Abstract
Interphase microtubules are organized into a radial array with centrosome in the center. This organization is a subject of cellular regulation that can be driven by protein phosphorylation. Only few protein kinases that regulate microtubule array in interphase cells have been described. Ste20-like protein kinase LOSK (SLK) was identified as a microtubule and centrosome-associated protein. In this study we have shown that the inhibition of LOSK activity by dominant-negative mutant K63R-ΔT or by LOSK depletion with RNAi leads to unfocused microtubule arrangement. Microtubule disorganization is prominent in Vero, CV-1, and CHO-K1 cells but less distinct in HeLa cells. The effect is a result neither of microtubule stabilization nor of centrosome disruption. In cells with suppressed LOSK activity centrosomes are unable to anchor or to cap microtubules, though they keep nucleating microtubules. These centrosomes are depleted of dynactin. Vero cells overexpressing K63R-ΔT have normal dynactin “comets” at microtubule ends and unaltered morphology of Golgi complex but are unable to polarize it at the wound edge. We conclude that protein kinase LOSK is required for radial microtubule organization and for the proper localization of Golgi complex in various cell types.
INTRODUCTION
The radial array of microtubules is typical for many mammalian cells. It organizes bidirectional organelle transport in the cytoplasm in the endocytotic and exocytotic direction. It is also required for the regulation of interaction of microtubule plus ends with cell periphery. Both functions are important for cell polarization, movement, and signal transduction (Hyman and Karsenti, 1996; Dujardin et al., 2003; Morrison, 2007). The degree of radiality of microtubules varies in different types of cells. For instance, in fish melanophores the system of microtubules is perfectly radial (Schliwa et al., 1978), whereas in myotubes it is unclear (Tassin et al., 1985; Musa et al., 2003). Moreover, among fibroblast-like cultured mammalian cells some (green monkey kidney Vero or Chinese hamster ovary CHO-K1) possess a distinct radial microtubule array (Bre et al., 1987), whereas others (human cervical carcinoma HeLa or mouse fibroblasts NIH 3T3) have rather chaotic microtubule arrangements (Bulinski and Borisy, 1980). Regardless of the tissue origin of cells, microtubules become more radial when cultured cells are sparse and less radial in confluent cultures. It seems that the status of microtubule organization is regulated by signal transduction pathways and depends on cell differentiation.
The focused microtubule arrays can also be formed in acentrosomal cell fragments as a result of interactions between microtubules and membrane vesicles, covered with motor proteins (Rodionov and Borisy, 1997; Malikov et al., 2005). In acentrosomal fragments of fish melanocytes microtubules are chaotic when pigment granules are dispersed. After the addition of adrenaline or other activation of melanosome aggregation microtubules assemble in a radial array. Thus, in this case the formation of radial microtubule array directly depends on signal transduction (Malikov et al., 2005).
The focused microtubule array in mammalian cells is usually organized by the centrosome and partly by the Golgi apparatus. Cellular centrosomes nucleate, anchor, cap, and release microtubules (Doxsey, 2001). All these centrosomal activities depend on certain proteins and can be regulated on the level of protein expression or activity. The latter can be modified by reversible phosphorylation. A number of protein kinases and phosphatases are associated with the centrosome; however, most of them regulate the centrosome duplication cycle, and only few were shown to be involved in centrosome-dependent microtubule organization. Recent works revealed that protein kinases can regulate both microtubule nucleation and anchoring at the centrosome. The association of phospho-beta catenin with centrosome enchances its microtubule-nucleating activity (Huang et al., 2007). Protein kinase Nek7 modulates the association of microtubule-nucleating γ-tubulin ring complexes (γTuRC) with the centrosome (Kim et al., 2007). This association is controlled by phosphorylation of GCP-WD protein (Luders et al., 2006). Protein kinases are also required for the accumulation of γTuRC in centrosome at the onset of mitosis (Abe et al., 2006). Protein kinase GSK-3β controls microtubule anchoring and capping by activation of dynein-dependent delivery of microtubule-anchoring protein ninein to the centrosome (Fumoto et al., 2006). Aurora A kinase regulates DTACC-Msps–mediated anchoring of microtubule minus ends on mitotic centrosomes (Barros et al., 2005). More protein kinases are expected to be involved in the organization of microtubule arrays.
LOSK (Long Ste20-like Kinase), a member of the germinal center kinase group, was identified as a microtubule and centrosome-associated protein (Zinovkina et al., 1997, 1998; Nadezhdina et al., 2001). We expected that it participates in the regulation of centrosomal function or microtubule organization. LOSK was described also by other authors under the name SLK (Ste20-Like Kinase; Itoh et al., 1997; Sabourin and Rudnicki, 1999; Yamada et al., 2000). LOSK/SLK (hereafter LOSK) is ubiquitously expressed in mammalian cells (Itoh et al., 1997; Zinovkina et al., 1998; Sabourin and Rudnicki, 1999; Storbeck et al., 2004), and its activity remains constant throughout the cell cycle though it slightly increases in mitosis (Ellinger-Ziegelbauer et al., 2000; O'Reilly et al., 2005). Either down- or up-regulation of this minor kinase results in apoptotic cell death (Sabourin et al., 2000; O'Reilly et al., 2005), indicating the importance of cell functions regulated by LOSK. However, natural LOSK substrates have not been identified yet.
In this work we studied the influence of LOSK activity on the microtubule system in cultured cells. The catalytic domain of LOSK was used to increase the activity of this kinase. Catalytic domain carrying K63R mutation was used as a dominant-negative construct to inhibit LOSK activity, and vector-based short hairpin RNA interference (shRNAi) was used to deplete LOSK from cells. We have shown that LOSK inhibition or depletion result in disruption of radial microtubule arrays, although the microtubule-nucleating activity of the centrosome is not altered. LOSK inhibition disturbs microtubule anchoring at the centrosome and causes the decrease of centrosomal dynactin content, whereas microtubule stability remains unaltered. LOSK inhibition does not alter GA integrity although it prevents GA polarization at a wound edge. We suggest that LOSK is involved in the signal transduction pathway that regulates the radial microtubule array in mammalian interphase cells.
MATERIALS AND METHODS
DNA Constructs
All LOSK cDNAs described below complied with the sequence of cDNA of KIAA0204 protein (human LOSK variant), NCBI Accession Number NM_014720. LOSK catalytic domain ΔT (amino acids 1-342, Figure 1A) cDNA was obtained from human brain cDNA library by PCR. To make the K63R mutation, the cDNA was mutated using QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). The XbaI internal site was used to create the deletion mutant ΔNΔT (amino acids 94-342, Figure 1A). The cDNA of LOSK (661-1018 amino acid fragment) was obtained using cDNA library of HeLa cells by PCR. A fragment of cDNA coding amino acids 779-1018 (M2f fragment, Figure 1A) was cut out using EcoRV internal site. Full-length cDNA of human LOSK was kindly provided by Kazusa DNA Research Institute (Japan). The LOSK amino acid 339-663 fragment (M1f, Figure 1A) and LOSK-Ct fragment (amino acids 944-1235, Figure 1A) were obtained by PCR using this cDNA. Full-length cDNA of human dynactin-1 (p150(Glued)) was kindly provided by Dr. W. Steffen (Medical School Hannover, Hannover, Germany). cDNAs were cloned to pQE30 (Qiagen, Hilden, Germany), pEGFPC2, pEGFPC1, and pDsRed-Monomer-C1 (Clontech, Palo Alto, CA), pGEX2T and pGEX4T vectors (GE Healthcare, Little Chalfont, United Kingdom). PEGFP-LamB plasmid for EGFP-lamin B expression in mammalian cells was kindly provided by Dr. S. Kurchashova (M.V. Lomonosov Moscow State University, Moscow, Russia). All the final constructs were verified by automatic sequencing.
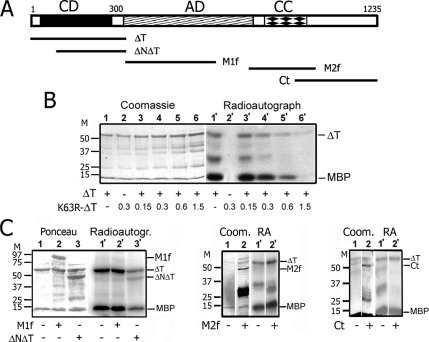
Figure 1.
