Abstract
HURP is a spindle-associated protein that mediates Ran-GTP-dependent assembly of the bipolar spindle and promotes chromosome congression and interkinetochore tension during mitosis. We report here a biochemical mechanism of HURP regulation by Aurora A, a key mitotic kinase that controls the assembly and function of the spindle. We found that HURP binds to microtubules through its N-terminal domain that hyperstabilizes spindle microtubules. Ectopic expression of this domain generates defects in spindle morphology and function that reduce the level of tension across sister kinetochores and activate the spindle checkpoint. Interestingly, the microtubule binding activity of this N-terminal domain is regulated by the C-terminal region of HURP: in its hypophosphorylated state, C-terminal HURP associates with the microtubule-binding domain, abrogating its affinity for microtubules. However, when the C-terminal domain is phosphorylated by Aurora A, it no longer binds to N-terminal HURP, thereby releasing the inhibition on its microtubule binding and stabilizing activity. In fact, ectopic expression of this C-terminal domain depletes endogenous HURP from the mitotic spindle in HeLa cells in trans, suggesting the physiological importance for this mode of regulation. We concluded that phosphorylation of HURP by Aurora A provides a regulatory mechanism for the control of spindle assembly and function.
INTRODUCTION
In mitosis, proper microtubule dynamics is central to spindle function and to chromosome congression and segregation. The assembly and function of the bipolar spindle is regulated by the Aurora A kinase (Glover et al., 1995; Hannak et al., 2001), whose activity is enhanced by binding of TPX2, a spindle-associated protein that protects active Aurora A from dephosphorylation of a phospho-residue in the T-loop by the protein phosphatase PP1 (Bayliss et al., 2003; Eyers et al., 2003; Tsai et al., 2003; Kufer et al., 2002). This mechanism of activation links Aurora A to the Ran pathway for spindle assembly, as TPX2 is released from an inhibitory association with importin α by the high Ran-GTP concentrations around chromatin (Gruss et al., 2001). Active Aurora A phosphorylates Eg5, a microtubule motor protein implicated in sorting randomly oriented microtubules around chromatin into parallel arrays (Giet and Prigent, 2000), although the biochemical and physiological consequence of this phosphorylation is unclear. To understand the molecular mechanism of Aurora A function in spindle assembly and dynamics, it is essential to identify direct targets of this kinase on the mitotic spindle and to understand the biochemical and molecular function of their phosphorylation. We report here a biochemical mechanism for Aurora A function through its phosphorylation of a spindle-associated protein, HURP.
During spindle assembly, HURP associates with chromatin-proximal regions of microtubules in prometaphase cells to reduce the turnover rate of tubulin subunits on the spindle and to stabilize kinetochore microtubules (Wong and Fang, 2006). The activity of HURP is required for proper kinetochore capture, efficient chromosome congression, and timely mitotic progression. Defects in these processes are permissive for inappropriate anaphase initiation and genomic instability.
HURP is a component of the chromatin-dependent pathway for spindle assembly. In particular, Ran-GTP regulates the binding of importin β to HURP and controls its association with the mitotic spindle (Sillje et al., 2006). Consistent with this, endogenous HURP preferentially localizes to a defined region of the mitotic spindle proximal to chromatin (Sillje et al., 2006; Wong and Fang, 2006). This mode of HURP regulation has also been reported in Xenopus egg extracts, in which HURP, TPX2, XMAP215, Eg5, and Aurora A were identified as components of a complex required for Ran-dependent assembly of the bipolar spindle (Koffa et al., 2006), although such a complex was not detected in human mitotic cells by coimmunoprecipitation using anti-HURP antibodies (our unpublished data).
Mitotic kinases also regulate HURP activity. Cyclin B/Cdk1 phosphorylates HURP and targets it to the SCF ligase for ubiquitination (Hsu et al., 2004), providing a potential mechanism to down-regulate HURP during mitotic exit. Conversely, it has been reported that mitotic phosphorylation by Aurora A may stabilize HURP (Yu et al., 2005). Furthermore, the expression of kinase-dead Aurora A disrupts a high-molecular-weight complex of HURP, suggesting that the Aurora A–dependent phosphorylation of HURP promotes the interaction between HURP and its binding partners (Yu et al., 2005; Koffa et al., 2006). Finally, Aurora A phosphorylation of HURP was found to be required for proliferation in low serum conditions (Yu et al., 2005). Thus, both the function and steady-state level of the HURP protein are regulated by posttranslational modifications.
We report here a biochemical mechanism for regulation of HURP in mitosis. Specifically, an N-terminal domain of HURP is sufficient to bind to and stabilize microtubules. However, the binding of the N-terminal domain to microtubules is inhibited by the C-terminal region of HURP, which, in turn, is regulated by Aurora A. We concluded that phosphorylation of HURP by Aurora A regulates an intra- or intermolecular interaction between the N- and C-terminal domains that controls HURP's microtubule binding and stabilizing activity on the spindle.
MATERIALS AND METHODS
Recombinant Proteins, Antibodies, and Cell Culture
HURP constructs (full length, aa1-280, aa281-625, and aa626-846) were subcloned into a modified version of pCS2+ containing an N-terminal monomeric red fluorescent protein (mRFP), green fluorescent protein (GFP), or FLAG tag. For recombinant protein production, HURP-N (aa1-280), HURP-M (aa281-625), and HURP-C (aa626-846) were subcloned into the pGEX vector (Amersham Biosciences, Piscataway, NJ), expressed in Escherichia coli, and purified by glutathione Sepharose 4B. For antibody production, the glutathione S-transferase (GST)-tagged HURP proteins were used to immunize rabbits, and antibodies were affinity-purified. The following antibodies were obtained from commercial sources: anti-GFP clone 3E6 (Invitrogen, Carlsbad, CA); anti-β-tubulin ascites clone 2-28-33, anti-γ-tubulin clone GTU-88, and anti-acetylated-α-tubulin (Sigma, St. Louis, MO); CREST (Antibodies, Davis, CA); anti-Hec1 (Genetex, San Antonio, TX). Rabbit antibodies against Mad2 and BubR1 were described previously (Fang, 2002).
