECFP for cells seeding on soft substrate for 4 h is 30% higher than that for cells seeding on control condition (Figure 4F). Taken together the results from different techniques, soft substrate increased the basal [Ca2+]i by 30–40%.
Soft Substrate Does Not Disturb Ca2+ Homeostasis of Cervical Cancer Cells
We also studied the regulation of Ca2+ signaling for cervical cancer cells cultured on different substrate rigidities. Culture of cervical cancer SiHa cells on various substrates did not change Ca2+ influx (Figure 5A) or basal [Ca2+]i (Figure 5B) or induce the activation of m- or μ-calpain (Figure 5C). In opposite to the findings in normal cervical epithelial cells, various culture conditions did not significantly change the ratio of cleaved to uncleaved α-spectrin or degrade the proteins of FAK and talin in cervical cancer SiHa cells (Figure 5D). Therefore, there were no significant changes in actin organization and cell morphology for cervical cancer SiHa cells cultured on control condition or soft substrate (Figure 5, E and F).
Figure 5.
Soft substrate does not disturb Ca2+ homeostasis of cervical cancer cells. (A) Soft substrate does not up-regulate Ca2+ influx in cervical cancer cells. Cervical cancer SiHa cells were cultured on various conditions for 4 h before [Ca2+]i measurement. To measure Ca2+ entry across the plasma membrane, cells were preincubated in Ca2+-free media plus 2 μM thapsigargin (TG) for 30 min to deplete the internal Ca2+ storage. Each point is mean ± SEM from at least 60 cells. (B) Soft substrate does not change the basal [Ca2+]i, which was calculated by Fura-2/AM measurement. Cervical cancer SiHa cells were cultured on the control condition or soft substrate for 4 h in the presence of 2 mM [Ca2+]o. Each column is mean ± SEM from at least 100 cells. (C) Soft substrate does not induce the activation of calpain in cervical cancer SiHa cells. Cell lysates were harvested at the indicated time points. A representative immunoblot of four different experiments. (D) Soft substrate does not induce the cleavage of α-spectrin or degradation of adhesion molecules in cervical cancer SiHa cells. SiHa cells were cultured on various conditions for 6–8 h. Cell lysates were harvested and analyzed by SDS-PAGE and Western blots. Small arrow and small arrowhead, the inactive and active catalytic μ-calpain subunit, respectively. Big arrow and big arrowhead, the full-length (280 kDa) and calpain-digested cleaved form (145 kDa) of α-spectrin, respectively. (E) Soft substrate shows no effect on the cellular structure of cervical cancer SiHa cells. Cells were cultured on control condition or on soft substrate for 10 h, and immunofluorescence study was performed to detect actin filaments and nucleus. Scale bars, 10 μm. (F) Morphology of cervical cancer SiHa cells cultured on control condition or on soft substrate for 10 h and detected by scanning electron microscope. Scale bars, 4 μm.
STIM1-mediated Ca2+ Influx Activates Calpain
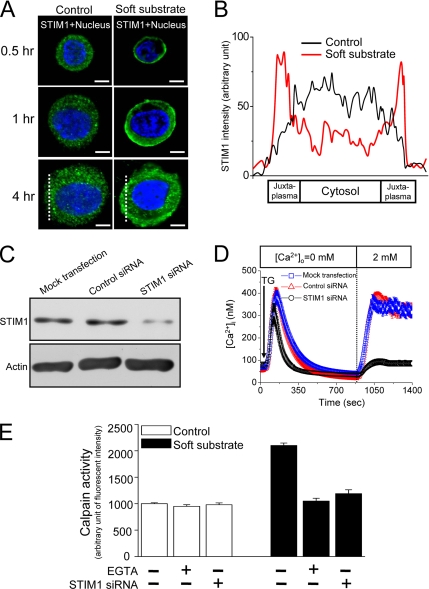
We have demonstrated that soft substrate up-regulated Ca2+ influx in response to depletion of intracellular Ca2+ stores (Figure 4A). Depletion of intracellular Ca2+ stores induces Ca2+ influx across the plasma membrane through SOC channels in a variety of mammalian cells (Bolotina, 2004). STIM1, a putative ER Ca2+ sensor, rapidly translocates to a plasma membrane-adjacent ER compartment upon the depletion of ER Ca2+ stores (Zhang et al., 2005). We therefore did the immunofluorescent staining to investigate if the substrate rigidity affects the cellular distribution of STIM1. In the control culture conditions, most fluorescent intensity of STIM1 scattered in cytosolic areas, which is independent of cell size (Figure 6A). In striking contrast, soft substrate induced the aggregation of STIM1 into puncta that localized to regions near or at the plasma membrane, as well as within the cytoplasm. These phenomena happen as early as 0.5 h after cells seeding and became more obviously 4 h after cells seeding on soft substrate (Figure 6, A and B). These results indicate that soft substrate but not cell size induces STIM1 translocation.
