Abstract
The barbed ends of actin filaments in striated muscle are anchored within the Z-disc and capped by CapZ; this protein blocks actin polymerization and depolymerization in vitro. The mature lengths of the thin filaments are likely specified by the giant “molecular ruler” nebulin, which spans the length of the thin filament. Here, we report that CapZ specifically interacts with the C terminus of nebulin (modules 160–164) in blot overlay, solid-phase binding, tryptophan fluorescence, and SPOTs membrane assays. Binding of nebulin modules 160–164 to CapZ does not affect the ability of CapZ to cap actin filaments in vitro, consistent with our observation that neither of the two C-terminal actin binding regions of CapZ is necessary for its interaction with nebulin. Knockdown of nebulin in chick skeletal myotubes using small interfering RNA results in a reduction of assembled CapZ, and, strikingly, a loss of the uniform alignment of the barbed ends of the actin filaments. These data suggest that nebulin restricts the position of thin filament barbed ends to the Z-disc via a direct interaction with CapZ. We propose a novel molecular model of Z-disc architecture in which nebulin interacts with CapZ from a thin filament of an adjacent sarcomere, thus providing a structural link between sarcomeres.
INTRODUCTION
In striated muscle, actin-containing thin filaments from adjacent sarcomeres overlap within the Z-disc in which their barbed ends are organized and anchored. Electron micrographs of longitudinal sections of mammalian skeletal muscle reveal that Z-discs contain an intricate network of “zigzag” bands (Rowe, 1973). Three-dimensional reconstruction and modeling of the Z-disc based on electron micrographs demonstrate that the zigzag bands are composed of sets of overlapping thin filament connectors called “Z-links,” which are predicted to be composed of α-actinin (Luther et al., 2002). These connectors allow the Z-disc to transmit force from one sarcomere to the next along the myofibril.
Drosophila melanogaster mutants that do not express α-actinin initially display relatively intact Z-discs in their striated muscle (Fyrberg et al., 1998). Later, severe muscle defects occur, and the larvae die. Thus, it seems that α-actinin is not absolutely required for Z-disc formation and function, but it is needed to maintain Z-disc stability in this organism. The giant sarcomeric protein titin has also been implicated in the assembly and maintenance of the Z-disc structure, whereas the specific contributions of other Z-disc components are currently unknown (Zou et al., 2006; Seeley et al., 2007).
Z-discs contain the barbed-end capping protein CapZ. CapZ binds with high affinity (Kd ≈ 1 nM) to the barbed ends of actin filaments, in which it effectively inhibits actin polymerization and depolymerization (Caldwell et al., 1989). Capping protein is an obligate α/β heterodimer, and efficient actin capping requires the C terminus of both subunits (Casella and Torres, 1994; Wear et al., 2003). Vertebrates express three conserved isoforms of each subunit. In muscle, the predominant isoform of the β-subunit is β1, which localizes to the Z-disc and is therefore called CapZ. The major β-subunit isoform in nonmuscle cells is β2; it is present in cardiomyocytes at low levels, but it localizes to the intercalated discs and the cell periphery, not to Z-discs (Schafer et al., 1994). How CapZ is targeted specifically to the Z-disc is not understood, but the ability of CapZ to interact with actin seems not to be necessary for its Z-disc localization (Schafer et al., 1995). Furthermore, the two β-subunit isoforms seem to have distinct functions in muscle cells. In mouse heart expression models, when the β1 subunit of CapZ is replaced with β2, the Z-disc is disorganized, truncated, and thickened, suggesting that the β2 subunit is not able to function at the Z-disc (Hart and Cooper, 1999). Also, in these experiments, expression of a dominant-negative β1 subunit unable to bind actin caused similar, but more severe, effects on Z-disc and sarcomere assembly (Hart and Cooper, 1999). The importance of CapZ in myofibrillogenesis has also been documented in primary cultures of skeletal myotubes, in which injection of an inhibitory antibody caused delays in thin filament and Z-disc assembly (Schafer et al., 1995). Thus, CapZ is an integral player in thin filament assembly and regulation within the Z-disc but the molecular mechanisms that underlie its Z-disc role remain unclear.
Thin filament assembly may also be regulated by the giant protein nebulin (600–900 kDa) (Wang and Williamson, 1980). Nebulin protein has been found in skeletal and cardiac muscle (although at lower levels in the latter), and transcripts have also been found in other tissues (Fock and Hinssen, 2002; Kazmierski et al., 2003; Bang et al., 2006). A single molecule of nebulin spans the entire length of the thin filament. The N terminus extends to the pointed end, in which it interacts with the pointed end capping protein tropomodulin (Wang and Wright, 1988; McElhinny et al., 2001). The C terminus of nebulin extends into the Z-disc, in which it interacts with α-actinin, myopalladin, and the intermediate filament protein desmin (Nave et al., 1990; Bang et al., 2001, 2002). Full-length human nebulin consists of 185 tandem copies of an ∼35-amino acid (aa) residue module, flanked by unique N- and C-terminal sequences. Each module has a conserved motif thought to interact with a single actin monomer. In addition, modules 9-162 are organized into sets of seven-module super-repeats, which contain a second conserved motif that matches the periodicity of, and is thought to organize, the tropomyosin/troponin complexes (Jin and Wang, 1991; Pfuhl et al., 1994; Labeit and Kolmerer, 1995; Wang et al., 1996).
Previous studies suggest that nebulin plays a role in regulating the final lengths of thin filaments at their pointed ends as well as the architecture of the Z-disc. Although the size and susceptibility of nebulin to proteolysis have made some functional approaches very difficult, the evidence for its role in determining the final lengths of thin filaments is strong (for reviews, see Trinick, 1994; McElhinny et al., 2003; Horowits, 2006). Two independent studies of nebulin knockout mice confirm the importance of nebulin in thin filament length regulation (Bang et al., 2006; Witt et al., 2006). In both studies, skeltal muscle thin filaments were shorter. In addition, wide Z-discs and nemaline rod bodies, which are composed of Z-disc material, were present, suggesting that nebulin contributes to Z-disc organization and/or maintenance. Previously, we showed that thin filament pointed ends grow to excessive lengths upon disassembly/reassembly in nebulin-deficient primary rat cardiomyocytes (McElhinny et al., 2005). In addition, α-actinin at the Z-disc was disorganized in these cells. The precise mechanism of action of nebulin and the potential contributions of the pointed end capping protein tropomodulin to nebulin function remain to be determined (Fowler et al., 2006).
Here, we report the discovery that CapZ specifically interacts with a segment of C-terminal nebulin (modules 160–164); this segment was previously considered to be located well outside of the Z-disc, within the I-band. The interaction of nebulin M160-M164 with CapZ does not alter the actin capping activity of CapZ in vitro. Furthermore, our data show that a knockdown of nebulin inhibits targeting of CapZ to the Z-disc. We also found evidence that nebulin has a role in regulating the lengths of thin filaments at their barbed ends, perhaps via its interaction with CapZ. We propose a novel molecular model of Z-disc architecture in which nebulin interacts with CapZ of a thin filament from an adjacent sarcomere, thus effectively cross-linking the thin filaments within the Z-disc, directly linking two sarcomeres and preventing growth of barbed ends into the adjacent sarcomere.
MATERIALS AND METHODS
Recombinant Protein Expression, Purification, and Biotinylation
A nebulin fragment encoding M160-M170 (Labeit and Kolmerer, 1995) was generated by Dr. Gloria Conover (University of Arizona, Tucson, AZ) (primer sequences: F-5′-GTA AAG GAA AGA GGA AGC TGT CAT GCT GTG-3′ and R-5′-CTG GCT GGC AAT GTC GGT AGC ATT CCT-3′) from mouse cDNA, and it was cloned into a modified pET-11 vector, which expresses the insert with an N-terminal 6X histidine tag (Novagen, Madison, WI). One exon within M160-M170, corresponding to a part of M168 and part of M169, was not amplified, likely due to alternative splicing. Smaller nebulin fragments corresponding to M160-M164 (forward [F]: 5′-GTA AAG GAA AGA GGA AGC TGT C-3′ and reverse [R]: 5′-GTT GTA TTT GGG TGT TTT GCC C-3′), M160-M161 (F: 5′-GTA AAG GAA AGA GGA AGC TGT C-3′ and R: 5′ GAT GGA GTA GTT GGA CTT GCC T-3′), M163-M164 (F: 5′-CCA GAC ATC AAG AAG GCC ACC C-3′ and R: 5′-GTT GTA TTT GGG TGT TTT GCC C-3′), and M165-M167 (F: 5′-CCG AAA GAC AGC CAG CTC TAC A-3′ and R: 5′-TTT CCC ACT CTG CAT CTG CTG A-3′) were subsequently subcloned from the M160-M170 construct (Supplemental Figure 1). All insert sequences were confirmed by DNA sequencing, and the expression constructs were transformed into Escherichia coli BL21-Codon Plus (DE3)-RIL competent cells (Stratagene, La Jolla, CA) for protein production. Other recombinant nebulin fragments, M2 and M50 (McElhinny et al., 2001), chicken Tmod1 (Babcock and Fowler, 1994), wild-type CapZ (Palmgren et al., 2001), and CapZ C-terminal deletion mutants (Wear et al., 2003) were expressed and purified as described previously.
