Abstract
Autophagy induced by nutrient depletion is involved in survival during starvation conditions. In addition to starvation-induced autophagy, the yeast Saccharomyces cerevisiae also has a constitutive autophagy-like system, the Cvt pathway. Among 31 autophagy-related (Atg) proteins, the function of Atg17, Atg29, and Atg31 is required specifically for autophagy. In this study, we investigated the role of autophagy-specific (i.e., non-Cvt) proteins under autophagy-inducing conditions. For this purpose, we used atg11Δ cells in which the Cvt pathway is abrogated. The autophagy-unique proteins are required for the localization of Atg proteins to the pre-autophagosomal structure (PAS), the putative site for autophagosome formation, under starvation condition. It is likely that these Atg proteins function as a ternary complex, because Atg29 and Atg31 bind to Atg17. The Atg1 kinase complex (Atg1–Atg13) is also essential for recruitment of Atg proteins to the PAS. The assembly of Atg proteins to the PAS is observed only under autophagy-inducing conditions, indicating that this structure is specifically involved in autophagosome formation. Our results suggest that Atg1 complex and the autophagy-unique Atg proteins cooperatively organize the PAS in response to starvation signals.
INTRODUCTION
Macroautophagy (autophagy) is a degradation process that sequesters cytoplasmic proteins and organelles into vacuoles/lysosomes for bulk degradation (Takeshige et al., 1992; Klionsky and Ohsumi, 1999). Autophagy accompanies dynamic membrane biogenesis that cannot be explained by classical secretory pathways, because it involves the formation of a unique double-membrane structure, called the autophagosome (Baba et al., 1994). Although autophagic degradation is generally considered nonselective, there are several examples of selective autophagy. In Saccharomyces cerevisiae, the cytoplasm to vacuole targeting (Cvt) pathway is now regarded as a unique, specialized transport route, although it is not conserved in all eukaryotes (Klionsky, 2005). The Cvt pathway is responsible for delivering the vacuolar hydrolases aminopeptidase I (API) and α-mannosidase from the cytoplasm to the vacuole (Klionsky and Ohsumi, 1999). The Cvt pathway is active in cells grown under nutrient-rich conditions, whereas autophagy is suppressed to undetectable level under such conditions. In contrast, autophagy is strongly induced by starvation (Takeshige et al., 1992). It has been demonstrated that the target of rapamycin (TOR) signaling pathway mediates the induction of autophagy by nutrient starvation (Noda and Ohsumi, 1998; Wullschleger et al., 2006).
So far, 31 autophagy-related (ATG) genes were identified and characterized. Of these, 18 ATG genes, ATG1–ATG10, ATG12–ATG14, ATG16–ATG18, ATG29, and ATG31, play roles in autophagosome formation (Klionsky et al., 2003; Kawamata et al., 2005; Kabeya et al., 2007). These Atg proteins are classified into several groups by their function: Atg1 kinase and its regulatory proteins (Atg1, Atg13, Atg17), two ubiquitin-like conjugation systems (Atg12 and Atg8 systems, Atg3, Atg4, Atg5, Atg7, Atg8, Atg10, Atg12, Atg16), a phosphatidylinositol 3-kinase complex (Vps34, Vps15, Atg6/Vps30, Atg14), and a group of several other proteins (Atg2, Atg9, Atg18) (Noda et al., 2002; Levine and Klionsky, 2004). Most of the Atg proteins are also required for the Cvt pathway (Noda et al., 2002; Levine and Klionsky, 2004).
Sets of Atg proteins specifically required for either the Cvt or autophagic pathway have been isolated. For example, Atg11/Cvt9 and Atg19/Cvt19, the API adapter and receptor protein, respectively, function only in the Cvt pathway (Shintani et al., 2002). Lack of these proteins does not affect autophagic activity at all. In contrast, three Atg proteins, Atg17, Atg29, and Atg31, are known to function specifically in autophagy but not in the Cvt (Kamada et al., 2000; Kawamata et al., 2005; Kabeya et al., 2007). These pathway-specific proteins likely determine the distinctions between the Cvt pathway and autophagy.
Fluorescence microscopy has shown that almost all Atg proteins gather onto the pre-autophagosomal structure (PAS), which is thought to be the site of the Cvt vesicle and autophagosome formation (Noda et al., 2000; Suzuki et al., 2001; Suzuki et al., 2007). Most Atg proteins are localized to the PAS under growth and starvation conditions. Recruitment of Atg proteins to the PAS is indispensable for formation of the Cvt vesicle and autophagosome (Shintani et al., 2001). Recently, we performed a systematic and comprehensive analysis of Atg proteins in the PAS organization during autophagy-inducible condition, and we proposed a hierarchy map of ATG genes (Suzuki et al., 2007). Localization of most Atg proteins to the PAS is impaired in the absence of ATG17, an autophagy-specific gene, thus Atg17 is situated at the basis of the PAS-organizing machinery. However, some Atg proteins are still observed at the PAS in atg17Δ cells, due to the ATG11-dependent Cvt pathway. The PAS completely disappeared in atg11Δatg17Δ, suggesting that Atg11 and Atg17 serve as alternative scaffolding proteins to organize the PAS. It is also reported that lack of the Cvt-specific factors, such as Atg11, diminished PAS localization of all Atg proteins under nutrient-rich conditions (Shintani and Klionsky, 2004). Because some Atg proteins localized to the PAS under starvation conditions in atg11Δ cells, PAS organization to create the Cvt vesicles requires Atg11 protein, but that to create autophagosome does not. These findings suggest that a strain lacking the Cvt pathway (such as atg11Δ) might give clues to understand how the PAS is generated to create autophagosomes but not the Cvt vesicles in response to starvation condition, and what are roles of the autophagy-specific Atg proteins in organization of the PAS responsible for autophagosome formation. In this study, we examined the mechanism underlying the Atg11-independent, Atg17-dependent formation of the PAS under starvation conditions.
We have previously reported the identification of Atg29 and Atg31 (Kawamata et al., 2005; Kabeya et al., 2007). It is reasonable to think that these proteins should have autophagy-restricted functions, which are required for autophagy but not for the Cvt pathway. Atg31 physically associates with Atg17, suggesting that Atg31 functions with Atg17 to regulate autophagy (Kabeya et al., 2007). Here, we report that Atg29 physically interacts with Atg17, suggesting the cooperative function of Atg17, Atg29, and Atg31. Our data suggest that Atg proteins play an essential role(s) in organizing the PAS responsible for autophagosome formation.
MATERIALS AND METHODS
Yeast Strains and Media
Growth media, culture conditions, and genetic manipulations were used as described previously (Kaiser et al., 1994; Kawamata et al., 2005). The yeast strains used in this study are listed in Table 1. Unless otherwise specified, strains were generated using one-step gene disruption or replacement methods as described previously (Longtine et al., 1998; Goldstein and McCusker, 1999). The atg1Δ, atg2Δ, atg8Δ, atg9Δ, atg11Δ, atg13Δ, atg14Δ, atg17Δ, atg29Δ, or atg31Δ strains were constructed as described previously (Kamada et al., 2000; Shintani et al., 2001; Suzuki et al., 2001, 2007; Kawamata et al., 2005; Kabeya et al., 2007). Replacement of kanMX with natMX was performed as described previously (Goldstein and McCusker, 1999). All deletion and epitope-tagged strains constructed in this study were confirmed by polymerase chain reaction (PCR).
Table 1.