HeLa cells were cultured in DMEM containing 10% fetal bovine serum (Invitrogen) and antibiotics. DNA transfection was performed using Effectene (Qiagen, Chatsworth, CA) or Lipofectamine 2000 (Invitrogen) as instructed by the manufacturers. Under these conditions, the GFP-HURP, GFP-HURP-N, and GFP-HURP-C proteins were expressed to 5, 30, and 20 times, respectively, above endogenous HURP levels, based on quantitative Western blot analysis (data not shown). At the individual cell level, expression of transgenes varies widely. Interestingly, GFP-HURP-N was always localized to the entire mitotic spindle, not just to the chromatin-proximal region, if its expression level was sufficiently high to allow its association with microtubules.
Immunofluorescence and FLIP
For immunofluorescence, HeLa cells on coverglasses were fixed with −20°C methanol for 5 min or 4% paraformaldehyde for 15 min at room temperature and permeabilized/blocked with PBS-BT (1× PBS/0.1% Triton X-100/3% BSA) for 30 min at room temperature. Images were acquired with OpenLab 4.0.3 (Improvision, Waltham, MA) under a Zeiss Axiovert 200M microscope (Thornwood, NY) using a 1.4 NA Plan-Apo 100× oil immersion objective with an Orca-ER CCD (Hamamatsu Photonics, Bridgewater, NJ). Z-stacks were deconvolved and processed using AutoDeblur 9.1 and AutoVisualize 9.1 (AutoQuant Imaging, Watervliet, NY).
For FLIP experiments, HeLa cells transiently expressing GFP-α-tubulin to <5% of endogenous α-tubulin levels and RFP-HURP and RFP-HURP-N protein to 5 and 30 times, respectively, above endogenous HURP levels were grown on 22-mm2 coverglasses and then placed in a sealed growth chamber heated to 37°C. Cytoplasmic GFP-α-tubulin was photobleached with a fiber-optically pumped dye laser, and images were acquired at 0.5-s intervals with SlideBook 4.0 (Intelligent Imaging Innovations, Denver, CO) on a Zeiss Axiovert 200M microscope with a 1.4 NA 100× oil immersion objective and a CoolSnap HQ CCD (Photometrics, Woburn, MA). Ten half-spindles for each transfection were analyzed by measuring the absolute GFP-α-tubulin fluorescence intensity in a defined circular area contained entirely within each half-spindle midway between the poles and the kinetochores. Fluorescence intensities for each half-spindle were normalized to their maximum intensity at the beginning of the time lapse, and individual half-lives for GFP-α-tubulin on the half-spindle were calculated by linear regression.
Kinase and Phosphatase Reactions
In vitro–translated 35S-FLAG-HURP proteins or purified recombinant GST-HURP-C were phosphorylated by 6.5 μM purified recombinant wild-type or kinase-dead (K169R) Aurora A with 200 μM ATP (with or without [γ-32P]ATP) in 1× kinase buffer (20 mM HEPES, pH 7.5, 5 mM MgCl2, 0.5 mM EGTA, 1 mM dithiothreitol, 0.05% Triton X-100, and 200 mM KCl) for 30 min at 30°C. In some experiments (see Figure 3, C–F), a variant of Aurora A (Aurora AΔN) with a deletion of the N-terminal 122 aa outside the kinase domain was used, as Aurora AΔN, which can be easily expressed and purified in E. coli, has the same substrate specificity, but higher kinase activity compared with the full-length Aurora A (Bayliss et al., 2003).
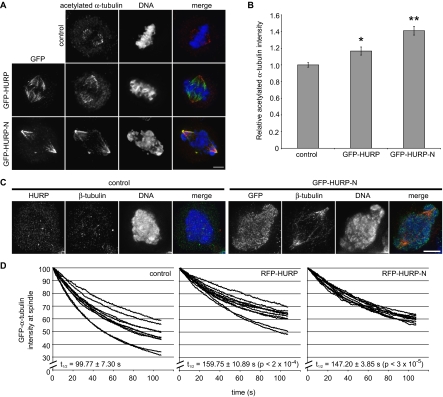
Figure 3.
Expression of HURP-N stabilized spindle microtubules. (A) Maximum projections from deconvolved z-stacks of representative HeLa cells transfected with control, GFP-HURP, and GFP-HURP-N and stained for GFP (green), acetylated α-tubulin (red), and DNA (blue). Scale bar, 5 μm. (B) Average acetylated α-tubulin immunofluorescence intensity on metaphase spindles stained as in A was quantified (n = 40 half-spindles from 20 cells). *p < 2 × 10−3; **p < 4 × 10−9 (one-tailed t test). Error bars, SE. (C) Maximum projections from deconvolved z-stacks of representative HeLa cells transfected with control and GFP-HURP-N and stained for HURP or GFP (green), β-tubulin (red), and DNA (blue). Cells were treated with 1 μg/ml nocodazole for 5 min at 37°C, washed with PBS, and fixed immediately. Scale bar, 5 μm. (D) HeLa cells were cotransfected of GFP-α-tubulin with a control vector, RFP-HURP, or RFP-HURP-N. The GFP fluorescence intensity was acquired every 0.5 s, whereas a photobleaching laser was focused to a diffraction-limited spot in the cytoplasm away from the spindle. Twelve half-spindles from six metaphase cells were quantified, and fluorescence signals for each half-spindle were normalized to their intensity at 0 s and plotted. Turnover half-lives for GFP-α-tubulin on each half-spindle were calculated by linear regression from experiments performed in duplicate on two different days. A mean and SE were then calculated and shown in the plots. p values are from one-tailed t tests.
