Abstract
Certain endoplasmic reticulum (ER)-associated degradation (ERAD) substrates with transmembrane domains are segregated from other ER proteins and sorted into a juxtanuclear subcompartment, known as the ER quality control compartment. Bap31 is an ER protein with three transmembrane domains, and it is assumed to be a cargo receptor for ER export of some transmembrane proteins, especially those prone to ERAD. Here, we show that Bap31 is a component of the ER quality control compartment and that it moves between the peripheral ER and a juxtanuclear ER or ER-related compartment distinct from the conventional ER–Golgi intermediate compartment. The third and second transmembrane domains of Bap31 are principally responsible for the movement to and recycling from the juxtanuclear region, respectively. This cycling was blocked by depolymerization of microtubules and disruption of dynein–dynactin function. Overexpression of Sar1p and Arf1 mutants affected Bap31 cycling, suggesting that this cycling pathway is related to the conventional vesicular transport pathways.
INTRODUCTION
The endoplasmic reticulum (ER) exhibits a reticular tubular network that extends from the nucleus to the cell periphery along microtubule tracks. It performs a variety of functions, including the synthesis, posttranslational modifications, quality control, and export of secretory and membrane proteins; the synthesis of lipids; stress response; Ca2+ storage; and apoptosis. Most, if not all, of these functions are managed by subdomains, such as the rough and smooth ER and transitional ER sites. Each subdomain contains a unique set of proteins that are responsible for its function and organization. ER subdomains are relatively stable, but they can be transformed into alternative structures in response to cellular conditions (for review, see Voeltz et al., 2002; Levine and Rabouille, 2005; Borgese et al., 2006;Vedrenne and Hauri, 2006).
Several studies showed the presence of a specialized ER subdomain that is related to ER-associated degradation (ERAD). ERAD is part of a quality control system that ensures the delivery of only correctly folded or assembled secretory and membrane proteins to their final destinations. Newly synthesized proteins that fail to fold or assemble correctly are retained in the ER, retrotranslocated to the cytosol, and degraded by the ubiquitin–proteasome system (for review, see Ellgaard and Helenius, 2003; Meusser et al., 2005; Römisch, 2005). Studies using mammalian cells demonstrated that transmembrane ERAD substrates are segregated into “ER quality control compartments,” which become discernible at the juxtanuclear region upon inhibition of ERAD by proteasome inhibitors (Kamhi-Nesher et al., 2001; Spiliotis et al., 2002). In Saccharomyces cerevisiae, ectopically expressed cystic fibrosis transmembrane conductance regulator (CFTR) is segregated from other ER proteins and accumulates in ER-associated compartments (Kiser et al., 2001; Zhang et al., 2001; Huyer et al., 2004). Degradation of CFTR in yeast is independent of ER-to-Golgi traffic, but it is dependent on the function of Sar1p/COPII (Fu and Sztul, 2003), which are components of COPII-coated vesicles involved in ER export (Bonifacino and Glick, 2004; Lee et al., 2004).
Bap31 is an integral ER membrane protein with three putative transmembrane domains (TMDs) and a dilysine motif at its C terminus (Kim et al., 1994), the latter of which is known to be able to act as a retrieval signal from post-ER compartments via COPI-coated vesicles (Cosson and Letourneur, 1994). Bap31 and its homologue Bap29 were originally discovered as B cell receptor-associated proteins (Kim et al., 1994). Although solid evidence demonstrated that Bap31 is involved in apoptosis (Breckenridge et al., 2003), accumulating evidence suggest that Bap31 in healthy cells functions as a cargo receptor for ER export of transmembrane proteins, such as cellubrevin, class I major histocompatibility complex (MHC) molecules, CFTR, membrane-bound immunoglobulin (Ig)G, tetraspanins, cytochrome P450 2C2, and the leukocyte integrin CD11b/CD18, some of which are well-known ERAD substrates (Annaert et al., 1997; Spiliotis et al., 2000; Lambert et al., 2001; Schamel et al., 2003; Paquet et al., 2004; Zen et al., 2004; Stojanovic et al., 2005; Ladasky et al., 2006; Szczesna-Skorupa and Kemper, 2006).
In the present study, we show that Bap31 is a component of the ER quality control compartment and that it cycles between the peripheral ER and a juxtanuclear ER or an ER-related compartment along microtubule tracks.