Protein kinase LOSK ΔT fragment possesses catalytic activity in vitro that can be inhibited with an excess of K63R-ΔT but not with other LOSK fragments. (A) The structure of the LOSK molecule and its fragments used in this work. Numbers indicate amino acids. CD, catalytic domain; AD, acid central domain with indefinite secondary structure; CC, predicted coiled-coil domain. (B) The inhibitory effect of K63R-ΔT to ΔT activity in vitro. Lanes 1–6, Coomassie-stained gel; lanes 1′–6′, radioautograph of the same gel. Each reaction mixture included 0.5 μg of MBP and 0.3 μg of ΔT, except lanes 2 and 2′ with K63R-ΔT alone. Lanes 3–6 and 3′–6′, the amount of K63R-ΔT (μg) added is indicated. (C). The influence of LOSK fragments on ΔT activity in vitro. ΔNΔT partially inhibited ΔT activity; M1f, M2f, and Ct had no effect. Note the highly deviant electrophoretic mobility of M1f (predicted molecular mass of fused with GST M1f is 63 kDa). Molecular mass markers are indicated (kDa).
For RNAi depletion of LOSK, the pG-Shin2 vector (kindly provided by Dr. S.-I. Kojima, Northwestern University, Chicago, IL) was used. This vector bears shRNA synthesis cassette under the H1 promoter and green fluorescent protein (GFP) cDNA under the cytomegalovirus (CMV) promoter (Kojima et al., 2004). DNA oligonucleotide primers were designed according to manufacturer's recommendations and included 19-nucleotide (nt) target sequences selected in LOSK open reading frame (ORF; 278–297 and 3202–3221 nt). The resulting constructs were named pG-Shin2-4.1 and pG-Shin2-6.1, respectively.
Restriction endonucleases and other cloning enzymes were purchased from Fermentas International (Burlington, Canada) or Sibenzyme (Novosibirsk, Russia). The plasmid DNA for transfection of culture cells was purified with Endo Free Plasmid Mega Kit, Spin Miniprep, and Midiprep kits (Qiagen, Chatsworth, CA).
Cell Growth, Transfection, Fixation, and Immunostaining
Green monkey kidney Vero epithelia-like cells, human cervical carcinoma HeLa cells, Chinese hamster ovary CHO-K1 fibroblast-like cells, green monkey kidney CV-1 fibroblast-like cells, and pig embryo kidney PE cells were cultured in medium containing 40% DMEM, 40% F10 medium, and 10% newborn calf serum (Paneco, Moscow, Russia) at 37°C and 5% CO2. Transfection was performed using either Maxifectin-M or Unifectin-56 reagent (Unifect, Moscow, Russia) according to the manufacturer's instructions. In some experiments cells were electrotransfected using a Multiporator (Eppendorf, Hamburg, Germany) according to the manufacturer's instructions, or cDNA was microinjected into cell cytoplasm using an Eppendorf Microinjector and micromanipulator. Experiments with cells and fixation were made at 18–22 h after transfection.
To induce microtubule regrowth, cells were chilled in an ice bath for 2 h and then 1.5 μg/ml nocodazole (Sigma, St. Louis, MO) was added, cells were warmed at 37°C, and nocodazole was washed out for the time indicated in the figure legends. Okadaic acid (EMD Biosciences-Calbiochem, Madison, WI) was added in 0.5 μM concentration for 2 h.
For actin staining, cells were fixed with 3% paraformaldehyde (PFA) freshly dissolved in phosphate-buffered saline (PBS). For immunostaining of γ-tubulin and pericentrin cells were fixed with 3% PFA at 37°C for 10 min and then treated with methanol at −20°C. For immunostaining of ninein, dynamitin, and p150(Glued), cells were fixed with methanol at −20°C for 10 min, immediately transferred to 3% PFA at 4°C for 15 min, and permeabilized with 0.1% Triton X-100 at 4°C.
Pictures of immunostained cells were taken using either an Axiophot microscope (Carl Zeiss Jena, Germany) and a 12-bit 1 MHz CCD MicroMax camera (Roper Scientific, Tucson, AZ) driven with WinView 32 software (Princeton Research Instruments, Princeton, NJ) or with a Nikon Diaphot 300 inverted microscope (Melville, NY) and slow-scan back-illuminated CCD camera (CH350, Roper Scientific) driven by Metamorph image acquisition and analysis software (Universal Imaging, West Chester, PA). The number of cells containing a distinct radial microtubule system or lacking it was estimated in randomly chosen fields. The intensity of centrosome fluorescence was calculated using 12–16-bit images of cells with ScionImage software (Scion, Frederick, MD).
EGFP-positive cells were purified using the FACSVantage SE system (BD Biosciences, San Jose, CA).
Kinase Activity Tests
The catalytic fragment of glutathione-S-transferase (GST) fused LOSK-ΔT was purified with a glutathione-agarose column (Sigma). The nonspecific LOSK substrate myelin basic protein (MBP) was purchased from Sigma. The kinase reactions were performed in 50 mM HEPES, pH 7.6, 10 mM MgCl2, 100 mM KCl, and 1 mM EGTA buffer supplemented with the proteins indicated in the figure legends and with 36 nM of γ32P-ATP (5000 Ci/mM, FEI Research Center, Obninsk, Russia). The reaction mixture was incubated for 1 h at room temperature. To stop the reaction, 0.5 volumes of 3× sample buffer (1% SDS, 50 mM Tris.HCl, pH 6.8, 10% glycerol, and 50 mM 2-mercaptoethanol) were added, and the mixture was heated at 100°C for 3 min and subjected to SDS-PAGE. Gels were stained with Coomassie G-250, dried, and radioautographed.
SDS-PAGE and Western Blotting
For SDS-PAGE, monolayer culture cells were washed with PBS, briefly washed with water, scraped in sample buffer, and heated at 100°C for 3 min. Fluorescence-activated cell sorting (FACS)-processed cells were immediately dissolved in 2× sample buffer and heated at 100°C for 5 min. SDS-PAGE of either cell homogenates or kinase reaction mixtures was run in 6–12% gradient gels. For Western blotting, proteins were electroblotted by the semidry method from gels to nitrocellulose membranes HyBond-C (GE Healthcare), blocked in tris-buffered saline (TBS) with 0.02% Tween 20 and 0.1% cold water fish gelatin (Sigma; TBS/Tween/gelatin) overnight, incubated with primary Abs diluted in TBS/Tween/gelatin, washed with TBS/Tween, incubated with goat either anti-rabbit IgG or anti-mouse IgG antibody conjugated with horseradish peroxidase (Imtech, Moscow, Russia) diluted in TBS/Tween/gelatin, and washed with TBS. Peroxidase was developed in 3,3′-diaminobenzidine solution with H2O2.
Tubulin Isolation and Microtubule Polymerization on Centrosomes In Vitro
Tubulin was isolated from rat brains using the protocol developed previously for calf or pig brains (Castoldi and Popov, 2003). Briefly, brains were homogenized in an equal volume of cold 50 mM KCl, 50 mM imidazole, pH 7.2, 1 mM MgCl2, 0.1 mM EDTA, homogenates were centrifuged at 4°C, and supernatants were brought to 4 M glycerol, 1 mM EGTA, 0.1 mM GTP, and 1 mM ATP and warmed at 37°C. Polymerized microtubules were sedimented, depolymerized in 0.5 M PIPES, pH 6.8, 1 mM MgCl2, 1 mM EGTA, and 1 mM GTP, clarified by centrifugation, and polymerized again at 37°C after addition of 5% DMSO. Microtubules were sedimented, dissolved in cold PEM buffer (50 mM PIPES, pH 6.8, 50 mM KCl, 1 mM EGTA, and 1 mM MgCl2,), and clarified by centrifugation, and aliquots were frozen in liquid nitrogen.
To study centrosome activity in vitro, cells were plated to poly-l-lysine–coated coverslips and at the next day were transfected by microinjection of a mixture of pEGFP-LamB and either LOSK-ΔT or LOSK-ΔT-K63R constructs. Twenty to 22 h after injection cells were treated with 1 μg/ml nocodazole for 3 h and permeabilized with 1% Triton X-100 at PEM buffer. Cell “ghosts” were incubated with 1.2 μg/ml rat brain tubulin in PEM buffer with 1 mM GTP at 37°C for 30 min, or with tubulin and purified GST-LOSK-ΔT, and then fixed with 1% glutaraldehyde (Merck, Rahway, NJ) for 5 min and immunostained with anti-tubulin antibody (Ab).