HURP and HURP fragments were dephosphorylated with λ-phosphatase (New England Biolabs, Beverly, MA) in 1× λ-phosphatase reaction buffer (50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 2 mM dithiothreitol, 0.1 mM EGTA, 0.01% Brij 35) supplemented with 2 mM MnCl2 for 30 min at 30°C. Reactions were stopped with the addition of 5 mM EGTA for 15 min at 30°C.
Microtubule Copelleting Assay
35S-HURP proteins translated in rabbit reticulocyte lysates were added to Taxol-stabilized microtubules in the presence of 2 mM GTP, 1× protease inhibitors, and 20 μM Taxol in 1× BRB80 buffer (80 mM PIPES, pH 6.8, 1 mM MgCl2, 1 mM EGTA). The reaction was incubated at 30°C for 30 min at room temperature and pelleted through a 40% glycerol cushion containing 20 μM Taxol and 1× protease inhibitors in 1× BRB80 at 100,000 × g for 20 min at 30°C. Pellets were washed three times with 1× BRB80 and analyzed by autoradiography.
Binding Assay
In vitro–translated 35S-FLAG-HURP-C was incubated overnight at 4°C with recombinant GST-HURP-N that had been bound to glutathione Sepharose beads. Beads were washed three times with PBS, and bound material was analyzed by autoradiography. Alternatively, purified recombinant GST-HURP-C was bound to glutathione Sepharose beads, incubated overnight at 4°C with in vitro–translated 35S-FLAG-HURP-N, and then washed three times with PBS. Bound material was analyzed by autoradiography.
RESULTS
Expression of the HURP Microtubule-binding Domain Leads to Mitotic Defects
To investigate the regulation of HURP localization, we determined the functional domain structure of HURP. Although the C-terminal region of HURP does not show significant homology to any known motifs, the conserved N-terminal domain of HURP may mediate its association with microtubules (Figure 1B), as this region (aa 66-220) has a weak homology to a domain in E-MAP-115 (aa 456-626). This region of E-MAP-115, although not directly involved in binding to microtubules, contains a sequence of 18 residues (aa 483-500) that is repeated in the N-terminus of E-MAP-115 responsible for its association with microtubules (Masson and Kreis, 1993). Indeed, the N-terminal domain of HURP binds to microtubules in vitro. In a copelleting assay using 35S-labeled proteins translated in vitro in rabbit reticulocyte lysates, GFP-HURP and GFP-HURP-N (aa 1-280) associated with microtubules, whereas GFP-HURP-M (aa 281-625) and GFP-HURP-C (aa 626-846) did not exhibit any microtubule-binding activity (Figure 1C).
Figure 1.
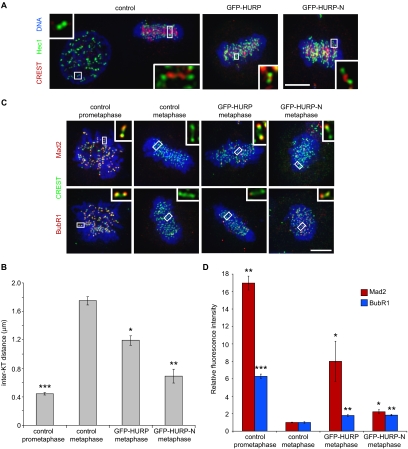
Expression of the HURP microtubule-binding domain altered spindle morphology. (A) Schematic representation of HURP. The central domain of HURP has homology to a family of guanylate kinase-associated proteins (GKAP), members of which have been implicated as scaffolding proteins for ion channels (Kim et al., 1997). (B) ClustalW alignment of the N-terminal domain of HURP. Conserved residues are indicated in bold and the region of homology to E-MAP-115 is indicated in the gray area. (C) In vitro–translated 35S-GFP-HURP proteins were analyzed in a copelleting assay with Taxol-stabilized microtubules. Input (20%), supernatants (sup), and pellets were analyzed by SDS-PAGE followed by autoradiography for the presence of HURP and by Western blotting for the presence of microtubules. (D) Maximum projections from deconvolved z-stacks of representative HeLa cells transfected with GFP-HURP or GFP-HURP-N and stained for GFP (green), β-tubulin (red), and DNA (blue). Scale bar, 5 μm. (E) Pole-to-pole distances were quantified from multiple experiments based on γ-tubulin immunofluorescence and spindle width quantified based on β-tubulin immunofluorescence in control (n = 32), GFP-HURP– (n = 30), and GFP-HURP-N– (n = 30) transfected HeLa cells at metaphase. *p < 3 × 10−7; **p < 2 × 10−8 (one-tailed t test). Error bars, SE.
Consistent with this result, ectopically expressed full-length GFP-HURP or GFP-HURP-N in HeLa cells colocalized with spindle microtubules. Expression of GFP-HURP (at five times above endogenous HURP levels) resulted in its localization to chromatin-proximal regions of the metaphase spindle (Figure 1D, top row), mimicking the pattern of endogenous HURP localization. Interestingly, expression of GFP-HURP-N (at 30 times above endogenous HURP levels) localized to the entire metaphase spindle (Figure 1D, bottom row). Furthermore, cells expressing GFP-HURP-N exhibited an obvious bundling of spindle microtubules, giving rise to a long, narrow mitotic spindle (Figure 1, D and E). The average pole-to-pole distance of GFP-HURP-N–expressing metaphase cells was 23% greater than that of control metaphase cells (GFP-HURP-N: 12.48 ± 0.35 μm; GFP-HURP: 9.89 ± 0.15 μm; control: 10.14 ± 0.12 μm), whereas the average width of the mitotic spindle at the metaphase plate was 14% narrower in GFP-HURP-N–expressing cells than in control cells (GFP-HURP-N: 7.02 ± 0.16 μm; GFP-HURP: 8.48 ± 0.13 μm; control: 8.33 ± 0.12 μm). These observations suggested that precise regulation of HURP levels and activity is essential for normal mitotic progression and that the spatial localization of HURP along the mitotic spindle may be regulated through either the deleted central GKAP-related or C-terminal domain, although we cannot exclude the possibility that the colocalization of HURP-N with the entire spindle is simply due to its high levels of expression.
