Abstract
Ca2+ is absorbed across intestinal epithelial monolayers via transcellular and paracellular pathways, and an active form of vitamin D3, 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3], is known to promote intestinal Ca2+ absorption. However, the molecules driving the paracellular Ca2+ absorption and its vitamin D dependency remain obscure. Because the tight junction proteins claudins are suggested to form paracellular channels for selective ions between neighboring cells, we hypothesized that specific intestinal claudins might facilitate paracellular Ca2+ transport and that expression of these claudins could be induced by 1α,25(OH)2D3. Herein, we show, by using RNA interference and overexpression strategies, that claudin-2 and claudin-12 contribute to Ca2+ absorption in intestinal epithelial cells. We also provide evidence showing that expression of claudins-2 and -12 is up-regulated in enterocytes in vitro and in vivo by 1α,25(OH)2D3 through the vitamin D receptor. These findings strongly suggest that claudin-2- and/or claudin-12-based tight junctions form paracellular Ca2+ channels in intestinal epithelia, and they highlight a novel mechanism behind vitamin D-dependent calcium homeostasis.
INTRODUCTION
Calcium plays a fundamental role in various physiological functions such as bone mineralization, blood coagulation, neuromuscular transmission, and muscle contraction, as well as cell–cell adhesion and intracellular signaling. Ca2+ is absorbed in the intestinal mucosa by two distinct routes, the transcellular and paracellular pathways (Bronner et al., 2003; Hoenderop et al., 2005). The transcellular Ca2+ transport in the intestine is thought to comprise a multistep process: uptake across the apical plasma membrane via the Ca2+ channel protein transient receptor potential vanilloid receptor channel (TRPV)6, intracellular transport by the Ca2+-binding proteins calbindins, and extrusion through plasma membrane Ca2+-ATPase PMCA1b (Hoenderop et al., 2005). In contrast, the molecular basis for paracellular Ca2+ absorption, which occurs throughout the intestine (Bronner et al., 1986), is largely unknown.
One of the most important hormones to enhance intestinal Ca2+ absorption is an active form of vitamin D3, 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3] (Norman, 1990; Hoenderop et al., 2005). Most of its actions are mediated by the vitamin D receptor (VDR), a member of the nuclear receptor superfamily (Mangelsdorf et al., 1995). In fact, VDR knockout (VDRKO) mice exhibit decreased Ca2+ uptake in the intestine with a concomitant reduction in expression of some of the aforementioned transcellular Ca2+ transport proteins (Yoshizawa et al., 1997; Li et al., 2001; Van Cromphaut et al., 2001; Song et al., 2002), indicating the significance of VDR in transcellular Ca2+ absorption. It is also suggested that vitamin D could promote paracellular Ca2+ transport across intestinal epithelial cells (Wasserman, 2004). However, it remains obscure which molecules driving paracellular Ca2+ absorption are targets for the vitamin D signaling.
Tight junctions are the apical-most constituent of the intercellular junctional complex in mammalian epithelial cell sheets, and they act as a semipermeable barrier to the paracellular transport of ions and solutes (Anderson and Cereijido, 2001). Among molecular components of tight junctions, claudins (Cldns) are the major transmembrane proteins, and they consist of 24 members of a gene family (Furuse et al., 1998; Tsukita et al., 2001). In addition, distinct sets of claudins are generally expressed in a cell- and tissue-specific manner. Recent studies have disclosed that claudins are the major determinant of the barrier function of tight junctions. Importantly, the first extracellular loop, in which there is a wide variation in the position and number of charged amino acids depending on each claudin, is shown to create paracellular pores (channels) for cations or anions between neighboring cells (Van Itallie and Anderson, 2006). Along this line, we assumed that the specific species of intestinal claudins might contribute to paracellular Ca2+ transport. We also hypothesized that expression of these claudins could be up-regulated by VDR. To examine this assumption, we compared the expression of putative cation-permissive claudins in the intestine of VDRKO mice with that of wild-type (WT) mice, and we used RNA interference (RNAi) and overexpression approaches in the vitamin D-responsive intestinal cell line Caco-2. We report here that expression of claudins-2 and -12 seems to be activated by VDR in vivo and in vitro and that these two claudins are essential for Ca2+ absorption between intestinal epithelial cells, providing a novel mechanism underlying vitamin D-dependent intestinal Ca2+ transport.
MATERIALS AND METHODS
Antibodies
Rabbit polyclonal antibodies (pAbs) against Cldn7, Cldn12, and Cldn15 were generated, and the specificity of these antibodies were verified as described previously (Satohisa et al., 2005; Fujita et al., 2006; Sakai et al., 2007). Preimmune sera from each rabbit before being immunized with antigens were also used as negative controls to confirm the selectivity of these pAbs. A rabbit pAbs against Cldn2 were obtained from Immuno-Biological Laboratories (Minneapolis, MN) and Zymed Laboratories (South San Francisco, CA). Mouse monoclonal antibodies (mAbs) against occludin and VDR and rabbit pAbs against actin, ezrin/radixin/moesin-binding phosphoprotein 50 (EBP50), and TRPV6 were purchased from Zymed Laboratories, Santa Cruz Biotechnology (Santa Cruz, CA), Sigma-Aldrich (St. Louis, MO), Affinity BioReagents (Golden, CO), and Alomone Labs (Jerusalem, Israel), respectively. A rat mAb against ezrin were obtained from Sanko Junyaku (Tokyo, Japan). The secondary antibodies used were horseradish peroxidase (HRP)-conjugated anti-rabbit or anti-mouse immunoglobulin (Ig)G (Dako Denmark, Glostrup, Denmark), Alexa Fluor 488 (green)-labeled anti-rabbit IgG (Invitrogen, Carlsbad, CA), Alexa Fluor 594 (red)-labeled anti-mouse IgG (Invitrogen), and fluorescein isothiocyanate-conjugated anti-rat IgG (DakoCytomation).
Animals and Tissue Preparation
VDRKO mice were generated and maintained as reported previously (Yoshizawa et al., 1997). Twelve-week-old wild-type and VDRKO mice were anesthetized with diethyl ether, and specimens of the intestine were obtained. All aspects of the study were approved by the animal use and care committee of Sapporo Medical University School of Medicine (Sapporo, Japan).
Cell Lines and Cell Culture
The human intestinal cell line Caco-2 (clone BBe; CRL-2102) was obtained from American Type Culture Collection. To establish Caco-2 cell lines expressing Cldn2, Cldn7, Cldn12, and Cldn15 (Caco-2: Cldn2, Caco-2: Cldn7, Caco-2: Cldn12 and Caco-2: Cldn15, respectively), cells were transfected with 10 μg of individual expression vectors containing the corresponding mouse claudin cDNAs (Ishizaki et al., 2003; Fujita et al., 2006) along with 1 μg of the puromycin-resistant gene expression vector pHRLpuro1 (Chiba et al., 1997a) by using Lipofectamine 2000 Reagent (Invitrogen) according to the manufacturer's protocols. Puromycin (5 μg/ml)-resistant clones were screened by immunofluorescence staining and immunoblotting.
Cells were plated in DMEM supplemented with 20% fetal bovine serum (heat-inactivated and charcoal-stripped), 100 U/ml penicillin, and 100 μg/ml streptomycin, and they were grown at 37°C in a humidified 5% CO2 atmosphere. They were refed every 2 d, and they were exposed to the vehicle or 1α,25(OH)2D3 (Sigma-Aldrich).
RNA Extraction and Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
For analysis of mRNA expression, total RNA was isolated from the intestine and cells using TRIzol reagent (Invitrogen), and RT-PCR was performed as reported previously (Chiba et al., 1997b, 2006). The PCR primers for cDNA were as follows: mouse VDR (GenBank/EMBL/DDBJ accession no. NM_009504), 5′-AACGCTATGACCTGTGAAGGC-3′ (nucleotides [nt] 250-270) and 5′-CCTGTACTTACGTCTGCACGA-3′ (nt 612-632); human Cldn2 (GenBank/EMBL/DDBJ accession no. NM_020384), 5′-GCTTCTACTGAGAGGTCTG-3′ (nt 306-324) and 5′-TTCTTCACACATACCCTG-3′ (nt 1006-1023); human Cldn7 (GenBank/EMBL/DDBJ accession no. NM_001307), 5′-AGGCATAATTTTCATCGTGG-3′ (nt 792-811) and 5′-GAGTTGGACTTAGGGTAAGAGCG-3′ (nt 1021-1043); human Cldn12 (GenBank/EMBL/DDBJ accession no. NM_012129), 5′-CTCCCCATCTATCTGGGTCA-3′ (nt 635-654) and 5′-GGTGGATGGGAGTACAATGG-3′ (nt 816-835); human Cldn15 (GenBank/EMBL/DDBJ accession no. NM_014343), 5′-AAATACGGCAGAAACGCCTA-3′ (nt 915-934) and 5′-CGACTTCCCAAGAGCAGTTC-3′ (nt 1109-1128). The primers for mouse claudins and those for the housekeeping gene 36B4 encoding the ribosomal protein were described previously (Kubota et al., 2001; Fujita et al., 2006).