MATERIALS AND METHODS
Antibodies
Monoclonal antibodies against prolyl-4-hydroxylase β-subunit, sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA2), KDEL (BiP), and multiubiquitin were purchased from Daiichi Fine Chemical (Tokyo, Japan), Calbiochem (San Diego, CA), Nventa Biopharmaceuticals (San Diego, CA), and Medical and Biological Laboratories (Nagoya, Aichi, Japan), respectively. Polyclonal antibodies against Sec61β and Derlin-1 were obtained from Upstate Biotechnology (Lake Placid, NY) and Sigma-Aldrich (St. Louis, MO), respectively. To raise a polyclonal antibody against Bap31, a His6-tagged C-terminal region of human Bap31 (amino acids 137-246) was expressed in Escherichia coli cells, purified, and used as an antigen. The rabbit polyclonal antibody against Bap31 was isolated by affinity chromatography on antigen-coupled beads. The sources of other antibodies were described previously (Hirose et al., 2004; Wakana et al., 2005; Arasaki et al., 2006).
Cell Culture
COS-7 and 293T cells were grown in DMEM supplemented with 50 IU/ml penicillin, 50 μg/ml streptomycin, and 10% fetal calf serum. HeLa cells were cultured in Eagle's minimal essential medium supplemented with the same materials. Establishment of stable tetracycline (Tet)-on HeLa cells was performed as described previously (Hirose et al., 2004; Nakajima et al., 2004). To express Bap31-monomeric red fluorescent protein (mRFP), Tet-on HeLa cells were incubated with 1 μg/ml doxycycline for 48 h.
Treatment of Cells with Chemical Reagents
H89 was obtained from D. Western Therapeutics Institute (Nagoya, Aichi, Japan). Brefeldin A (BFA) and nocodazole (Noc) were purchased from Sigma-Aldrich. For H89 experiments, cells were treated with 50 μM H89 for 15 min unless otherwise stated. To test the reversibility of the H89 effect, H89 was washed out, and the cells were incubated with medium containing 20 μg/ml cycloheximide for 3 h. In the BFA/washout experiments, cells were first incubated with 10 μM BFA for 30 min, washed to remove BFA, and then incubated with medium containing 20 μg/ml cycloheximide. Fetal calf serum was omitted during incubation of cells with chemical reagents including lactacystin. To depolymerize microtubules, Noc was used at a concentration of 5 μg/ml.
Immunoprecipitation
293T cells coexpressing FLAG-tagged valosin-containing protein (VCP) and hemagglutinin (HA)-tagged Bap31 or reticulon 3 were lysed in buffer consisting of 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA, 1 mM dithiothreitol, 1% Nonidet P-40, 0.1% sodium deoxycholate, 1 μg/ml leupeptin, 2 μM pepstatin A, 2 μg/ml aprotinin, and 1 mM phenylmethylsulfonyl fluoride, and then they were centrifuged at 17,000 × g for 10 min. The supernatants were immunoprecipitated with a monoclonal antibody (mAb) against FLAG, and the precipitated proteins were analyzed by immunoblotting with a mAb against FLAG and a polyclonal antibody against HA or Bap31.
Immunofluorescence Microscopy
For immunofluorescence microscopy, cells were fixed with 4% paraformaldehyde for 20 min at room temperature and observed with a Fluoview 300 laser scanning microscope (Olympus, Tokyo, Japan), as described previously (Tagaya et al., 1996).
Live Cell Imaging
To transiently express N-acetylglucosaminyl transferase I-green fluorescent protein (NAGFP) and Bap31-mRFP, Tet-on HeLa cells were transfected with the corresponding plasmids and incubated with 1 μg/ml doxycycline for 24 h. Live cell images were acquired continuously with time intervals between frames of 10 s by using a fluorescence imaging system (Till Photonics, Gräfelfing, Germany).
Immunoelectron Microscopic Analysis
Localization of Bap31 was analyzed by immunoelectron microscopy by using a pre-embedding gold enhancement method. H89-treated HeLa cells were fixed in 4% paraformaldehyde in 0.1 M sodium phosphate buffer, pH 7.4, for 2 h, incubated in 0.1 M sodium phosphate buffer containing 0.25% saponin for 30 min, and then in 0.1 M sodium phosphate buffer containing 0.005% saponin, 10% bovine serum albumin, 10% normal goat serum, and 0.1% cold water fish skin gelatin (blocking solution) for 30 min. The cells were exposed to an anti-Bap31 in blocking solution overnight, and then they were incubated with the Fab' fragment of a goat anti-mouse IgG conjugated to colloidal gold (1.4 nm in diameter) in blocking solution for 2 h. The cells were fixed with 1% glutaraldehyde in 0.1 M sodium phosphate buffer for 10 min. The gold labeling was intensified with a gold enhancement kit (GoldEnhance-EM; Nanoprobes, Stony Brook, NY) for 3 min according to the manufacturer's protocol. After washing, the cells were postfixed in 0.1 M sodium phosphate buffer containing 1% OsO4 and 1.5% potassium ferrocyanide for 60 min, dehydrated in a series of graded ethanol solution, and embedded in epoxy resin. Ultrathin sections were cut horizontally to the cell layer and observed under a Hitachi H7600 electron microscope.