Yeast strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| SEY6210 | MATα ura3 leu2 his3 trp1 lys2 suc2 | Robinson et al. (1988) |
| TMK4 | SEY6210 atg29Δ::kanMX | Kawamata et al. (2005) |
| TMK174 | SEY6210 ATG29-myc-kanMX | This study |
| PJ69-4A | MATa his3-200 leu2-3,112 trp1-901 ura3-52 gal4Δ | |
| gal80Δ GAL2-ADE2 LYS2::GAL1-HIS3 met2::GAL7-lacZ | James et al. (1996) | |
| TMK327 | PJ69-4A atg29Δ::kanMX | This study |
| BY4741 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | Giaever et al. (2002) |
| TMK181 | BY4741 atg29Δ::kanMX | Kawamata et al. (2005) |
| TMK190 | SEY6210 ATG29-GFP-kanMX | Kawamata et al. (2005) |
| TMK214 | TMK190 atg17Δ::his3MX | This study |
| TMK400 | TMK190 atg11Δ::LEU2 | This study |
| TMK200 | TMK190 atg1Δ::his3MX | This study |
| TMK464 | TMK400 atg17Δ::URA3 | This study |
| TMK553 | BY4741 ATG29-GFP-kanMX | This study |
| YKY163 | TMK553 atg11Δ::LEU2 atg31Δ::natMX | This study |
| TMK607 | TMK400 atg1Δ::his3MX | This study |
| TMK680 | TMK400 atg13Δ::URA3 | This study |
| TMK698 | TMK400 atg2Δ::HIS3 | This study |
| TMK728 | TMK400 atg9Δ:: his3MX | This study |
| TMK730 | TMK400 atg14Δ:: his3MX | This study |
| YKY161 | BY4741 ATG31-GFP-kanMX atg11Δ::LEU2 | This study |
| YKY157 | YKY161 atg17Δ::HIS3 | This study |
| YKY159 | YKY161 atg29Δ::natMX | This study |
| YKY155 | BY4741 ATG31-GFP-kanMX atg11Δ::URA3 atg1Δ::LEU2 | This study |
| YKY173 | YKY161 atg13Δ::URA3 | This study |
| YKY6 | BY4741 ATG17-GFP-his3MX | Kabeya et al. (2007) |
| TMK672 | YKY6 atg11Δ::kanMX atg1Δ::LEU2 | This study |
| TMK576 | BY4741 ATG17-GFP-kanMX | This study |
| TMK580 | TMK576 atg1Δ::LEU2 | This study |
| TMK600 | TMK576 atg29Δ::natMX | This study |
| TMK625 | TMK576 atg11Δ::LEU2 | This study |
| TMK623 | TMK625 atg29Δ::natMX | This study |
| YKY179 | TMK625 atg31Δ::natMX | This study |
| TMK686 | TMK625 atg13Δ::URA3 | This study |
| TMK696 | TMK625 atg2Δ::HIS3 | This study |
| TMK725 | TMK625 atg9Δ::his3MX | This study |
| TMK727 | TMK625 atg14Δ::his3MX | This study |
| TMK689 | BY4741 ATG2-GFP-kanMX atg11Δ::LEU2 | This study |
| TMK617 | TMK689 atg29Δ::natMX | This study |
| TMK584 | BY4741 ATG2-GFP-kanMX atg29Δ::natMX | This study |
| TMK690 | BY4741 ATG5-GFP-kanMX atg11Δ::LEU2 | This study |
| TMK618 | TMK690 atg29Δ::natMX | This study |
| TMK588 | BY4741 ATG5-GFP-kanMX atg29Δ::natMX | This study |
| TMK688 | BY4741 ATG1-GFP-kanMX atg11Δ::LEU2 | This study |
| TMK616 | TMK688 atg29Δ::natMX | This study |
| TMK731 | TMK688 atg13Δ::URA3 | This study |
| TMK732 | TMK688 atg17Δ::URA3 | This study |
| YKY175 | TMK688 atg31Δ::natMX | This study |
| TMK695 | BY4741 ATG13-GFP-kanMX atg11Δ::LEU2 | This study |
| TMK621 | TMK695 atg29Δ::natMX | This study |
| TMK765 | TMK695 atg1Δ::his3MX | This study |
| TMK766 | TMK695 atg17Δ::his3MX | This study |
| YKY177 | TMK695 atg31Δ::natMX | This study |
| OND88 | W303-1B pho8::pho8Δ60 | This study |
| YYK762 | OND88 atg11Δ::LEU2 | This study |
| YYK865 | SEY6210 ATG29-YFP-his3MX atg8Δ::TRP1 atg11Δ::LEU2 | This study |
| BJ2168 | MATa leu2 trp1 ura3-52 prb1-1122 prc1-407 pep4-3 | Yeast Stock Center |
| YYK806 | BJ6218 HA-ATG1 atg29Δ::kanMX | This study |
| YYK850 | BJ2168 HA-ATG1 ATG29-myc-natMX | This study |
| YYK868 | YYK850 atg11Δ::LEU2 | This study |
| YYK870 | YYK850 atg11Δ::LEU2 atg17Δ::kanMX | This study |
| YYK872 | YYK850 atg11Δ::LEU2 atg31Δ::kanMX | This study |
| YYK874 | BJ2168 HA-ATG1 ATG31-myc-hphMX | |
| atg11Δ::LEU2 atg29Δ::kanMX | This study | |
| YYK878 | BJ2168 HA-ATG1 ATG31-myc-hphMX | |
| atg11Δ::LEU2 atg17Δ::URA3 atg29Δ::kanMX | This study |
Plasmid Construction
The plasmids used in this study are listed in Table 2. To construct pTM20, a PCR fragment containing ATG29-13Myc was obtained by PCR using genomic DNA from TMK174 as a template, digested with SphI, and subcloned into pRS314x. To generate plasmids harboring ATG29 for two-hybrid analyses, each ATG29 variant was amplified by PCR using genomic DNA as a template and primers containing EcoRI (forward) and PstI (reverse). PCR fragments containing full-length or truncated Atg29 were digested with EcoRI and PstI, and then they were subcloned into pBGD-C2 digested with the corresponding enzymes. Similarly, plasmids harboring ATG17 were constructed by inserting fragments containing full-length ATG17 or truncated ATG17 variants into pGAD-C1 at the BamHI and ClaI sites. pTM41 was constructed by subcloning a SphI fragment bearing Gal4AD-ATG17 (Kabeya et al., 2005) into pGADU-C1 digested with the corresponding enzymes. To generate point mutations in ATG29, Trp54, Pro64, Phe67, or Arg71 were changed to Arg, His, Leu, and Gly, respectively, using a QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA)) according to the recommendations of the manufacturer. Restriction analysis and DNA sequencing verified these different mutations. All primer sequences and detailed maps for the constructs described here can be provided upon request.
Table 2.