To confirm that amplifications were in the linear range, PCR was performed using three different numbers of cycles between 21 and 36, depending on the gene analyzed. Aliquots of PCR products were loaded onto 2% agarose gel, and then they were analyzed after staining with ethidium bromide. The mRNA signals were quantified using Image 1.62c software (Scion, Frederick, MD).
Gel Electrophoresis and Immunoblotting
The mouse intestinal tissue and Caco-2 cells grown on 60-mm tissue culture plates were washed twice with ice-cold phosphate-buffered saline (PBS), sonicated for 20 s in ice-cold NaHCO3 buffer (1 mM NaHCO3 and 1 mM phenylmethylsulfonyl fluoride [PMSF], pH 7.5), and put on ice for 30 min. They were mixed with 2× SDS sample buffer and boiled for 5 min. Total cell lysates were then resolved by one-dimensional SDS-polyacrylamide gel electrophoresis (PAGE) and electrophoretically transferred onto a polyvinylidene difluoride membrane (Immobilon; Millipore, Billerica, MA). The membrane was saturated with PBS containing 4% skim milk, and then it was incubated for 1 h at room temperature with primary antibodies in PBS. After rinsing in PBS containing 0.1% Tween 20, the membrane was incubated for 1 h at room temperature with HRP-conjugated anti-rabbit or anti-mouse IgG (diluted 1:1000) in PBS. It was then rinsed again, and finally it was analyzed using an ECL Western blotting detection system (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom). The blots were stripped with Restore Western blot stripping solution (Nacalai Tesque, Kyoto, Japan) according to the manufacturer's instructions, and immunoprobed with an anti-actin antibody. When immunoblotted signals were faint, ECL Advance Western blotting detection system (GE Healthcare) was used according to the manufacturer's recommendations. Signals in immunoblots were quantified using Image 1.62c software.
Immunohistochemistry
Ten-micrometer-thick frozen sections of the mouse intestine were fixed in 95% ethanol for 30 min at 4°C and in 100% acetone for 1 min at room temperature. After being washed three times with PBS, they were incubated for 1 h at room temperature with primary antibodies and rinsed again with PBS, followed by reaction for 1 h at room temperature with appropriate secondary antibodies. For immunohistochemistry of Caco-2 cells, cells grown on coverslips were fixed in 1% formaldehyde in PBS for 10 min. After being washed three times with PBS, they were treated with 0.2% Triton X-100 in PBS for 10 min, rinsed again with PBS, and preincubated in PBS containing 5% skim milk. They were subsequently incubated with primary and secondary antibodies as described above. All samples were examined using a laser-scanning confocal microscope (MRC 1024; Bio-Rad, Hercules, CA) and a PlanApo 60× numerical aperture 1.40 oil immersion objective (Nikon, Tokyo, Japan). Photographs were recorded using a Dell computer (PowerEdge 2200) and OS/2 WARP software (IBM, White Plains, NY), and they were processed with Photoshop 6.0 (Adobe Systems, Mountain View, CA). Observations were made at room temperature.
Measurement of Transepithelial Electrical Resistance (TER) and Calcium Transport Studies
Caco-2 cells were grown on 1.0-cm2 polycarbonate Transwell-Clear membranes (0.4-μm pore size; Corning Life Sciences, Acton, MA) coated with rat tail collagen. TER was measured using an EVOM voltohmmeter with ENDOHM-12 (WPI, Sarasota, FL) on a heating plate (FHP-30S; Fine Tokyo, Japan) adjusted to 37°C. The values are expressed in standard units of ohms per square centimeter. For calculation, the resistance of blank filters was subtracted from that of filters covered with cells.
Calcium transport across Caco-2 monolayers was assessed as reported previously (Giuliano and Wood, 1991). In brief, cells were washed twice with PBS, and transport buffer (140 mM NaCl, 5.8 mM KCl, 0.34 mM Na2HPO4, 0.44 mM NaH2PO4, 1 mM MgCl2, 1 mM CaCl2, 25 mM glucose, and 20 mM HEPES, pH 7.4) was poured into the inner and outer chambers. After adding 1 μCi/ml 45Ca2+ to the insert, cells were incubated at 37°C, and samples were collected from the opposite compartment at 15, 30, 60, and 120 min. Radioactivity of 45Ca2+ was measured using a scintillation counter (LS6500; Beckman).
Electrophysiological Measurement
For electrophysiological measurements, Caco-2 cells were grown on Snapwell filters (0.4-μm pore size; Corning Life Sciences) coated with rat tail collagen. The permeabilities of Na+ and Cl− across Caco-2 monolayers were determined using an Ussing chamber with an ECV4000 Precision V/I Clamp (WPI) according to the procedure of Hou et al. (2005).
RNAi and Transfection
Stealth small interfering RNA (siRNA) duplex oligonucleotides against human Cldn2 and Cldn12 were synthesized by Invitrogen. The sequences were as follows: Cldn2 RNAi #1, sense (5′-AUUUCAUGCUGUCAGGCACCAGUGG-3′) and antisense (5′-CCACUGGUGCCUGACAGCAUGAAAU-3′); Cldn2 RNAi #2, sense (5′-AGAAAUAAUGCCCAAGUAAAGAGCC-3′) and antisense (5′-GGCUCUUUACUUGGGCAUUAUUUCU-3′); Cldn2 RNAi #3, sense (5′-AGAGGAUGAUUCCAGCUAUCAGGGA-3′) and antisense (5′-UCCCUGAUAGCUGGAAUCAUCCUCU-3′); Cldn7 RNAi #1, sense (5′-AUAGGAGCUCAUCUGCCACUGCGGG-3′) and antisense (5′-CCCGCAGUGGCAGAUGAGCUCCUAU-3′); Cldn7 RNAi #2, sense (5′-AUUAGGGCUCGAGUGGCCUGCAAGG-3′) and antisense (5′-CCUUGCAGGCCACUCGAGCCCUAAU-3′); Cldn7 RNAi #3, sense (5′-AUACGGGCCUUCUUCACUUUGUCGU-3′) and antisense (5′-ACGACAAAGUGAAGAAGGCCCGUAU-3′); Cldn12 RNAi #1, sense (5′-UAUAAGGGAAGUCAUAAACAGGCCC-3′) and antisense (5′-GGGCCUGUUUAUGACUUCCCUUAUA-3′), Cldn12 RNAi #2, sense (5′-AUUUCAAUGGCAGAGAGGCGAGAGC-3′) and antisense (5′-GCUCUCGCCUCUCUGCCAUUGAAAU-3′); and Cldn12 RNAi #3, sense (5′-UUCUCGUUUCUGUUGAAUGUGAUCA-3′) and antisense (5′-UGAUCACAUUCAACAGAAACGAGAA-3′). Cells were transfected with 20 pmol of siRNAs or a Stealth RNAi negative control by using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's protocols, and they were treated 12 h after transfection followed by exposure to 1α,25(OH)2D3.
Statistical Analysis
All measured values are presented as the mean ± SD. Statistical significance of differences was evaluated using the unpaired Student's t test.