Antibodies
Microtubules were immunostained with anti-α-tubulin DM-1A mouse monoclonal Ab or with DM-1A conjugated with fluorescein isothiocyanate (FITC; Sigma). Monoclonal Abs to acetylated tubulin and to actin were purchased from Sigma, and anti-GFP monoclonal Ab was purchased from Rusbiolink (Moscow, Russia). Anti-dynamitin was mouse monoclonal Ab 611002 (BD Biosciences), anti-p150(Glued) was mouse monoclonal Ab 610473 (BD Biosciences), anti-mannosidase II was rabbit polyclonal Ab 12277 (AbCam, Cambridge, United Kingdom), anti-ninein Ab was rabbit polyclonal Ab 4447 (AbCam), and anti-pericentrin was rabbit polyclonal Ab 4448 (AbCam). Anti-γ-tubulin rabbit polyclonal Abs were kindly provided by Dr. R. Uzbekov (M.V. Lomonosov Moscow State University), anti-dynein heavy chain (DHC) rabbit polyclonal antibody were kindly provided by Dr. R. Vallee (Columbia University, New York, NY). F-actin was stained with rhodamine-phalloidin (Sigma-Aldrich).
The rabbit polyclonal affinity-purified pol3D2 antibody against the LOSK structural domain was described previously (Zinovkina et al., 1997, 1998). To develop an anti-LOSK N-terminal domain antibody polKIA, LOSK-ΔT-pQE30 was expressed in Escherichia coli; 6His-LOSK-ΔT protein was purified in Ni-NTA column (Qiagen) and used for rabbit immunization and serum affinity fractionation.
Goat anti-mouse IgG (H+L) ML and goat anti-rabbit IgG (H+L) ML antibodies conjugated with either rhodamine (TRITC) or fluorescein (FITC) were purchased from Jackson ImmunoResearch Europe (Newmarket, United Kingdom) and Kirkegaard & Perry Laboratories Gaithersburg, MD; ML).
RESULTS
In Vitro Assay of LOSK Enzymatic Activity
Both full-length endogenous LOSK and its recombinant catalytic domain expressed in E. coli exhibited apparent catalytic activity in vitro (Sabourin et al., 2000; Wagner et al., 2002; Potekhina et al., 2003). To analyze the function of LOSK, we generated a dominant-negative form K63R-ΔT by introduction of a K63R mutation in the ΔT construct. This mutation of the invariant lysine in the catalytic kinase domain usually makes protein dominant-negative because it competes with the wild-type form for substrate binding (Li et al., 1995). GST-fused K63R-ΔT was expressed in E. coli and purified. This protein did not exhibit any activity—neither autophosphorylation nor MBP phosphorylation (Figure 1B, columns 2 and 2′). Moreover, the addition of the increased amount of GST-K63R-ΔT gradually inhibited MBP phosphorylation by enzymatically active GST-ΔT (Figure 1B, columns 3–6 and 3′–6′). The fivefold excess of mutated kinase fully inhibited kinase the activity (Figure 1B, columns 6 and 6′). Another LOSK fragment GST-ΔNΔT also lacked its own enzymatic activity and partially inhibited GST-ΔT (Figure 1C).
The C-terminal structural domain of LOSK was supposed to inhibit its kinase activity (Sabourin et al., 1999, 2000). To prove it we examined the influence of two central molecule fragments (M1f and M2f) and the C-terminal part Ct on ΔT activity in kinase reaction in vitro. A large excess of neither Ct, nor M1f, nor M2f significantly influenced ΔT activity, and these fragments lacked LOSK phosphorylation sites (Figure 1C). This data shows that unfortunately the expression of the LOSK C-terminal part in cells could not be used as an inhibitor of this activity.
Neither pig brain tubulin nor chicken gizzard actin was phosphorylated by LOSK in vitro (data not shown).
Full-Length LOSK Partially Colocalizes with Microtubules, Whereas the N-terminal LOSK Fragment Is Diffusely Distributed in Cytoplasm
In our previous work we have shown that the protein kinase LOSK was associated with microtubules and centrosome in CHO-K1 cell culture and in fish sperm (Zinovkina et al., 1997, 1998; Nadezhdina et al., 2001). We proved it with immunofluorescence staining, immunoelectron microscopy, and cosedimentation with microtubules. In this study we demonstrate additional proof that LOSK is associated with microtubules by expressing full-length LOSK fused with enhanced GFP (EGFP) in Vero and HeLa cells. Full-length EGFP-fused LOSK was distributed in cytoplasm and it colocalized partly with microtubules (Figure 2A, white arrows). Some microtubules especially at cell periphery lacked the full-length LOSK (black arrows in Figure 2A).
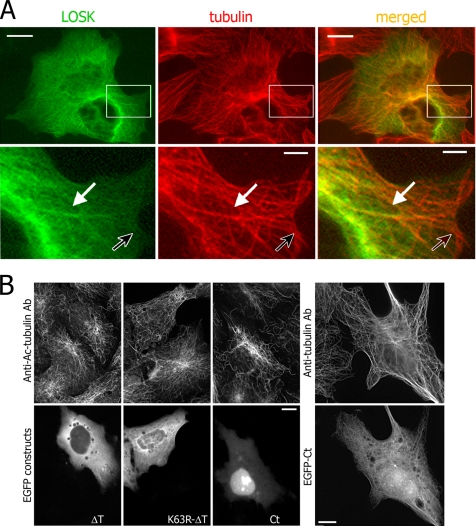
Figure 2.
Protein kinase LOSK interacts with cellular microtubules. (A) EGFP-fused full-length LOSK expressed in HeLa cells is partially distributed along microtubules. Top panels, overview of the cell; bottom panels, higher magnification of boxed areas. The white arrow points to distinctive structures; the black arrow points to LOSK-lacking microtubules. (B and C) Acetylated tubulin, which specifies stabilized microtubules, is not accumulated in cells expressing either K63R-ΔT or ΔT and is slightly increased in Ct-expressing cells. At high expression level, the Ct fragment is partially distributed along microtubules. Scale bars, 10 μm (2 μm in bottom panels in A).
In transfected cells active ΔT, truncated ΔNΔT, or mutated K63R-ΔT kinase fragments were diffusely distributed throughout the cytoplasm (Figures 2B and 3). We did not see any fluorescent fibrils or centrosome-like speckles in transfected cells, except occasional fluorescent protein inclusions (data not shown). Even if cells were treated with low doses of detergents, expression products were completely removed from the cells (data not shown). Thus, the N-terminal LOSK domain had no affinity either to microtubules or to the centrosome; however, N-terminal LOSK fragments located in the cytoplasm could compete with natural LOSK for any cytoplasmic substrates.
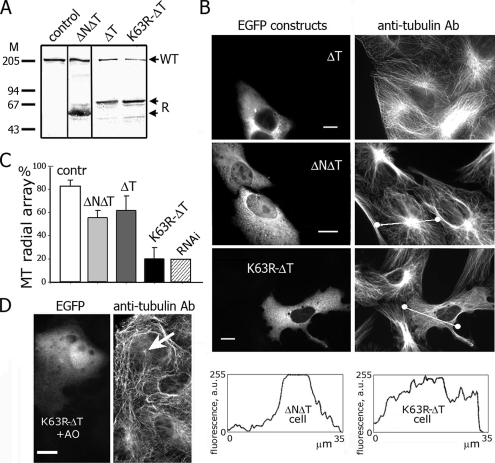
Figure 3.
The expression of dominant-negative K63R-ΔT in Vero cells alters radial microtubule arrays. (A) Immunoblotting of cell homogenates with anti-LOSK Ab polKIA. Lane 1, nontransfected cells, lanes 2–4, cells transfected with ΔT (2), K63R-ΔT (3), and ΔNΔT (4). Molecular mass markers are indicated (kDa). WT, cellular wild-type LOSK; R, recombinant LOSK fragments. (B) Vero cells expressing active ΔT and inactive ΔNΔT showed unaltered microtubule arrays, and cells expressing K63R-ΔT had disorganized microtubules. Bottom, scans of fluorescence intensity along lines shown in the cell images. (C) The frequency of cells containing distinct radial microtubule arrays. Fifty cells were counted in three independent experiments. Error bars, SD. (D) Microtubule aster (arrow) is visible in cell expressing K63R-ΔT after treatment with okadaic acid. Scale bars, 10 μm.