Plasmids used in this study
| Name | Description | Source or reference |
|---|---|---|
| pRS313 | HIS3, CEN6-ARSH4, ampr | Sikorski and Hieter (1989) |
| pRS314 | TRP1, CEN6-ARSH4, ampr | Sikorski and Hieter (1989) |
| pRS316 | URA3, CEN6-ARSH4, ampr | Sikorski and Hieter (1989) |
| pRS314x | TRP1, CEN6-ARSH4, ampr | Moriya and Isono (1999) |
| pTN3 | pKT10, PHO8Δ60 | Noda et al. (1995) |
| pGBD-C(X) | TRP1, 2μARS, GAL4-BD(1-147), ampr | James et al. (1996) |
| pGBDU-C(X) | URA3, 2μARS, GAL4-BD(1-147), ampr | James et al. (1996) |
| pGAD-C(X) | LEU2, 2μARS, GAL4-AD(768-881), ampr | James et al. (1996) |
| pTM4 | pRS314x, ATG29 | This study |
| pTM20 | pRS314x, ATG29-Myc | This study |
| pTM25 | pGBD-C2, ATG29(1-213) | This study |
| pTM28 | pGBD-C2, ATG29(1-162) | This study |
| pTM29 | pGBD-C2, ATG29(1-132) | This study |
| pTM30 | pGBD-C2, ATG29(1-69) | This study |
| pTM31 | pGBD-C2, ATG29(44-213) | This study |
| pTM32 | pGBD-C2, ATG29(44-162) | This study |
| pTM33 | pGBD-C2, ATG29(44-132) | This study |
| pTM34 | pGBD-C2, ATG29(44-104) | This study |
| pTM35 | pGBD-C2, ATG29(97-213) | This study |
| pTM36 | pGBD-C2, ATG29(97-162) | This study |
| pTM37 | pGBD-C2, ATG29(97-132) | This study |
| pTM38 | pGBD-C2, ATG29(136-213) | This study |
| pTM41 | pGADU, ATG17 | This study |
| pTM42 | pGBD-C2, ATG29W54R | This study |
| pTM43 | pGBD-C2, ATG29P64H | This study |
| pTM44 | pGBD-C2, ATG29F67L | This study |
| pTM45 | pGBD-C2, ATG29R71G | This study |
| pTM50 | pRS316, ATG29F67L | This study |
| pTM51 | pRS316, ATG29R71G | This study |
| pTM52 | pRS314x, ATG29W54R-Myc | This study |
| pTM53 | pRS314x, ATG29P64H-Myc | This study |
| pTM54 | pRS314x, ATG29F67L-Myc | This study |
| pTM55 | pRS314x, ATG29R71G-Myc | This study |
| pTM108 | pRS313, ATG29 | This study |
| pTM109 | pRS313, ATG29F67L | This study |
| pTM110 | pRS313, ATG29R71G | This study |
| pYOK6 | pGAD-C1, ATG17(1-417) | This study |
| pYOK7 | pGAD-C1, ATG17(1-354) | This study |
| pYOK8 | pGAD-C1, ATG17(1-209) | This study |
| pYOK9 | pGAD-C1, ATG17(1-99) | This study |
| pYOK10 | pGAD-C1, ATG17(97-417) | This study |
| pYOK11 | pGAD-C1, ATG17(97-354) | This study |
| pYOK12 | pGAD-C1, ATG17(97-209) | This study |
| pYUK13 | pGAD-C1, ATG17(207-417) | This study |
| pYOK14 | pGAD-C1, ATG17(207-354) | This study |
| pYOK15 | pGAD-C1, ATG17(349-417) | This study |
| pYOK16 | pRS314 ATG13(1-738) | This study |
| pYOK17 | pRS314 ATG13(1-448) | This study |
| pYOK18 | pRS316, ATG1 | This study |
| pYOK19 | pRS316, ATG1D211A | This study |
| pYOK20 | pRS316, ATG1K54A | This study |
| pRS314 (CFP-ATG8) | pRS314, CFP-ATG8 | Suzuki et al. (2001) |
Yeast Two-Hybrid Analysis and Mutant Screening
Two-hybrid assays were carried out essentially as described previously (James et al., 1996). Briefly, pGAD- and pGBD-based plasmids were cotransformed into host strain PJ69-4A. Transformant colonies were replica-plated onto either SC plates lacking tryptophan, leucine, and histidine containing 3 mM 3-aminotriazole (3-AT) or SC plates lacking tryptophan, leucine, and adenine, and then they were tested for growth.
Atg29 mutants unable to interact with Atg17 were isolated as follows. A segment of ATG29 corresponding to amino acid residues 44-104 was mutagenized by PCR in the presence of 0.5 mM MnCl2, by using pTM34 as a template. The PCR products were cotransformed with a linearized pGBD-C2 plasmid digested with EcoRI and PstI into host strain TMK327 (PJ69-4A atg29Δ) harboring plasmid pTM41 (GAL4ADU-ATG17). Transformants were selected for growth on SC-Trp-Ura, and then they were replica-plated onto SC-Trp-Ura-Ade plates to identify Atg17-nonbinding mutants. Of ∼300 colonies examined, 35 colonies unable to grow on SC-Leu-Trp-Ade were selected as candidates. These cells were streaked on SC-Trp plates supplemented with 5-fluoro-orotic acid at a final concentration of 1 mg/ml to select against the URA3-based ATG17 plasmid, and then they were incubated at 30°C. atg29 mutants were recovered from these cells by isolating plasmids and determining the nucleotide sequence of atg29 by DNA sequencing.
Fluorescence Microscopy
Intracellular localization of fluorescent proteins was examined using an inverted fluorescence microscope (IX-71 or IX-81; Olympus, Tokyo, Japan) as described previously (Suzuki et al., 2001, 2007). Images were acquired using MetaMorph software (Molecular Devices, Sunnyvale, CA), and they were processed using Adobe PhotoShop software (Adobe Systems, Mountain View, CA). The percentages of cells with green fluorescent protein (GFP) fluorescent dot signals at the PAS were determined by counting 100∼200 cells for each sample (Figures 1–3).
Figure 1.
Atg29 interaction with Atg17 is essential for autophagy. (A) The Atg17 binding site in Atg29. The indicated segments of Atg29 were fused to the Gal4 DNA-binding domain (BD). Binding to Atg17 fused to the Gal4-activating domain was evaluated by growth on −Ade plates for 3 d. (B) The Atg29 binding sites in Atg17. The indicated segments of Atg17 were fused to the Gal4 (transcriptional) activation domain (AD). Binding to Atg29 was tested by growth on −His plates containing 3 mM 3-AT. Atg17 interacts with Atg29 via coiled-coil domain 2. (C) Mutant Atg29s (Atg29W54R, Atg29P64H, Atg29F67L, or Atg29R71G) did not associate with Atg17. Two-hybrid assays were carried out as described in A. (D) Mutant Atg29s failed to interact with Atg17 in vivo. atg29Δ (TMK4) cells carrying the indicated myc-tagged ATG29 plasmids (wild-type and mutants) were grown in YEPD, and then they were treated with 0.2 μg/ml rapamycin for 3 h. Cell extracts were immunoprecipitated with anti-myc antibody. Coimmunoprecipitated proteins were analyzed by immunoblotting with anti-myc (top) or anti-Atg17 antibodies (middle). The bottom row indicates the amount of Atg17 in the total lysates. (E) The binding of Atg29 and Atg17 is essential for autophagy. Wild-type (BY4741 + pTN3) or atg29Δ (TMK181 + pTN3) cells carrying pTM108 (Atg29), pTM109 (Atg29F67L), pTM110 (Atg29R71G), or pRS313 (empty vector) were cultured in SCD, and then they were transferred to SD(−N) for 4 h, lysed, and assayed for ALP activity. The bars represent the SD of three independent experiments. (F) Atg17 is required for proper Atg29 localization to the PAS. WT (TMK190) or atg17Δ (TMK214) cells were grown in SD medium containing casamino acid supplemented with adenine, tryptophan, and uracil, and then they were treated with 0.2 μg/ml rapamycin. Growing (0 h) and rapamycin-treated (3 h) cells were observed by fluorescence microscopy. The percentages of cells with fluorescent dot signals at the PAS were determined. Bar, 5 μm. (G) Atg29 is not required for proper Atg17 localization to the PAS. WT (TMK576) or atg29Δ (TMK600) cells were tested as in F.
Figure 2.
Deletion of ATG29 in atg11Δ cells affects PAS localization of Atg proteins. Localization of GFP-tagged Atg17, Atg29, and Atg31 was analyzed in the indicated atg mutant cells. Similarly, localization of Atg2 and Atg5 was observed. Rapamycin-treated (3 h) cells were observed by fluorescence microscopy. The percentages of cells with fluorescent dot signals at the PAS were determined. Bar, 5 μm.
Figure 3.