RESULTS
Claudins 2 and 12 Are down-Regulated in the Intestine of VDRKO Mice
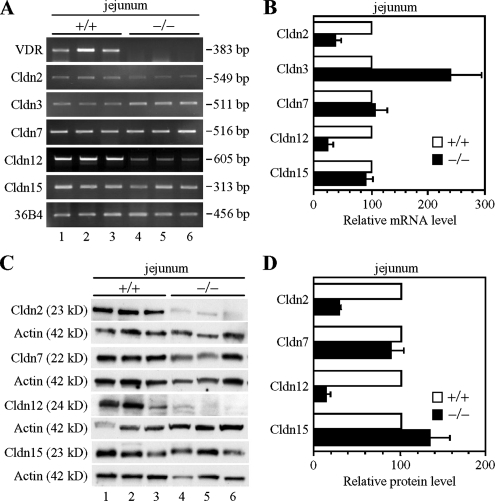
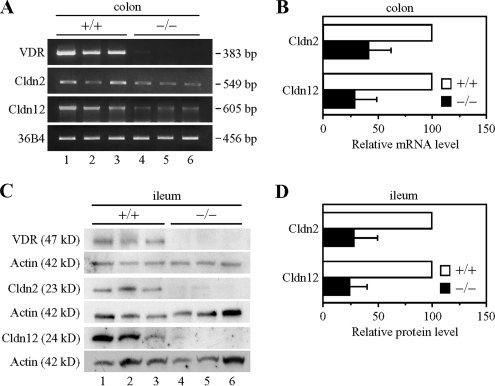
Among claudin species that are abundantly expressed in the intestine, Cldn2, Cldn7, Cldn12, and Cldn15 are suggested to act as paracellular channels for cations (Amasheh et al., 2002; Colegio et al., 2002; Van Itallie et al., 2003; Alexandre et al., 2005; Fujita et al., 2006; Hou et al., 2006). Therefore, we first compared, by RT-PCR and Western blot analyses, the mRNA and protein levels of these claudins throughout the intestinal tract in VDRKO mice with those in WT mice. The lack of VDR transcripts and proteins in the intestine of VDRKO mice was confirmed by RT-PCR and immunoblot experiments (Figures 1A and 2, A and C; data not shown). As shown in Figure 1, A and B, expression levels of Cldn3 transcripts were higher in the jejunum in VDRKO mice than in WT mice, in good agreement with the finding that the gene expression of Cldn3 is decreased in the rat small intestine after 1α,25(OH)2D3 treatment (Kutuzova and Deluca, 2004). In contrast, the mRNA expression of Cldn2 and Cldn12, but not Cldn7 or Cldn15, was reduced in the VDRKO mouse jejunum compared with that of the WT mouse (∼3- and 4-fold decreases, respectively). Similarly, levels of Cldn2 and Cldn12 proteins, but not Cldn7 or Cldn15 proteins, were much lower in the VDRKO mouse jejunum than in that of WT mice (Figure 1, C and D) (∼3- and 7-fold less, respectively). Cldn2 and Cldn12 were also down-regulated in the duodenum, ileum, and colon of the VDRKO mouse at both mRNA and protein levels (Figure 2, A–D; data not depicted).
Figure 1.
Reduced expression of claudins-2 and -12 in the jejunum of VDRKO mice. (A) Gene expression of Cldns in the jejunum of wild-type (+/+; lanes 1–3) and VDRKO mice (−/−; lanes 4–6) at 12 wk of age. One microgram of total RNA from the jejunum was subjected to RT-PCR analysis for the indicated genes. PCR was performed for 20 (36B4), 21 (Cldn3 and Cldn7), 25 (Cldn15), 28 (Cldn2), 32 (Cldn12), or 36 (VDR) cycles. (B) RT-PCR analysis was performed as described in A for at least five independent experiments. The mRNA levels were normalized to the corresponding 36B4 levels and expressed relative to the amount present in the wild-type mice, which was taken as 100. Values represent the mean ± SD (error bars; n = 5 for Cldn7 and n = 6 for other Cldns). (C) Expression of Cldn proteins in the jejunum of each genotype at 12 wk of age. Twenty-five micrograms of whole cell extract from the jejunum was separated by SDS-PAGE and immunoblotted with the corresponding antibodies followed by chemiluminescence detection. Each blot was stripped and reimmunoprobed with an anti-actin antibody. (D) Western blot analysis was performed as in C for at least four independent experiments. The protein levels are normalized to the corresponding actin levels and expressed relative to the amount present in wild-type mice, which was taken as 100. Values represent the mean ± SD (error bars; n = 4 for Cldn15, n = 6 for Cldn2 and Cldn7, and n = 7 for Cldn12).
Figure 2.
Down-regulation of claudins-2 and -12 in the ileum and colon of VDRKO mice. (A) Gene expression of claudin-2 (Cldn2) and Cldn12 in the colon of wild-type (+/+; lanes 1–3) and VDRKO mice (−/−; lanes 4–6) at 12 wk of age. One microgram of total RNA from the jejunum was subjected to RT-PCR analysis for the indicated genes. PCR was performed for 20 (36B4), 23 (Cldn2), or 36 (Cldn12 and VDR) cycles. (B) RT-PCR analysis was performed as described in A for at least four independent experiments. The mRNA levels are normalized to the corresponding 36B4 levels and expressed relative to the amount present in the wild-type mice, which was taken as 100. Values represent the mean ± SD (error bars; n = 4 for Cldn12 and n = 6 for Cldn2). (C) Expression of Cldn2 and Cldn12 proteins in the ileum of each genotype at 12 wk of age. Twenty-five micrograms of whole cell extract from the ileum was separated by SDS-PAGE and immunoblotted with the corresponding antibodies followed by chemiluminescence detection. Each blot was stripped and reimmunoprobed with an anti-actin antibody. (D) Western blot analysis was performed as described in C for at least five independent experiments. The protein levels are normalized to the corresponding actin levels and expressed relative to the amount present in wild-type mice, which was taken as 100. Values represent the mean ± SD (error bars; n = 5 for Cldn2 and n = 8 for Cldn12).
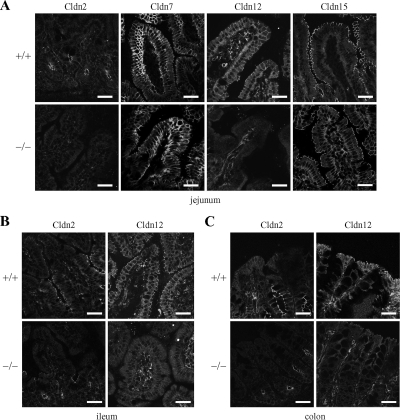
We subsequently determined, by immunofluorescent analysis, differences in the distribution of these claudins in the intestinal epithelial cells of WT and VDRKO mice (Figure 3, A–C). In the jejunum, ileum, and colon of WT mice, Cldn2 was localized at the apical-most edges of lateral membranes of crypt epithelia as reported previously (Rahner et al., 2001; Holmes et al., 2006). In VDRKO mice, however, it was not detected on the jejunal mucosa and only weakly observed in the ileum and colon compared with WT mice. Cldn12 was expressed at the apical-most sites of lateral membranes of epithelia in the jejunum, ileum, and colon of WT mice as reported previously (Fujita et al., 2006), whereas it was not detectable on intestinal epithelial cells of VDRKO mice. In contrast, the distribution of Cldn7 and Cldn15 in the intestine of VDRKO mice was indistinguishable from that of WT mice (Figure 3A; data not shown). Thus, the expression of both Cldn2 and Cldn12 seemed to be reduced throughout segments of the intestinal tract in VDRKO mice as far as we could determine.
Figure 3.
Distribution of claudins in intestinal mucosa of wild-type (+/+) and VDRKO (−/−) mice. Sections of the jejunum (A), ileum (B), and colon (C) of mice at 12 wk of age were subjected to immunostaining with the corresponding antibodies. Bar, 30 μm.
Expression of Claudins-2 and -12 Is Induced by Vitamin D in Caco-2 Cells
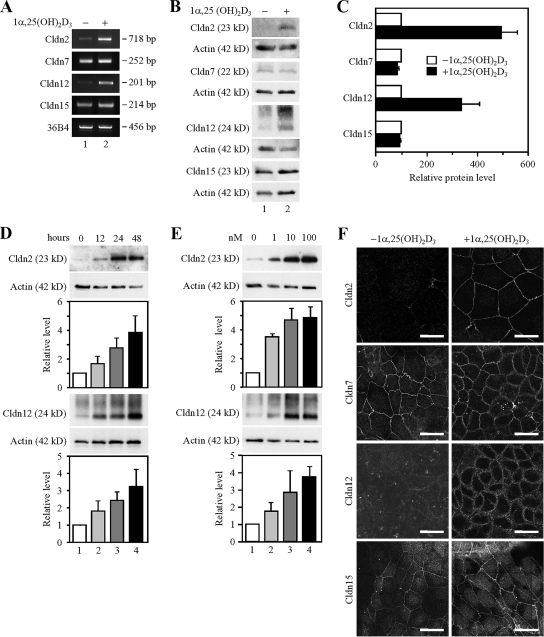
We next analyzed the effects of 1α,25(OH)2D3 on the expression of Cldn2, Cldn7, Cldn12, and Cldn15 in the well-established human cell line Caco-2, because it exhibits a small intestinal phenotype. As expected, the mRNA and protein expression of Cldn2 and Cldn12, but not that of Cldn7 or Cldn15, was induced in the cells by treatment for 48 h with 10−7 M 1α,25(OH)2D3 (∼4- to 5-fold and 3- to 4-fold increases in their protein levels, respectively) (Figure 4, A–C). In addition, the levels of Cldn2 and Cldn12 proteins were elevated by 1α,25(OH)2D3 in time- and dose-dependent manners (Figure 4, D and E). Note that multiple bands were observed in the immunoblots by using the anti-Cldn12 pAb used (also see Figures 6A and 7A), as reported previously for COS-7 cells transfected with the Cldn12 expression vector (Fujita et al., 2006), suggesting that this pAb also recognizes posttranslationally modified Cldn12.