Figure 6.
STIM1-mediated Ca2+ influx activates calpain. (A) Soft substrate induces the translocation of STIM1. Normal cervical epithelial cells were cultured on different substrates for 0.5, 1, and 4 h, and the confocal images were then taken by immunofluorescent staining. A representative image of five different experiments. Scale bar, 8 μm. Dashed lines indicate the area of quantitative analyses of STIM1 fluorescent intensity shown in B. (B) Quantitative fluorescent analysis of cellular STIM1 distribution. Each trace is the mean of fluorescent intensity from five different experiments. (C and D) Knockdown of STIM1 protein inhibits store-operated Ca2+ influx. Normal cervical epithelial cells were transfected with vehicle alone (Mock), control nontargeting siRNA (Control siRNA, 100 nM), or STIM1 siRNA (100 nM) for 48 h. Then at 48 h after transfection, the cells were harvested for analysis of protein expression by immunoblotting (C), and the single-cell [Ca2+]i measurement was studied (D). TG, 2 μM thapsigargin. (E) Soft substrate–induced μ-calpain activation was inhibited by blockade of Ca2+ influx with 1.5 mM EGTA and knockdown of STIM1 by siRNA. Normal cervical epithelial cells were cultured on collagen gel–coated dish (control) or on collagen gel (soft substrate) for 4 h. Intracellular calpain activities were measured by using a fluorogenic membrane-permeable calpain substrate t-Boc-LM-CMAC. Each column represents mean ± SEM (n = 6). EGTA, ethyleneglycol-bis(2-aminoethylether)-N,N,N′,N′-tetra-acetic acid.
We further used siRNA to knockdown STIM1 expression in determining whether STIM1-mediated Ca2+ influx contributed to soft substrate–induced calpain activation. Endogenous STIM1 protein levels and the activation of store-operated Ca2+ entry were specifically reduced in the presence of a STIM1-specific double-stranded RNA oligomer (Figure 6, C and D), indicating that STIM1 has a plasma membrane role in the activation of SOC channels. Furthermore, chelating [Ca2+]o by EGTA or down-regulated STIM1-mediated Ca2+ influx by siRNA significantly inhibited soft substrate–induced activation of μ-calpain (Figure 6E). Thus, these data indicate that enhanced Ca2+ entry mediated by STIM1 translocation plays an important role in the regulation of soft substrate-activated calpain activity.
Interaction of STIM1 and SOC Channels
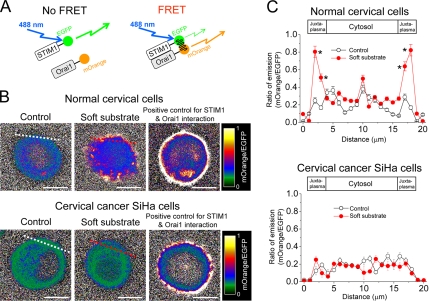
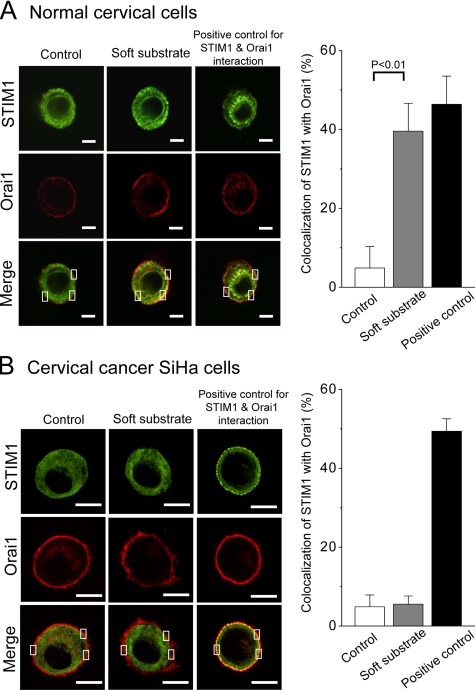
STIM1 has recently been shown to be essential for the regulation of Orai1, a pore subunit of the SOC channel (Marchant, 2005). To test whether soft substrate enhances the interaction between STIM1 and membrane SOC channel, we developed a live-cell FRET approach to visualize and quantify the interaction between EGFP-conjugated STIM1 and mOrange-conjugated Orai1 (Figure 7A). When EGFP–STIM1 and mOrange–Orai1 fusion proteins do not interact, illumination with blue (∼488 nm) light results in stronger EGFP fluorescence. As a result of Ca2+ depletion–induced interactions between EGFP–STIM1 and mOrange–Orai1, EGFP, and mOrange are brought into proximity and excitation energy is transferred, resulting in stronger mOrange fluorescence. The pseudocolored FRET images indicate that the increased emissive ratio of mOrange to EGFP happens in the juxta-membrane regions for normal cervical cells seeding on soft substrate (Figure 7B). We did the statistical