CapZ was biotinylated at a molar ratio of 1:60 by using aminohexanoyl-biotin N-hydroxysuccinimide according to the manufacturer's instructions (Zymed Laboratories, South San Francisco, CA). Biotinylated CapZ was dialyzed against a storage buffer (0.1 M KCl and 10 mM HEPES, pH 7.5), aliquoted, frozen in liquid nitrogen, and stored at −80°C until use.
Blot Overlays
Adult rat psoas muscle was frozen in liquid nitrogen, ground into a fine powder, and then the proteins were solubilized in preheated (70°C) 2× Laemmli sample buffer. The sample was resolved on a 4–20% gradient SDS polyacrylamide gel, and then it was transferred to nitrocellulose membranes (0.2 μm; PerkinElmer Life and Analytical Sciences, Boston, MA). Efficient transfer was determined by Ponceau S staining. The membrane was then blocked with binding buffer (20 mM HEPES, pH 7.4, 80 mM KCl, 2 mM MgCl2, 0.05% Tween, and 2% bovine serum albumin [BSA]) for 2 h at 37°C, washed with binding buffer without BSA, and incubated with 0.06–0.25 μg/ml (0.9–3.7 nM) biotinylated CapZ (α1β1) or nonmuscle CP (α1β2) diluted in binding buffer overnight at 4°C. After additional washes, CapZ binding was detected chemiluminescently by incubation with horseradish peroxidase (HRP)-conjugated streptavidin (Pierce Chemical, Rockford, IL) diluted 1:20,000 in binding buffer for 1 h at 4°C, followed by SuperSignal West Pico Chemiluminescent Substrate (Pierce Chemical).
Solid Phase Binding Assays
These assays were performed essentially as described in McElhinny et al. (2001). Unless otherwise noted, wells were coated overnight at 4°C with 200 nM nebulin fragments diluted in 0.1 M carbonate buffer, pH 9.6. The wells were then washed and blocked with binding buffer (20 mM HEPES, pH 7.4, 250 mM KCl, 2 mM MgCl2, 0.1% Tween, and 0.2% BSA) for 1 h at 4°C and incubated with 125 nM of biotinylated CapZ diluted in binding buffer for 1 h at 4°C, followed by alkaline phosphatase-conjugated ImmunoPure streptavidin (Pierce Chemical) diluted 1:10,000 in binding buffer for 1 h at 4°C. The wells were then washed extensively with binding buffer without Tween and BSA, and they were incubated with 1 mg/ml 4-nitrophenyl phosphate disodium salt hexahydrate (Sigma-Aldrich, St. Louis, MO) in substrate buffer (0.1 M glycine, 1 mM MgCl2, and 1 mM ZnCl2, pH 10.4) for 30 min at 37°C. An interaction was determined by a colorimetric reaction at A405 on a Tecan plate reader using Winselect software (Phenix, Hayward, CA). All solid-phase binding assays were performed in Costar 96-well High binding, Easywash, enzyme immunoassay/radioimmunoassay plates (Corning, Corning, NY) with 100-μl volumes. Dissociation constants were determined from nonlinear regression curves fitted using the one-site binding equation Y = Bmax × X/(Kd + X) with Prism 4 software (GraphPad, San Diego, CA).
SPOTs Membranes
A SPOTs membrane containing 63 consecutive peptides (13 mer with 5 mer overlaps) representing human nebulin M159-M171 (Labeit and Kolmerer, 1995) was purchased from Sigma Genosys (The Woodlands, TX). The membrane was blocked with binding buffer (20 mM HEPES, pH 7.4, 80 mM KCl, 2 mM MgCl2, 0.05% Tween, and 2% BSA) for 3 h at 37°C, incubated with 0.5 μg/ml (7.4 nM) biotinylated CapZ overnight at 4°C, washed three times, and incubated with streptavidin-conjugated HRP diluted 1:20,000 in binding buffer for 1 h at 4°C. An interaction was determined after addition of SuperSignal West Pico Chemiluminescent Substrate (Pierce Chemical).
Cell Culture and Small Interfering RNA (siRNA) Treatment
Primary cultures of chick skeletal myotubes were prepared as described previously (Almenar-Queralt et al., 1999). A nebulin-specific siRNA was designed and generated using Silencer Construction kit (Ambion, Austin, TX) (target cDNA sequence: 5′-GTA GCT GAC TCT CCA ATT A-3′). As a control, a random siRNA was also generated (target cDNA sequence: 5′-CTC GAC TAG AGT CTG TCT A-3′). Skeletal myotubes were transfected with 50 nM siRNA using the lipid-based reagent Effectene (QIAGEN, Valencia, CA) according to the manufacturer's instructions 12 to 24 h after plating. Two to 3 d after transfection, the cells were incubated in relaxing buffer [150 mM KCl, 5 mM MgCl2, 10 mM 3-(N-morpholino)propanesulfonic acid, pH 7.4, 1 mM EGTA, and 4 mM ATP] for 15 min, and then they were fixed with 0.5–2% paraformaldehyde in relaxing buffer for 15 min.
Microinjection
Two to 3 d after siRNA treatment, cells were microinjected with rhodamine-labeled G-actin (Cytoskeleton, Denver, CO) resuspended to 2 mg/ml in 5 mM Tris, pH 8.0, 10 μM MgCl2, 0.2 mM ATP, and 1 mM dithiothreitol by using an Eppendorf Transjector, fixed 1 h after injection (i.e., short time interval to mark the ends of the filaments only) in 4% paraformaldehyde in phosphate-buffered saline (PBS), and stained as described below.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR) and Western Blotting
RT-PCR was performed as described previously (McElhinny et al., 2005). Total RNA was extracted from chick skeletal myotubes 1 d after siRNA treatment, and cDNA was synthesized from 1 μg of total RNA. Five microliters of template cDNA (diluted 1:50) was used in a 20-μl PCR reaction with 30 cycles. Primers included nebulin (F: 5′-CTT GGG CTG CTT CCT TTA TG-3′ and R: 5′-TCA AAT GGG TTT TTA GTT CCT GA-3′, which amplified a 170-base pair product), capping protein (F: 5′-CTT CTC CGC ACA TAG CCA AT-3′ and R: 5′-CTC TTC AAA GCC TCC ACC AG-3′, which simultaneously amplified a 300-base pair β1-specific product and a 190-base pair β2-specific product), cardiac α-actin (F: 5′-GAG CGT GGC TAT TCC TTT GT-3′ and R: 5′-TCC TGA GTG GGA AGT AAA TG-3′, which amplified a 578-base pair product) (Lin-Jones and Hauschka, 1997), skeletal α-actin (F: 5′-GAG CGT GGC TAT TCC TTT GT-3′ and R: 5′-ATC CTG AGT GTG GTT GGC AA-3′, which amplified a 573-base pair product) (Lin-Jones and Hauschka, 1997), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (F: 5′-GGC ACT GTC AAG GCT GAG AAC G-3′ and R: 5′-GGA GCT GAG ATG ATA ACA CGC TTA G-3′, which amplified a 200-base pair product). Western blotting was also performed as described previously using a rabbit anti-N-terminal nebulin antibody (1357L; ∼2 μg/ml) (McElhinny et al., 2005), a monoclonal anti-CapZ β1-specific antibody (1E5; 1:500), a monoclonal anti-nonmuscle CP β2-specific antibody (3F2; 1:100), and a rabbit anti-pan actin antibody (0.25 μg/ml) (Sigma-Aldrich).
Immunofluorescence Microscopy
To observe sarcomeric components, cells were stained as described previously (Gregorio and Fowler, 1995). The fixed cells were permeablized in 0.2% Triton X-100/PBS, blocked with 2% BSA plus 1% normal donkey serum/PBS, and incubated for 1 h with primary antibodies diluted in PBS. The primary antibodies included a polyclonal anti-C-terminal nebulin M176-M181 antibody (1:50; kindly provided by Siegfried Labeit, Universitatsklinikum Mannheim, Mannheim, Germany), a monoclonal anti-CapZ β1-specific antibody (1E5; 1:100), a polyclonal anti-titin Z1-Z2 antibody (1:100), a monoclonal anti-α-actinin antibody (1:15,000) (Sigma-Aldrich), a monoclonal anti-myomesin antibody (1:100; generously provided by E. Ehler, King's College London, London, United Kingdom; and J. C. Perriard, ETH Zurich, Zurich, Switzerland), and a monoclonal anti-cardiac actin antibody (1:10; American Research Products, Belmont, MA). Alexa Fluor 488-conjugated phalloidin was used to stain F-actin (Invitrogen, Carlsbad, CA). The cells were then washed with PBS for 15 min, and then they were incubated with secondary antibodies/PBS for 30 min. The secondary antibodies, obtained from Invitrogen (Carlsbad, CA) and Jackson Immunoresearch Laboratories (West Grove, PA), included: Alexa Fluor 488-conjugated goat anti-mouse IgG (1:1000), Alexa Fluor 350-conjugated goat anti-mouse IgG (1:300), and Texas Red-conjugated donkey anti-rabbit IgG (1:600). Coverslips were mounted onto slides with Aqua Poly/Mount (Polysciences, Warrington, PA). Note, we analyzed >500 nebulin siRNA treated cells and stringently compared distribution patterns in cells with a broad range of shapes, widths, or both. To triple-label cells, the anti-CapZ antibody (1E5) was directly labeled using the Zenon One Alexa Fluor 594 Mouse IgG1 Labeling kit according to the manufacturer's instructions (Invitrogen). The cells were analyzed and images captured using a Deltavision deconvolution microscope (Applied Precision, Issaquah, WA) with a 100× objective (1.3 numerical aperture) and a CoolSnap HQ charge-coupled device camera (Photometrics, Tucson, AZ). The images were then processed using Adobe Photoshop CS. Actin filament lengths were measured from deconvolved images of cells stained with an anti-cardiac actin antibody (which stains the I-band region of the thin filaments) by using ImageJ 1.37 software (National Institutes of Health, Bethesda, MD). The same cells were costained with a Z-disc anti-titin N terminal (Z1-Z2) antibody, and plot profiles of the staining intensity along the longitudinal axis of the myofibrils were generated using ImageJ. Z-disc to Z-disc distances, and thus sarcomere lengths, were determined by measuring the distance from one peak of intensity to the next. Statistical analyses were performed using Excel (Microsoft, Redmond, WA).