The level of acetylated tubulin, characteristic for stabilized microtubules, was not significantly altered in Vero cells expressing either ΔT or K63R-ΔT: in ΔT-expressing cells it increased by 10 ± 5% (n = 127) and in K63R-ΔT-expressing cells by 10 ± 7% (n = 106; Figure 2, B and C). So, these LOSK fragments did not influence the stability of microtubules. When expressed in cells, M1f and M2f were also diffusely distributed in the cytoplasm, had no effect on microtubules and did neither bind to nor stabilize microtubules (data not shown). On the contrary, cells, expressing the C-terminal LOSK fragment Ct showed an increased level of acetylated tubulin for 28 ± 9% (n = 57). Acetylated tubulin–containing microtubules were organized in radial arrays (Figure 2, B and C). At low levels of expression Ct was mainly localized in the nuclei, whereas at increased levels (accompanied with protein inclusions) it accumulated in the cytoplasm and partially bound to microtubules similarly to full-length LOSK (Figure 2B). We suggested that the microtubule-binding site of LOSK was situated in the Ct fragment, within amino acids 944-1235 and that the Ct fragment slightly stabilized microtubules like some “classic” microtubule-associated proteins. Remarkably, this stabilization did not influence the formation of radial microtubule arrays.
Disruption of Radial Microtubule Arrays in Vero Cells Overexpressing the Dominant-Negative Catalytic Domain of LOSK
Because endogenous LOSK was found to be associated with microtubules, we supposed that it could regulate microtubule organization via its kinase activity. We compared the microtubule system in Vero cells expressing dominant-negative K63R-ΔT (inhibited LOSK activity) with cells expressing active ΔT (increased LOSK activity) and inactive ΔNΔT (partially inhibited LOSK activity).
Is the inhibitory effect obtained by expressing K63R-ΔT constructs in cultured cells? The amount of recombinant LOSK fragments in cell lysates was at least threefold higher than that of endogenous 210-kDa LOSK (Figure 3A). Because <20% of cells expressed recombinant protein in these experiments, most of transfected cells contained approximately a 15-fold excess of inhibitory fragments. Therefore, in cells expressing K63R-ΔT the activity of cellular LOSK was expected to be fully inhibited.
A quantitative analysis showed that ∼80% of nontransfected Vero cells possessed radial microtubule arrays with distinct microtubule-organization centers (Figure 3C). Expression of ΔT caused a slight decrease of the number of cells with radial arrays; ΔNΔT had a more prominent effect, whereas in most cells expressing K63R-ΔT the microtubules were distributed chaotically (Figures 3, B and C). The effect was independent of the monolayer density (data not shown). The same result was obtained with DsRed-monomer–fused K63R-ΔT (data not shown). We measured tubulin fluorescence intensity along linear tracks across cells (indicated in the pictures). These tracks crossed the brightest part of the cytoplasm. If the microtubule system was radial, the intensity plot was bell-shaped. In cells with chaotic microtubules the plot had flattened a shape, with irregular peaks (Figure 3B, bottom), indicating random tubulin and microtubule distribution. Earlier we had published preliminary results indicating that the inhibition of LOSK activity entailed microtubule chaotization in Vero cells (Burakov et al., 2005).
Cells expressing K63R-ΔT became rough, with protrusions unlike intact Vero cells. However, the expression of neither active kinase domain nor its inactive mutant considerably influenced actin stress fibers. The percentage of cells with well-developed stress fibers or lacking them was unchanged by either ΔT or K63R-ΔT expression (data not shown). This observation confirms the data of Sabourin et al. (2000) that the C-terminal LOSK domain disturbs the actin system and the N-terminal domain does not. Similarly, expression of K63R-ΔT did not influence focal contacts visualized with paxillin immunostaining (data not shown). Both observations suggest a specific influence of LOSK kinase activity on the microtubule system.
Residual LOSK activity could remain in transfected cells. To check this possibility, we treated cells with okadaic acid. It did not influence radial microtubule arrays in control cells, though in K63R-ΔT–expressing cells one tiny aster with few microtubules was seen among peripheral chaotic microtubules (Figure 3D). Perhaps, this partial rescue of radial microtubule arrays reflected residual activity of LOSK or some minor kinases that phosphorylate the same site(s).
Depletion of LOSK by RNAi Also Disrupts Radial Microtubule Arrays
To confirm that the inhibitory effect of the dominant-negative LOSK construct on radial microtubule arrays was specific, we depleted LOSK by RNAi. Transfected cells expressing shRNA were detected by EGFP fluorescence, and we determined LOSK levels in cells by immunostaining and by immunoblotting after EGFP-expressing cell purification by FACS. We found that in the case of the pG-Shin2-4.1 construct at 7–8 d after transfection of either Vero or HeLa cells the intensity of LOSK staining decreased dramatically indicating LOSK knockdown (Figure 4A). At 9–10 d after transfection all transfected cells had died (data not shown). The later result confirmed the observations of O'Reilly et al. (2005) that LOSK was essential for cell viability. Neither the empty vector nor the alternative construct pG-Shin2-6.1 influenced cell viability or LOSK levels. By immunoblotting data (Figure 4B) the residual LOSK level was ∼5%.
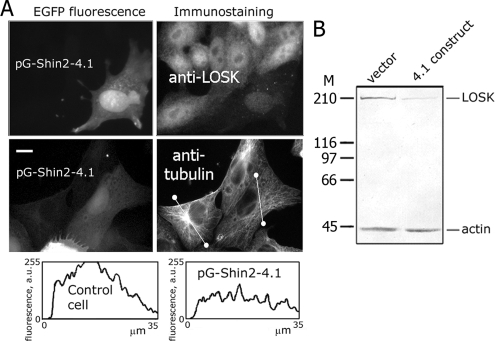
Figure 4.
Depletion of LOSK in cells by RNAi alters radial microtubule arrays. (A) Immunostaining of LOSK (top right) and microtubules (middle right) in cells at day 8 after transfection with pG-Shin2-4.1. Bottom right, scans of fluorescence intensity along lines shown in the cell images. Scale bar, 10 μm. (B) Immunoblotting of LOSK and actin in cells transfected with either empty vector or pG-Shin2-4.1 and selected with FACS at day 8 after transfection. Molecular mass markers are indicated (kDa).
We performed immunostaining of microtubules in Vero cells at day 8 after transfection with pG-Shin2-4.1 and found that they usually had disrupted radial arrays of microtubules similar to K63R-ΔT–expressing cells (Figures 3C and 4A). Their microtubules were distributed chaotically, without distinct centers, and the plot of tubulin fluorescence taken across the cell was almost horizontal (Figure 4A).
Expression of either full-length LOSK or K63R-ΔT or ΔT as well as LOSK depletion with RNAi was fatal for cells within 1 or 2 d (data not shown). This LOSK feature made rescue experiments with knockdown cells difficult. The time curves of cell death induced by either ΔT or K63R-ΔT were similar with ∼40% dead cells 1 d after transfection (data not shown). Remarkably, ΔT-expressing cells died, but their microtubule array was normal. It seemed that cell death was not the reason of microtubule array disruption caused by LOSK inhibition. For data adequacy we did not estimate microtubule arrangement in dying cells with condensed or fragmented nuclei.
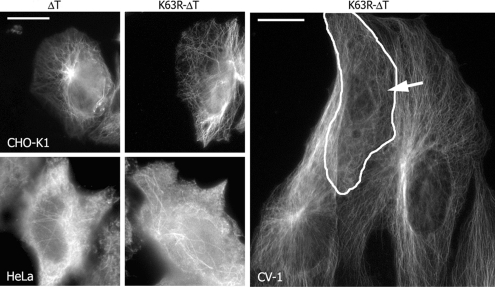
The Dominant-Negative Catalytic LOSK Domain Disrupts Radial Microtubule Arrays in a Variety of Cell Types
LOSK kinase is a highly conservative protein, especially in its catalytic domain (Potekhina et al., 2003). It was found in all cells and tissues examined. We used fibroblast- or epithelial-like cell cultures derived from human or from other mammals to express human cDNAs. Vero cells were the most appropriate due to their distinctly radial system of microtubules and a high level of K63R-ΔT expression. CHO-K1 cells (Chinese hamster ovary fibroblast-like cells) had a distinctly radial system of microtubules. K63R-ΔT expression effectively caused microtubule chaotization in CHO-K1 cells (Figure 5). Ninety-five percent of control CHO-K1 cells exhibited aster-like microtubules around centrosomes. The same was observed in 94% of cells expressing ΔT, whereas only 57% of the cells transfected with K63R-ΔT had a radial system of microtubules.