analyses on the ratio of emission for normal cervical cells and cervical cancer cells that were cultured on different substrate rigidities. For normal cervical cells cultured on the soft substrate, there is a significant increase of emission ratio of mOrange to EGFP in the juxta-membrane region. In contrast, there is no significant change in the emission ratio of mOrange to EGFP for cervical cancer cells cultured either on control condition or on soft substrates (Figure 7C). The confocal images suggest that soft substrate induce the aggregation and translocation of STIM1 toward the cell periphery to colocalize with Orai1 on the opposing plasma membrane of normal cervical cells (Figure 8A). The quantitative analyses of confocal images confirm that soft substrate significantly increases the colocalization of STIM1 with Orai1 in normal cervical cells but not in cervical cancer cells (Figure 8). Thus the evidences from FRET measurement as well as image analyses support the hypothesis that soft substrate up-regulates the interaction of STIM1 with Orai1 in normal cervical cells but not in cervical cancer cells.
Figure 7.
The interaction between STIM1 and Orai1 detected by FRET measurement. (A) Model of FRET measurement. EGFP and mOrange were conjugated with STIM1 and Orai1, respectively, and used as a FRET pair. An optional 488 nm laser was used to directly excite EGFP without exciting mOrange. When EGFP–STIM1 and mOrange–Orai1 fusion proteins do not interact, illumination with blue (∼488 nm) light results in stronger EGFP fluorescence. As a result of Ca2+ depletion–induced interactions between EGFP–STIM1 and mOrange–Orai1, EGFP and mOrange are brought into proximity and excitation energy is transferred, resulting in stronger mOrange fluorescence. (B) Pseudocolor FRET images of emission ratio of mOrange to EGFP. Representative FRET images of normal cervical epithelial cells and cervical cancer cells cultured on different substrate rigidities for 4 h. The detailed information of FRET measurement was described in Materials and Methods. Cells treated with 2 μM thapsigargin were used as positive control for STIM1 and Orai1 interaction. Dashed lines indicate the area of quantitative analyses of emission ratio of mOrange to EGFP shown in C. Scale bar, 10 μm. (C) Quantitative analyses of emission ratio of mOrange to EGFP. Each point represents mean ± SEM from at least 15 cells. * p < 0.01, compared with control condition.
Figure 8.
The interaction between STIM1 and Orai1 detected by confocal images. Soft substrate stimulates the interaction between STIM1 and Orai1 in normal cervical epithelial cells (A) but not in cervical cancer SiHa cells (B). DNA plasmids of EGFP–STIM1 and mOrange–Orai1 were cotransfected into cells and then cultured on different substrate rigidities for 4 h. Soft substrate induced the aggregation and translocation of STIM1 toward the cell periphery to colocalize with Orai1 on the opposing plasma membrane of normal cervical epithelial cells. Cells treated with 2 μM thapsigargin were used as positive control for STIM1 and Orai1 interaction. Left panel, the representative confocal images. The inset indicates that a pixel-by-pixel colocalization analysis by FV-1000 software was used to assess levels of STIM1 colocalization with Orai1. The quantitative results are shown in the right panels. Scale bar, (A) 6 μm; (B) 10 μm.