Cytochalasin D Staining
Cells were stained as described above except permeabilization was accomplished by the addition of 0.2 mg/ml saponin (Sigma-Aldrich) in every step. Following our general staining protocol, the cells were incubated with 275 nM green fluorescent BODIPY FL cytochalasin D (Invitrogen) for 10 min at 4°C followed by a 15-min wash in PBS. Coverslips were mounted onto slides with VECTASHIELD HardSet mounting media (Vector Laboratories, Burlingame, CA).
Tryptophan Fluorescence and Actin Polymerization Assays
Intrinsic tryptophan fluorescence assays were performed as described previously (Wear and Cooper, 2004) with minor modifications. Briefly, emission spectra (300–400 nm) of CapZ or nebulin M182-C were recorded with excitation at 292 nm. Nebulin M160-M164 does not contain any tryptophan residues. CapZ maximal fluorescence values, at 334 nm, in the presence of varying nebulin concentrations were fit with an equation for saturation binding using Prism 4 (GraphPad Software). Nebulin M182-C has six tryptophan residues, which was incorporated into the fitting equation used to determine binding affinities. CapZ and its C-terminal deletion mutants were used at a fixed concentration of 75 nM with varying concentrations of nebulin. The CapZ α-subunit truncation resulted in one tryptophan deletion, whereas no tryptophans were removed in the β-subunit deletion. The values on the y-axes in the figures reflect the difference of two larger numbers, the fluorescence of capping protein itself and the capping protein/nebulin complex. The difference in fluorescence values for the truncation mutants with the concentrations of nebulin shown reflected a much smaller change in y values than observed for wild-type capping protein and nebulin. Actin polymerization assays were performed as described previously (Wear and Cooper, 2004).
RESULTS
CapZ Interacts with Full-Length Endogenous Nebulin
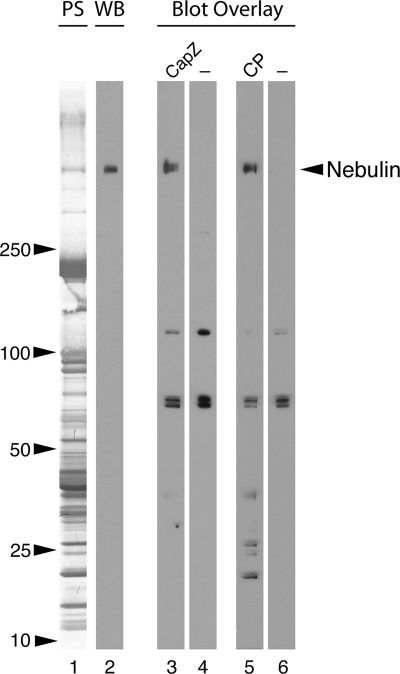
Based on the observation that the actin filament pointed end capping protein tropomodulin Tmod1 interacts with N-terminal nebulin (McElhinny et al., 2001) and the prediction by us, and others (Littlefield and Fowler, 1998; Fowler et al., 2006) that actin filament capping proteins work together with putative actin filament rulers to regulate thin filament assembly, we sought to determine whether CapZ interacts with the C-terminal region of nebulin. First, to determine whether CapZ binds full-length nebulin, blot overlays were performed on lysates of rat psoas muscle. The lysate was electrophoresed on a large 4–20% SDS polyacrylamide gel, which resolved proteins of a wide range of molecular weights (Figure 1, lane 1). After transfer to nitrocellulose, the lysate was probed with biotinylated recombinant CapZ (the Z-disc isoform, α1β1) or CP (the nonmuscle isoform, α1β2); both proteins bound to a high-molecular-weight protein (Figure 1, lanes 3 and 5) with the same mobility as nebulin (Figure 1, lane 2) along with lower-molecular-weight proteins of unknown identity.
Figure 1.
Endogenous nebulin interacts with CapZ in a blot overlay assay. Rat psoas muscle proteins were resolved on a 4–20% SDS-polyacrylamide gel, transferred to nitrocellulose (Ponceau S stain, lane 1), and probed with anti-nebulin IgG (Western blot, lane 2). A parallel blot was incubated with biotinylated CapZ (α1β1) (lane 3) or biotinylated nonmuscle CP (α1β2) (lane 5), followed by HRP-streptavidin. Both capping protein isoforms bound to a protein of the same molecular weight as nebulin, as well as to some lower-molecular-weight proteins of unknown identity. Controls were incubated with HRP-streptavidin alone which bound to three other bands (lanes 4 and 6).
CapZ Binds C-Terminal Nebulin within Modules 160–164
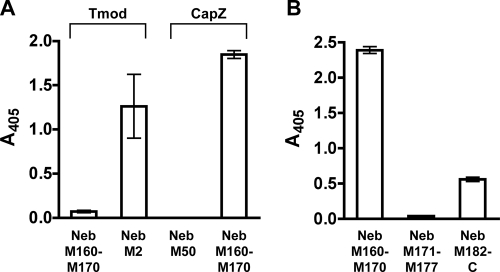
In striated muscle, CapZ binds the barbed ends of the actin filaments, which are believed to overlap within the Z-disc, thus placing CapZ at both edges of the Z-disc. Previous immunoelectron microscopy (immunoEM) and sequence alignment studies suggest that the C-terminal end of nebulin extends into the Z-disc, with the single repeats M177-M181 located at the edge or outside of the Z-disc (see model, Figure 11A) (Millevoi et al., 1998; Moncman and Wang, 2000). This model places M177-M181 in proximity (at the edge of the Z-disc) to the predicted location of CapZ at the barbed end of an opposing thin filament, from the adjacent sarcomere. Therefore, we tested two recombinant C-terminal nebulin fragments representing M171 to M177, and M182 to the C terminus, for binding to CapZ, in solid-phase binding assays. M160-M170, a region of nebulin predicted to be located outside the Z-disc in the I-band, was also tested. Nebulin M178-M181 was not tested because it could not be cloned; these modules are variably spliced (Millevoi et al., 1998). Each nebulin fragment was immobilized onto a microtiter plate, and were incubated with biotinylated CapZ. Surprisingly, CapZ bound to nebulin M160-M170, whereas significantly less binding was detected with the other C-terminal nebulin fragments in this assay (Figure 2B). Nonmuscle CP (α1β2) also bound to nebulin M160-M170 in this assay (data not shown). As a positive control, nebulin M2 (located at the N terminus) was immobilized and incubated with biotinylated Tmod1 (the pointed end capping protein). As negative controls, nebulin M160-M170 was incubated with biotinylated Tmod1, and nebulin M50 (located in the central region of the thin filament) was incubated with biotinylated CapZ; negligible binding was observed in each case (Figure 2A).
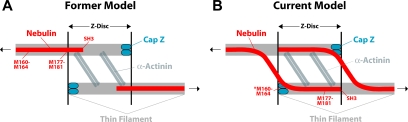
Figure 11.
Models of the architecture of nebulin and CapZ at the Z-disc. (A) In the former model, based on immunoEM mapping, nebulin extends only a short way into the Z-disc (Millevoi et al., 1998). According to this model, nebulin M160-M164 is far from CapZ at the end of the same filament. (B) In our current model, based on our binding data, CapZ at the barbed end of one thin filament interacts with nebulin M160-M164 bound to an adjacent thin filament. Nebulin thus cross-links thin filaments from adjacent sarcomeres. See Discussion for further details.
Figure 2.
CapZ binds C-terminal nebulin within modules 160–170 in solid-phase binding assays. (A) Various nebulin fragments were immobilized on microtiter plates, and then they were incubated with biotinylated CapZ or Tmod1. Binding is indicated by a colorimetric reaction measured at A405. Nebulin M2 is located at the pointed end of the thin filament, in which it interacts with Tmod1; nebulin M50 is located in the central region of the nebulin filament. (B) Various C-terminal nebulin fragments were immobilized on microtiter plates and incubated with biotinylated CapZ. Values are the mean of duplicates: bars represent the range of values.