Figure 4.
Induction of the expression of claudins-2 and -12 by 1α,25(OH)2D3 in Caco-2 cells. (A) Expression of Cldn mRNAs in Caco-2 cells treated for 48 h with the vehicle (lane 1) or 100 nM 1α,25(OH)2D3 (lane 2). One microgram of total RNA from the cells was subjected to RT-PCR analysis for indicated genes. PCR was performed for 22 (36B4), 30 (Cldn12 and Cldn15) or 32 (Cldn2 and Cldn7) cycles. (B) Expression of Cldn proteins in Caco-2 cells grown for 48 h in the absence (lane 1) or presence of 100 nM 1α,25(OH)2D3 (lane 2). Twenty-five micrograms of whole cell extract was separated by SDS-PAGE and immunoblotted with antibodies against the corresponding claudins, followed by chemiluminescence detection. The blots were stripped and immunoprobed with an anti-actin antibody. (C) Western blot analysis was performed as in C for three independent experiments. The protein levels are normalized to the corresponding actin levels and expressed relative to the amount present in the cells cultured without 1α,25(OH)2D3, which was taken as 100. Values represent the mean ± SD (error bars; n = 3). (D) Expression of Cldn2 and Cldn12 proteins in Caco-2 cells exposed to 100 nM 1α,25(OH)2D3 for 0, 12, 24, and 48 h (lanes 1–4, respectively). Western blot analysis was performed as described in B for three independent experiments, and the protein levels are expressed as described in C (error bars; n = 3). (E) Expression of Cldn2 and Cldn12 proteins in Caco-2 cells treated for 48 h with 0, 1, 10, and 100 nM 1α,25(OH)2D3 (lanes 1–4, respectively). Western blot analysis was performed as described in B for three independent experiments, and the protein levels are expressed as described in C (error bars; n = 3). (E) Staining pattern of Cldn2, Cldn7, Cldn12, and Cldn15 in Caco-2 cells grown for 48 h in the absence or presence of 100 nM 1α,25(OH)2D3. Cells were subjected to immunostaining with the corresponding antibodies, and they were observed under a laser-scanning confocal microscope. Bars, 20 μm.
Figure 6.
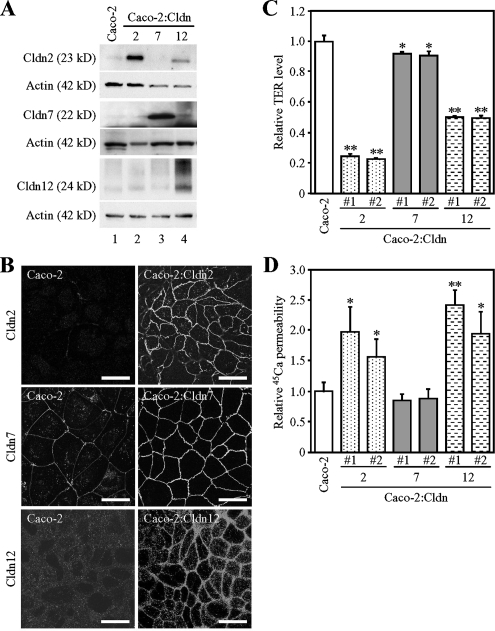
Overexpression of claudins-2 and -12 promotes calcium transport in Caco-2 cells. (A) Expression of claudin-2 (Cldn2), Cldn7, and Cldn12 proteins in Caco-2 and Caco-2:Cldn transfectants. Twenty-five micrograms of whole cell extract from the cells was separated by SDS-PAGE and immunoblotted with antibodies against the corresponding claudins, followed by chemiluminescence detection. The blots were stripped and reimmunoprobed with an anti-actin antibody. (B) Staining pattern of Cldn2, Cldn7, and Cldn12 in Caco-2 and Caco-2:Cldn transfectants. Cells were subjected to immunostaining with the corresponding claudin antibodies. Bar, 20 μm. (C and D) Relative levels of TER (C) and 45Ca2+ transport (D) in Caco-2 and Caco-2:Cldn transfectants. The values of TER and 45Ca2+ permeability in Caco-2:Cldn transfectants (clones #1 and #2) are expressed relative to the level in the mock-transfected cells, which was taken as 1, and they represent the mean ± SD (error bars; n = 4). *p < 0.05 and **p < 0.01 compared with values of the mock-transfected cells.
Figure 7.
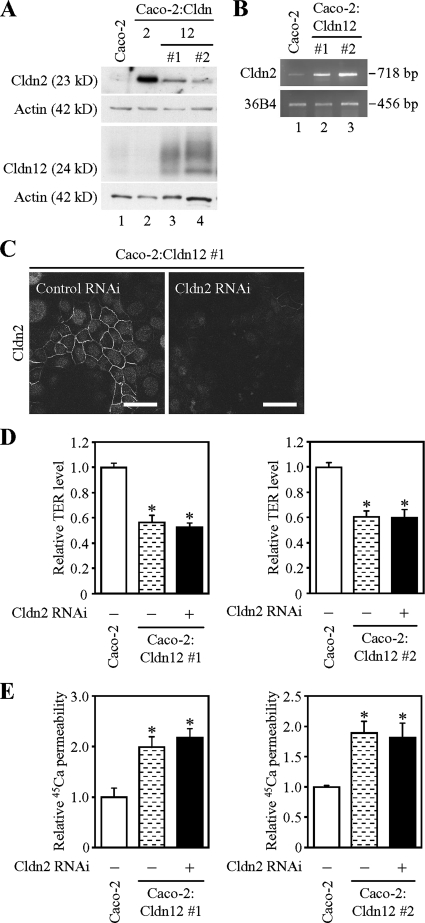
Weak induction of claudin-2 does not contribute to increased calcium transport in Caco-2 cells overexpressing claudin-12. (A and B) Slight induction of endogenous Cldn2 protein (A) and mRNA (B) in Caco-2:Cldn12 cells (clones #1 and #2). (A) Twenty-five micrograms of whole cell extract from the cells was separated by SDS-PAGE and immunoblotted with antibodies against the corresponding claudins, followed by chemiluminescence detection. The blots were stripped and reimmunoprobed with anti-actin antibody. (B) One microgram of total RNA from the cells was subjected to RT-PCR analysis for indicated genes. PCR was performed for 23 (36B4) or 30 (Cldn2) cycles. (C) Knockdown of endogenous Cldn2 expression in Caco-2:Cldn12 cells (clone #1) by RNAi. Cells were transfected with negative control siRNA (left) or siRNA against Cldn2 (#1; right), incubated for 12 h after transfection, and then they were refed and grown for 48 h. They were subjected to immunostaining with the corresponding antibodies, and they were observed under a laser-scanning confocal microscope. Bars, 20 μm. (D and E) Effects of suppressed endogenous Cldn2 on TER (D) and 45Ca2+ permeability in Caco-2:Cldn12 cells (clones #1 and #2). Cells were transfected and grown as described in C, and then they were subjected to measurement of TER (D) and calcium transport studies (E). The values are expressed relative to the level in Caco-2 cells transfected with negative control siRNA, which was taken as 1, and they represent the mean ± SD (error bars; n = 6 [left] and n = 3 [right]). *p < 0.01 compared with values of the mock-transfected cells.