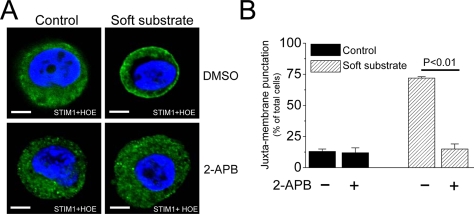
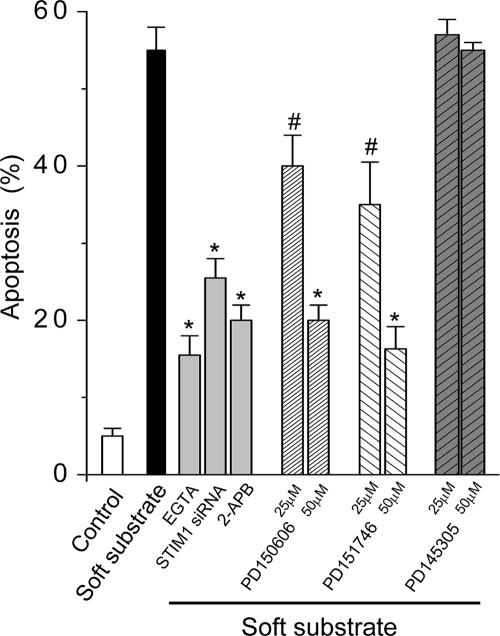
There is a distinct mode of activating STIM1 translocation, i.e., through depletion of ER Ca2+ stores by inositol 1,4,5-trisphosphate (IP3) signaling (Lis et al., 2007). The IP3 receptor inhibitor, 2-aminoethoxydiphenyl borate (2-APB; Lis et al., 2007), prevented the puncta formation of STIM1 near the plasma membrane in the soft substrate (Figure 9), indicating that IP3 signaling is the upstream regulation to trigger ER STIM1 activation and translocation. More importantly, soft substrate–induced apoptosis was significantly inhibited by chelating [Ca2+]o with EGTA, down-regulation of STIM1-mediated Ca2+ influx by siRNA, IP3 receptor inhibitor (2-APB), or calpain inhibitors targeting the Ca2+-binding site (Figure 10).
Figure 9.
IP3 signaling is required for STIM1 redistribution induced by soft substrate. (A) Soft substrate induces the puncta formation of STIM1, which is abolished by the inhibitor of IP3 receptor. Normal cervical epithelial cells were cultured on collagen gel–coated dish (control) or on collagen gel (soft substrate) for 4 h in the absence or presence of IP3 receptor inhibitor (20 μM 2-APB). The confocal images were then taken by immunofluorescent staining. (B) Quantitative analyses of juxta-membrane punctuation of STIM1. Each column represents mean ± SEM from five different experiments.
Figure 10.
Inhibition of Ca2+ signaling decreases soft substrate–induced apoptosis. Normal cervical epithelial cells were cultured on collagen gel–coated dish (control) or on collagen gel (soft substrate) for 24 h in the absence or presence of various chemicals (1.5 mM EGTA, 20 μM 2-APB, 50 μM PD150606, PD151746, and PD145305) or STIM1 siRNA. Apoptotic analysis was assessed by flow cytometry of PI staining. Each column represents mean ± SEM from three different experiments. # p < 0.05; * p < 0.01, compared with soft substrate–induced apoptosis.
DISCUSSION
This study highlights the functional significance of STIM1 in response to the physical properties of extracellular matrix. We identify the Ca2+ transport system that contributes to the soft substrate–induced apoptotic cascades. Changes in cytosolic Ca2+ levels regulate a variety of fundamental cellular functions. In nonexcitable cells, a major pathway of Ca2+ entry involves receptor-mediated depletion of [Ca2+]i stores followed by the activation of SOC channels in the plasma membrane. The last couple of years have seen significant breakthroughs in the study of SOC channel, i.e., identification of STIM1 as the store Ca2+ sensor and Orai1 as the pore-forming essential subunit of SOC channel. Soft substrate induces the aggregation and translocation of STIM1 toward the cell periphery to colocalize with Orai1 on the opposing plasma membrane, demonstrated by FRET measurement and confocal imaging. The enhancing STIM1 translocation correlates with the up-regulation of Ca2+ entry in soft substrate which subsequently disrupts the integrity of Ca2+-signaling complexes.