The PAS localization of Atg17 or Atg29 does not depend on Atg2, Atg9, or Atg14 in atg11Δ cells. Localization of Atg17-GFP and Atg29-GFP in the indicated atg mutants (treated with rapamycin for 3 h) was observed by fluorescence microscopy. The percentages of cells with fluorescent dot signals at the PAS were determined. Bars, 5 μm.
Immunoprecipitation
Immunoprecipitation of Atg29 or Atg1 were performed as described previously (Kamada et al., 2000). Cells grown to mid-log phase in YEPD were treated with 0.2 μg/ml rapamycin for 3 h, collected, washed once with stop mix buffer (0.9% NaCl, 1 mM NaN3, 10 mM EDTA, and 50 mM potassium fluoride), and suspended in ice-cold lysis buffer (1× phosphate-buffered saline [PBS], pH 7.4, 1 mM EDTA, 1 mM EGTA, 2 mM Na3VO4, 50 mM KF, 15 mM sodium pyrophosphate, pH 7.5, 15 mM p-nitrophenylphosphate, 20 μg/ml leupeptin, 20 μg/ml benzamidine, 10 μg/ml pepstatin A, 40 μg/ml aprotinin, and 1 mM phenylmethylsulfonyl fluoride). An equal volume of glass beads (ø = 0.5 mm) was added to this suspension, and cells were broken by vigorous vortexing for 10 min at 4°C. A half volume of lysis buffer containing 1% Tween 20 was added to a final concentration of 0.5% Tween 20, and the mixture was incubated for 10 min with mild rotation. The beads and cell debris were removed by two consecutive 10-min centrifugations at 10,000 × g and 4°C. Glycerol was added to the resulting lysate to a final concentration of 30% before storage at −20°C. Protein concentrations were estimated using a bicinchoninic acid kit (Pierce Chemical, Rockford, IL). For immunoprecipitation analysis, cell lysates were brought up to 1 ml with immunoprecipitation buffer (1× PBS, pH 7.4, 1 mM EDTA, 1 mM EGTA, 2 mM Na3VO4, 50 mM KF, 15 mM NaPPi, pH 7.5, 15 mM pNPP, and 0.5% Tween 20), and then they were incubated with anti-myc (9E10, for Atg29-Myc) or anti-hemagglutinin (HA) (16B12 [Covance Research Products, Princeton, NJ] for HA-Atg1) antibodies for 1.5 h at 4°C with gentle rotation. Twenty microliters of 50% suspension of protein G-Sepharose 4 FF (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom) was added to the extract/antibody mixture, followed by rotation for 1.5 h at 4°C. Immunocomplexes were washed three times with 1 ml of wash buffer (1× PBS, pH 7.4, 2 mM Na3VO4, and 1% NP-40). Proteins bound to the beads were suspended in 2× sample buffer (100 mM Tris-Cl, pH 6.8, 4% SDS, 20% glycerol, 0.4% bromophenol blue, and 200 mM dithiothreitol), eluted by boiling, and subjected to SDS-polyacrylamide gel electrophoresis.
Other Procedures
Immunoblot analyses were performed as described previously (Kamada et al., 2000; Kabeya et al., 2005). Alkaline phosphatase (ALP) assays were performed as described previously (Noda et al., 1995). Pho8Δ60 was expressed from the 2μ plasmid (pTN3; Figure 1), or it was genomically expressed from the chromosome (Figure 1E).
RESULTS
Association of Atg29 with Atg17 Is Required for Autophagy
To understand the mechanism of Atg17 for the PAS assembly, we focused on Atg29, an autophagy-specific protein. We performed yeast two-hybrid analyses of the interaction between Atg29 and Atg17. Atg29 was found to associate with Atg17, suggesting that Atg29 functions with Atg17 (Figure 1A). Because our previous studies have shown that Atg17 physically associates with Atg1 and Atg13, we investigated whether Atg29 interacts with Atg1 or Atg13 by the yeast two-hybrid assay. No interaction between Atg29 and Atg1 or Atg13 was detected in these experiments, suggesting that Atg29 does not possess an intrinsic binding site for Atg1 or Atg13 (data not shown).
To determine the domain of Atg29 required for interaction with Atg17, we constructed and examined a series of truncated forms of Atg29. We found that Atg17 binds to the central region of Atg29 (residues 44-104), which contains a putative coiled-coil motif (Kawamata et al., 2005) (Figure 1A). We also performed the reverse experiment, determining the domain of Atg17 required for interaction with Atg29. Atg17 possesses five putative coiled-coil domains, CC1–CC5 (Cheong et al., 2005). An Atg17 fragment containing CC2 domain (residues 97-209) turned out to be sufficient to bind Gal4BD-Atg29 (Figure 1B). Together, it is likely that Atg17 and Atg29 interact with each other through their coiled-coil domains. Next, we isolated Atg29 mutants that do not bind to Atg17 by two-hybrid assay. We introduced random mutations within the coiled-coil domain region by using PCR-based mutagenesis, and we screened for mutants unable to interact with Atg17 as described in Materials and Methods. Among ∼300 clones screened, we obtained three mutants (W54R, P64H, and F67L/R71G) that completely lost their ability to bind Atg17. We confirmed that introduction of the W54R, P64H, F67L, or R71G mutation into full-length Atg29 abolishes their interaction with Atg17 in two-hybrid analysis (Figure 1C).
We next examined the Atg17–Atg29 interaction by coprecipitation experiments. We generated a myc-tagged version of Atg29 (Atg29-myc), which was confirmed to complement the autophagic defect of atg29Δ cells (data not shown). Atg29-myc efficiently precipitated endogenous Atg17, confirming that Atg17 and Atg29 physically interact in vivo (Figure 1D). Rapamycin treatment mimics starvation condition and triggers autophagy (Noda and Ohsumi, 1998). Atg17–Atg29 interaction was detected in the cells treated with or without rapamycin. Atg29W54R, Atg29P64H, Atg29F67L, or Atg29R71G did not associate with Atg17 under either growth or starvation condition. Atg17–Atg29 association was not affected by disruption of other ATG genes, e.g., ATG1, ATG13, ATG8, or ATG11 (data not shown). We next estimated the autophagic activity of these mutants by an ALP assay (Noda et al., 1995). Wild-type Atg29 rescued the autophagic defect of atg29Δ, compared with vector control (Figure 1E). In contrast, autophagy was not induced by the atg29F67L or atg29R71G mutants, but it could be partially recovered when these mutants were overexpressed (data not shown). Atg29 localizes to the PAS (Kawamata et al., 2005), and this localization required ATG17 (Figure 1F). Atg17–GFP distribution was not affected in atg29Δ cells under growth or starvation conditions (Figure 1G).
We have recently reported that Atg31, another autophagy-specific protein, binds to Atg17 (Kabeya et al., 2007). Atg31 also interacts with Atg29 (Kabeya and Ohsumi, unpublished data), suggesting that Atg17, Atg29, and Atg31 form a ternary complex. The PAS localization of Atg31 is also dependent on ATG17, but not vice versa. These results suggest that Atg29 and Atg31 associate together with Atg17 and function at the PAS to generate autophagosome.