We then examined the localization of Cldn2, Cldn7, Cldn12, and Cldn15 in Caco-2 cells by immunostaining (Figure 4F). Cldn2 and Cldn12 proteins were hardly detected in the cells grown without 1α,25(OH)2D3, whereas they were concentrated on the cell boundaries in the cells exposed to 10−7 M 1α,25(OH)2D3 for 48 h. By contrast, Cldn7 and Cldn15 were observed along the cell borders regardless of the presence or absence of 1α,25(OH)2D3. In addition, these four claudin species were colocalized, at least in part, with occludin at tight junctions in Caco-2 cells (Supplemental Figure S1). Hence, expression of Cldn2 and Cldn12 was apparently up-regulated by 1α,25(OH)2D3 in Caco-2 cells, as in intestinal epithelial cells in mice.
Claudins-2 and-12 Are Required for Vitamin D-induced Calcium Absorption in Caco-2 Cells
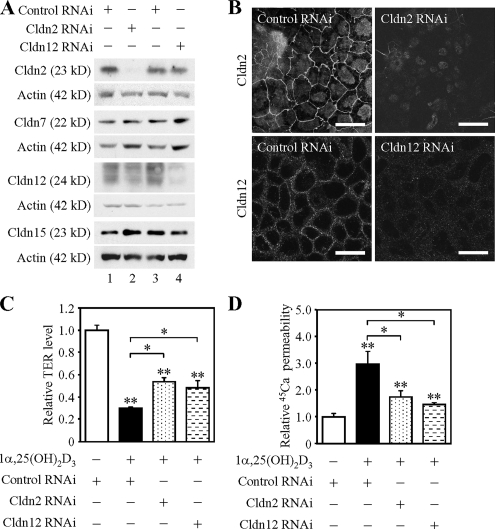
Treatment of Caco-2 cells with 1α,25(OH)2D3 is known not only to decrease TER, an indicator of the paracellular barrier to ion conductance but also to increase Ca2+ transport (Giuliano and Wood, 1991; Chirayath et al., 1998; Fleet and Wood, 1999; also see Figure 5, C and D). Therefore, we subsequently verified whether expression of endogenous Cldn2 and/or Cldn12 was required for 1α,25(OH)2D3-induced Ca2+ absorption. To this end, we used an RNAi strategy to knock down the expression of Cldn2 and Cldn12. Caco-2 cells were transfected with three distinct siRNAs against human Cldn2, those against human Cldn12 or negative control siRNA, and then they were incubated for 12 h after transfection followed by treatment for 48 h with 10−7 M 1α,25(OH)2D3. Western blot and immunofluorescence analyses showed that two different Cldn2 siRNAs (#1 and #3) and a Cldn12 siRNA (#1) effectively diminished the expression of Cldn2 and Cldn12, respectively (Figure 5, A and B; data not shown). Neither Cldn7 nor Cldn15 expression was affected by the Cldn2 or Cldn12 siRNAs, indicating the specific knockdown effects of these siRNAs. Interestingly, when the expression Cldn2 or Cldn12 was suppressed, reduction of TER values by 1α,25(OH)2D3 was partially but significantly attenuated (Figure 5C and Supplemental Figure S2A). More importantly, the knockdown of Cldn2 or Cldn12 expression resulted in a significant decrease in 1α,25(OH)2D3-induced 45Ca2+ transport (Figure 5D and Supplemental Figure S2B). Note that suppression of Cldn2 or Cldn12 expression did not alter expression of the transcellular Ca2+ channel protein TRPV6 or distribution of apical markers (ezrin and EBP50) (Supplemental Figure S3, A and C). By contrast, the specific knockdown of Cldn7 expression did not significantly alter the levels of TER or 45Ca2+ permeability in Caco-2 cells grown in the presence and absence of 1α,25(OH)2D3 (Supplemental Figure S4; data not shown). Together, these results indicated that 1α,25(OH)2D3 induced paracellular Ca2+ transport at least in part through the upregulation of both Cldn2 and Cldn12 expression.
Figure 5.
Knockdown of claudins-2 and -12 impairs vitamin D-induced calcium transport across Caco-2 cells. (A) Suppression of claudin-2 (Cldn2) and Cldn12 expression in Caco-2 cells by RNAi. Cells were transfected with negative control siRNA (lanes 1 and 3) or siRNAs against Cldn2 (#1) (lane 2) and Cldn12 (#1) (lane 4), incubated for 12 h after transfection, and then treated for 48 h with 100 nM 1α,25(OH)2D3. Twenty-five micrograms of whole cell extract from the cells was separated by SDS-PAGE and immunoblotted with the corresponding antibodies, followed by chemiluminescence detection. The blots were stripped and immunoprobed with an anti-actin antibody. (B) Disappearance of Cldn2 and Cldn12 along cell borders in Caco-2 cells by RNAi. Cells were transfected and treated as described in A, subjected to immunostaining with the corresponding antibodies, and observed under a laser-scanning confocal microscope. Bars, 20 μm. (C and D) Suppression of Cldn2 and Cldn12 expression in Caco-2 cells prevents 1α,25(OH)2D3-dependent decrease in TER levels and increase in 45Ca2+ transport. Cells were transfected and treated as described in A, subjected to measurement of TER (C) and calcium transport studies (D). The values of TER and 45Ca2+ permeability are expressed relative to the level in cells transfected with negative control siRNA and grown without 1α,25(OH)2D3, which was taken as 1, and they represent the mean ± SD (error bars; n = 4). *p < 0.05 and **p < 0.01 compared with values of cells grown without 1α,25(OH)2D3.
Overexpression of Claudins-2 and -12 Results in Enhancement of Calcium Transport across Caco-2 Cells
The question that arises from the aforementioned results is whether expression of exogenous Cldn2 and/or Cldn12 is enough to promote transepithelial Ca2+ transport in Caco-2 cells. To address this issue, mouse Cldn2, Cldn7, Cldn12, and Cldn15 cDNAs were introduced into Caco-2 cells along with the puromycin-resistance gene. Cells were then screened by Western blot and immunofluorescence analyses, and stable clones were obtained for Caco-2 cell lines expressing Cldn2, Cldn7, Cldn12, and Cldn15 (Caco-2:Cldn2, Caco-2:Cldn7, Caco-2:Cldn12, and Caco-2:Cldn15, respectively) (Figure 6, A and B, and Supplemental Figure S5A). In Caco-2:Cldn2, Caco-2:Cldn7, and Caco-2:Cldn15 cells, the level of endogenous claudins was not altered compared with that in the mock-transfected Caco-2 cells (Figure 6A; data not shown). Unexpectedly, however, expression of endogenous Cldn2, but not Cldn7 or Cldn15, was slightly increased in Caco-2:Cldn12 cells (Figure 6A; data not shown; see also Figure 7, A and B). Immunofluorescence microscopy revealed that in these Caco-2:Cldn cell lines, cell–cell contact sites were rich in overexpressed Cldn2, Cldn7, Cldn12, and Cldn15 (Figure 6B and Supplemental Figure S5A).
We subsequently measured TER and 45Ca2+ permeability in control Caco-2 cells and Caco-2:Cldn transfectants. As shown in Figure 6C, the TER values were obviously lower in Caco-2:Cldn2 (clones 1 and 2) and Caco-2:Cldn12 cells (clones 1 and 2) than in control Caco-2 cells (∼4-fold and 2-fold decreases, respectively). Conversely, the TER levels were marginally altered in two independent clones of Caco-2:Cldn7 cells, and they were slightly elevated in Caco-2:Cldn15 cells (Figure 6D and Supplemental Figure S5B). Interestingly, the values of 45Ca2+ transport were elevated in two different clones of both Caco-2:Cldn2 and Caco-2:Cldn12 cells compared with control cells (an ∼2-fold increase) (Figure 6D), although their levels were lower than those in 1α,25(OH)2D3-treated Caco-2 cells (see Figure 5D and Supplemental Figure S2B). These results indicated that expression Cldn2 and/or Cldn12 at least partially replaced the effects of 1α,25(OH)2D3 on Ca2+ transport. In contrast, the levels of 45Ca2+ permeability in Caco-2:Cldn7 and Caco-2:Cldn15 cells were indistinguishable from those in control cells (Figure 6D and Supplemental Figure S5C). Neither TRPV6 expression nor localization of apical markers (ezrin and EBP50) in Caco-2 cells was affected by overexpression of Cldn2 or Cldn12 (Supplemental Figure S3, B and D), strongly suggesting that these claudins contribute to paracellular Ca2+ transport, but not to transcellular Ca2+ flux.
Slight induction of endogenous Cldn2 expression was observed in two independent Caco-2:Cldn12 clones at both mRNA and protein levels (Figure 7, A and B). Therefore, to verify that weak Cldn2 expression could influence the permeability property in Caco-2:Cldn12 cells, we subsequently suppressed endogenous Cldn2 expression by using RNAi (Figure 7C). As shown in Figure 7, D and E, the values of TER and 45Ca2+ permeability were not largely altered in Caco-2:Cldn12 cells when Cldn2 expression was knocked down.