Figure 5.
K63R-ΔT expression disrupts the organization of radial microtubule arrays in CHO-K1 and CV-1 cells but does not influence microtubules in HeLa cells. A transfected CV-1 cell is encircled and marked with an arrow. CHO-K1 and HeLa cells were chilled in ice and then warmed during 15 min; CV-1 cells were simply grown at 37°C. Scale bar, 10 μm.
Microtubules in normal interphase HeLa cells (human cervical carcinoma) do not display distinct radial arrangement, and no alteration was seen under the expression either of DT or K63R-ΔT (Figure 5). Moreover, HeLa interphase centrosomes even in microtubule recovery experiments did not form radial asters of microtubules, suggesting that they were initially inactive (Figure 5).
In most transfected CV-1 cells (another green monkey kidney line) LOSK fragments were expressed at a very low level. However, in cells expressing K63R-ΔT at a high level, chaotization of microtubules was observed (Figure 5). Thus, the protein kinase LOSK regulates microtubule radial organization in various types of cultured cells.
The Inhibition of LOSK Activity Does Not Influence Either Centrosome Integrity or Its Microtubule-Nucleation Ability, Whereas Microtubule Anchoring/Capping at the Centrosome Is Abolished
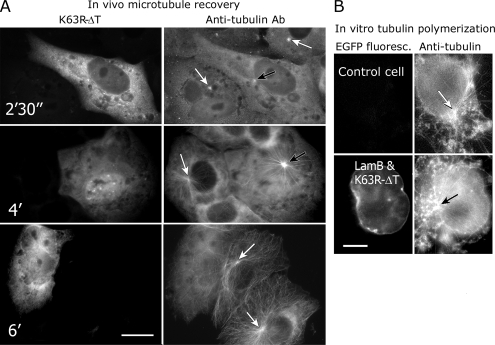
As mentioned above, the formation of radial microtubule arrays depends mostly on the activity of centrosome. We tested the influence of K63R-ΔT on the ability of the centrosome to nucleate and to anchor microtubules both in vivo and in vitro. Microtubule recovery experiments were performed as described in Materials and Methods. Both control and K63R-ΔT–expressing Vero cells formed single asters of short microtubules at 2 min after nocodazole washout (Figure 6A). These asters grew and microtubules reached the cell margin at 4–5 min both in control and in transfected cells (Figure 6A). However, already 6 min after nocodazole washout microtubules in transfected cells became chaotic, whereas in control cells they were still organized in asters (Figure 6A). Similar results were obtained using cells with RNAi-depleted LOSK (data not shown). Thus, in cells with suppressed LOSK activity centrosomes were unable to anchor or to cap microtubules, whereas their microtubule-nucleation activity remained intact.
Figure 6.
K63R-ΔT expression in Vero cells inhibits anchoring/capping of microtubules at the centrosome but does not inhibit microtubule nucleation. (A) Vero cells were treated with nocodazole for 2 h and then washed for the time indicated in the pictures. White arrows point to microtubule asters in control cells and black arrows to microtubule asters in cells expressing K63R-ΔT. (B) Vero cells expressing EGFP-lamin B and K63R-ΔT were treated with nocodazole, permeabilized with Triton X-100, and incubated with purified tubulin at 37°C. Microtubules organize as asters around centrosomes both in expressing and control cells. Scale bar, 10 μm.
We also tested the centrosome activity in transfected cells in vitro. In our preliminary experiments we found that LOSK-ΔT and its mutated variant were completely washed out from the cells after Triton X-100 treatment, so that the transfected cells could not be distinguished from the control cells. Therefore, we used simultaneous synthesis of LOSK fragments and EGFP-LaminB—a nuclear envelope protein resistant to Triton X-100 treatment in cells. We microinjected cells with mixture of pEGFP-LamB and either ΔT or K63R-ΔT. The effect of LOSK fragments on the microtubule system was independent from the expression of EGFP-LaminB (data not shown). The centrosomes in cells expressing EGFP-Lamin B and K63R-ΔT after nocodazole treatment of cells and Triton X-100 permeabilization effectively induced the polymerization of purified tubulin in vitro and also organized newly formed microtubules in an aster-like manner (Figure 6B). The size of asters was similar to that of control cells (Figure 6B). These data confirmed our previous result that centrosome's ability to nucleate microtubules was not affected by inhibition of LOSK.
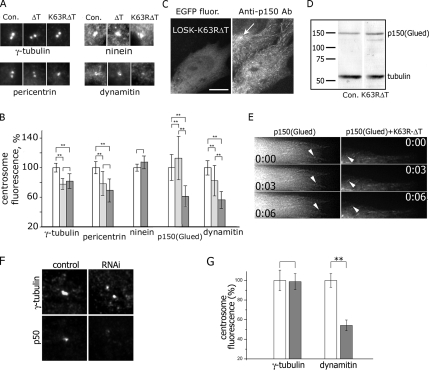
To reveal why the ability of centrosome to anchor or to cap microtubules was suppressed, we examined the centrosomal content of major proteins responsible for centrosome integrity and microtubule organization: γ-tubulin, pericentrin, ninein, and dynactin subunits p150(Glued) and dynamitin. Cells expressing K63R-ΔT had the same amount of ninein in centrosomes compared with control, and the amount of γ-tubulin and pericentrin was 20% decreased both in ΔT and K63R-ΔT–expressing cells (Figure 7, A–C). Thus, the inability of the centrosome to anchor and cap microtubules in K63R-ΔT–expressing cells did not depend on the amount of these centrosomal proteins. However, we found that centrosomes in cells expressing K63R-ΔT were depleted with dynactin, both its p150(Glued) and dynamitin components (Figure 7, A–C). Expression of active kinase ΔT induced an increase of centrosomal p150(Glued) (Figure 7C). However, immunostaining revealed unaltered p150(Glued) “comets” at microtubule plus ends in K63R-ΔT–expressing cells (Figure 7C). To estimate the total dynactin content in K63R-ΔT expressing cells, we immunoblotted 100% transfected HeLa cells: the amount of neither p150(Glued) nor dynamitin was decreased by the inhibition of LOSK (Figure 7D and data not shown). Finally, we expressed in Vero cells DsRed-K63R-ΔT and EGFP-p150(Glued) simultaneously and observed p150(Glued) “comets” that were undistinguishable in control and DsRed-K63R-ΔT–expressing cells (Figure 7E and Video).
Figure 7.
The influence of LOSK on the content of major centrosomal proteins: the decrease of dynactin levels under either LOSK inhibition or depletion. (A) Pictures of typical centrosomes (matching average values shown in part B of this figure) immunostained with Abs to proteins indicated below the pictures. Con, control cells. (B) Immunostaining of K63R-ΔT–expressing cells with Ab to p150(Glued). The arrow points to the centrosome in a control (nontransfected) cell. Notice p150(Glued) “comets” at microtubule ends. Scale bar, 10 μm. (C) Bar graphs of fluorescence intensity of immunostained centrosomes. Thirty to 50 centrosomes were measured for each point; differences at *p < 0.05 and **p < 0.01. White bars, control cells; light gray, ΔT; dark gray, K63R-ΔT. (D) Immunoblotting of control (Con.) and K63R-ΔT–expressing cells treated with Ab to p150(Glued) and tubulin. Molecular mass markers are indicated (kDa). (E) Live imaging of a cell expressing either EGFP-p150(Glued) alone or a mixture of DsRed-Monomer-K63R-ΔT and EGFP-p150(Glued). Arrowheads point to “comets”; numbers indicate seconds. See also Video 1. (F) Double immunostaining of dynamitin (p50) and γ-tubulin in control and LOSK-depleted (RNAi) cells. (G) Bar graphs of fluorescence intensity of immunostained centrosomes. Centrosomes (n = 28–43) were measured for each point. White bars, control cells; gray bars, RNAi. For γ-tubulin data, p = 0.93, for p50 data p = 0.00017. Scale bar, 10 μm.