Here we demonstrate that soft substrate of collagen gel causes the activation of Ca2+-dependent protease μ-calpain, which leads to apoptosis in normal cervical epithelial cells. In addition, soft substrate–induced apoptosis is cell-type specific. This conclusion is supported by the following findings: 1) Culture on soft substrate of collagen gel inhibited the growth of normal cervical epithelial cells but not cervical cancer cells. 2) Positive annexin-V staining, an early apoptotic marker, happened as early as 4 h after normal cervical epithelial cells cultured on soft substrate. 3) The analyses of PI staining confirmed that soft substrate–induced cell death of normal cervical epithelial cells through the apoptotic pathways. 4) Ca2+-activated cystine protease, μ-calpain, was cleaved on soft substrate, detected by Western blotting. The image studies showed the increasing retention of synthetic fluorescent calpain substrate, confirming the activation of calpain activity in soft substrate. 5) PD150606, a specific inhibitor of calpains by blocking of the calpain Ca2+-binding site, inhibited soft substrate–induced calpain activation and apoptosis in a dose-dependent manner. PD151746, the 20-fold selective inhibitor for μ-calpain directed to Ca2+-binding site, showed the similar effects. 6) Knockdown of μ-calpain protein levels with siRNA significantly inhibited ∼70% apoptosis induced by soft substrate. This supports the data obtained using pharmacological calpain inhibitors and provides further evidence that μ-calpain is involved in soft substrate–induced apoptosis.
This study highlights the importance of Ca2+ homeostasis for epithelial cells in response to the physical properties of extracellular matrix. Soft substrate disrupted the integrity of Ca2+-signaling complexes in normal cervical epithelial cells, by up-regulation of STIM1-mediated Ca2+ influx. The store-operated Ca2+ influx mediated by STIM1 translocation is important for the replenishment of Ca2+ stores and is also involved in many signaling processes by virtue of the ability of [Ca2+]i to act as a second messenger (Soboloff et al., 2006). Blockade of Ca2+ influx by EGTA or STIM1 knockdown by siRNA almost abolished soft substrate–induced activation of μ-calpain and significantly inhibited soft substrate–induced apoptosis. Thus, loss of Ca2+ homeostasis followed by calpain activation significantly contributes to soft substrate–induced epithelial cell apoptosis. STIM1 siRNA, IP3 receptor inhibitor 2-APB, and blocking calcium influx by EGTA inhibited 64, 75, and 82% of soft substrate–induced apoptosis, respectively. These data indicate that IP3-dependent STIM1 translocation is an important signaling, but not the only pathway for soft substrate–induced apoptosis. There is convincing evidence that the alteration of Ca2+ homeostasis is associated with cell survival and death (Rizzuto et al., 2003). Recent studies have established some of the biochemical mechanisms (e.g., the proteolytic enzymes) by which intracellular Ca2+ overload can trigger either necrotic or apoptotic cell death, and several researches have shown that prevention of Ca2+ overload by pretreatment with either Ca2+ chelators, receptor antagonists, or channel blockers can rescue cells that would otherwise die (Orrenius et al., 2003). Similarly, a Ca2+-based theory of carcinogenesis has been proposed. For example, dysregulation of Ca2+ homeostasis has been demonstrated to initiate oncogenesis that leads to cell malignant transformation (Jaffe, 2005).
The cellular behavior on soft extracellular matrix is characteristic of important phenotypes; e.g., cell growth on soft agar gels is to differentiate cancer cells from normal cells. The responses to substrate rigidity also play an important role in distinguishing the growth behavior of normal cells from that of transformed cells (Wang et al., 2000). Here we confirm that soft substrate of collagen gel induced apoptosis in normal cervical epithelial cell, but not in cervical cancer cells. More importantly, we show that soft substrate differentially regulated the interaction of STIM1 with SOC channels during the malignant transformation of human cervical epithelial cells. An understanding of how cells in tissues, including fibroblasts, myocytes, neurons, and other cell types, sense matrix stiffness is just emerging with quantitative studies of cells adhering to gels (or to other cells) with which elasticity can be tuned to approximate that of tissues (Engler et al., 2004; Discher et al., 2005, Paszek et al., 2005). Key roles in signal pathways are played by adhesion complexes and cytoskeleton, whose contractile forces are transmitted through transcellular structures. The feedback of local matrix stiffness on cell state likely has important implications for cellular function, such as development, differentiation, regeneration, and malignant transformation (Georges and Janmey, 2005; Hung and Ingber, 2005; Guo et al., 2006). Further studies need to clarify the relationship between cell tension, matrix mechanics, and tumor development.