Expression of HURP-N Generates Interkinetochore Tension Defects
To investigate the consequence of altered HURP activity, we measured interkinetochore distance as an indication of tension across sister kinetochores in HeLa cells (Figure 2, A and B). The interkinetochore distance in control cells increased from prometaphase (0.44 ± 0.02 μm) to metaphase (1.75 ± 0.06 μm). However, interkinetochore distances in metaphase cells expressing full-length or HURP-N were significantly shorter than control metaphase cells (GFP-HURP: 1.19 ± 0.07 μm; GFP-HURP-N: 0.69 ± 0.10 μm). Given that reduced tension activates spindle checkpoint, we analyzed the checkpoint status. The checkpoint proteins Mad2 and BubR1, which monitor microtubule-kinetochore attachment and tension across sister kinetochores, respectively (Chan et al., 1999; Skoufias et al., 2001), localized to kinetochores in control prometaphase cells, but disappeared at metaphase (Figure 2, C and D). Consistent with a partial loss of tension, BubR1 levels were elevated on kinetochores in metaphase cells expressing GFP-HURP or GFP-HURP-N. Surprisingly, Mad2 levels were also elevated on kinetochores in metaphase cells expressing full-length or HURP-N, suggesting that these kinetochores were only partially populated with microtubules or that they transiently lost some of their kinetochore microtubules. Thus, the up-regulation of HURP activity results in reduced kinetochore-microtubule attachment and a loss of sister kinetochore tension, both of which activate the spindle checkpoint.
Figure 2.
Expression of HURP-N reduced the interkinetochore tension and activated the spindle checkpoint. (A) Maximum projections from deconvolved z-stacks of representative HeLa cells transfected with control, GFP-HURP, and GFP-HURP-N and stained for Hec1 (Alexa 680, shown as green), CREST (Alexa 594, shown as red), and DNA (blue). CREST staining is less intense during prophase compared with metaphase. Insets show single z-slices of the boxed regions. Scale bar, 5 μm. (B) Interkinetochore distance was quantified based on Hec1 and CREST staining for 40–80 kinetochore pairs from 8 to 16 HeLa cells. *p < 2 × 10−6; **p < 4 × 10−8; ***p < 3 × 10−9 (one-tailed t test). Error bars, SE. (C) Maximum projections from deconvolved z-stacks of representative HeLa cells transfected with control, GFP-HURP, and GFP-HURP-N and stained for Mad2 or BubR1 (Alexa 680, shown as red), CREST (Alexa 594, shown as green), and DNA (blue). Insets show single z-slices of the boxed regions. Scale bar, 5 μm. (D) Mad2 (n = 80 kinetochores from 16 cells) and BubR1 (n = 120 kinetochores from 24 cells) signals were quantified in control, GFP-HURP, and GFP-HURP-N–transfected HeLa cells. *p < 4 × 10−3; **p < 4 × 10−7; ***p < 3 × 10−11 (one-tailed t test). Error bars, SE.
HURP-N Stabilizes the Mitotic Spindle and Reduces Tubulin Subunit Turnover
The thick, highly bundled spindle microtubules found in HeLa cells expressing HURP-N (Figure 1D) suggested that this domain stabilizes microtubules. Consistent with this hypothesis, the average immunofluorescence intensity of acetylated α-tubulin on the mitotic spindle, a marker for stabilized microtubules (Piperno et al., 1987; de Pennart et al., 1988), increased from control cells to cells expressing HURP to cells expressing HURP-N (Figure 3, A and B). Spindle microtubule stability was independently assayed in triplicate by incubation of HeLa cells with 1 μg/ml nocodazole for 5 min. In control cells, the nocodazole treatment entirely depolymerized the mitotic spindle (Figure 3C), whereas the mitotic spindle in HURP-N cells was resistant to nocodazole. Thus, expression of HURP-N is sufficient to hyperstabilize the mitotic spindle.
The consequence of HURP-N expression on spindle dynamics was analyzed in a fluorescence-loss-in-photobleaching (FLIP) experiment that measures the turnover rate of α/β-tubulin heterodimers on the mitotic spindle. GFP-α-tubulin was cotransfected with a control vector, RFP-HURP (expressed at five times above endogenous HURP levels), or RFP-HURP-N (expressed at 30 times above endogenous HURP levels) into HeLa cells, and the cytoplasm of metaphase cells was photobleached continuously, whereas time-lapse images were captured every 0.5 s to record the decrease in GFP fluorescence on the spindle (Figure 3D and Supplementary Videos 1 and 2). The half-life of GFP-α-tubulin on the control metaphase spindle was 99.77 ± 7.30 s; this was increased to 159.75 ± 10.89 and 147.20 ± 3.85 s in the RFP-HURP and RFP-HURP-N metaphase spindles, respectively. Interestingly, the half-lives of GFP-α-tubulin on the spindles of RFP-HURP– and RFP-HURP-N–expressing cells are similar, suggesting that these two proteins have a similar effect on the turnover rate of the mitotic spindle kinetically, even though HURP-N has a stronger stabilizing effect on spindle microtubules at steady state. Thus, HURP and HURP-N stabilize the mitotic spindle by generating a more static population of microtubules that exchanges tubulin subunits with the cytoplasm at a lower rate compared with control cells.