The dominant-negative kinase form might cause nonspecific effects when overexpressed in cells. To confirm the specificity of its effect on centrosomal dynactin, we studied the content of dynactin in LOSK-depleted cells transfected with pG-Shin2-4.1. LOSK depletion correlated with a strong reduction of dynactin subunits [both p150(Glued) and dynamitin] in the centrosome (Figure 7, F and G, and data not shown). To ensure centrosome position in cells, we double-immunostained cells with antibodies to γ-tubulin and dynactin subunits. The ratio of γ-tubulin fluorescence to dynamitin increased dramatically after LOSK depletion (Figure 7G). The dynactin “comets” were unaltered in these cells (data not shown).
Thus, LOSK might regulate the intracellular distribution of dynactin, destabilizing its binding to the centrosome. Because dynactin ensures Golgi complex integrity (Quintyne et al., 1999) and its intracellular movements, we decided to study the influence of LOSK inhibition on Golgi apparatus.
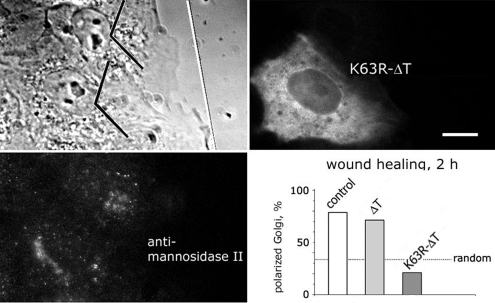
LOSK Inhibition Hinders Golgi Complex Polarization at the Wound Edge
We immunostained Vero cells with an Ab to the Golgi complex marker mannosidase II and found that Golgi complex compactness was unaltered in cells expressing either ΔT or K63R-ΔT (data not shown and Figure 8). In Vero cells expressing the dynein/dynactin inhibiting p150(Glued) fragment CC1, Golgi complex was dispersed (Zhapparova et al., 2007). These data indicate that alterations of LOSK activity did not dramatically affect Golgi-bound dynein/dynactin. We also studied Golgi complex relocalization during cell polarization at a wound edge with the method described by Watson and Stephens (2006). We found that at 2 h after scratching the monolayer, most cells had their Golgi complex oriented to the wound (Figure 8). Expression of catalytically active ΔT did not influence Golgi reorientation. However, although K63R-ΔT–expressing cells protruded their lamellae toward the wound, the Golgi complex did not follow, and only a few cells had a wound-oriented Golgi complex (Figure 8). The distribution of focal adhesions as revealed by paxillin immunostaining was the same in control and in LOSK-inhibited cells (data not shown). Hence, Golgi complex mislocalization was not the result of substrate adhesion disturbance, so that it can be concluded that the activity of protein kinase LOSK is required for the formation of radial arrays of microtubules and for the Golgi complex polarization at a wound edge.
Figure 8.
K63R-ΔT expression inhibits the polarization of the Golgi complex at a wound edge. Vero cells at wound edge 2 h after scratching of the monolayer. In the phase contrast image the direction of the scratch is shown as white line. Angle shape marks indicate leading cell thirds. In the bar graph one of three independent experiments is shown, n = 75, 42, and 38, respectively. Scale bar, 10 μm.
DISCUSSION
In this work we have shown that the inhibition of LOSK activity either by the expression of its dominant-negative construct or by knockdown induced disruption of radial microtubule arrays. We found that the disruption of radial microtubule arrays was caused by a loss of microtubule anchoring/capping activity of the centrosome in Vero, CV-1, and CHO-K1 culture cells. Cells with inhibited LOSK activity demonstrated a decrease of centrosomal dynactin content and impaired Golgi complex polarization at a wound edge. The inhibition of LOSK did not influence either microtubule nucleation at the centrosome or the stability of microtubules. What are the potential targets of LOSK activity? LOSK might regulate centrosomes either by direct phosphorylation of some centrosomal proteins or indirectly, by the phosphorylation and modulation of the activity of kinase/phosphatase involved in the regulation of the centrosome. We cannot discriminate between these possibilities, because LOSK natural downstream substrates remain obscure.
The process of microtubule anchoring/capping on the centrosome seems to be very complicated. Defocused microtubule arrays in cells with normal microtubule-nucleation activity of the centrosome have been described in a number of works. The list of proteins whose inhibition (by antibodies, RNAi, dominant-negative constructs, etc.) results in the chaotization of microtubules includes centrosomal proteins PCM-1 (Dammermann and Merdes, 2002), ninein (Dammermann and Merdes, 2002; Delgehyr et al., 2005), BBS4 (Kim et al., 2004), Cep135 (Uetake et al., 2004), 4.1R isoform (Perez-Ferreiro et al., 2004), Nlp (Casenghi et al., 2005), and CAP350 and FOP (Yan et al., 2006). The same effect of microtubule array defocusing can be achieved by the inhibition of cytoplasmic dynein and its cofactor dynactin (Ma et al., 1999; Quintyne et al., 1999; Askham et al., 2002; Quintyne and Schroer, 2002; Zhapparova et al., 2007) or with dynein-binding protein Nudel (Guo et al., 2006). Dynein and dynactin play multiple roles in microtubule organization. They organize radial microtubule arrays in noncentrosomal cell fragments (Malikov et al., 2005). Dynein delivers some centrosomal proteins to the centrosome (Young et al., 2000; Dammermann and Merdes, 2002; Casenghi et al., 2005; Fumoto et al., 2006). Dynactin along with EB-1 is a part of pericentriolar scaffold of centrosomes (Quintyne et al., 1999; Askham et al., 2002; Yan et al., 2006) and binds to functionally important proteins such as Cep135, BBS4, and CAP350/FOP (Kim et al., 2004; Uetake et al., 2004; Yan et al., 2006). A pool of dynein is also associated with the centrosome and might interact with dynactin (Quintyne and Schroer, 2002). Both dynein and dynactin bind to microtubules and even promote microtubule polymerization (Waterman-Storer et al., 1995; Malikov et al., 2004). It is likely that dynactin and its interacting proteins form a lattice in the centrosome that somehow anchors and caps microtubules.
The effect of LOSK-induced disruption of microtubule arrays is morphologically similar to the effects of inhibition of specific centrosomal proteins and dynein/dynactin. LOSK distributed mostly in cytoplasm is not a specific centrosomal protein. However, it was found in isolated sperm basal bodies and in centrosomes in cell culture (Zinovkina et al., 1997; Nadezhdina et al., 2001). Among potential centrosomal targets of LOSK (probably indirect) the tempting one is dynactin that localizes both in centrosomes and in the cytoplasm. Our data show that the expression of dominant-negative K63R-ΔT decreased the dynactin content on centrosomes—at least its p150(Glued) and dynamitin subunits. Moreover, dynactin comets at microtubule ends remain unaltered indicating that dynactin is intact but dissociates from the centrosome. The association of dynactin with the centrosomal microtubule-anchoring protein Nlp is destabilized by the mitotic kinase Plk1 (Casenghi et al., 2005) and it cannot be excluded that LOSK enhances a similar interaction in interphase.
In this work we have also shown that LOSK is involved in Golgi complex reorientation during cell polarization at a wound edge. Similar, but less pronounced effects were observed in cells depleted either with the dynactin p150(Glued) subunit or with other microtubule plus-end tracking proteins CLIP-170 and EB-1 (Watson and Stephens, 2006). Because LOSK inhibition does not induce Golgi complex dispersion, it seems most likely that LOSK does not regulate either dynein activity itself or dynein–dynactin interaction.
The results stated in this article indicate the presence of a signal pathway that regulates radial microtubule arrays in interphase cells. Protein kinase LOSK is likely to be one of the members of this pathway, whereas other participants remain obscure. Which signaling pathways might LOSK be involved in? LOSK has homology with Xenopus xPlkk1 kinase which regulates the Xenopus homolog of Polo-like kinase Plx1. Therefore LOSK was supposed to regulate the activity of Plk1 (Ellinger-Ziegelbauer et al., 2000), but it turned out that the Plk1-activating site was not phosphorylated by LOSK and LOSK had no effect on Plk1 activity in vitro (Kelm et al., 2002). LOSK is indirectly slightly down-regulated in vivo by overexpression of v-Src, probably via phosphorylation of its casein kinase II sites in the M1f fragment. However, activation of casein kinase II had no discernible effect on LOSK in vivo (Chaar et al., 2006). Cell polarization depends on Cdc42-regulated signal transduction pathway, which involves the activation of the Par6/aPKC complex and inhibition of GSK-3beta (Schlessinger et al., 2007); it seems probable that LOSK participates in these events. Currently we are working on the identification of natural substrate(s) of LOSK among centrosomal and microtubular proteins that could shed light on the regulation of microtubule anchoring–capping activity of the centrosome.