Autophagy-Unique Atg Protein Complex Is Required for Recruitment of Atg Proteins to the PAS for Autophagosome Formation
It has been recently reported that Atg11 and Atg17 play important roles in the recruitment of other Atg proteins to the PAS (Suzuki et al., 2007). ATG11 is a Cvt-specific gene, and it is dispensable for autophagy (Kim et al., 2001), and in atg11Δ cells the Cvt pathway is abrogated because the PAS is not organized under growth condition (Shintani and Klionsky, 2004). Thus, we used atg11Δ cells to learn about the targeting of autophagy-unique Atg proteins to the PAS specifically involved in autophagosome formation under starvation condition. In atg11Δ cells, Atg29-GFP, scattered throughout the cytosol under growth condition (Figure 6A), was recruited to the PAS after rapamycin treatment (Figure 2). The PAS localization of Atg29-GFP disappeared in rapamycin-treated atg11Δatg17Δ cells, confirming that Atg17 is responsible for the localization of Atg29 to the PAS in atg11Δ cells. Atg31 was also required for the PAS localization of Atg29. Because the PAS localization of Atg31-GFP was abolished in atg11Δatg17Δ and atg11Δatg29Δ cells, Atg29 was indispensable for the PAS targeting of Atg31 (Figure 2). We next examined Atg17-GFP in atg11Δatg29Δ and atg11Δatg31Δ cells. Atg29 or Atg31 was not required for the localization of Atg17 in the presence of Atg11 (Figure 1G) (Kawamata et al., 2005; Kabeya et al., 2007); however, unexpectedly PAS localization of Atg17-GFP disappeared in atg11Δatg29Δ and atg11Δatg31Δ mutants (Figure 2). These results suggest that Atg17, Atg29, and Atg31 are mutually essential for the PAS localization. We reported previously that any Atg protein does not target to the PAS in atg11Δatg17Δ cells (Suzuki et al., 2007). Thus, we reexamined this using atg11Δatg29Δ strain. Although Atg2-GFP and Atg5-GFP normally localized to the PAS in atg11Δ and atg29Δ cells, their PAS localization was severely impaired in atg11Δatg29Δ cells, indicating that not only Atg17 but also Atg29 is essential for the PAS localization of Atg proteins (Figure 2). These results suggest that at least in atg11Δ cells the autophagy-specific protein complex plays an important role in starvation-induced assembly of Atg proteins to the PAS responsible for autophagosome formation (but not for the Cvt vesicle).
Figure 6.
Assembly of Atg proteins to the PAS is regulated in response to nutrient conditions. (A) Recruitment of Atg17 or Atg29 to the PAS is induced by starvation in atg11Δ cells. Atg29-GFP and Atg17-GFP were observed in wild-type or atg11Δ cells under both growth and rapamycin-treated (3 h) conditions. (B) Atg29 localization in response to nutrient conditions in wild-type or in atg11Δ cells. Cells grown in SD medium containing casamino acid supplemented with adenine, tryptophan, and uracil were shifted to SD(−N). Nitrogen-starved cells (−N, 2 h) were supplied with 2× SD medium containing casamino acid supplemented with adenine, tryptophan, and uracil, and then they were incubated for 10 min. (C) PAS recruitment of Atg8 in response to starvation. atg11Δatg8Δ cells (YYK865) harboring the CFP-ATG8 (pRS314) were observed as described in B. CFP-Atg8 and Atg29-YFP were colocalized in starved cells. (Noted that CFP signal was observed in the vacuole indicating the induction of autophagy.) (D) Accumulation of Atg17 and Atg29 to the PAS in atg1D211Aatg11Δ is starvation specific. The localization of Atg17-GFP and Atg29-GFP was analyzed as described in B. (E) Comparison of autophagic activity of wild-type cells and atg11Δ cells in response to cellular nutrient condition. Wild-type (OND88) or atg11Δ (YYK762) cells genomically expressing Pho8Δ60 protein were cultured in YEPD, and then they were shifted to SD(−N) at time 0. After SD(−N) for 4 h, nitrogen-starved cells were supplied with nutrients as described in B. After 1 or 2 h, cells were corrected and subjected to ALP assay. The bars represent the SD of three independent experiments. Bars (A–D), 5 μm.
Atg1 Complex Also Plays an Essential Role in the Localization of Atg Proteins under Starvation Condition
Next, we examined the effect of another ATG gene disruption on localization of Atg17 and Atg29 in atg11Δ cells in the presence of rapamycin. Although Atg17-GFP and Atg29-GFP localized to the PAS in atg1Δ and atg11Δ cells, none of them localized to the PAS in atg1Δatg11Δ cells (Figure 3). In contrast, they significantly localized to the PAS in atg2Δatg11Δ, atg9Δatg11Δ, and atg11Δatg14Δ cells. According to the previous report (Suzuki et al., 2007), ATG1 and ATG9 are situated next to ATG17 in the hierarchy of protein assembly required for PAS formation, ATG14 is located downstream of ATG9, and ATG2 is located downstream of ATG1 and ATG14 in the ATG gene hierarchy map. In addition to the autophagy-specific proteins, Atg1 plays an important role in organization of the PAS.
Because Atg1 binds to Atg13 in response to starvation (Kamada et al., 2000), we tested whether Atg1–Atg13 complex is involved in the starvation-responsive recruitment of Atg proteins to the PAS. As well as ATG1, ATG13 was found to be required for the PAS localization of the autophagy-specific proteins (Figure 4A). Then, we tested localization of Atg1 and Atg13 in atg1Δatg11Δ, atg11Δatg13Δ, atg11Δatg17Δ, atg11Δatg29Δ, and atg11Δatg31Δ mutants. The results, shown in Figure 4B, are summarized in Table 3. We found that these proteins are equally required for PAS targeting. In conclusion, these five proteins (Atg1–Atg13 complex and Atg17–Atg29–Atg31 autophagy-unique proteins) participate in the recruitment of Atg proteins to the ATG11-independent PAS for autophagosome formation under starvation condition.
Figure 4.
All of five proteins (Atg1, Atg13, Atg17, Atg29, and Atg31) play important role in their localization at the Atg11-independent PAS. (A) In atg11Δ cells, both Atg1 and Atg13 maintain Atg17-GFP, Atg29-GFP, and Atg31-GFP at the PAS. Rapamycin-treated (3 h) cells were observed by fluorescence microscopy. (B) Localization of Atg1-GFP and Atg13-GFP in the indicated atg cells was examined as described in A. Bars, 5 μm.
Table 3.
Summary of localization of Atg1-GFP, Atg13-GFP, Atg17-GFP, Atg29-GFP, and Atg31-GFP in atg11Δ, atg11Δatg1Δ, atg11Δatg13Δ, atg11Δatg17Δ, atg11Δatg29Δ, or atg11Δatg31Δ cells under vegetative (above) or starvation (below) conditions
| Strain | atg11Δ | atg11Δatg1Δ | atg11atg13Δ | atg11Δatg17Δ | atg11Δatg29Δ | atg11Δatg31Δ |
|---|---|---|---|---|---|---|
| Atg1-GFP | − | / | − | − | − | − |
| Atg13-GFP | − | − | / | − | − | − |
| Atg17-GFP | − | − | − | / | − | − |
| Atg29-GFP | − | − | − | − | / | − |
| Atg31-GFP | − | − | − | − | − | / |
| atg11Δ | atg11Δatg1Δ | atg11atg13Δ | atg11Δatg17Δ | atg11Δatg29Δ | atg11Δatg31Δ | |
|---|---|---|---|---|---|---|
| Atg1-GFP | + | / | − | − | − | − |
| Atg13-GFP | + | − | / | − | − | − |
| Atg17-GFP | + | − | − | / | − | − |
| Atg29-GFP | + | − | − | − | / | − |
| Atg31-GFP | + | − | − | − | − | / |
+, PAS localization observed; −, no PAS localization observed.