Overexpressed Claudin-2, but Not Claudin-12, Promotes Na+ Permeability across Caco-2 Cells
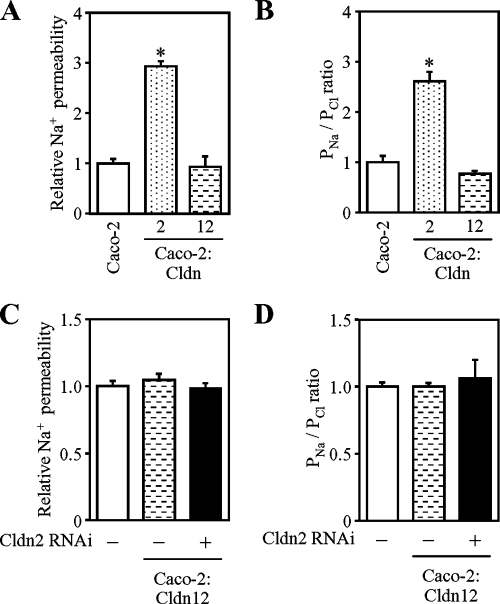
Because Cldn2 and Cldn12 seemed to participate in Ca2+ transport, we next analyzed the permeability of Na+ and Cl− in Caco-2:Cldn2 and Caco-2:Cldn12 cells (Figure 8, A and B). Overexpression of Cldn2 in Caco-2 cells enhanced Na+ permeation (3-fold), but not Cl− permeation, resulting in a threefold increase of PNa/PCl. In contrast, forced expression of Cldn12 did not basically change the permeability of Na+ or Cl− in Caco-2 cells. Thus, differences in Na+ permeability between Caco-2:Cldn2 and Caco-2:Cldn12 cells might contribute to the TER values in these cells (Figure 6C). Nor did suppression of weak induction of endogenous Cldn2 in Caco-2:Cldn12 cells influence Na+ or Cl− conductance (Figure 8, C and D).
Figure 8.
Overexpression of claudin-2, but not claudin-12, increases Na+ permeability in Caco-2. (A and B) Na+ permeability (A) and relative levels of Na+/Cl− permeability (B; PNa/PCl) in Caco-2, Caco-2:Cldn2 (clone #2) and Caco-2:Cldn12 (clone #1). (C and D) Effects of suppressed endogenous Cldn2 on Na+ permeability (C) and Na+/Cl− (D) in Caco-2:Cldn12 cells (clones #1). Cells were transfected with negative control siRNA (left) or siRNA against Cldn2 (#1, right), incubated for 12 h after transfection, and then they were refed and grown for 48 h. They were then subjected to the measurement. The values are expressed relative to the level in Caco-2 cells transfected with negative control siRNA, which was taken as 1, and they represent the mean ± SD (error bars; n = 4). *p < 0.01 compared with values of the mock-transfected cells.
DISCUSSION
Claudins form the backbone of tight junctions, and they contribute to the barrier properties, including the paracellular charge and size selectivity (Tsukita et al., 2001; Van Itallie and Anderson, 2006). In this study, we found that, among members of the claudin family, Cldn2 and Cldn12 were targets for vitamin D signaling. This was obvious because Cldn2 and Cldn12, but not Cldn7 or Cldn15, were down-regulated in the duodenum, jejunum, ileum, and colon of VDRKO mice, which are known to exhibit reduced Ca2+ uptake in the intestine (Van Cromphaut et al., 2001; Song et al., 2002), at both mRNA and protein levels. Our immunofluorescence analysis also showed that signals of these two claudins were decreased or disappeared in intestinal epithelial cells of VDRKO mice. In addition, expression of Cldn2 and Cldn12 was induced by 1α,25(OH)2D3 treatment in intestinal epithelial cell line Caco-2, further supporting our conclusion.
We previously demonstrated that two types of nuclear receptors, retinoid receptors and hepatocyte nuclear receptor 4α (HNF4α), triggered expression of Cldn6 and Cldn7 in embryonal carcinoma cell line F9 (Kubota et al., 2001; Chiba et al., 2003; Satohisa et al., 2005). Subsequently, Battle et al. (2006) showed that Cldn1 was absent in hepatocytes in mice with conditional KO of the HNF4α gene. More recently, Gareus et al. (2007) found that retinoid receptors and the kinase IκB kinase complex 1 cooperatively regulated the expression of retinoid target genes, including Cldn23, in keratinocytes. Furthermore, comparison of gene expression profiling between wild-type and Sertoli cell-specific androgen receptor (AR)-knockout mice revealed that AR mediated activation of the Cldn3 expression in Sertoli cells (Meng et al., 2005). Thus, taken collectively with our present work, expression of different claudin species seems to be up-regulated by distinct members of the nuclear receptor superfamily in a cell-specific manner.
The most important conclusion of the present study is that Cldn2 and Cldn12 facilitate the paracellular conductance and Ca2+ transport in intestinal epithelial cells. This conclusion was drawn from results using RNAi and overexpression strategies in Caco-2 cells, in which endogenous Cldn2 and Cldn12 expression was hardly detected along cell interfaces and was induced upon 1α,25(OH)2D3 treatment. The suppression of either Cldn2 or Cldn12 expression by RNAi in Caco-2 cells hindered 1α,25(OH)2D3-induced passage of electricity and 45Ca2+, indicating that these claudin species were at least in part necessary for vitamin D-triggered calcium absorption. Furthermore, overexpression of Cldn2 and Cldn12, but not Cldn7 or Cldn15, led to significant increases in the electrical conductivity and 45Ca2+ transport across Caco-2 cells. These results highlighted that Cldn2 and Cldn12 were not only required but also sufficient to promote the paracellular conductance and calcium absorption in intestinal epithelial cells. In this sense, it should be noted that, among the different segments of the intestinal tract, Cldn2 and Cldn12 are most abundantly expressed in the ileum (Fujita et al., 2006; Holmes et al., 2006), where the majority (65–88%) of dietary calcium is absorbed (for review, see Wasserman, 2004). It is also noteworthy that, in both mice and humans, Cldn2 has three negatively charged amino acids (positions 53, 65, and 75) within the first extracellular loop (Van Itallie et al., 2003), and that Cldn12 contains four negatively charged residues (positions 62, 66, 71 and 74) in the same domain (Fujita et al., 2006), suggesting that these claudins form paracellular pores for cations. Hence, although a relative contribution of paracellular and transcellular Ca2+ transport has not been established (McCormick, 2002; Bronner et al., 2003; Wasserman, 2004; Hoenderop et al., 2005), we propose that Cldn2- and/or Cldn12-based tight junctions create paracellular Ca2+ channels in enterocytes to maintain calcium homeostasis together with the transcellular pathway.
Several lines of evidence have suggested that Cldn16/paracellin-1 (PCLN-1) plays a key role in the reabsorption of Mg2+ and Ca2+. First, positional cloning has identified that familial hypomagnesemia with hypercalciuria and nephrocalcinosis (FHHNC), an autosomal recessive disease showing severe Mg2+ and Ca2+ wasting, is caused by mutations in the Cldn16/PCLN-1 gene (Simon et al., 1999). It is also known that Cldn16 is highly expressed at tight junctions of epithelial cells in the thick ascending loop of Henle, where the reabsorption of divalent cations occurs (Simon et al., 1999). Second, disease-associated mutations in patients with FHHNC do indeed affect the intracellular traffic and paracellular Mg2+ transport functions of Cldn16 (Kausalya et al., 2006). Third, overexpression of Cldn16 enhances Mg2+ and Ca2+ transport in Madin-Darby canine kidney (MDCK) cells (Ikari et al., 2004, 2006). Fourth, using the pig renal epithelial cell line LLC-PK1 and transgenic mice, Cldn16, which also seems to cause profound effects on permeability of monovalent cations, is shown to drive the reabsorption of Mg2+ and Ca2+ (Hou et al., 2005, 2007). However, Cldn16 seems not to be expressed along the intestinal tract (Fujita et al., 2006; Holmes et al., 2006), suggesting that other claudin species may contribute to Ca2+ absorption in the intestine. These reports, taken together with our present findings, indicate that distinct claudins, Cldn2 and Cldn12 in the intestine and Cldn16 in the kidney, are most likely involved in paracellular Ca2+ transport.