Supplementary Material
ACKNOWLEDGMENTS
The authors are grateful to Galina Kuznetsova and Olga Deryabina for technical assistance; to Drs. Vallee, Steffen, and Kurchashova and the Kazusa DNA Research Institute for the antibodies and DNAs. The authors appreciate fruitful discussions with Drs. Nicolai Gusev, Yulia Komarova, and Anna Akhmanova. This work was financially supported by the Program of the Presidium of Russian Academy of Science “Molecular and Cellular Biology”; Russian Foundation for Basic Research Grant 02-04-48783 to L.Z. and 05-04-49015 to E.N.; and German DAAD Leonhard-Euler-Program to A.B., O.Z., E.P., S.K., D.W., and E.N.
Abbreviations used:
- DHC
dynein heavy chain
- EGFP
enhanced green fluorescent protein
- GST
glutathione-S-transferase
- LOSK
long Ste20-like kinase
- MBP
myelin basic protein.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-12-1156) on February 20, 2008.
REFERENCES
- Abe Y., Ohsugi M., Haraguchi K., Fujimoto J., Yamamoto T. LATS2-Ajuba complex regulates gamma-tubulin recruitment to centrosomes and spindle organization during mitosis. FEBS Lett. 2006;580:782–788. doi: 10.1016/j.febslet.2005.12.096. [DOI] [PubMed] [Google Scholar]
- Askham J. M., Vaughan K. T., Goodson H. V., Morrison E. E. Evidence that an interaction between EB1 and p150(Glued) is required for the formation and maintenance of a radial microtubule array anchored at the centrosome. Mol. Biol. Cell. 2002;13:3627–3645. doi: 10.1091/mbc.E02-01-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros T. P., Kinoshita K., Hyman A. A., Raff J. W. Aurora A activates D-TACC-Msps complexes exclusively at centrosomes to stabilize centrosomal microtubules. J. Cell Biol. 2005;170:1039–1046. doi: 10.1083/jcb.200504097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bre M. H., Kreis T. E., Karsenti E. Control of microtubule nucleation and stability in Madin-Darby canine kidney cells: the occurrence of noncentrosomal, stable detyrosinated microtubules. J. Cell Biol. 1987;105:1283–1296. doi: 10.1083/jcb.105.3.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulinski J. C., Borisy G. G. Immunofluorescence localization of HeLa cell microtubule-associated proteins on microtubules in vitro and in vivo. J. Cell Biol. 1980;87:792–801. doi: 10.1083/jcb.87.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burakov A. V., Kovalenko O. V., Potekhina E. S., Nadezhdina E. S., Zinovkina L. A. LOSK (SLK) protein kinase activity is necessary for microtubule organization in the interphase cell centrosome. Dokl. Biol. Sci. 2005;403:317–319. doi: 10.1007/s10630-005-0123-9. [DOI] [PubMed] [Google Scholar]
- Casenghi M., Barr F. A., Nigg E. A. Phosphorylation of Nlp by Plk1 negatively regulates its dynein-dynactin-dependent targeting to the centrosome. J. Cell Sci. 2005;118:5101–5108. doi: 10.1242/jcs.02622. [DOI] [PubMed] [Google Scholar]
- Castoldi M., Popov A. V. Purification of brain tubulin through two cycles of polymerization-depolymerization in a high-molarity buffer. Protein Expr. Purif. 2003;32:83–88. doi: 10.1016/S1046-5928(03)00218-3. [DOI] [PubMed] [Google Scholar]
- Chaar Z., O'Reilly P., Gelman I., Sabourin L. A. V-src-dependent downregulation of the Ste20-like kinase SLK by casein kinase II. J. Biol. Chem. 2006;281:28193–28199. doi: 10.1074/jbc.M605665200. [DOI] [PubMed] [Google Scholar]
- Dammermann A., Merdes A. Assembly of centrosomal proteins and microtubule organization depends on PCM-1. J. Cell Biol. 2002;159:255–266. doi: 10.1083/jcb.200204023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgehyr N., Sillibourne J., Bornens M. Microtubule nucleation and anchoring at the centrosome are independent processes linked by ninein function. J. Cell Sci. 2005;118:1565–1575. doi: 10.1242/jcs.02302. [DOI] [PubMed] [Google Scholar]
- Doxsey S. Re-evaluating centrosome function. Nat. Rev. Mol. Cell. Biol. 2001;2:688–698. doi: 10.1038/35089575. [DOI] [PubMed] [Google Scholar]
- Dujardin D. L., Barnhart L. E., Stehman S. A., Gomes E. R., Gundersen G. G., Vallee R. B. A role for cytoplasmic dynein and LIS1 in directed cell movement. J. Cell Biol. 2003;163:1205–1211. doi: 10.1083/jcb.200310097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinger-Ziegelbauer H., Karasuyama H., Yamada E., Tsujikawa K., Todokoro K., Nishida E. Ste20-like kinase (SLK), a regulatory kinase for polo-like kinase (Plk) during the G2/M transition in somatic cells. Genes Cells. 2000;5:491–498. doi: 10.1046/j.1365-2443.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- Fumoto K., Hoogenraad C. C., Kikuchi A. GSK-3beta-regulated interaction of BICD with dynein is involved in microtubule anchorage at centrosome. EMBO J. 2006;25:5670–5682. doi: 10.1038/sj.emboj.7601459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J., Yang Z., Song W., Chen Q., Wang F., Zhang Q., Zhu X. Nudel contributes to microtubule anchoring at the mother centriole and is involved in both dynein-dependent and -independent centrosomal protein assembly. Mol. Biol. Cell. 2006;17:680–689. doi: 10.1091/mbc.E05-04-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P., Senga T., Hamaguchi M. A novel role of phospho-beta-catenin in microtubule regrowth at centrosome. Oncogene. 2007;26:4357–4371. doi: 10.1038/sj.onc.1210217. [DOI] [PubMed] [Google Scholar]
- Hyman A. A., Karsenti E. Morphogenetic properties of microtubules and mitotic spindle assembly. Cell. 1996;84:401–410. doi: 10.1016/s0092-8674(00)81285-4. [DOI] [PubMed] [Google Scholar]
- Itoh S., Kameda Y., Yamada E., Tsujikawa K., Mimura T., Kohama Y. Molecular cloning and characterization of a novel putative STE20-like kinase in guinea pigs. Arch. Biochem. Biophys. 1997;340:201–207. doi: 10.1006/abbi.1997.9893. [DOI] [PubMed] [Google Scholar]
- Kelm O., Wind M., Lehmann W. D., Nigg E. A., et al. Cell cycle-regulated phosphorylation of the Xenopus polo-like kinase Plx1. J. Biol. Chem. 2002;277:25247–25256. doi: 10.1074/jbc.M202855200. [DOI] [PubMed] [Google Scholar]
- Kim J. C., et al. The Bardet-Biedl protein BBS4 targets cargo to the pericentriolar region and is required for microtubule anchoring and cell cycle progression. Nat. Genet. 2004;36:462–470. doi: 10.1038/ng1352. [DOI] [PubMed] [Google Scholar]
- Kim S., Lee K., Rhee K. NEK7 is a centrosomal kinase critical for microtubule nucleation. Biochem. Biophys. Res. Commun. 2007;360:56–62. doi: 10.1016/j.bbrc.2007.05.206. [DOI] [PubMed] [Google Scholar]
- Kojima S., Vignjevic D., Borisy G. G. Improved silencing vector co-expressing GFP and small hairpin RNA. Biotechniques. 2004;36:74–79. doi: 10.2144/04361ST02. [DOI] [PubMed] [Google Scholar]
- Li W., Yu J. C., Shin D. Y., Pierce J. H. Characterization of a protein kinase C-delta (PKC-delta) ATP binding mutant. An inactive enzyme that competitively inhibits wild type PKC-delta enzymatic activity. J. Biol. Chem. 1995;270:8311–8318. doi: 10.1074/jbc.270.14.8311. [DOI] [PubMed] [Google Scholar]
- Luders J., Patel U. K., Stearns T. GCP-WD is a γ-tubulin targeting factor required for centrosomal and chromatin-mediated microtubule nucleation. Nat. Cell Biol. 2006;8:137–147. doi: 10.1038/ncb1349. [DOI] [PubMed] [Google Scholar]