α-Spectrin plays an important role in the maintenance of actin architecture. It is irreversibly cleaved by the proteolytic enzyme calpain and caspase 3 at different specific cleavage sites, leading to the disruption of cytoskeleton (Büki et al., 2000). Cleavage of spectrin causes the membrane to form blebs and results in cell death (Castillo and Babson, 1998). Here we found that soft substrate caused breakdown of α-spectrin mediated by activation of calpain as early as 6–8 h after normal cervical epithelial cells cultured on soft substrate. The downstream targets of μ-calpain on adhesion molecules such as talin and FAK were degraded as well. These findings establish a relationship between Ca2+ signaling, calpain activation, and cellular structure and function in epithelial cells responding to the stiffness of their substrate.
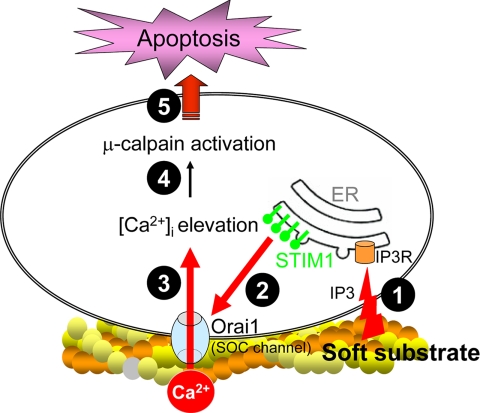
Taken together, the regulatory mechanisms of soft substrate–induced normal epithelial cell apoptosis can be summarized as follows (Figure 11). Soft substrate induces the aggregation and translocation of STIM1 toward the cell periphery to colocalize with Orai1, an essential pore subunit of SOC channel. The cellular redistribution of STIM1 depends on IP3 signaling. The up-regulated interaction between STIM1 and Orai1 enhances SOC entry, which results in increased [Ca2+]i. Thus, soft substrate causes the dysregulation of Ca2+ homeostasis in normal cervical epithelial cells. The disturbance of Ca2+ homeostasis triggers the activation of μ-calpain, which mediates the disruption of cytoskeletal organization and the subsequent apoptosis. Further studies are to investigate the differential mechanical sensing machineries leading to IP3-dependent STIM1 translocation during the malignant transformation of human cervical epithelial cells.
Figure 11.
Schematic diagram of soft substrate–induced normal epithelial cell apoptosis. Soft substrate stimulates the aggregation and translocation of STIM1, the ER Ca2+ sensor, toward the cell periphery to colocalize with Orai1, an essential pore subunit of the store-operated Ca2+ (SOC) channel. The cellular redistribution of STIM1 depends on inositol 1,4,5-trisphosphate (IP3) signaling. The up-regulated interaction between STIM1 and Orai1 enhances Ca2+ entry across the plasma membrane which leads to the increased cytosolic Ca2+ ([Ca2+]i). This disturbed Ca2+ homeostasis results in the activation of μ-calpain, which cleaves α-spectrin, induces actin disorganization, and causes apoptosis.
ACKNOWLEDGMENTS
This work was partly supported by National Science Council (NSC96-2120-M-006-004 to M.R.S. and M.J.T.) Taiwan, Center of Excellence for Clinical Trial and Research (DOH-TD-B-111-004), Department of Health, Executive Yuan, Taiwan and Center for Gene Regulation and Signal Transduction Research, National Cheng Kung University, Taiwan.
Abbreviations used:
- [Ca2+]i
intracellular Ca2+
- [Ca2+]o
extracellular Ca2+
- ER
endoplasmic reticulum
- SOC channel
store-operated Ca2+ channel
- STIM1
stromal interacting molecule 1.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-11-1170) on March 12, 2008.