To further define the CapZ binding site(s) within nebulin, a custom SPOTs membrane was used. The membrane, which contains a series of overlapping 13-mer peptide spots representing human nebulin M159-M171, was probed with biotinylated CapZ. Biotinylated CapZ strongly bound to two spots, one spot corresponding to a peptide within M160 (PQILLAKTVSNLV) and the other spot to a peptide within M164 (DIEMAKKAAKLSS) (Figure 3). Other less intense spots were also detected; these may indicate weak interactions or most likely, nonspecific binding.
Figure 3.
CapZ binds to peptides within nebulin modules 160 and 164. A membrane spotted with overlapping 13 residue peptides that span nebulin M159-M171 was incubated with biotinylated CapZ. CapZ specifically bound to two spots, one spot corresponding to a peptide within M160 (PQILLAKTVSNLV, top box), and the other spot corresponding to a peptide within M164 (DIEMAKKAAKLSS, bottom box).
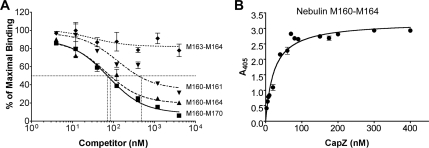
Based on the results obtained with the SPOTs membrane, we subcloned a nebulin fragment that encompassed both identified interacting peptides (M160-M164), and nebulin fragments that contained only one of the peptides (M160-M161 and M163-M164). To test the ability of these fragments to bind CapZ, the capacity of each nebulin fragment to displace the binding of CapZ to nebulin M160-M170 was determined. Nebulin M160-M170 (40 nM) was immobilized on a microtiter plate and incubated with equimolar biotinylated CapZ plus increasing concentrations of nebulin fragments. A 100-fold excess of nebulin M160-M170 effectively displaced 95% of CapZ binding, with a 50% inhibitory concentration (IC50) of 70 nM (Figure 4A, squares). Nebulin M160-M164 displaced CapZ binding nearly as well, with an IC50 value of 80 nM (Figure 4A, triangles). In contrast, nebulin M160-M161 was not nearly as effective a competitor (IC50 = 400 nM), and nebulin M163-M164 caused only ∼25% displacement at the highest concentration tested (4 μM) (Figure 4A, inverted triangles and diamonds, respectively). As a negative control, the addition of high concentrations of a nebulin module (M50), located in the proximity of the central region of the thin filament, resulted in negligible displacement of CapZ binding (data not shown).
Figure 4.
Nebulin modules 160–164 account for the majority of binding to CapZ in a competitive binding assay. (A) 40 nM recombinant nebulin fragment containing M160-M170 was immobilized on a microtiter plate and incubated with 40 nM biotinylated CapZ plus increasing concentrations of various nebulin fragments (4 nM–4 μM) followed by alkaline phosphatase-conjugated streptavidin. Binding is indicated by a colorimetric reaction measured at A405. The fraction of maximal binding versus the concentration of inhibitor was determined. Both nebulin M160-M170 and M160-M164 displaced nearly all of the binding of CapZ to nebulin M160-M170 (squares and triangles, respectfully), whereas two smaller nebulin fragments (M160-M161 and M163-M164) were not nearly as effective competitors (inverted triangles and diamonds, respectfully). (B) 100 nM nebulin M160-M164 was immobilized on a microtiter plate and incubated with increasing concentrations of biotinylated CapZ followed by alkaline phosphatase-conjugated streptavidin. Binding is indicated by a colorimetric reaction measured at A405. A representative experiment is shown. Values are the mean of duplicates; bars represent the range of values. In this assay, the calculated Kd ranged from 23 to 40 nM.
To estimate a dissociation constant (Kd) for the interaction of CapZ with nebulin M160-M164, another solid-phase binding assay was used. Nebulin M160-M164 (100 nM) was immobilized on a microtiter plate and incubated with increasing concentrations of biotinylated CapZ (Figure 4B). The interaction was saturable, with a Kd of ∼20–50 nM.
Neither of the Two Actin Binding Regions of CapZ Is Necessary for Its Interaction with Nebulin Modules 160–164
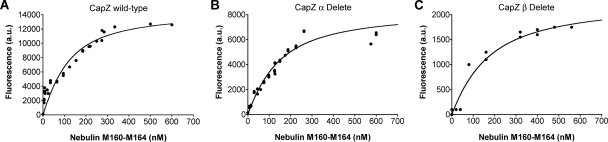
Together, the α and β subunits of the CapZ heterodimer form a structure that resembles a mushroom, with two actin binding regions on its top surface (Yamashita et al., 2003). Each actin binding region corresponds to the C-terminal segment of one subunit (Wear et al., 2003). To determine the importance of these regions for binding nebulin, we performed tryptophan fluorescence assays with truncation mutants of CapZ lacking these regions. A constant concentration of CapZ was incubated with an increasing concentration of nebulin M160-M164. Both truncation mutants bound to nebulin M160-M164 with an affinity comparable with that of wild-type CapZ (Figure 5). This indicates that the binding site is likely located within the main body of the CapZ heterodimer and that nebulin and actin do not share the same binding site within the C-terminal actin binding regions of CapZ. In addition, the Kd determined for the interaction of wild-type CapZ and nebulin M160-M164 in these assays (∼75 nM) is comparable with the Kd obtained using solid-phase binding assays (∼20–50 nM; see above; Figure 4B).
Figure 5.
Neither of the two actin binding regions of CapZ is necessary to bind nebulin M160-M164 in tryptophan fluorescence assays. Here, the binding of nebulin modules 160–164 to CapZ actin binding mutants was tested. (A) Increasing concentrations of nebulin M160-M164 were added to 75 nM of wild-type CapZ. M160–164 has no tryptophan residues, so the change in fluorescence represents a change in the fluorescence of CapZ, which has eight Trp residues. The fluorescence value of CapZ decreased with increasing M160-M164. The y-axis values are the fluorescence in absence of M160-M164 minus the fluorescence at the concentration of M160–164 indicated on the x-axis. The solid line is the fit of the data to a single-site binding model, with a Kd of 75 nM. (B) C-terminal truncation of the CapZ α subunit. (C) C-terminal truncation of the β subunit. The results are similar in each case.
Interaction with Nebulin Modules 160–164 Does Not Affect the Actin Filament Capping Activity of CapZ In Vitro
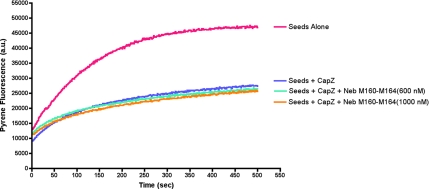
One possible function of the nebulin–CapZ interaction could be to modulate the ability of CapZ to cap the barbed end of the thin filament. To determine the effect of nebulin M160-M164 binding on the actin capping activity of CapZ, in vitro actin polymerization assays were performed. Actin was polymerized from the barbed-end using pyrene-labeled G-actin and spectrin-actin seeds. The seeds nucleate the polymerization of actin filaments, which is marked by an increase in pyrene fluorescence over time (Figure 6, pink curve). Because CapZ is an efficient inhibitor of actin polymerization at the barbed ends, addition of CapZ to the reaction resulted in a decreased rate of filament formation (Figure 6, blue curve). Addition of up to 1 μM nebulin M160-M164 (a concentration much greater than the estimated Kd of the CapZ-nebulin M160-M164 interaction) did not change the ability of CapZ to inhibit actin polymerization (Figure 6, teal and orange curves). This result is consistent with our observation above that neither of the two actin binding regions of CapZ is necessary to bind nebulin M160-M164.
Figure 6.
The interaction of nebulin modules 160–164 with CapZ does not modulate actin filament capping activity of CapZ in an in vitro actin polymerization assay. Pyrene fluorescence of actin filaments nucleated by F-actin seeds is plotted versus time. CapZ at 2 nM effectively inhibits polymerization (pink vs. blue curves). Up to 1 μM nebulin M160-M164 (a concentration much greater than the Kd of the CapZ-nebulin M160-M164 interaction) does not modulate the ability of CapZ to inhibit actin polymerization (teal and orange curves).
CapZ Does Not Bind Specifically to Nebulin Modules 182-C in Two Different Assays
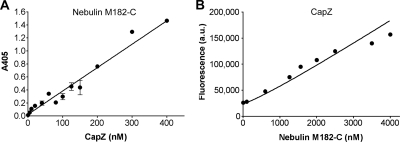
Our initial solid-phase binding assays indicated that CapZ might also interact with the most C-terminal region of nebulin tested (M182 to the end) (Figure 2B); this fragment is composed of the final four nebulin repeats, a unique serine-rich region, and a Src homology (SH)3 domain at the very C terminus. In a recent report, CapZ copurified with nebulin fragments containing the SH3 domain after simultaneous expression of all three polypeptides in bacteria (Witt et al., 2006). To test the specificity of the binding of CapZ to nebulin M182-C and to estimate a binding constant, we performed solid-phase binding assays as above. Nebulin M182-C (200 nM) was immobilized on a microtiter plate and incubated with increasing concentrations of biotinylated CapZ. Saturation was not observed, at CapZ concentrations up to 400 nM (Figure 7A). The potential interaction of CapZ with nebulin M182-C was also tested by trypotophan fluorescence assays. CapZ (75 nM) was incubated with increasing amounts of nebulin M182-C in solution (Figure 7B). The plot of fluorescence versus nebulin M182-C concentration was linear, indicating that binding did not occur or that fluorescence did not change with binding. Thus, the interaction of CapZ and nebulin M182-C is not significant in our assays.