Atg17–Atg29 and Atg1–Atg13 Interactions Are Essential for the PAS Organization
As shown above, ATG29 and ATG31 are necessary for the PAS localization of Atg1, Atg13, and Atg17 in atg11Δ cells under autophagy-inducing conditions. To further examine whether association of Atg29 to Atg17 is required for the PAS targeting, we examined the localization of Atg proteins in cells expressing Atg29F67L and Atg29R71G. Expression of Atg29WT completely rescued the PAS localization of Atg1-GFP, Atg13-GFP, and Atg17-GFP in atg11Δatg29Δ cells treated with rapamycin (Figure 5A). In contrast, Atg29F67L and Atg29R71G failed to recruit any of these GFP-tagged Atg proteins to the PAS. Thus, the Atg17–Atg29 association is indispensable for Atg1, Atg13, and Atg17 localization.
Figure 5.
Atg17–Atg29 and Atg1–Atg13 interactions are indispensable for their recruitment to the PAS. (A) Atg29 mutant (Atg29F67L and Atg29R71G) is defective for the ATG11-independent PAS localization of Atg1, Atg13, or Atg17. atg11Δatg29Δ cells expressing GFP-tagged Atg1, Atg13, or Atg17 were transformed with a plasmid expressing either Atg29wild-type, Atg29F67L, or Atg29F71G. Cells were treated with rapamycin for 3 h before observation. (B) Atg131-448 does not recruit Atg29 to the PAS in atg11Δ cells. atg11Δatg13Δ cells expressing Atg29-GFP were transformed with a plasmid expressing either Atg13wild type or Atg131-448. Cells were observed after rapamycin treatment for 3 h. (C) Atg1 kinase activity is not essential for the PAS targeting of Atg17 and Atg29 in atg11Δ. atg11Δatg1Δ cells expressing Atg17-GFP or Atg29-GFP were transformed with a plasmid expressing either wild-type Atg1 or Atg1 kinase-deficient mutant (Atg1D211A or Atg1K54A). Cells were observed after rapamycin-treatment for 3 h. Bars, 5 μm.
In atg11Δatg13Δ cells, Atg29-GFP was dispersed in the cytosol (Figure 4A). A C-terminally truncated mutant of Atg13 (Atg131-448) lacks binding activity with Atg1, resulting in an autophagy defect, whereas the Cvt pathway was not affected in this mutant (Kamada et al., 2000). Atg131–448 did not recruit Atg1-GFP, Atg17-GFP, or Atg29-GFP to the PAS in atg11Δatg13Δ cells (Figure 5B; data not shown). Therefore, the Atg1–Atg13 interaction is also essential for the PAS localization of these Atg proteins. Taking these results together, it seems likely that the PAS localization of Atg1, Atg13, Atg17, and Atg29 is mediated by the interactions among them.
Atg1 is a protein kinase essential for autophagy. We tested whether Atg1 kinase activity is required for the PAS targeting of Atg17 and Atg29 in atg11Δ. We observed localization of Atg17-GFP or Atg29-GFP in atg1Δatg11Δ cells expressing either Atg1WT or a kinase-deficient Atg1D211A and Atg1K54A (Matsuura et al., 1997; Kamada et al., 2000). We confirmed that both Atg1 K54A and Atg1D211A interact normally with Atg13 and Atg17 as well as Atg1WT (Kamada and Ohsumi, unpublished results). Atg1WT complemented the starvation-induced targeting of Atg17-GFP and Atg29-GFP to the PAS in atg1Δatg11Δ cells (Figure 5C). PAS localization of Atg17-GFP and Atg29-GFP was also observed in both atg1K54A atg11Δ cells and atg1D211Aatg11Δ cells (Figure 5C). Thus, not the protein kinase activity but Atg1 protein itself is required for the recruitment of these two proteins. It is noteworthy that Atg17- and Atg29-GFP abnormally accumulated at the PAS in atg1K54Aatg11Δ and atg1D211Aatg11Δ than in ATG1WTatg11Δ (Figure 5C), suggesting that the proper subcellular redistribution of autophagy-specific Atg proteins requires Atg1 kinase activity.
The PAS Generating Autophagosomes Is Organized in Response to Nutrient Condition
In atg11Δ cells, the organization of the PAS, which is specifically needed for autophagy, should be regulated by nutrient conditions. As expected, Atg17-GFP and Atg29-GFP in growing atg11Δ cells were recruited to the PAS only after rapamycin treatment or nitrogen depletion (Figure 6, A and B). The protein amounts of Atg17 and Atg29 were not dramatically affected by rapamycin treatment (Figure 1D), suggesting that the recruitment of Atg17 and Atg29 to the PAS is highly stimulated by starvation. We further observed that cyan fluorescent protein (CFP)-Atg8 was also recruited to the PAS in response to nitrogen starvation, colocalizing with Atg29-yellow fluorescent protein (YFP) (Figure 6C). When nitrogen-starved atg11Δ cells were supplied with a nitrogen source, such as amino acids and ammonium sulfate, PAS-localized Atg8 and Atg29 quickly disappeared within 10 min (Figure 6, B and C), indicating that recruitment of Atg proteins to the PAS is tightly regulated by nutrient conditions. In atg1D211Aatg11Δ cells, the accumulated Atg17-GFP and Atg29-GFP were also quickly dispersed into the cytosol by addition of a nitrogen source within 10 min, suggesting that Atg1 kinase activity is not required for perception of nutrient signal (Figure 6D).
Based on GFP observation, Atg17 and Atg29 seemed to stay statically at the PAS irrespective of nutrient condition in wild-type (ATG11) cells, because the Atg11-dependent PAS was formed under nutrient-rich condition (Figure 6, A and B). However, the above-mentioned results using atg11Δ cells suggested that recruitment of Atg proteins to the PAS is dynamically regulated by cellular nutrient status. To examine whether Atg proteins assemble to the PAS in response to starvation even in wild-type cells, excluding the possibility that our observation is an artifact of deletion of ATG11, we carried out several experiments. Autophagy was induced both in nitrogen-depleted wild-type and atg11Δ cells (Kim et al., 2001) (Figure 6E). When nutrients were supplied to the starved cells (at 4 h after starvation), autophagic activity was immediately terminated in both strains (Figure 6E). This result indicates that the PAS responsible for autophagosome formation is organized only under starvation conditions. Furthermore, because the autophagic activity is terminated immediately after nutrient resupply, the results also imply that in wild-type (ATG11) cells, the autophagy-specific PAS changes to the ATG11-dependent, Cvt-specific PAS according to the availability of a nutrient signal.
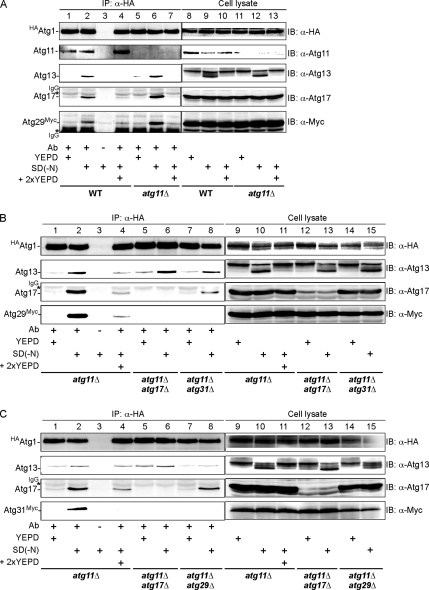
Next, we analyzed Atg protein assembly by an immunoprecipitation experiment. When HA-tagged Atg1 was immunoprecipitated from cell lysates, coprecipitation of Atg13, Atg17, Atg29, and Atg31 was strongly enhanced by nitrogen starvation (Figure 7, A–C). When starved cells were shifted to nutrient-rich medium, these proteins quickly dissociated within 10 min. Atg1–Atg11 interaction was not affected by nutrient condition. It is noteworthy that this regulation was observed in both wild-type and atg11Δ cells. These results support that autophagy-specific Atg proteins assemble to and disassemble from the PAS in response to nutrient conditions, even though GFP-fusions of Atg proteins apparently stay statically at the PAS in wild-type cells. Starvation-induced Atg1 interaction toward Atg29 and Atg31 was abolished in atg11Δatg17Δ, atg11Δatg31Δ, or atg11Δatg29Δ cells (Figure 7, B and C). Together, these results indicate that Atg1-Atg13 and autophagy-specific Atg proteins (Atg17, Atg29, and Atg31) assemble together to the PAS in response to starvation for induction of autophagy.