The Binding of HURP to Microtubules Is Regulated through Phosphorylation of its C-Terminal Domain by Aurora A
Because HURP is a target of Aurora A (Yu et al., 2005), we investigated whether this kinase regulates the affinity of HURP for microtubules. First, we confirmed that Aurora A phosphorylates HURP in vitro. On incubation with recombinant Aurora A, in vitro–translated 35S-FLAG-HURP, and 35S-FLAG-HURP-C migrated slower in SDS-PAGE, whereas no band shift was observed upon incubation with kinase-dead Aurora A (Figure 4A). These mobility shifts resulted from phosphorylation, as confirmed by treatment with λ-phosphatase. To rule out the possibility that the mobility shift induced by Aurora A is indirectly mediated through an unknown kinase present in the rabbit reticulocyte translation lysates, we directly assayed the phosphorylation of purified recombinant HURP proteins by Aurora A. Among the three purified recombinant domains of HURP tested, only GST-HURP-C was directly phosphorylated by Aurora A to a significant amount in the presence of [γ-32P]ATP (Figure 4B). Thus, these results are consistent with the interpretation that Aurora A phosphorylates the C-terminal domain of HURP.
Figure 4.
HURP-C regulates the binding of HURP-N to microtubules in an Aurora A–dependent manner. (A) In vitro–translated 35S-FLAG-HURP proteins were incubated with recombinant kinase-dead (K169R mutant, KD) or wild-type (WT) Aurora A in the presence of unlabeled ATP, treated with or without λ-phosphatase (PPase), and then analyzed by SDS-PAGE and autoradiography. (B) Purified recombinant GST-TPX2 or GST-HURP proteins were incubated with recombinant Aurora A in the presence of [γ-32P]ATP and analyzed by autoradiography for phosphorylation and by Coomassie blue staining for the amounts of recombinant proteins. (C) In vitro–translated 35S-FLAG-HURP was dephosphorylated with λ-phosphatase, treated with EGTA to inactivate phosphatase, and subsequently incubated with recombinant Aurora A. Modified FLAG-HURP was then added to Taxol-stabilized microtubules in a copelleting assay. Input (20%), supernatants (sup), and pellets were analyzed by SDS-PAGE and autoradiography for HURP and by Western blotting for microtubules. (D) In vitro–translated, nonradioactive FLAG-HURP-C was dephosphorylated with λ-phosphatase and then treated with or without active Aurora A. In vitro–translated 35S-FLAG-HURP-N was then incubated with the modified FLAG-HURP-C and analyzed in a copelleting assay with Taxol-stabilized microtubules. Input (20%), supernatants (sup), and pellets were analyzed by SDS-PAGE and autoradiography for HURP-N and by Western blotting for microtubules. (E) Recombinant purified GST-HURP-C was incubated with Aurora A and subsequently treated with or without λ-phosphatase. Modified GST-HURP-C was bound to glutathione beads, then incubated with 35S-FLAG-HURP-N. Input (20%), supernatants (sup), and pellets were analyzed by SDS-PAGE and autoradiography for bound HURP-N and by Coomassie blue staining for equivalent pulldown of GST-HURP-C. (F) In vitro–translated 35S-FLAG-HURP-C was incubated with recombinant Aurora A, treated with or without λ-phosphatase, and then incubated with purified recombinant GST-HURP-N or GST that had been bound to glutathione beads. Input (20%), supernatants (sup), and pellets were analyzed by SDS-PAGE and autoradiography for bound HURP-C and by Coomassie blue staining for equivalent pulldown of GST-HURP-N.
We next determined whether this posttranslational modification regulates the binding of HURP to microtubules. In vitro–translated 35S-FLAG-HURP was incubated with Taxol-stabilized microtubules in a copelleting assay. Although FLAG-HURP weakly copelleted with microtubules, this binding was abolished upon treatment of FLAG-HURP with λ-phosphatase, suggesting that FLAG-HURP may be phosphorylated by an unknown kinase during in vitro translation (Figure 4C). Interestingly, phosphorylation of hypophosphorylated FLAG-HURP with active, but not kinase-dead Aurora A, greatly enhanced its association with microtubules. This increased association was not due to the formation of the HURP-Aurora A complex, and the subsequent binding of this complex to microtubules, because we did not coimmunoprecipitate HURP and Aurora A in our assay (data not shown).
Because the C-terminal domain of HURP can be a target of Aurora A, we tested whether the phosphorylation state of HURP-C regulated the microtubule-binding activity of HURP-N. First, as expected, in vitro–translated 35S-FLAG-HURP-N associated with microtubules in a copelleting assay (Figure 4D). However, when 35S-FLAG-HURP-N was preincubated with in vitro–translated FLAG-HURP-C that was dephosphorylated with λ-phosphatase, the microtubule-binding domain of HURP no longer copelleted with microtubules. This indicates that hypophosphorylated HURP-C inhibits the microtubule-binding activity of HURP-N, although we cannot formally rule out a possible effect of other proteins in rabbit reticulocyte lysates. Interestingly, this inhibitory activity of HURP-C was abrogated upon its phosphorylation by Aurora A, suggesting that Aurora A–dependent phosphorylation controls the ability of HURP to bind to microtubules.
These data are consistent with a model in which the C-terminal domain of HURP, when hypophosphorylated, inhibits the N-terminal domain from binding to microtubules. In a glutathione bead pulldown assay, we found that 35S-FLAG-HURP-N binds directly to recombinant GST-HURP-C treated with or without kinase-dead Aurora A (Figure 4E). However, this interaction was abrogated when HURP-C was first phosphorylated by active Aurora A before its incubation with HURP-N, whereas the interaction was rescued when the phosphorylated HURP-C was subsequently dephosphorylated with λ-phosphatase. In this binding assay, the only target of Aurora A phosphorylation was GST-HURP-C and not HURP-N, because recombinant GST-HURP-C was purified away from Aurora A and λ-phosphatase before its incubation with 35S-HURP-N. This mode of interaction between HURP-N and HURP-C was also confirmed in a binding assay of the reverse direction: 35S-HURP-C interacts directly with recombinant GST-HURP-N on glutathione beads, and this interaction was unaffected by treatment of HURP-C with kinase-dead Aurora A (Figure 4F). On the other hand, HURP-C phosphorylated by active Aurora A no longer bound to recombinant GST-HURP-N, whereas phosphorylated HURP-C that was subsequently incubated with λ-phosphatase regained the ability to associate with HURP-N. Thus, Aurora A regulates the microtubule binding activity of HURP by controlling an intra- or intermolecular interaction between C- and N-terminal HURP.