Figure 7.
CapZ does not significantly bind to nebulin modules 182-C in solid-phase binding and tryptophan fluorescence assays. (A) Nebulin M182-C at 200 nM was immobilized on a microtiter plate, and then it was incubated with increasing concentrations of biotinylated CapZ. A representative experiment is shown. Values are the mean of duplicates; bars represent the range of values. The interaction between CapZ and M182-C was not saturable and a dissociation constant could not be calculated. (B) Increasing concentrations of nebulin M182-C were added to 75 nM CapZ, and tryptophan fluorescence was measured. Nebulin M182-C has six tryptophan residues, which accounts for the increase in fluorescence with increasing nebulin M182-C concentration. The linearity of the data is consistent with either no binding at all or binding without a change in fluorescence.
Nebulin Is Responsible for the Localization of CapZ at the Z-Disc
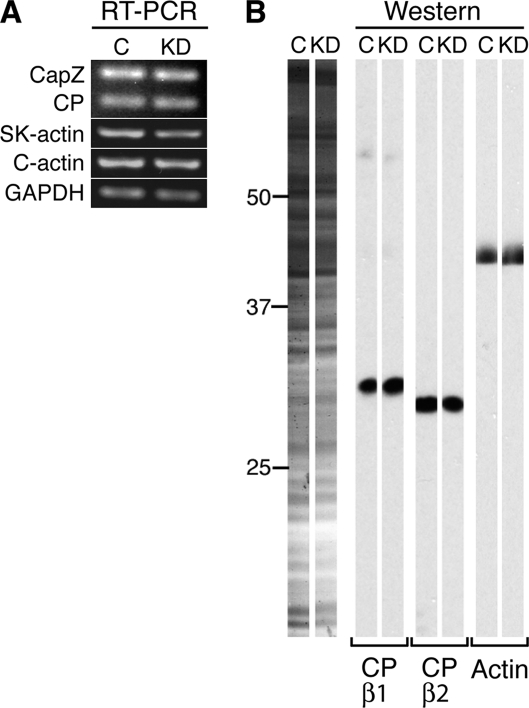
To explore the function of the nebulin-CapZ interaction in cells, we knocked down nebulin in primary cultures of chick skeletal myotubes. A nebulin-specific siRNA reduced nebulin transcript levels ≥70% after 1 d, compared with a scrambled control (Figure 8C) as determined by RT-PCR. Nebulin protein levels were reduced by ≥90% after 2 d (Figure 8D), and it remained low for another 2 d (data not shown), based on immunoblots. Transcript and protein levels of CapZ, nonmuscle CP, and actin did not significantly change (Figure 9).
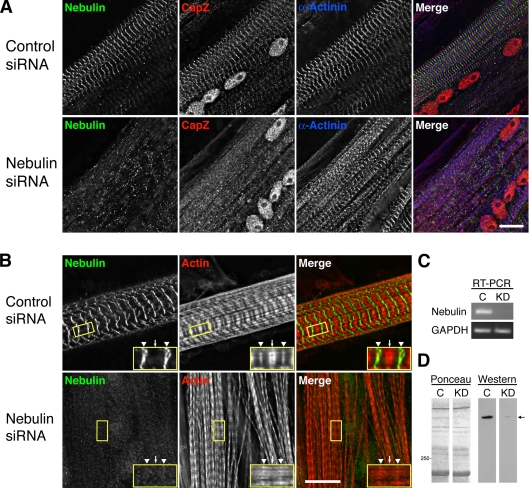
Figure 8.
Knockdown of nebulin in primary cultures of chick skeletal myotubes results in a loss of CapZ at the Z-disc. (A) Myotubes were triple stained with antibodies to N-terminal nebulin, CapZ, and α-actinin 3 d after siRNA treatment. Treatment with nebulin-specific siRNA resulted in a dramatic decrease in nebulin staining and a reduction in the amount of CapZ at the Z-disc, with α-actinin only partially perturbed. (B) Knockdown of nebulin also resulted in phalloidin staining along the entire thin filament, not just at the Z-disc (arrowheads) and pointed ends (arrows) as observed in control cells. Myotubes were costained with an anti-C-terminal nebulin antibody. Bar, 10 μm. (C) RT-PCR consistently showed a ≥70% reduction in nebulin transcript levels in chick skeletal myotubes 1 d after treatment with nebulin-specific (KD) versus control (C) siRNA, whereas GAPDH transcript levels were similar in both samples. (D) Western blot analysis revealed a ≥90% decrease in nebulin protein levels (arrow) in chick skeletal myotubes two days after treatment with nebulin-specific (KD) versus control (C) siRNA by using an anti-N-terminal nebulin antibody.
Figure 9.
(A) RT-PCR of cDNA from chick skeletal myotubes 1 d after treatment with siRNA. Transcript levels of CapZ, nonmuscle CP, skeletal α-actin, cardiac α-actin and GAPDH were not significantly changed in myotubes treated with nebulin-specific (KD) versus control (C) siRNA. (B) Western blot analysis revealed no significant change in CapZ, nonmuscle CP, or actin protein levels in chick skeletal myotubes two days after treatment with nebulin-specific (KD) versus control (C) siRNA. Control and knockdown samples from a single blot were cut into strips and probed separately.
Immunofluorescence microscopy was used to observe sarcomeric components within myotubes with reduced levels of nebulin 2–3 d after transfection. To confirm the knockdown, cells were stained with an anti-N-terminal nebulin antibody. Nearly every cell treated with nebulin-specific siRNA had either undetectable or very faint nebulin staining, whereas the control-treated cells had intense bands of nebulin staining (Figure 8A). Two other anti-nebulin antibodies specific to the C terminus and I-band region of nebulin also indicated a knockdown (Figure 8B and data not shown, respectively).
Knockdown of nebulin altered the staining pattern of actin filaments revealed by fluorescent phalloidin. In control cultured skeletal myotubes, phalloidin stained a thin band within the Z-disc and a thicker band at the pointed ends of the thin filaments (Figure 8B, arrowheads and arrow, respectively), with relatively less staining of the intervening region of the I-band, as described by others (e.g., Bukatina et al., 1984; Wilson et al., 1987; Szczesna and Lehrer, 1993; Ao and Lehrer, 1995; Zhukarev et al., 1997). In cells with reduced nebulin, however, phalloidin stained along the entire length of the thin filament in the I-band. Therefore, our results suggest that nebulin blocks phalloidin binding sites along the length of the actin thin filament. Knockdown of nebulin also resulted in an increase in the number of cells with nonstriated actin, an ∼10% decrease in sarcomere lengths (Z-to-Z line distance) and an ∼20% decrease in thin filament lengths (data not shown); the latter result is similar to what was reported previously in skeletal muscle isolated from nebulin knockout mice (Bang et al., 2006; Witt et al., 2006).
Remarkably, nebulin knockdown also resulted in a decrease of anti-CapZ staining intensity at the Z-disc, indicating that nebulin is required for Z-disc localization of CapZ (Figure 8A). Two other integral Z-disc proteins, α-actinin (Figure 8A) and N-terminal titin (data not shown), were only slightly affected by the knockdown of nebulin based on immunofluorescence staining, revealing that the Z-discs were intact. However, myofibrils exhibited some degree of lateral misalignment in the nebulin knockdown cells (Figure 8A; more clearly visualized in Figure 10A).
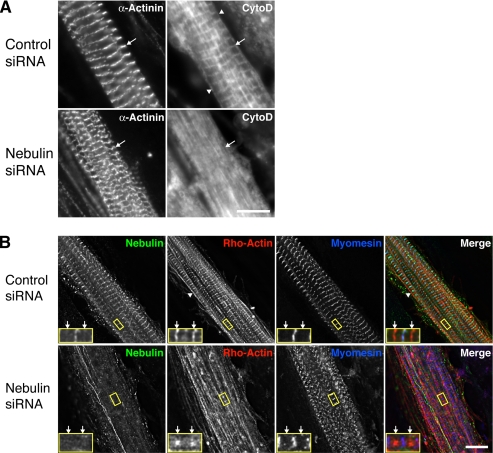
Figure 10.
Knockdown of nebulin in chick skeletal myotubes results in a dramatic loss of actin barbed end uniformity within the Z-disc. (A) Three days after siRNA treatment, primary cultures of chick skeletal myotubes were costained with fluorescently conjugated cytochalasin D, which binds the barbed ends of actin filaments, and an antibody to α-actinin. Control myotubes exhibited striations that colocalized with α-actinin staining at the Z-disc, whereas the cytochalasin D striations were nearly absent in cells treated with nebulin-specific siRNA (arrows). Staining was also observed at the sarcolemma (arrowheads). (B) Chick skeletal myotubes were microinjected with rhodamine-labeled G-actin 3 d after siRNA treatment and stained 1 h later with antibodies to N-terminal nebulin and myomesin. Rho-actin clearly marked the barbed ends of thin filaments (arrows) in control treated cells, whereas cells with reduced nebulin levels exhibited a reduction in barbed end labeling and a less organized, more nonstriated rho-actin distribution. The sarcolemma was also labeled by rho-actin (arrowheads). Bars, 10 μm.