Figure 7.
Association of Atg1, Atg13, Atg17, Atg29, and Atg31 is regulated by nutrient condition. (A) Interaction of Atg1 with Atg13, 17, and Atg29 is enhanced by starvation. Wild-type (YYK850) or atg11Δ (YYK868) cells grown in YEPD (lanes 1, 5, 8, and 11) were transferred to SD(−N) for 2 h (lanes 2, 3, 6, 9, and 12). Starvation was terminated by adding 2× YEPD for 10 min (lanes 4, 7 10, and 13). HA-Atg1 was immunoprecipitated by anti-HA ascite (Ab), and immunocomplex (lanes 1–7) was analyzed by immunoblot with the indicated antibodies. Total cell extracts also are shown (lanes 8–13). Note that all Atg proteins were chromosomally expressed under their own promoter. (B) Atg17 and Atg31 are required for starvation-induced Atg1–Atg29 association. atg11Δ (YYK868), atg11Δ atg17Δ (YYK870) and atg11Δatg31Δ (YYK872) cells were analyzed as described in A. (C) Atg1–Atg31 association is disrupted in atg11Δatg17Δ and atg11Δatg29Δ cells. The experiment described in A was carried out using atg11Δatg29Δ (YYK874) harboring plasmid pTM4 (atg11Δ), atg11Δatg17Δ atg29Δ (YYK878) harboring plasmid pTM4 (atg11Δatg17Δ), or atg11Δatg29Δ (YYK874).
DISCUSSION
In this study, we investigated PAS dynamics by observation of the GFP-tagged autophagy-specific proteins Atg17, Atg29, and Atg31. Previously, we found the PAS through the localization analysis of Atg proteins under vegetative condition (Suzuki et al., 2001). Originally, the PAS was defined as a GFP–Atg8 signal a punctate structure to which almost all Atg proteins localized (Suzuki et al., 2001). In S. cerevisiae, the Cvt pathway, which is an autophagy-related biosynthetic pathway, constitutively works in the presence of nutrients. Because most Atg proteins are required for both autophagy and the Cvt pathway, they assemble to organize the PAS under growth (i.e., nutrient-rich) conditions, and they seem to stay at the PAS under starvation condition. Therefore, it was difficult to precisely describe the dynamics of Atg proteins under autophagy-inducing conditions, such as nitrogen starvation and rapamycin treatment. Accumulating evidence shows that Atg11 (a Cvt pathway-specific protein) is important for gathering Atg proteins to the PAS under growth conditions. For example, Atg11 has an intrinsic binding activity toward Atg17 (Cheong et al., 2005), thus autophagy-specific proteins presumably localized to the Atg11-dependent PAS under nutrient-rich condition. Here, we used atg11Δ cells to disconnect the autophagic pathway from the Cvt pathway. This enabled us to observe Atg proteins localizing at the PAS in an autophagy-dependent manner, specialized to form autophagosomes (but not the Cvt vesicle). Therefore, our results suggest the starvation-induced PAS responsible for autophagosome formation. They also confirm the existence of the Atg11-dependent, Cvt-specific PAS (Shintani and Klionsky, 2004). The Cvt-specific PAS seems to be present under conditions permissive for growth. So far, we know of no marker that can distinguish the Cvt-specific PAS from the autophagy-specific PAS in wild-type cells, but the former is unable to form an autophagosome at all (Figure 6).
We found interesting characteristics of the PAS responsible for autophagosome formation. First, Atg proteins assemble to organize the PAS in response to nutrient starvation, and they quickly disassemble after resupply of nutrients (Figures 6 and 7). Our results suggest that the PAS is a dynamic structure in which many Atg proteins continuously assemble and disassemble. For example, although GFP-fusions of Atg1, Atg13, and autophagy-unique Atg protein complex (Atg17, Atg29, and Atg31) seem to statically localize at the PAS in wild-type cells, their physical association is drastically changed by nutrient conditions. Second, Atg1-Atg13 and autophagy-specific Atg proteins act as an organizing center of the starvation-induced PAS. Our previous report showed that Atg17 is the most fundamental protein in PAS organization (Suzuki et al., 2007). Here, we show that Atg17 forms a ternary complex with Atg29 and Atg31 and that this association is indispensable for recruitment of Atg proteins to the PAS to generate autophagosomes. Atg1-Atg13 is also required for recruitment of autophagy-specific Atg complex to the PAS. We reported previously that the Atg17-GFP localization to the PAS is not disrupted in atg1Δ ATG11 or atg13Δ ATG11 cell (Suzuki et al., 2007). We obtained a similar result about the PAS targeting of Atg29 and Atg31 (Figure 3; data not shown), indicating that Atg11 can recruit Atg17 and Atg29 to the PAS in an Atg1- and Atg13-independent manner. Because Atg11 has an intrinsic binding activity toward a couple of Atg proteins (e.g., Atg9 and Atg17), we assume that Atg9 and Atg17 accumulated to the (presumably Atg11-dependent) PAS in atg1Δ cells.
Tor signaling controls Atg1–Atg13 interaction through the phosphorylation state of Atg13. In growth condition, Atg13, when highly phosphorylated in a Tor-dependent manner, has low affinity for Atg1 (Figure 7) (Kamada et al., 2000). In starvation condition, Atg13 is immediately dephosphorylated and it associates with Atg1 kinase. This is one of the earliest and most important steps in induction of autophagy. In agreement with our previous study (Kamada et al., 2000), our present results indicate that assembly of Atg1, Atg13, and the autophagy-specific protein complex is tightly regulated by nutrient condition, and it is likely that Tor-dependent phosphorylation of Atg13 is responsible for this regulation. Studies using atg13 and atg29 mutants strongly support the idea that Atg1–Atg13 and Atg17–Atg29 associations are indispensable for the assembly of Atg proteins to the PAS. The Cvt pathway is observed in atg131-448 cells (Kamada et al., 2000), and it does not require either ATG17, ATG29, or ATG31, confirming that these associations are involved in the autophagy-specific process. Because Atg17–Atg29 binding is detected in atg1Δ and atg13Δ mutants (our unpublished results), and Atg1–Atg13 binding is detected in starved atg11Δatg17Δ mutants (Figure 7B), Atg17–Atg29–Atg31 complex formation and Atg1–Atg13 association are prerequisites for subsequent recruitment to the PAS.
Atg1–Atg13 association also enhances kinase activity of Atg1 (Kamada et al., 2000). We have presented evidence that kinase activity of Atg1 is essential for autophagy, demonstrating autophagy is not induced in Atg1K54A or Atg1D211A (kinase-deficient mutants) or Atg1M102A (ATP analogue-sensitive mutant) (Matsuura et al., 1997; Kamada et al., 2000; Kabeya et al., 2005). In the present study, we show that Atg1 protein itself has another role in recruiting Atg proteins to the PAS to generate autophagosome. Atg17 and Atg29 accumulated to abnormally high levels at the PAS in atg1 kinase-deficient cells (Figure 3) and recruitment of Atg2 to the PAS requires Atg1 kinase activity (Sekito and Ohsumi, unpublished data), suggesting that kinase activity of Atg1 plays an essential role in assembly of other proteins, such as Atg2, to the PAS for autophagosome formation. We hypothesize that the kinase activity is required for the subsequent step(s) after assembly of Atg1–Atg13 and autophagy-unique Atgs, possibly phosphorylating target protein(s). Our findings clearly indicated that assembly of Atg proteins to the PAS responsible for autophagosome formation is mediated by autophagy-specific Atg proteins. Organization of the PAS should be acutely controlled in response to cellular nutrient environment.