Expression of HURP-C Depletes Endogenous HURP from the Mitotic Spindle
To test our hypothesis that C-terminal HURP regulates HURP activity in vivo, we expressed GFP-HURP-C in HeLa cells. Strikingly, an excess of C-terminal HURP (expressed at 20 times above endogenous HURP levels) resulted in depletion of endogenous HURP from the mitotic spindle (Figure 5A). This is consistent with our in vitro observation that HURP-C, when bound to the microtubule-binding domain, inhibits the association between the N-terminal domain of HURP and microtubules. We reasoned that the loss of HURP localization in the chromatin-proximal regions of the mitotic spindle due to expression of HURP-C should phenocopy the mitotic defects in HURP-knockdown cells (Wong and Fang, 2006). In fact, cells expressing HURP-C contained unaligned chromosomes that were not attached to any kinetochore microtubules (Figure 5B), a phenotype frequently observed in HURP-knockdown cells (Wong and Fang, 2006). As with siRNA-mediated HURP depletion, these unattached kinetochores, as well as the kinetochores on chromosomes aligned at the metaphase plate, were also only under partial tension, as indicated by a decrease in interkinetochore distance compared with control metaphase kinetochores (Figure 5, C and D). Finally, the loss of HURP activity due to expression of HURP-C results in activation of the spindle checkpoint, because Mad2 and BubR1 levels are elevated on kinetochores in cells expressing HURP-C (Figure 5, E and F). The fact that each of these phenotypes is similar to the defects in HURP depleted cells supports our conclusion that the C-terminal domain of HURP regulates HURP activity by controlling the binding of the N-terminal domain to spindle microtubules in vivo.
Figure 5.
Expression of HURP-C displaced the endogenous HURP from the mitotic spindle and phenocopied HURP depletion. (A) Maximum projections from deconvolved z-stacks of representative HeLa cells transfected with GFP or GFP-HURP-C and stained for the endogenous HURP using an antibody generated against HURP-N (red), GFP (green), and DNA (blue). Scale bar, 5 μm. (B and C) Maximum projection from deconvolved z-stacks of representative GFP-HURP-C–transfected HeLa cells stained for Hec1 (Alexa 680, shown as green), β-tubulin or CREST (Alexa 594, shown as red), and DNA (blue). Insets show single z-slices of the boxed regions. Scale bar, 5 μm. (D) Interkinetochore distance was quantified based on Hec1 and CREST staining for 40 kinetochore pairs in 8 HeLa cells. Prometa, prometaphase. *p < 8 × 10−8 (one-tailed t test). Error bars, SE. (E) Maximum projection from deconvolved z-stacks of GFP-HURP-C–transfected HeLa cells stained for Hec1 (green), Mad2 or BubR1 (red), and DNA (blue). Insets show single z-slices of the boxed regions. Scale bar, 5 μm. (F) Mad2 and BubR1 signals on 60 kinetochores from 12 prometaphase (prometa) or metaphase cells were quantified in control and GFP-HURP-C–transfected HeLa cells. For the unaligned chromosomes in GFP-HURP-C–expressing cells, one pair of kinetochores in each of eight cells with an unaligned chromosome was quantified and then a mean and SE were calculated from the 16 kinetochores. *p < 2 × 10−4; **p < 2 × 10−6; +p < 3 × 10−8 (one-tailed t test). Error bars, SE.
DISCUSSION
HURP is a microtubule-binding protein that stabilizes the mitotic spindle in the vicinity of chromatin (Wong and Fang, 2006). We have identified an N-terminal domain of HURP sufficient for binding to microtubules. Mis-regulation of the microtubule-stabilizing activity of HURP either by overexpression of HURP or its microtubule-binding domain generates defects in spindle morphology and function and activates the spindle checkpoint due to deficiencies in kinetochore capture and tension across sister-kinetochores. Interestingly, the microtubule-binding activity of the N-terminal domain is regulated by the C-terminal region of HURP and by the Aurora A kinase. Specifically, HURP-C binds to HURP-N and prevents it from associating with microtubules. When HURP-C is phosphorylated by Aurora A, the interaction between HURP-N and HURP-C is abolished, thereby allowing the binding of HURP-N to microtubules. Thus, Aurora A controls the microtubule-binding and stabilization activity of HURP in mitosis.
Aurora A Regulates HURP Activity
We showed here that Aurora A regulates the affinity of HURP toward microtubules and, therefore, its microtubule-stabilizing activity (Figure 4). We hypothesize that hypophosphorylated HURP-C, possibly in an intramolecular manner, competes against microtubules for the same binding site in HURP-N. Interestingly, this highly charged N-terminal domain that contains a cluster of basic residues has been previously shown to be required for the induction of a novel microtubule sheet conformation that correlates with the microtubule stabilizing activity of HURP (Santarella et al., 2007). It is likely that phosphorylation of HURP-C by Aurora A directly modifies its surface residues involved in binding or results in a conformation change that makes it incompatible with binding to HURP-N, thereby freeing HURP-N to bind to microtubules. In fact, phosphorylation has also been shown to affect the binding of HURP to other proteins, as the expression of kinase-dead Aurora A disrupts a high-molecular-weight complex of HURP (Yu et al., 2005).