REFERENCES
- Beningo K. A., Lo C. M., Wang Y. L. Flexible polyacrylamide substrata for the analysis of mechanical interactions at cell-substratum adhesions. Methods Cell Biol1. 2002;69:325–339. doi: 10.1016/s0091-679x(02)69021-1. [DOI] [PubMed] [Google Scholar]
- Benjamin M., Hillen B. Mechanical influences on cells, tissues and organs—mechanical morphogenesis. Eur. J. Morphol. 2003;41:3–7. doi: 10.1076/ejom.41.1.3.28102. [DOI] [PubMed] [Google Scholar]
- Bershadsky A. D., Balaban N. Q., Geiger B. Adhesion-dependent cell mechanosensitivity. Annu. Rev. Cell Dev. Biol. 2003;19:677–695. doi: 10.1146/annurev.cellbio.19.111301.153011. [DOI] [PubMed] [Google Scholar]
- Bolotina V. M. Store-operated channels: diversity and activation mechanisms. Sci. STKE. 2004;243:pe34. doi: 10.1126/stke.2432004pe34. [DOI] [PubMed] [Google Scholar]
- Büki A., Okonkwo D. O., Wang K. K., Povlishock J. T. Cytochrome c release and caspase activation in traumatic axonal injury. J. Neurosci. 2000;20:2825–2834. doi: 10.1523/JNEUROSCI.20-08-02825.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo M. R., Babson J. R. Ca2+-dependent mechanisms of cell injury in cultured cortical neurons. Neuroscience. 1998;86:1133–1144. doi: 10.1016/s0306-4522(98)00070-0. [DOI] [PubMed] [Google Scholar]
- Chen C. S., Mrksich M., Huang S., Whitesides G. M., Ingber D. E. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- Chicurel M. E., Chen C. S., Ingber D. E. Cellular control lies in the balance of forces. Curr. Opin. Cell Biol. 1998;10:232–239. doi: 10.1016/s0955-0674(98)80145-2. [DOI] [PubMed] [Google Scholar]
- Chiu W. T., Wang Y. H., Tang M. J., Shen M. R. Soft substrate induces apoptosis by the disturbance of Ca2+ homeostasis in renal epithelial LLC-PK1 cells. J. Cell Physiol. 2007;212:401–410. doi: 10.1002/jcp.21037. [DOI] [PubMed] [Google Scholar]
- Chou C. Y., Shen M. R., Wu S. N. Volume-sensitive chloride channels associated with human cervical carcinogenesis. Cancer Res. 1995;55:6077–6083. [PubMed] [Google Scholar]
- Cukierman E., Pankov R., Stevens D. R., Yamada K. M. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- Discher D. E., Janmey P., Wang Y. L. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- Debiasi R. L., Squier M. K., Pike B., Wynes M., Dermody T. S., Cohen J. J., Tyler K. L. Reovirus-induced apoptosis is preceded by increased cellular calpain activity and is blocked by calpain inhibitors. J. Virol. 1999;73:695–701. doi: 10.1128/jvi.73.1.695-701.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler A. J., Griffin M. A., Sen S., Bönnemann C. G., Sweeney H. L., Discher D. E. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J. Cell Biol. 2004;166:877–887. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler A. J., Sen S., Sweeney H. L., Discher D. E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Fettucciari K., Fetriconi I., Mannucci R., Nicoletti I., Bartoli A., Coaccioli S., Marconi P. Group B Streptococcus induces macrophage apoptosis by calpain activation. J. Immunol. 2006;176:7542–7556. doi: 10.4049/jimmunol.176.12.7542. [DOI] [PubMed] [Google Scholar]
- Georges P. C., Janmey P. A. Cell type-specific response to growth on soft materials. J. Appl. Physiol. 2005;98:1547–1553. doi: 10.1152/japplphysiol.01121.2004. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Guo W. H., Frey M. T., Burnham N. A., Wang Y. L. Substrate rigidity regulates the formation and maintenance of tissues. Biophys. J. 2006;90:2213–2220. doi: 10.1529/biophysj.105.070144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda S., Marumoto T., Hirota T., Nitta M., Arima Y., Ogawa M., Saya H. Activation of m-Calpain is required for chromosome alignment on the metaphase plate during mitosis. J. Biol. Chem. 2004;279:10615–10623. doi: 10.1074/jbc.M308841200. [DOI] [PubMed] [Google Scholar]
- Hung S., Ingber D. E. Cell tension, matrix mechanics, and cancer development. Cancer Cell. 2005;8:175–176. doi: 10.1016/j.ccr.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Jaffe L. F. A calcium-based theory of carcinogenesis. Adv. Cancer Res. 2005;94:231–263. doi: 10.1016/S0065-230X(05)94006-2. [DOI] [PubMed] [Google Scholar]
- Jiang S. T., Yang T. P., Huang J. J., Hsu C. C., Tang M. J. Age effect of type I collagen on morphogenesis of Madin-Darby canine kidney cells. Kidney Int. 2000;57:1539–1548. doi: 10.1046/j.1523-1755.2000.00998.x. [DOI] [PubMed] [Google Scholar]