ACKNOWLEDGMENTS
We thank members of the Ohsumi laboratory for helpful discussion, and C. Kondo, M. Oku, and National Institute for Basic Biology Center for Analytical Instruments for technical assistance. This work was supported in part by grants-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Abbreviations used:
- ALP
alkaline phosphatase
- Cvt
cytoplasm to vacuole targeting
- PAS
preautophagosomal structure.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-10-1048) on February 20, 2008.
REFERENCES
- Baba M., Takeshige K., Baba N., Ohsumi Y. Ultrastructural analysis of the autophagic process in yeast: detection of autophagosomes and their characterization. J. Cell Biol. 1994;124:903–913. doi: 10.1083/jcb.124.6.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong H., Yorimitsu T., Reggiori F., Legakis J. E., Wang C. W., Klionsky D. J. Atg17 regulates the magnitude of the autophagic response. Mol. Biol. Cell. 2005;16:3438–3453. doi: 10.1091/mbc.E04-10-0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaever G., et al. Functional profiling of the Saccharomyces cerevisiae genome. Nature. 2002;418:387–391. doi: 10.1038/nature00935. [DOI] [PubMed] [Google Scholar]
- Goldstein A. L., McCusker J. H. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast. 1999;15:1541–1553. doi: 10.1002/(SICI)1097-0061(199910)15:14<1541::AID-YEA476>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- James P., Halladay J., Craig E. A. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics. 1996;144:1425–1436. doi: 10.1093/genetics/144.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabeya Y., Kamada Y., Baba M., Takikawa H., Sasaki M., Ohsumi Y. Atg17 functions in cooperation with Atg1 and Atg13 in yeast autophagy. Mol. Biol. Cell. 2005;16:2544–2553. doi: 10.1091/mbc.E04-08-0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabeya Y., Kawamata T., Suzuki K., Ohsumi Y. Cis1/Atg31 is required for autophagosome formation in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 2007;356:405–410. doi: 10.1016/j.bbrc.2007.02.150. [DOI] [PubMed] [Google Scholar]
- Kaiser C., Michaelis S., Mitchell A. Methods in Yeast Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- Kamada Y., Funakoshi T., Shintani T., Nagano K., Ohsumi M., Ohsumi Y. Tor-mediated induction of autophagy via an Apg1 protein kinase complex. J. Cell Biol. 2000;150:1507–1513. doi: 10.1083/jcb.150.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamata T., Kamada Y., Suzuki K., Kuboshima N., Akimatsu H., Ota S., Ohsumi M., Ohsumi Y. Characterization of a novel autophagy-specific gene, ATG29. Biochem. Biophys. Res. Commun. 2005;338:1884–1889. doi: 10.1016/j.bbrc.2005.10.163. [DOI] [PubMed] [Google Scholar]
- Kim J., Kamada Y., Stromhaug P. E., Guan J., Hefner-Gravink A., Baba M., Scott S. V., Ohsumi Y., Dunn W. A., Jr, Klionsky D. J. Cvt9/Gsa9 functions in sequestering selective cytosolic cargo destined for the vacuole. J. Cell Biol. 2001;153:381–396. doi: 10.1083/jcb.153.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky D. J. The molecular machinery of autophagy: unanswered questions. J. Cell Sci. 2005;118:7–18. doi: 10.1242/jcs.01620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky D. J., et al. A unified nomenclature for yeast autophagy-related genes. Dev. Cell. 2003;5:539–545. doi: 10.1016/s1534-5807(03)00296-x. [DOI] [PubMed] [Google Scholar]
- Klionsky D. J., Ohsumi Y. Vacuolar import of proteins and organelles from the cytoplasm. Annu. Rev. Cell Dev. Biol. 1999;15:1–32. doi: 10.1146/annurev.cellbio.15.1.1. [DOI] [PubMed] [Google Scholar]
- Levine B., Klionsky D. J. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev. Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- Longtine M. S., McKenzie A., 3rd, Demarini D. J., Shah N. G., Wach A., Brachat A., Philippsen P., Pringle J. R. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Matsuura A., Tsukada M., Wada Y., Ohsumi Y. Apg1p, a novel protein kinase required for the autophagic process in Saccharomyces cerevisiae. Gene. 1997;192:245–250. doi: 10.1016/s0378-1119(97)00084-x. [DOI] [PubMed] [Google Scholar]
- Moriya H., Isono K. Analysis of genetic interactions between DHH1, SSD1 and ELM1 indicates their involvement in cellular morphology determination in Saccharomyces cerevisiae. Yeast. 1999;15:481–496. doi: 10.1002/(SICI)1097-0061(199904)15:6<481::AID-YEA391>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Noda T., Kim J., Huang W. P., Baba M., Tokunaga C., Ohsumi Y., Klionsky D. J. Apg9p/Cvt7p is an integral membrane protein required for transport vesicle formation in the Cvt and autophagy pathways. J. Cell Biol. 2000;148:465–480. doi: 10.1083/jcb.148.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda T., Matsuura A., Wada Y., Ohsumi Y. Novel system for monitoring autophagy in the yeast Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 1995;210:126–132. doi: 10.1006/bbrc.1995.1636. [DOI] [PubMed] [Google Scholar]
- Noda T., Ohsumi Y. Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J. Biol. Chem. 1998;273:3963–3966. doi: 10.1074/jbc.273.7.3963. [DOI] [PubMed] [Google Scholar]
- Noda T., Suzuki K., Ohsumi Y. Yeast autophagosomes: de novo formation of a membrane structure. Trends Cell Biol. 2002;12:231–235. doi: 10.1016/s0962-8924(02)02278-x. [DOI] [PubMed] [Google Scholar]
- Robinson J. S., Klionsky D. J., Banta L. M., Emr S. D. Protein sorting in Saccharomyces cerevisiae: isolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Mol. Cell Biol. 1988;8:4936–4948. doi: 10.1128/mcb.8.11.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani T., Huang W. P., Stromhaug P. E., Klionsky D. J. Mechanism of cargo selection in the cytoplasm to vacuole targeting pathway. Dev. Cell. 2002;3:825–837. doi: 10.1016/s1534-5807(02)00373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani T., Klionsky D. J. Cargo proteins facilitate the formation of transport vesicles in the cytoplasm to vacuole targeting pathway. J. Biol. Chem. 2004;279:29889–29894. doi: 10.1074/jbc.M404399200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani T., Suzuki K., Kamada Y., Noda T., Ohsumi Y. Apg2p functions in autophagosome formation on the perivacuolar structure. J. Biol. Chem. 2001;276:30452–30460. doi: 10.1074/jbc.M102346200. [DOI] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Kirisako T., Kamada Y., Mizushima N., Noda T., Ohsumi Y. The pre-autophagosomal structure organized by concerted functions of APG genes is essential for autophagosome formation. EMBO J. 2001;20:5971–5981. doi: 10.1093/emboj/20.21.5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Kubota Y., Sekito T., Ohsumi Y. Hierarchy of Atg proteins in pre-autophagosomal structure organization. Genes Cells. 2007;12:209–218. doi: 10.1111/j.1365-2443.2007.01050.x. [DOI] [PubMed] [Google Scholar]
- Takeshige K., Baba M., Tsuboi S., Noda T., Ohsumi Y. Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J. Cell Biol. 1992;119:301–311. doi: 10.1083/jcb.119.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullschleger S., Loewith R., Hall M. N. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]