Nebulin Aids in Restricting the Barbed Ends of Actin Filaments to the Z-Disc
Direct observation of the barbed ends of actin filaments in myocytes is difficult because they overlap within the Z-disc, resulting in continuous staining of actin markers such as phalloidin or anti-actin antibodies. Thus, to determine the effect of the knock down of nebulin, and the subsequent reduction of CapZ at the Z-disc, on the actin filaments within the Z-disc, we developed a novel approach to visualize only the barbed ends of the actin filaments. Fluorescently labeled cytochalasin D, a fungal toxin that binds with high affinity (2–50 nM) to the barbed end of actin filaments (Cooper, 1987; Urbanik and Ware, 1989), was used to stain fixed chick skeletal myotubes. Control myotubes showed striations that colocalized with α-actinin at the Z-disc, which is consistent with specific labeling of the barbed ends of the actin filaments (Figure 10A, arrows). The edges/membranes of the cells were also labeled with cytochalasin D (arrowheads), probably due to the actin cytoskeleton of the sarcolemma. In contrast, cytochalasin D staining was largely diffuse, with only a few detectable striations, in myotubes treated with nebulin-specific siRNA. Costaining for α-actinin confirmed that the Z-discs were intact in the nebulin siRNA-treated myocytes.
Using an independent approach, we microinjected live cells with rhodamine (rho)-labeled actin subunits, under conditions in which subunits specifically incorporate at the thin filament ends. Control myotubes displayed distinct bands of rho-actin at the Z-disc (barbed ends) and within the H zone (pointed ends), indicating that the ends of the filaments were uniformly aligned (Figure 10B, arrows denote Z-disc). Rho-actin also incorporated at the sarcolemma (Figure 10B, arrowheads), similar to cytochalasin D. In cells with reduced nebulin levels, however, rho-actin was markedly less organized at the barbed and pointed ends, with decreased Z-disc and H zone labeling, displaying a more “nonstriated”/nonuniform appearance. Staining for the thick filament-associated protein myomesin suggested that the thick filaments were not significantly affected by the knock down of nebulin. Z-disc structure was also not significantly disrupted by the knockdown of nebulin or by the incorporation of rho-actin, as determined by staining for α-actinin (Supplemental Figure 2).
Together, these results show that the positions of the barbed ends in nebulin-deficient sarcomeres are not restricted to the Z-disc, as they should be. The architecture of the sarcomere was otherwise relatively normal, in terms of the existence and the position of Z-discs, a thick filament-associated protein and thin filaments. Thus, nebulin is needed to maintain the uniform alignment of the barbed ends of the actin filaments at the Z-disc, consistent with the idea that the nebulin/CapZ interaction is critical for sarcomere function.
DISCUSSION
In this study, we found that the barbed end actin filament capping protein CapZ directly interacts with the actin filament length regulator nebulin. CapZ binds to modules 160–164 of the C-terminal region of nebulin with an affinity (25–75 nM) comparable with that of the interaction of the pointed end capping protein tropomodulin with N-terminal nebulin (15–30 nM) (McElhinny et al., 2001). Our results indicate that the binding of nebulin M160-M164 to CapZ does not affect the capping activity of CapZ in vitro, which is consistent with our finding that neither of the two actin binding regions of CapZ is necessary for the CapZ–nebulin interaction. Strikingly, we found that nebulin is required for the localization of CapZ to the Z-disc, and for the uniform assembly of the barbed ends of the actin filaments within the Z-disc. Our identification and characterization of the interactions of integral Z–disc components allows us to propose a novel molecular model of the layout of the filament systems within the Z-disc.
Nebulin M160-M164 contains two binding sites for CapZ, based on peptide array analysis. Competition experiments suggest that each binding site contributes to the interaction of nebulin with CapZ. An alternative interpretation is that the fragment containing M164 is only necessary for proper folding of the M160-M164 fragment. Other binding sites may also exist within nebulin M160-M164, which are not represented in M160-M161 or M163-M164.
We found that nebulin binding to CapZ did not affect the actin capping activity of CapZ. An important implication of this result is that nebulin, CapZ and the actin filament barbed end should be able to interact directly and simultaneously at the Z-disc, which is a key element of the model we propose below.
Knockdown of nebulin in chick skeletal myotubes resulted in a striking decrease in CapZ staining at the Z-disc, indicating that nebulin is important for the proper targeting of CapZ to the Z-disc. Myofibrillogenesis was otherwise remarkably normal, although the increased presence of nonstriated thin filaments (indicative of elongated and/or disorganized filaments), decreased sarcomere lengths and myofibril misalignment suggests that myofibrillogenesis may have been slightly delayed. Nebulin knockdown also caused a decrease in thin filament lengths comparable with that observed in the skeletal muscle of nebulin knockout mice (Bang et al., 2006; Witt et al., 2006), which is consistent with nebulin functioning to determine the final lengths of the thin filaments.
Using nebulin gene knockout mice, Witt et al. (2006) found a much more subtle CapZ phenotype than the phenotype that we see here with siRNA-mediated knockdown. In their study, immunofluorescence microscopy revealed that CapZ was less intense and more broadly distributed at the Z-disc. In addition, some Z-disc were found to be very wide by electron microscopy, suggesting that the complete loss of nebulin over the life of the animal affected the structure of the Z-disc. Our results, with effects of knockdown observed over the course of a few days, showed a more pronounced loss of CapZ at the Z-disc with essentially normal staining patterns for α-actinin and N-terminal titin. Of course, the long-term consequences of a mouse gene knockout may include the appearance of secondary effects or the masking of primary effects due to compensation.
Nebulin seems to be necessary to target CapZ to the Z-disc, but our results suggest that other interactions may be important. In blot-overlay and solid-phase binding assays CapZ (α1β1) and nonsarcomeric CP (α1β2), both bound to nebulin equally well. In myocytes with both CapZ and CP, only CapZ is found at the Z-disc (Schafer et al., 1994). Therefore, another mechanism or interacting partner may provide this isoform-specific targeting of CapZ.
If nebulin is a ruler molecule for the thin filament, it should specify the locations of both ends of the filament. We found that the barbed ends of the thin filaments were not uniformly confined to the Z-disc in the absence of nebulin, by using two different approaches for marking barbed ends (incorporation of rho-actin subunits and staining with fluorescently labeled cytochalasin D). Complicating the interpretation of the rho-actin labeling, however, is that the knockdown of nebulin also resulted in the loss of pointed end uniformity shown by the absence of distinct rho-actin incorporation and alternations in the staining pattern of phalloidin in the H zone. This suggests that the lengths of the filaments at the pointed ends are also altered and perhaps even to the extent that rho-actin could be marking the pointed ends of very short thin filaments near the Z-disc. However, combined with the results of the cytochalasin D staining, which does not mark actin filament pointed ends, our data indicate that the thin filament barbed ends are indeed misassembled. The loss of uniform positioning of barbed ends may be due to filament elongation, misalignment, or both. The observation that Z-discs were still present and positioned properly, as assessed by staining for α-actinin and the N-terminal region of titin, argues against gross misalignment of the thin filaments and favors the possibility that the barbed ends elongated beyond the Z-disc, due to the absence of capping by CapZ at the Z-disc. Thus, nebulin does seem to function as a ruler, regulating the length of thin filaments at their pointed ends, as described previously (McElhinny et al., 2005; Bang et al., 2006; Witt et al., 2006), and at their barbed ends (this study). In addition, these results also indicate that aspects of Z-disc structure do not depend on actin filament barbed end organization.
Our results also indicate that nebulin inhibits the binding of phalloidin to the I-band region of the thin filament. Failure of phalloidin to stain the I-band with the expected intensity is a well-known phenomenon (Bukatina et al., 1984; Wilson et al., 1987; Szczesna and Lehrer, 1993; Ao and Lehrer, 1995; Zhukarev et al., 1997). Removal of myosin and/or tropomyosin and troponin does not change this staining pattern (Zhukarev et al., 1997). These previous results led to the proposal that nebulin prevents phalloidin from binding to the thin filament in this region (Ao and Lehrer, 1995; Zhukarev et al., 1997). Our results reported here support that idea. Nebulin and phalloidin both bind directly to the actin filament, so perhaps their binding sites overlap.
One molecule of nebulin has been proposed to run along the entire length of the thin filament, from the pointed end, in which it interacts with tropomodulin, to the barbed end, in which it inserts into the Z-disc (Wang and Wright, 1988; Wright et al., 1993; Herrera et al., 2000; McElhinny et al., 2001). A study using immunoEM to map the layout of the nebulin molecule within the Z-disc (Millevoi et al., 1998) concluded that 1) nebulin modules 177–181 are located at the Z-disc periphery, 2) the C-terminal SH3 domain only partially inserts (∼25 nm) into the Z-disc, and 3) nebulin molecules from adjacent sarcomeres do not overlap within the Z-disc. The results of the Millevoi et al. (1998) study predict that nebulin modules 160–164, which we identify here as the CapZ binding site, should be located outside the Z-disc, within the I-band, as illustrated in Figure 11A.