- Ma S., Trivinos-Lagos L., Graf R., Chisholm R. L. Dynein intermediate chain mediated dynein-dynactin interaction is required for interphase microtubule organization and centrosome replication and separation in Dictyostelium. J. Cell Biol. 1999;147:1261–1274. doi: 10.1083/jcb.147.6.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malikov V., Kashina A., Rodionov V. Cytoplasmic dynein nucleates microtubules to organize them into radial arrays in vivo. Mol. Biol. Cell. 2004;15:2742–2749. doi: 10.1091/mbc.E03-10-0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malikov V., Cytrynbaum E. N., Kashina A., Mogilner A., Rodionov V. Centering of a radial microtubule array by translocation along microtubules spontaneously nucleated in the cytoplasm. Nat. Cell Biol. 2005;7:1213–1218. doi: 10.1038/ncb1332. [DOI] [PubMed] [Google Scholar]
- Morrison E. E. Action and interactions at microtubule ends. Cell Mol. Life Sci. 2007;64:307–317. doi: 10.1007/s00018-007-6360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musa H., Orton C., Morrison E. E., Peckham M. Microtubule assembly in cultured myoblasts and myotubes following nocodazole induced microtubule depolymerisation. J. Muscle Res. Cell Motil. 2003;24:301–308. [PMC free article] [PubMed] [Google Scholar]
- Nadezhdina E. S., Zinovkina L. A, Fais D., Chentsov Iu.S. Spermatozoa of the loach Misgurnus fossilis as a test system for identification of new centrosome proteins. Ontogenez. 2001;32:41–50. [PubMed] [Google Scholar]
- O'Reilly P. G., Wagner S., Franks D. J., Cailliau K., Browaeys E., Dissous C., Sabourin L. A. The Ste20-like kinase SLK is required for cell cycle progression through G2. J. Biol. Chem. 2005;280:42383–42390. doi: 10.1074/jbc.M510763200. [DOI] [PubMed] [Google Scholar]
- Perez-Ferreiro C. M., Vernos I., Correas I. Protein 4.1R regulates interphase microtubule organization at the centrosome. J. Cell Sci. 2004;117:6197–6206. doi: 10.1242/jcs.01544. [DOI] [PubMed] [Google Scholar]
- Potekhina E. S., Zinovkina L. A., Nadezhdina E. S. Enzymatic activity of protein kinase LOSK: possible regulatory role of the structural domain. Biochemistry (Moscow) 2003;68:188–195. doi: 10.1023/a:1022649428881. [DOI] [PubMed] [Google Scholar]
- Rodionov V. I., Borisy G, G. Self-centering activity of cytoplasm. Nature. 1997;386:170–173. doi: 10.1038/386170a0. [DOI] [PubMed] [Google Scholar]
- Quintyne N. J., Gill S. R., Eckley D. M., Crego C. L., Compton D. A., Schroer T. A. Dynactin is required for microtubule anchoring at centrosomes. J. Cell Biol. 1999;147:321–334. doi: 10.1083/jcb.147.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintyne N. J., Schroer T. A. Distinct cell cycle-dependent roles for dynactin and dynein at centrosomes. J. Cell Biol. 2002;159:245–254. doi: 10.1083/jcb.200203089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabourin L. A., Rudnicki M. A. Induction of apoptosis by SLK, a Ste20-related kinase. Oncogene. 1999;18:7566–7575. doi: 10.1038/sj.onc.1203119. [DOI] [PubMed] [Google Scholar]
- Sabourin L. A., Tamai K., Seale P., Wagner J., Rudnicki M. A. Caspase 3 cleavage of the Ste20-related kinase SLK releases and activates an apoptosis-inducing kinase domain and an actin-disassembling region. Mol. Cell. Biol. 2000;20:684–696. doi: 10.1128/mcb.20.2.684-696.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliwa M., Osborn M., Weber K. Microtubule system of isolated fish melanophores as revealed by immunofluorescence microscopy. J. Cell Biol. 1978;76:229–236. doi: 10.1083/jcb.76.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger K., McManus E. J., Hall A. Cdc42 and noncanonical Wnt signal transduction pathways cooperate to promote cell polarity. J. Cell Biol. 2007;30:355–361. doi: 10.1083/jcb.200701083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storbeck C. J., Daniel K., Zhang Y. H., Lunde J., Scime A., Asakura A., Jasmin B., Korneluk R. G., Sabourin L. A. Ste20-like kinase SLK displays myofiber type specificity and is involved in C2C12 myoblast differentiation. Muscle Nerve. 2004;29:553–564. doi: 10.1002/mus.20000. [DOI] [PubMed] [Google Scholar]
- Tassin A. M., Maro B., Bornens M. Fate of microtubule-organizing centers during myogenesis in vitro. J. Cell Biol. 1985;100:35–46. doi: 10.1083/jcb.100.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetake Y., Terada Y., Matuliene J., Kuriyama R. Interaction of Cep135 with a p50 dynactin subunit in mammalian centrosomes. Cell Motil. Cytoskelet. 2004;58:53–66. doi: 10.1002/cm.10175. [DOI] [PubMed] [Google Scholar]
- Waterman-Storer C. M., Karki S., Holzbaur E. L. The p150Glued component of the dynactin complex binds to both microtubules and the actin-related protein centractin (Arp-1) Proc. Natl. Acad. Sci. USA. 1995;92:1634–1638. doi: 10.1073/pnas.92.5.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson P., Stephens D. J. Microtubule plus-end loading of p150(Glued) is mediated by EB1 and CLIP-170 but is not required for intracellular membrane traffic in mammalian cells. J. Cell Sci. 2006;119:2758–2767. doi: 10.1242/jcs.02999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S., Flood T. A., O'Reilly P., Hume K., Sabourin L. A. Association of the Ste20-like kinase (SLK) with the microtubule. Role in Rac1-mediated regulation of actin dynamics during cell adhesion and spreading. J. Biol. Chem. 2002;277:37685–37692. doi: 10.1074/jbc.M205899200. [DOI] [PubMed] [Google Scholar]
- Yamada E., Tsujikawa K., Itoh S., Kameda Y., Kohama Y., Yamamoto H. Molecular cloning and characterization of a novel human STE20-like kinase, hSLK. Biochim. Biophys. Acta. 2000;1495:250–262. doi: 10.1016/s0167-4889(99)00164-0. [DOI] [PubMed] [Google Scholar]
- Yan X., Habedanck R., Nigg E. A. A complex of two centrosomal proteins, CAP350 and FOP, cooperates with EB1 in microtubule anchoring. Mol. Biol. Cell. 2006;17:634–644. doi: 10.1091/mbc.E05-08-0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A., Dictenberg J. B., Purohit A., Tuft R., Doxsey S. J. Cytoplasmic dynein-mediated assembly of pericentrin and gamma tubulin onto centrosomes. Mol. Biol. Cell. 2000;11:2047–2056. doi: 10.1091/mbc.11.6.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhapparova O. N., Burakov A. B., Nadezhdina E. S. The centrosome keeps nucleating microtubules but loses the ability to anchor them after the inhibition of dynein–dynactin complex. Biochemistry (Moscow) 2007;72:1233–1240. doi: 10.1134/s0006297907110090. [DOI] [PubMed] [Google Scholar]
- Zinovkina L. A., Poltaraus A. B., Solovyanova O. B., Nadezhdina E. S. Chinese hamster protein homologous to human putative protein kinase KIAA0204 is associated with nuclei, microtubules and centrosomes in CHO-K1 cells. FEBS Lett. 1997;414:135–139. doi: 10.1016/s0014-5793(97)00952-6. [DOI] [PubMed] [Google Scholar]
- Zinovkina L. A., Poltaraus A. B., Solov'ianova O. B., Nadezhdina E. S. A proposed new mammalian cell protein kinase, associated with microtubules. (article in Russian) Mol. Biol. (Mosk.) 1998;32:341–348. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.