Aurora A may also control HURP function in the context of the Ran pathway. Importin β inhibits HURP through a direct association, and the high levels of Ran-GTP near the chromatin dissociate this inhibitory complex (Sillje et al., 2006). Thus, the microtubule-binding domain of HURP is dually regulated by two independent mechanisms. Although Aurora A may relieve one inhibitory mechanism through phosphorylation of the C-terminal domain, it is in the vicinity of chromatin that Ran-GTP relieves the second mode of inhibition by importin β. Because active Aurora A is localized to the spindle poles, HURP may be phosphorylated by this kinase near the centrosomes and subsequently transported toward the high Ran-GTP concentrations near chromatin to satisfy the two potential requirements for HURP association with microtubules. Alternatively, cytoplasmic Aurora A may phosphorylate HURP and the chromatin-proximal Ran-GTP promotes phosphorylated HURP to associate with mitotic spindle. Whether the Aurora A and Ran-GTP regulatory mechanisms in vivo act independently, redundantly with each other, or even synergistically remains to be investigated.
Furthermore, regulatory mechanisms for HURP localization and function in mitotic cells are likely to be more complex than simply a combination of these two pathways, because the known regulation from Aurora A phosphorylation and from the Ran pathway cannot fully account for the uniquely restricted localization pattern of endogenous HURP to chromatin-proximal spindle microtubules (Kalab et al., 2006; Wong and Fang, 2006). Consistent with this, inhibition of Aurora A by the small molecule VX-680 or depletion of Aurora A by siRNA did not globally alter the localization of HURP in mitotic cells (data not shown).
Mis-Regulation of HURP Generates Mitotic Defects and Genomic Instability
It has been reported that HeLa cells depleted of HURP or ch-TOG bypass the spindle checkpoint in the presence of persistently unaligned chromosomes (Koffa et al., 2006; Wong and Fang, 2006). We found that a low concentration of nocodazole (5 ng/ml) also generated unaligned chromosomes in mitosis, and cancer-derived HeLa and Hct116 cells initiated anaphase despite the presence of unaligned chromosomes, whereas the spindle checkpoint remained active in the nontransformed RPE-1 cells, arresting these cells at metaphase until all kinetochores were captured (data not shown; Wong and Fang, 2006). This suggests that nontransformed cells have a more robust spindle checkpoint that is lost in tumor cells, thereby promoting improper anaphase initiation and aneuploidy during tumorigenesis. We found that cells overexpressing HURP are deficient in tension across sister kinetochores and have stochastic loss of microtubule-kinetochore attachment (Figure 2). In a permissive background of checkpoint bypass in tumor cells, anaphase initiation in a cell with excess HURP protein may lead to mis-segregation of chromosomes in the presence of unaligned chromosomes. Thus, the combined effects of defective tension arising from HURP overexpression along with an insensitive spindle checkpoint may tip the balance toward genomic instability and aneuploidy, hallmarks of cancer cells.
As HURP was initially characterized as a transcript that is up-regulated in hepatocellular carcinomas (Tsou et al., 2003), it is possible that mis-regulated HURP expression is oncogenic. Indeed, HURP expression was correlated with recurrence of urinary bladder transitional cell carcinoma (Huang et al., 2003). Consistent with a proproliferative function, overexpression of HURP in 293T or NIH3T3 cells stimulated anchorage-independent growth as well as proliferation in low serum conditions (Tsou et al., 2003; Wang et al., 2006). Interestingly, Aurora A is also identified as an oncogene and is overexpressed in many tumors (Giet et al., 2005). As Aurora A positively regulates the microtubule-binding activity of HURP, it is possible that hyperactivation of Aurora A in tumor cells may also promote cell proliferation through enhanced HURP function. In summary, our discovery of the Aurora A-HURP pathway provides a novel biochemical mechanism that controls the assembly, dynamics, and function of the mitotic spindle and points to a potential link between mis-regulation of HURP and Aurora A and genomic instability during tumorigenesis.
Supplementary Material
ACKNOWLEDGMENTS
We thank Angela Barth, Elias Spiliotis, and Soichiro Yamada of the W. James Nelson (Stanford University) laboratory for the GFP-α-tubulin construct and for assistance with FLIP experiments. J.W. is a recipient of the Howard Hughes Medical Institute Predoctoral Fellowship. This work was supported by National Institutes of Health Grant GM-062852 and by the Burroughs-Wellcome Career Award in Biomedical Sciences to G.F.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-10-1088) on March 5, 2008.
REFERENCES
- Bayliss R., Sardon T., Vernos I., Conti E. Structural basis of Aurora-A activation by TPX2 at the mitotic spindle. Mol. Cell. 2003;12:851–862. doi: 10.1016/s1097-2765(03)00392-7. [DOI] [PubMed] [Google Scholar]
- Chan G. K., Jablonski S. A., Sudakin V., Hittle J. C., Yen T. J. Human BUBR1 is a mitotic checkpoint kinase that monitors CENP-E functions at kinetochores and binds the cyclosome/APC. J. Cell Biol. 1999;146:941–954. doi: 10.1083/jcb.146.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pennart H., Houliston E., Maro B. Post-translational modifications of tubulin and the dynamics of microtubules in mouse oocytes and zygotes. Biol. Cell. 1988;64:375–378. doi: 10.1016/0248-4900(88)90012-3. [DOI] [PubMed] [Google Scholar]
- Eyers P. A., Erikson E., Chen L. G., Maller J. L. A novel mechanism for activation of the protein kinase Aurora A. Curr. Biol. 2003;13:691–697. doi: 10.1016/s0960-9822(03)00166-0. [DOI] [PubMed] [Google Scholar]