Cldn2 is well known to function as paracellular channel to Na+ in renal epithelial cells without altering Cl− conductance. For example, overexpression of Cldn2 in MDCK strain I cells lacking endogenous Cldn2 increases paracellular Na+ permeability and decreases TER (Furuse et al., 2001; Amasheh et al., 2002). Similar effects are observed when Cldn2 is overexpressed in LLC-PK1 cells expressing little endogenous Cldn2 (Van Itallie et al., 2003). Conversely, knockdown of endogenous Cldn2 expression in MDCK strain II cells reduces paracellular Na+ permeation and elevates TER (Hou et al., 2006). We have revealed that forced expression of Cldn2 in Caco-2 cells results in enhancement of permeability of Na+ and Ca2+, indicating that Cldn2 forms paracellular channel for both cations in intestinal epithelial cells. In contrast, overexpression of Cldn12 promoted conductance of Ca2+, but not of Na+, suggesting that Cldn12, unlike Cldn2 (this study) and Cldn16 (Hou et al., 2005, 2007), may preferentially serve as a paracellular channel to Ca2+ in enterocytes.
Another issue that should be discussed is weak induction of endogenous Cldn2 expression by Cldn12. Overexpression of Cldn2, Cldn7, and Cldn15 in Caco-2 cells did not alter the levels of endogenous claudins, in good agreement with previous results using MDCK and LLC-PK1 cells (Furuse et al., 2001; Van Itallie et al., 2003; Alexandre et al., 2005; Hou et al., 2006). In contrast, increased expression of endogenous Cldn2 was detected in two independent Caco-2:Cldn12 clones, but not in Caco-2:Cldn7 and Caco-2:Cldn15 cells, at both transcription and protein levels. In this regard, it is worth noting that forced expression of Cldn8 in MDCK strain II cells results in down-regulation of endogenous Cldn2 protein (Yu et al., 2003). Although it is unclear by which mechanism Cldn12 activates Cldn2 expression, we excluded, by RNAi experiments, the possibility that slight induction of Cldn2 influenced the permeability of both Ca2+ and Na+ as well as TER in Caco-2:Cldn12 cells.
In conclusion, we have shown that expression of Cldn2 and Cldn12 is up-regulated in intestinal epithelial cells by 1α,25(OH)2D3 via VDR. We also found that these two claudin species acted as paracellular Ca2+ channels in intestinal mucosa. These findings identify essential roles of Cldn2 and Cldn12 in the vitamin D-dependent intestinal Ca2+ absorption, providing new insight into Ca2+ homeostasis. Further studies will be required to determine whether expression of Cldn2 and Cldn12 genes are directly and/or indirectly regulated by VDR. It will also be important to generate and characterize Cldn2- and/or Cldn12-deficient mice, as well as to elucidate possible contributions of these claudins to pathological conditions such as intestinal malabsorption and impaired Ca2+ absorption. Moreover, it will be interesting to investigate whether the luminal Ca2+ concentration in the intestine might affect expression of Cldn2 and Cldn12.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Immuno-Biological Laboratories for cooperation in generation of anti-claudin-7, anti-claudin-12, and anti-claudin-15 antibodies; Dr. T. Onaga for technical advice; N. Sakai, K. Takeda, and M. Nakazawa as well as the staff of the animal facility for technical assistance; and K. Barrymore for help with manuscript.
This work was supported by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan, the Akiyama Foundation, and the Takeda Science Foundation.
Abbreviations used:
- Cldn
claudin
- EBP50
ezrin/radixin/moesin-binding phosphoprotein 50
- FHHNC
familial hypomagnesemia with hypercalciuria and nephrocalcinosis
- KO
knockout
- PCLN-1
paracellin-1
- siRNA
small interfering RNA
- TER
transepithelial electrical resistance.
- VDR
vitamin D receptor
- WT
wild type.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-09-0973) on February 20, 2008.
REFERENCES
- Anderson J. M., Cereijido M. Introduction: evolution of ideas on the tight junction. In: Cereijido M., Anderson J. M., editors. Tight junctions. Boca Raton, FL: CRC Press; 2001. pp. 1–18. [Google Scholar]
- Alexandre M. D., Lu Q., Chen Y. H. Overexpression of claudin-7 decreases the paracellular Cl− conductance and increases the paracellular Na+ conductance in LLC-PK1 cells. J. Cell Sci. 2005;118:2683–2693. doi: 10.1242/jcs.02406. [DOI] [PubMed] [Google Scholar]
- Amasheh S., Meiri N., Gitter A. H., Schöneberg T., Mankertz J., Schulzke J. D., Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J. Cell Sci. 2002;115:4969–4976. doi: 10.1242/jcs.00165. [DOI] [PubMed] [Google Scholar]
- Battle M. A., Konopka G., Parviz F., Gaggl A. L., Yang C., Sladek F. M., Duncan S. A. Hepatocyte nuclear factor 4α orchestrates expression of cell adhesion proteins during the epithelial transformation of the developing liver. Proc. Natl. Acad. Sci. USA. 2006;103:8419–8424. doi: 10.1073/pnas.0600246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner F., Pansu D., Stein W. D. An analysis of intestinal calcium transport across the rat intestine. Am. J. Physiol. Gastrointestinal. Liver Physiol. 1986;250:G561–G569. doi: 10.1152/ajpgi.1986.250.5.G561. [DOI] [PubMed] [Google Scholar]
- Bronner F., Slepchenko B., Wood R. J., Pansu D. The role of passive transport in calcium absorption. J. Nutr. 2003;133:1426. doi: 10.1093/jn/133.5.1426. [DOI] [PubMed] [Google Scholar]
- Chiba H., Clifford J., Metzger D., Chambon P. Specific and redundant functions of retinoid X receptor/retinoic acid receptor heterodimers in differentiation, proliferation, and apoptosis of F9 embryonal carcinoma cells. J. Cell Biol. 1997a;139:735–747. doi: 10.1083/jcb.139.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba H., Clifford J., Metzger D., Chambon P. Distinct retinoid X receptor-retinoic acid receptor heterodimers are differentially involved in the control of expression of retinoid target genes in F9 embryonal carcinoma cells. Mol. Cell. Biol. 1997b;17:3013–3020. doi: 10.1128/mcb.17.6.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba H., Gotoh T., Kojima T., Satohisa S., Kikuchi K., Osanai M., Sawada N. Hepatocyte nuclear factor (HNF)-4α triggers formation of functional tight junctions and establishment of polarized epithelial morphology in F9 embryonal carcinoma cells. Exp. Cell Res. 2003;286:288–297. doi: 10.1016/s0014-4827(03)00116-2. [DOI] [PubMed] [Google Scholar]
- Chiba H., Sakai N., Murata M., Osanai M., Ninomiya T., Kojima T., Sawada N. The nuclear receptor hepatocyte nuclear factor 4α acts as a morphogen to induce the formation of microvilli. J. Cell Biol. 2006;175:971–980. doi: 10.1083/jcb.200608012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirayath M. V., Gajdzik L., Hulla W., Graf J., Cross H. S., Peterlik M. Vitamin D increases tight-junction conductance and paracellular Ca2+ transport in Caco-2 cell cultures. Am. J. Physiol. Gastrointest. Liver Physiol. 1998;274:G389–G396. doi: 10.1152/ajpgi.1998.274.2.G389. [DOI] [PubMed] [Google Scholar]
- Colegio O. R., Van Itallie C. M., McCrea H. J., Rahner C., Anderson J. M. Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am. J. Physiol. Cell Physiol. 2002;283:C142–C147. doi: 10.1152/ajpcell.00038.2002. [DOI] [PubMed] [Google Scholar]
- Fleet J. C., Wood R. J. Specific 1,25(OH)2D3-mediated regulation of transcellular calcium transport in Caco-2 cells. Am. J. Physiol. Gastrointest. Liver Physiol. 1999;276:G958–G964. doi: 10.1152/ajpgi.1999.276.4.G958. [DOI] [PubMed] [Google Scholar]
- Fujita H., Chiba H., Yokozaki H., Sakai N., Sugimoto K., Wada T., Kojima T., Yamashita T., Sawada N. Differential expression and subcellular localization of claudin-7, -8, -12, -13, and -15 along the mouse intestine. J. Histochem. Cytochem. 2006;54:933–944. doi: 10.1369/jhc.6A6944.2006. [DOI] [PubMed] [Google Scholar]