Our results lead us to suggest an alternative model, illustrated in Figure 11B, in which nebulin M160-M164 is located in the Z disc. First, we found that M160-M164 binds directly to CapZ. CapZ is expected to reside at the barbed ends of the thin filaments, which define the edge of the Z disc (e.g., Narita et al., 2006). However, the localization of CapZ within a sarcomere has never been directly shown at the EM level. Second, desmin, an intermediate filament that localizes to the periphery of the Z-disc, interacts within nebulin M160-M164 (Bang et al., 2002; Conover, Henderson, and Gregorio, unpublished data). Finally, in unpublished studies, we find that nebulin M160-M170 interacts with α-actinin in a yeast two-hybrid assay (Mount-Patrick, Pappas, and Gregorio, unpublished data). Together, these data indicate that nebulin M160-M164 is located at the Z-disc, not outside it.
In our model, we propose that the M160-M164 region of nebulin interacts with a molecule of CapZ at the barbed end of a thin filament from the adjacent sarcomere (Figure 11B). The conventional notion has been that a molecule of nebulin on a particular thin filament extends through the Z-disc to interact with the barbed end of the same thin filament. However, if this were the case, and nebulin M160-M164 binds to CapZ, then 21 actin binding nebulin repeats (M165-M185, predicted to span nearly 120 nm) would extend past the barbed end of the thin filament and into the adjacent sarcomere. Alternatively, the C-terminal end of nebulin could extend past the barbed end and fold back into the Z-disc. However, in either configuration, almost three nebulin super-repeats of modules 142–162, which are thought to organize the tropomyosin/troponin complexes, would be located within the Z-disc. This seems unlikely because tropomyosins and troponins are absent from the Z-disc (Stromer and Goll, 1972; Ohtsuki, 1975).
Our model also predicts that the C-terminal segment of nebulin crosses from one thin filament to another within the Z-disc, suggesting that nebulin may be a component of the “Z-links” that connect actin filaments laterally. These Z-link connections between thin filaments of neighboring sarcomeres should provide support and strength to the Z-disc. The architecture of nebulin in our model may also help to establish the extent of thin filament overlap within the Z-disc, by specifying the distance between CapZ molecules, and thus the barbed ends of the actin filaments, from adjacent sarcomeres. Accordingly, the width of the Z-disc may correspond to the number of C-terminal nebulin repeats expressed (Millevoi et al., 1998).
In conclusion, we propose that nebulin acts in concert with both the pointed end and barbed end-capping proteins (Tmod and CapZ, respectively) to regulate thin filament assembly. Interactions between rulers and capping molecules may be a general mechanism for regulating the length of actin filaments or other biological polymers.
Supplementary Material
ACKNOWLEDGMENTS
We thank Gloria Conover (University of Arizona, Tucson, AZ) for the original cloning of nebulin M160-M170; Alexandria Lau (University of Arizona) for preparing the chick skeletal myotube cultures and for performing RT-PCR experiments; Sarah Mount-Patrick, Verena Mentrup, and Catherine Schwach (University of Arizona) for also preparing the chick skeletal myotube cultures and other technical assistance; Anke Zieseniss (University of Arizona) for generating anti-titin Z1Z2 antibodies and for assistance with ELISA and Western blot assays; Samantha Whitman (University of Arizona) for assistance with RT-PCR experiments; and Martin Wear and Kyoungtae Kim (Washington University, St. Louis, MO) for capping protein preparations. We also thank Abby McElhinny, Takehiro Tsukada, Paul St. John, and JMST graduate student discussion group (University of Arizona) for helpful discussions. Recombinant human nebulin fragments corresponding to modules 171–177 and 182-C terminus were generously provided by Drs. Dittmar Labeit and Siegfried Labeit (Universitatsklinikum Mannheim, Mannheim, Germany). This work was supported by National Institutes of Health grants HL-57461 and HL-083146 (to C.C.G.) and GM-38542 (to J.A.C.); a National Science Foundation IGERT in Genomics at the University of Arizona, an American Heart Association Predoctoral Fellowship, and an Achievement Rewards for College Scientists Foundation (Phoenix Chapter) scholarship (to C.T.P.) and National Institutes of Health grant T32 HL007873 (to N.B.).
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-07-0690) on February 13, 2008.
REFERENCES
- Almenar-Queralt A., Gregorio C. C., Fowler V. M. Tropomodulin assembles early in myofibrillogenesis in chick skeletal muscle: evidence that thin filaments rearrange to form striated myofibrils. J. Cell Sci. 1999;112:1111–1123. doi: 10.1242/jcs.112.8.1111. [DOI] [PubMed] [Google Scholar]
- Ao X., Lehrer S. S. Phalloidin unzips nebulin from thin filaments in skeletal myofibrils. J. Cell Sci. 1995;108:3397–3403. doi: 10.1242/jcs.108.11.3397. [DOI] [PubMed] [Google Scholar]
- Babcock G., Fowler V. M. Isoform specific interaction of tropomodulin with skeletal muscle and erythrocyte tropomyosins. J. Biol. Chem. 1994;269:27510–27518. [PubMed] [Google Scholar]
- Bang M. L., Gregorio C., Labeit S. Molecular dissection of the interaction of desmin with the C-terminal region of nebulin. J. Struct. Biol. 2002;137:119–127. doi: 10.1006/jsbi.2002.4457. [DOI] [PubMed] [Google Scholar]
- Bang M. L., Li X., Littlefield R., Bremner S., Thor A., Knowlton K. U., Lieber R. L., Chen J. Nebulin-deficient mice exhibit shorter thin filament lengths and reduced contractile function in skeletal muscle. J. Cell Biol. 2006;173:905–916. doi: 10.1083/jcb.200603119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang M. L., Mudry R. E., McElhinny A. S., Trombitas K., Geach A. J., Yamasaki R., Sorimachi H., Granzier H., Gregorio C. C., Labeit S. Myopalladin, a novel 145-kilodalton sarcomeric protein with multiple roles in Z-disc and I-band protein assemblies. J. Cell Biol. 2001;153:413–427. doi: 10.1083/jcb.153.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukatina A. E., Sonkin B. Y., Alievskaya L. L., Yashin V. A. Sarcomere structures in the rabbit psoas muscle as revealed by fluorescent analogs of phalloidin. Histochemistry. 1984;81:301–304. doi: 10.1007/BF00495644. [DOI] [PubMed] [Google Scholar]
- Caldwell J. E., Heiss S. G., Mermall V., Cooper J. A. Effects of CapZ, an actin capping protein of muscle, on the polymerization of actin. Biochemistry. 1989;28:8506–8514. doi: 10.1021/bi00447a036. [DOI] [PubMed] [Google Scholar]
- Casella J. F., Torres M. A. Interaction of Cap Z with actin. The NH2-terminal domains of the alpha 1 and beta subunits are not required for actin capping, and alpha 1 beta and alpha 2 beta heterodimers bind differentially to actin. J. Biol. Chem. 1994;269:6992–6998. [PubMed] [Google Scholar]
- Cooper J. A. Effects of cytochalasin and phalloidin on actin. J. Cell Biol. 1987;105:1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fock U., Hinssen H. Nebulin is a thin filament protein of the cardiac muscle of the agnathans. J. Muscle Res. Cell Motil. 2002;23:205–213. doi: 10.1023/a:1020909902462. [DOI] [PubMed] [Google Scholar]
- Fowler V. M., McKeown C. R., Fischer R. S. Nebulin: does it measure up as a ruler? Curr. Biol. 2006;16:R18–R20. doi: 10.1016/j.cub.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Fyrberg C., Ketchum A., Ball E., Fyrberg E. Characterization of lethal Drosophila melanogaster alpha-actinin mutants. Biochem. Genet. 1998;36:299–310. doi: 10.1023/a:1018789227987. [DOI] [PubMed] [Google Scholar]
- Gregorio C. C., Fowler V. M. Mechanisms of thin filament assembly in embryonic chick cardiac myocytes: tropomodulin requires tropomyosin for assembly. J. Cell Biol. 1995;129:683–695. doi: 10.1083/jcb.129.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart M. C., Cooper J. A. Vertebrate isoforms of actin capping protein beta have distinct functions in vivo. J. Cell Biol. 1999;147:1287–1298. doi: 10.1083/jcb.147.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera A. H., Elzey B., Law D. J., Horowits R. Terminal regions of mouse nebulin: sequence analysis and complementary localization with N-RAP. Cell Motil. Cytoskeleton. 2000;45:211–222. doi: 10.1002/(SICI)1097-0169(200003)45:3<211::AID-CM4>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Horowits R. Nebulin regulation of actin filament lengths: new angles. Trends Cell Biol. 2006;16:121–124. doi: 10.1016/j.tcb.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Jin J. P., Wang K. Nebulin as a giant actin-binding template protein in skeletal muscle sarcomere. Interaction of actin and cloned human nebulin fragments. FEBS Lett. 1991;281:93–96. doi: 10.1016/0014-5793(91)80366-b. [DOI] [PubMed] [Google Scholar]