- Lis A., Peinelt C., Beck A., Parvez S., Monteilh-Zoller M., Fleig A., Penner R. CRACM1, CRACM2, and CRACM3 are store-operated Ca2+ channels with distinct functional properties. Curr. Biol. 2007;17:794–800. doi: 10.1016/j.cub.2007.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo C. M., Wang H. B., Dembo M., Wang Y. L. Cell movement is guided by the rigidity of the substrates. Biophys. J. 2000;79:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant J. S. Cellular signaling: STIMulating calcium entry. Curr. Biol. 2005;15:R493–R495. doi: 10.1016/j.cub.2005.06.035. [DOI] [PubMed] [Google Scholar]
- Nagai T., Yamada S., Tominaga T., Ichikawa M., Miyawaki A. Expanded dynamic range of fluorescent indicators for Ca2+ by circularly permuted yellow fluorescent proteins. Proc. Natl. Acad. Sci. USA. 2004;101:10554–10559. doi: 10.1073/pnas.0400417101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrenius S., Zhivotovsky B., Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat. Rev. Mol. Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- Paszek M. J., et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Pelham R. J., Jr, Wang Y. L. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R., Pinton P., Ferrari D., Chami M., Szabadkai G., Magalhaes P. J., Di Virgili F., Pozzan T. Calcium and apoptosis: facts and hypotheses. Oncogene. 2003;22:8619–8627. doi: 10.1038/sj.onc.1207105. [DOI] [PubMed] [Google Scholar]
- Roos J., et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross P. E., Cahalan M. Ca2+ influx pathways mediated by swelling or stores depletion in mouse thymocytes. J. Gen. Physiol. 1995;106:415–444. doi: 10.1085/jgp.106.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M. R., Chou C. Y., Chiu W. T. Streptomycin and its analogues are potent inhibitors of the hypotonicity-induced Ca2+ entry and Cl− channel activity. FEBS Lett. 2003;554:494–500. doi: 10.1016/s0014-5793(03)01231-6. [DOI] [PubMed] [Google Scholar]
- Soboloff J., Spassova M. A., Dziadek M. A., Gill D. L. Calcium signals mediated by STIM and Orai proteins—a new paradigm in inter-organelle communication. Biochim. Biophys. Acta. 2006;1763:1161–1168. doi: 10.1016/j.bbamcr.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Vogel V., Sheetz M. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 2006;7:265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- Wang H. B., Dembo M., Wang Y. L. Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. Am. J. Physiol. 2000;279:C1345–C1350. doi: 10.1152/ajpcell.2000.279.5.C1345. [DOI] [PubMed] [Google Scholar]
- Wang K. K. Calpain and caspase: can you tell the difference? Trends Neurosci. 2000;23:20–26. doi: 10.1016/s0166-2236(99)01536-2. [DOI] [PubMed] [Google Scholar]
- Wang Y. K., Wang Y. H., Wang C. Z., Sung J. M., Chiu W. T., Lin S. H., Chang Y. H., Tang M. J. Rigidity of collagen fibrils controls collagen gel-induced down-regulation of focal adhesion complex proteins mediated by integrin. J. Biol. Chem. 2003;278:21886–21892. doi: 10.1074/jbc.M300092200. [DOI] [PubMed] [Google Scholar]
- Wang C. Z., Hsu Y. M., Tang M. J. Function of discoidin domain receptor I in HGF-induced branching tubulogenesis of MDCK cells in collagen gel. J. Cell Physiol. 2005;203:295–304. doi: 10.1002/jcp.20227. [DOI] [PubMed] [Google Scholar]
- Wang Y. H., Chiu W. T., Wang Y. K., Wu C. C., Chen T. L., Teng C. F., Chang W. T., Chang H. C., Tang M. J. Deregulation of AP-1 proteins in collagen gel-induced epithelial cell apoptosis mediated by low substratum rigidity. J. Biol. Chem. 2007;282:752–763. doi: 10.1074/jbc.M604801200. [DOI] [PubMed] [Google Scholar]
- Wu C. C., Ding S. J., Wang Y. H., Tang M. J., Chang H. C. Mechanical properties of collagen gels derived from rats of different ages. J. Biomater. Sci. Polym. Ed. 2005;16:1261–1275. doi: 10.1163/156856205774269494. [DOI] [PubMed] [Google Scholar]
- Xu L., Deng X. Tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone induces phosphorylation of μ- and m-calpain in association with increased secretion, cell migration, and invasion. J. Biol. Chem. 2004;279:53683–53690. doi: 10.1074/jbc.M409889200. [DOI] [PubMed] [Google Scholar]
- Zhang S. L., Yu Y., Ross J., Kozak J. A., Deerinck T. J., Ellisman M. H., Stauderman K. A., Cahalan M. D. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]