- Furuse M., Fujita K., Hiiragi T., Fujimoto K., Tsukita S. Claudin-1 and -2, novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J. Cell Biol. 1998;141:1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M., Furuse K., Sasaki H., Tsukita S. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J. Cell Biol. 2001;153:263–272. doi: 10.1083/jcb.153.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareus R., Huth M., Breiden B., Nenci A., Rosch N., Haase I., Bloch W., Sandhoff K., Pasparakis M. Normal epidermal differentiation but impaired skin-barrier formation upon keratinocyte-restricted IKK1 ablation. Nat. Cell. Biol. 2007;9:461–469. doi: 10.1038/ncb1560. [DOI] [PubMed] [Google Scholar]
- Giuliano A. R., Wood R. J. Vitamin D-regulated calcium transport in Caco-2 cells: unique in vitro model. Am. J. Physiol. Gastrointest. Liver Physiol. 1991;23:G207–G212. doi: 10.1152/ajpgi.1991.260.2.G207. [DOI] [PubMed] [Google Scholar]
- Hoenderop J. G., Nilius R., Bindels R. J. Calcium absorption across epithelia. Physiol. Rev. 2005;85:373–422. doi: 10.1152/physrev.00003.2004. [DOI] [PubMed] [Google Scholar]
- Holmes J. L., Van Itallie C. M., Rasmussen J. E., Anderson J. M. Claudin profiling in the mouse during postnatal intestinal development and along the gastrointestinal tract reveals complex expression patterns. Gene Expr. Patterns. 2006;6:581–588. doi: 10.1016/j.modgep.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Hou J., Paul D. L., Goodenough D. A. Paracellin-1 and the modulation of ion selectivity of tight junctions. J. Cell Sci. 2005;118:5109–5118. doi: 10.1242/jcs.02631. [DOI] [PubMed] [Google Scholar]
- Hou J., Gomes A. S., Paul D. L., Goodenough D. A. Study of claudin function by RNA interference. J. Biol. Chem. 2006;281:36117–36123. doi: 10.1074/jbc.M608853200. [DOI] [PubMed] [Google Scholar]
- Hou J., Shan Q., Wang T., Gomes A. S., Yan Q., Paul D. L., Goodenough D. A. Transgenic RNAi depletion of claudin-16 and the renal handling of magnesium. J. Biol. Chem. 2007;282:17114–17122. doi: 10.1074/jbc.M700632200. [DOI] [PubMed] [Google Scholar]
- Ikari A., Hirai N., Shiroma M., Harada H., Sakai H., Hayashi H., Suzuki Y., Degawa M., Takagi K. Association of paracellin-1 with ZO-1 augments the reabsorption of divalent cations in renal epithelial cells. J. Biol. Chem. 2004;279:54826–54832. doi: 10.1074/jbc.M406331200. [DOI] [PubMed] [Google Scholar]
- Ikari A., Matsumoto S., Harada H., Takagi K., Hayashi H., Suzuki Y., Degawa M., Miwa M. Phosphorylation of paracellin-1 at Ser217 by protein kinase A is essential for localization in tight junctions. J. Cell Sci. 2006;119:1781–1789. doi: 10.1242/jcs.02901. [DOI] [PubMed] [Google Scholar]
- Ishizaki T., Chiba H., Kojima T., Fujibe M., Soma T., Miyajima H., Nagasawa K., Wada I., Sawada N. Cyclic AMP induces phosphorylation of claudin-5 immunoprecipitates and expression of claudin-5 gene in blood-brain-barrier endothelial cells via protein kinase A-dependent and –independent pathways. Exp. Cell Res. 2003;290:275–288. doi: 10.1016/s0014-4827(03)00354-9. [DOI] [PubMed] [Google Scholar]
- Kausalya P. J., Amasheh S., Günzel D., Wurps H., Müller D., Fromm M., Hunziker W. Disease-associated mutations affect intracellular traffic and paracellular Mg2+ transport function of claudin-16. J. Clin. Invest. 2006;116:878–891. doi: 10.1172/JCI26323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota H., Chiba H., Takakuwa Y., Osanai M., Tobioka H., Kohama G., Mori M., Sawada N. Retinoid X receptor α and retinoic acid receptor γ mediate expression of genes encoding tight junction proteins and barrier function in F9 cells during visceral endodermal differentiation. Exp. Cell Res. 2001;263:163–172. doi: 10.1006/excr.2000.5113. [DOI] [PubMed] [Google Scholar]
- Kutuzova G. D., Deluca H. F. Gene expression profiles in rat intestine identify pathways for 1, 25-dihydroxyvitamin D3 stimulated calcium absorption and clarify its immunomodulatory properties. Arch. Biochem. Biophys. 2004;432:152–166. doi: 10.1016/j.abb.2004.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. C., Bolt M. J., Cao L. P., Sitrin M. D. Effects of vitamin D receptor inactivation on the expression of calbindins and calcium metabolism. Am. J. Physiol. Endocrinol. Metab. 2001;281:E558–E564. doi: 10.1152/ajpendo.2001.281.3.E558. [DOI] [PubMed] [Google Scholar]
- McCormick C. C. Passive diffusion does not play a major role in the absorption of dietary calcium in normal adults. J. Nutr. 2002;132:3428–3430. doi: 10.1093/jn/132.11.3428. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J., Holdcraft R. W., Shima J. E., Griswold M. D., Braun R. E. Androgens regulate the permeability of the blood-testis barrier. Proc. Natl. Acad. Sci. USA. 2005;102:16696–16700. doi: 10.1073/pnas.0506084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman A. W. Intestinal calcium absorption: a vitamin D-hormone mediated adaptive response. Am. J. Clin. Nutr. 1990;51:290–300. doi: 10.1093/ajcn/51.2.290. [DOI] [PubMed] [Google Scholar]
- Rahner C., Mitic L. L., Anderson J. M. Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology. 2001;120:411–422. doi: 10.1053/gast.2001.21736. [DOI] [PubMed] [Google Scholar]
- Sakai N., Chiba H., Fujita H., Akashi Y., Osanai M., Kojima T., Sawada N. Expression patterns of claudin family of tight-junction proteins in the mouse prostate. Histochem. Cell Biol. 2007;127:457–462. doi: 10.1007/s00418-007-0269-7. [DOI] [PubMed] [Google Scholar]
- Satohisa S., Chiba H., Osanai M., Ohno S., Kojima T., Saito T., Sawada N. Behavior of tight-junction, adherens-junction and cell polarity proteins during HNF-4α-induced epithelial polarization. Exp. Cell Res. 2005;310:66–78. doi: 10.1016/j.yexcr.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Simon D. B., et al. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science. 1999;285:103–106. doi: 10.1126/science.285.5424.103. [DOI] [PubMed] [Google Scholar]
- Song Y., Kato S., Fleet J. C. Vitamin D receptor (VDR) knockout mice reveal VDR-independent regulation of intestinal calcium absorption and ECaC2 and calbindin D9k mRNA. J. Nutr. 2002;133:374–380. doi: 10.1093/jn/133.2.374. [DOI] [PubMed] [Google Scholar]
- Tsukita S., Furuse M., Itoh M. Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- Van Cromphaut S. J., et al. Duodenal calcium absorption in vitamin D receptor-knockout mice: functional and molecular aspects. Proc. Natl. Acad. Sci. USA. 2001;98:13324–13329. doi: 10.1073/pnas.231474698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Itallie C. M., Anderson J. M. Claudins and epithelial paracellular transport. Annu. Rev. Physiol. 2006;68:403–429. doi: 10.1146/annurev.physiol.68.040104.131404. [DOI] [PubMed] [Google Scholar]
- Van Itallie C. M., Fanning A. S., Anderson J. M. Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins. Am. J. Physiol. Cell Physiol. 2003;285:F1078–F1084. doi: 10.1152/ajprenal.00116.2003. [DOI] [PubMed] [Google Scholar]
- Wasserman R. H. Vitamin D and the dual processes of intestinal calcium absorption. J. Nutr. 2004;134:3137–3139. doi: 10.1093/jn/134.11.3137. [DOI] [PubMed] [Google Scholar]
- Yoshizawa T., et al. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat. Genet. 1997;16:391–396. doi: 10.1038/ng0897-391. [DOI] [PubMed] [Google Scholar]
- Yu A. S., Enck A. H., Lencer W. I., Schneeberger E. E. Claudin-8 expression in Madin-Darby Canine kidney cells augments the paracellular barrier to cation permeation. J. Biol. Chem. 2003;278:17350–17359. doi: 10.1074/jbc.M213286200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.