- Fang G. Checkpoint protein BubR1 acts synergistically with Mad2 to inhibit anaphase-promoting complex. Mol. Biol. Cell. 2002;13:755–766. doi: 10.1091/mbc.01-09-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giet R., Petretti C., Prigent C. Aurora kinases, aneuploidy and cancer, a coincidence or a real link? Trends Cell Biol. 2005;15:241–250. doi: 10.1016/j.tcb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Giet R., Prigent C. The Xenopus laevis aurora/Ip11p-related kinase pEg2 participates in the stability of the bipolar mitotic spindle. Exp. Cell Res. 2000;258:145–151. doi: 10.1006/excr.2000.4903. [DOI] [PubMed] [Google Scholar]
- Glover D. M., Leibowitz M. H., McLean D. A., Parry H. Mutations in aurora prevent centrosome separation leading to the formation of monopolar spindles. Cell. 1995;81:95–105. doi: 10.1016/0092-8674(95)90374-7. [DOI] [PubMed] [Google Scholar]
- Gruss O. J., Carazo-Salas R. E., Schatz C. A., Guarguaglini G., Kast J., Wilm M., Le Bot N., Vernos I., Karsenti E., Mattaj I. W. Ran induces spindle assembly by reversing the inhibitory effect of importin alpha on TPX2 activity. Cell. 2001;104:83–93. doi: 10.1016/s0092-8674(01)00193-3. [DOI] [PubMed] [Google Scholar]
- Hannak E., Kirkham M., Hyman A. A., Oegema K. Aurora-A kinase is required for centrosome maturation in Caenorhabditis elegans. J. Cell Biol. 2001;155:1109–1116. doi: 10.1083/jcb.200108051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu J. M., Lee Y. C., Yu C. T., Huang C. Y. Fbx7 functions in the SCF complex regulating Cdk1-cyclin B-phosphorylated hepatoma up-regulated protein (HURP) proteolysis by a proline-rich region. J. Biol. Chem. 2004;279:32592–32602. doi: 10.1074/jbc.M404950200. [DOI] [PubMed] [Google Scholar]
- Huang Y. L., Chiu A. W., Huan S. K., Wang Y. C., Ju J. P., Lu C. L. Prognostic significance of hepatoma-up-regulated protein expression in patients with urinary bladder transitional cell carcinoma. Anticancer Res. 2003;23:2729–2733. [PubMed] [Google Scholar]
- Kalab P., Pralle A., Isacoff E. Y., Heald R., Weis K. Analysis of a RanGTP-regulated gradient in mitotic somatic cells. Nature. 2006;440:697–701. doi: 10.1038/nature04589. [DOI] [PubMed] [Google Scholar]
- Kim E., Naisbitt S., Hsueh Y. P., Rao A., Rothschild A., Craig A. M., Sheng M. GKAP, a novel synaptic protein that interacts with the guanylate kinase-like domain of the PSD-95/SAP90 family of channel clustering molecules. J. Cell Biol. 1997;136:669–678. doi: 10.1083/jcb.136.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffa M. D., Casanova C. M., Santarella R., Kocher T., Wilm M., Mattaj I. W. HURP is part of a Ran-dependent complex involved in spindle formation. Curr. Biol. 2006;16:743–754. doi: 10.1016/j.cub.2006.03.056. [DOI] [PubMed] [Google Scholar]
- Kufer T. A., Sillje H. H., Korner R., Gruss O. J., Meraldi P., Nigg E. A. Human TPX2 is required for targeting Aurora-A kinase to the spindle. J. Cell Biol. 2002;158:617–623. doi: 10.1083/jcb.200204155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson D., Kreis T. E. Identification and molecular characterization of E-MAP-115, a novel microtubule-associated protein predominantly expressed in epithelial cells. J. Cell Biol. 1993;123:357–371. doi: 10.1083/jcb.123.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G., LeDizet M., Chang X. J. Microtubules containing acetylated alpha-tubulin in mammalian cells in culture. J. Cell Biol. 1987;104:289–302. doi: 10.1083/jcb.104.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarella R. A., Koffa M. D., Tittmann P., Gross H., Hoenger A. HURP wraps microtubule ends with an additional tubulin sheet that has a novel conformation of tubulin. J. Mol. Biol. 2007;365:1587–1595. doi: 10.1016/j.jmb.2006.10.064. [DOI] [PubMed] [Google Scholar]
- Sillje H. H., Nagel S., Korner R., Nigg E. A. HURP is a Ran-importin beta-regulated protein that stabilizes kinetochore microtubules in the vicinity of chromosomes. Curr. Biol. 2006;16:731–742. doi: 10.1016/j.cub.2006.02.070. [DOI] [PubMed] [Google Scholar]
- Skoufias D. A., Andreassen P. R., Lacroix F. B., Wilson L., Margolis R. L. Mammalian mad2 and bub1/bubR1 recognize distinct spindle-attachment and kinetochore-tension checkpoints. Proc. Natl. Acad. Sci. USA. 2001;98:4492–4497. doi: 10.1073/pnas.081076898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai M. Y., Wiese C., Cao K., Martin O., Donovan P., Ruderman J., Prigent C., Zheng Y. A Ran signalling pathway mediated by the mitotic kinase Aurora A in spindle assembly. Nat. Cell Biol. 2003;5:242–248. doi: 10.1038/ncb936. [DOI] [PubMed] [Google Scholar]
- Tsou A. P., et al. Identification of a novel cell cycle regulated gene, HURP, overexpressed in human hepatocellular carcinoma. Oncogene. 2003;22:298–307. doi: 10.1038/sj.onc.1206129. [DOI] [PubMed] [Google Scholar]
- Wang Y. C., Lee Y. H., Huang G. C., Lin Y. H., Fan-Chiang M. H., Chiu A. W., Huang Y. L. Enhanced transformation and chemosensitivity of NIH3T3 cells transduced with hepatoma up-regulated protein. Biochem. Biophys. Res. Commun. 2006;340:244–249. doi: 10.1016/j.bbrc.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Wong J., Fang G. HURP controls spindle dynamics to promote proper interkinetochore tension and efficient kinetochore capture. J. Cell Biol. 2006;173:879–891. doi: 10.1083/jcb.200511132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C. T., Hsu J. M., Lee Y. C., Tsou A. P., Chou C. K., Huang C. Y. Phosphorylation and stabilization of HURP by Aurora-A: implication of HURP as a transforming target of Aurora-A. Mol. Cell. Biol. 2005;25:5789–5800. doi: 10.1128/MCB.25.14.5789-5800.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.