- Kazmierski S. T., Antin P. B., Witt C. C., Huebner N., McElhinny A. S., Labeit S., Gregorio C. C. The complete mouse nebulin gene sequence and the identification of cardiac nebulin. J. Mol. Biol. 2003;328:835–846. doi: 10.1016/s0022-2836(03)00348-6. [DOI] [PubMed] [Google Scholar]
- Labeit S., Kolmerer B. The complete primary structure of human nebulin and its correlation to muscle structure. J. Mol. Biol. 1995;248:308–315. doi: 10.1016/s0022-2836(95)80052-2. [DOI] [PubMed] [Google Scholar]
- Lin-Jones J., Hauschka S. D. Skeletal and cardiac α-actin isoforms exhibit unanticipated temporal and tissue-specific gene expression patterns in developing avian limbs and embryos. Dev. Biol. 1997;189:322–334. doi: 10.1006/dbio.1997.8677. [DOI] [PubMed] [Google Scholar]
- Littlefield R., Fowler V. M. Defining actin filament length in striated muscle: rulers and caps or dynamic stability? Annu. Rev. Cell Dev. Biol. 1998;14:487–525. doi: 10.1146/annurev.cellbio.14.1.487. [DOI] [PubMed] [Google Scholar]
- Luther P. K., Barry J. S., Squire J. M. The three-dimensional structure of a vertebrate wide (slow muscle) Z-band: lessons on Z-band assembly. J. Mol. Biol. 2002;315:9–20. doi: 10.1006/jmbi.2001.5217. [DOI] [PubMed] [Google Scholar]
- McElhinny A. S., Kazmierski S. T., Labeit S., Gregorio C. C. Nebulin: the nebulous, multifunctional giant of striated muscle. Trends Cardiovasc. Med. 2003;13:195–201. doi: 10.1016/s1050-1738(03)00076-8. [DOI] [PubMed] [Google Scholar]
- McElhinny A. S., Kolmerer B., Fowler V. M., Labeit S., Gregorio C. C. The N-terminal end of nebulin interacts with tropomodulin at the pointed ends of the thin filaments. J. Biol. Chem. 2001;276:583–592. doi: 10.1074/jbc.M005693200. [DOI] [PubMed] [Google Scholar]
- McElhinny A. S., Schwach C., Valichnac M., Mount-Patrick S., Gregorio C. C. Nebulin regulates the assembly and lengths of the thin filaments in striated muscle. J. Cell Biol. 2005;170:947–957. doi: 10.1083/jcb.200502158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millevoi S., Trombitas K., Kolmerer B., Kostin S., Schaper J., Pelin K., Granzier H., Labeit S. Characterization of nebulette and nebulin and emerging concepts of their roles for vertebrate Z-discs. J. Mol. Biol. 1998;282:111–123. doi: 10.1006/jmbi.1998.1999. [DOI] [PubMed] [Google Scholar]
- Moncman C. L., Wang K. Architecture of the thin filament-Z-line junction: lessons from nebullete and nebulin homologies. J. Muscle Res. Cell Motil. 2000;21:153–169. doi: 10.1023/a:1005697226465. [DOI] [PubMed] [Google Scholar]
- Narita A., Takeda S., Yamashita A., Maeda Y. Structural basis of actin filament capping at the barbed-end: a cryo-electron microscopy study. EMBO J. 2006;25:5626–5633. doi: 10.1038/sj.emboj.7601395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave R., Furst D. O., Weber K. Interaction of alpha-actinin and nebulin in vitro. Support for the existence of a fourth filament system in skeletal muscle. FEBS Lett. 1990;269:163–166. doi: 10.1016/0014-5793(90)81144-d. [DOI] [PubMed] [Google Scholar]
- Ohtsuki I. Distribution of troponin components in the thin filament studied by immunoelectron microscopy. J. Biochem. 1975;77:633–639. doi: 10.1093/oxfordjournals.jbchem.a130765. [DOI] [PubMed] [Google Scholar]
- Palmgren S., Ojala P. J., Wear M. A., Cooper J. A., Lappalainen P. Interactions with PIP2, ADP-actin monomers, and capping protein regulate the activity and localization of yeast twinfilin. J. Cell Biol. 2001;155:251–260. doi: 10.1083/jcb.200106157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfuhl M., Winder S. J., Pastore A. Nebulin, a helical actin binding protein. EMBO J. 1994;13:1782–1789. doi: 10.1002/j.1460-2075.1994.tb06446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe R.W.D. The ultrastructure of Z discs from white, intermediate, and red fibers of mammalian striated muscles. J. Cell Biol. 1973;57:261–277. doi: 10.1083/jcb.57.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer D. A., Hug C., Cooper J. A. Inhibition of CapZ during myofibrillogenesis alters assembly of actin filaments. J. Cell Biol. 1995;128:61–70. doi: 10.1083/jcb.128.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer D. A., Korshunova Y. O., Schroer T. A., Cooper J. A. Differential localization and sequence analysis of capping protein beta-subunit isoforms of vertebrates. J. Cell Biol. 1994;127:453–465. doi: 10.1083/jcb.127.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley M., Huang W., Chen Z., Wolff W. O., Lin X., Xu X. Depletion of zebrafish titin reduces cardiac contractility by disrupting the assembly of Z-discs and A-bands. Circ. Res. 2007;100:238–245. doi: 10.1161/01.RES.0000255758.69821.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromer M. H., Goll D. E. Studies on purified-actinin. II. Electron microscopic studies on the competitive binding of -actinin and tropomyosin to Z-line extracted myofibrils. J. Mol. Biol. 1972;67:489–494. doi: 10.1016/0022-2836(72)90465-2. [DOI] [PubMed] [Google Scholar]
- Szczesna D., Lehrer S. S. The binding of fluorescent phallotoxins to actin in myofibrils. J. Muscle Res. Cell Motil. 1993;14:594–597. doi: 10.1007/BF00141556. [DOI] [PubMed] [Google Scholar]
- Trinick J. Titin and nebulin protein rulers in muscle? Trends Biochem. Sci. 1994;19:405–408. doi: 10.1016/0968-0004(94)90088-4. [DOI] [PubMed] [Google Scholar]
- Urbanik E., Ware B. R. Actin filament capping and cleaving activity of cytochalasins B, D, E, and H. Arch. Biochem. Biophys. 1989;269:181–187. doi: 10.1016/0003-9861(89)90098-2. [DOI] [PubMed] [Google Scholar]
- Wang K., Knipfer M., Huang Q. Q., van Heerden A., Hsu L. C., Gutierrez G., Quian X. L., Stedman H. Human skeletal muscle nebulin sequence encodes a blueprint for thin filament architecture. Sequence motifs and affinity profiles of tandem repeats and terminal SH3. J. Biol. Chem. 1996;271:4304–4314. doi: 10.1074/jbc.271.8.4304. [DOI] [PubMed] [Google Scholar]
- Wang K., Williamson C. L. Identification of an N2-line protein of striated muscle. Proc. Natl. Acad. Sci. USA. 1980;77:3254–3258. doi: 10.1073/pnas.77.6.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Wright J. Architecture of the sarcomere matrix of skeletal muscle: immunoelectron microscopic evidence that suggests a set of parallel inextensible nebulin filaments anchored at the Z line. J. Cell Biol. 1988;107:2199–2212. doi: 10.1083/jcb.107.6.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wear M. A., Cooper J. A. Capping protein binding to S100B: implications for the tentacle model for capping the actin filament barbed end. J. Biol. Chem. 2004;279:14382–14390. doi: 10.1074/jbc.M313412200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wear M. A., Yamashita A., Kim K., Maeda Y., Cooper J. A. How capping protein binds the barbed end of the actin filament. Curr. Biol. 2003;13:1531–1537. doi: 10.1016/s0960-9822(03)00559-1. [DOI] [PubMed] [Google Scholar]
- Wilson P., Fuller E., Forer A. Irradiations of rabbit myofibrils with an ultraviolet microbeam. II. Phalloidin protects actin in solution but not in myofibrils from depolymerization by ultraviolet light. Biochem. Cell Biol. 1987;65:376–385. doi: 10.1139/o87-047. [DOI] [PubMed] [Google Scholar]
- Witt C. C., Burkart C., Labeit D., McNabb M., Wu Y., Granzier H., Labeit S. Nebulin regulates thin filament length, contractility, and Z-disc structure in vivo. EMBO J. 2006;25:3843–3855. doi: 10.1038/sj.emboj.7601242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J., Huang Q. Q., Wang K. Nebulin is a full-length template of actin filaments in the skeletal muscle sarcomere: an immunoelectron microscopic study of its orientation and span with site-specific monoclonal antibodies. J. Muscle Res. Cell Motil. 1993;14:476–483. doi: 10.1007/BF00297210. [DOI] [PubMed] [Google Scholar]
- Yamashita A., Maeda K., Maeda Y. Crystal structure of CapZ: structural basis for actin filament barbed end capping. EMBO J. 2003;22:1529–1538. doi: 10.1093/emboj/cdg167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhukarev V., Sanger J. M., Sanger J. W., Goldman Y. E., Shuman H. Distribution and orientation of rhodamine-phalloidin bound to thin filaments in skeletal and cardiac myofibrils. Cell Motil. Cytoskeleton. 1997;37:363–377. doi: 10.1002/(SICI)1097-0169(1997)37:4<363::AID-CM7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Zou P., Pinotsis N., Lange S., Song Y. H., Popov A., Mavridis I., Mayans O. M., Gautel M., Wilmanns M. Palindromic assembly of the giant muscle protein titin in the sarcomeric Z-disc. Nature. 2006;439:229–233. doi: 10.1038/nature04343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.