Fluorescence Loss in Photobleaching
The stable cell lines expressing green fluorescent protein (GFP)-fused proteins were subjected to two bleach cycles. Each cycle consisted of 64 bleaching scans at high zoom with high laser intensity (488-nm laser) followed by one imaging scan at low zoom with low laser intensity. The duration of each cycle was 1 min 50 s. Last imaging scan was performed at 1 min 10 s after the second bleach cycle. The total elapsed time was 4 min 50 s. Photobleaching experiments were conducted with a TCS SP2 AOBS laser scanning microscope (Leica Microsystems, Wetzlar, Germany).
Plasmid Construction and Transfection
The cDNAs encoding full-length Bap31 and full-length Bap29 were inserted into pTRE (Clontech, Mountain View, CA) together with the cDNA of mRFP (a gift from Dr. R. Y. Tsien) (Campbell et al., 2002) so as to express proteins with the C-terminal mRFP in Tet-on HeLa cells in the presence of doxycycline. The cDNAs encoding chimeras between Bap31 and Bap29 were generated by overlap extension polymerase chain reaction (PCR) and inserted into pTRE together with the cDNA of mRFP. The plasmids for FLAG-dynamitin, FLAG-Sar1p mutants, and HA-reticulon 3 were constructed as described previously (Hirose et al., 2004; Shimoi et al., 2005; Wakana et al., 2005). The cDNA encoding Arf1[Q71L] was inserted into pEGFP-N1 (Clontech) and pcDNA3-based plasmid encoding mRFP to express proteins with the C-terminal GFP and mRFP, respectively. The plasmids for NAGFP, GFP-CFTRΔF508, and GFP-galactosyltransferase were kind gifts from Drs. R. Pepperkok, R. R. Kopito, and J. Lippincott-Schwartz, respectively. The plasmids for FLAG-VCP wild-type (WT) and FLAG-VCP K524M were prepared in this laboratory (Nagahama et al., 2003). Transfection was carried out using Lipofectamine Plus (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. Unless otherwise stated, transfected cells were incubated for 18 h and used for analysis.
RESULTS
Bap31 Is a Component of the ER Quality Control Compartment
Although Bap31 exhibited a typical reticular ER pattern in most COS-7 cells (Figure 1A, left), it was found to accumulate at the juxtanuclear region in a few percent of cells (Figure 1A, right, arrows). This juxtanuclear Bap31 localization was reminiscent of the ER quality control compartment, which becomes discernible at the juxtanuclear region upon inhibition of ERAD by proteasome inhibitors (Kamhi-Nesher et al., 2001; Spiliotis et al., 2002). To examine whether Bap31 is present in the ER quality control compartment, the distribution of Bap31 was determined after incubation of cells with a proteasome inhibitor, lactacystin, for 5 h. As shown in Figure 1B, Bap31 (top right), but not other ER proteins (BiP and calnexin, middle row), accumulated at the juxtanuclear region in 60–70% of lactacystin-treated cells. A similar result was obtained when another proteasome inhibitor, MG132, was used (bottom row). To demonstrate that Bap31 indeed accumulates in the quality control compartment, cells expressing an ERAD substrate, GFP-CFTRΔF508, were treated with MG132, and the distribution of Bap31 was compared with that of the accumulated ERAD substrate. As shown in Figure 1C, Bap31 was colocalized with GFP-CFTRΔF508 at the juxtanuclear region in MG132-treated cells. These results suggest that Bap31 is a component of the ER quality control compartment. To verify that the region where Bap31 accumulated does not correspond to the Golgi apparatus or the ER–Golgi intermediate compartment (ERGIC), we examined the effects of Golgi-disrupting reagents BFA and Noc on Bap31 localization in lactacystin-treated cells. Previous studies showed that the quality control compartment is insensitive to BFA, but sensitive to Noc (Kamhi-Nesher et al., 2001; Spiliotis et al., 2002). As shown in Figure 1D, the accumulated Bap31 in lactacystin-treated cells was redistributed by Noc, but not by BFA. To compare the distribution of Bap31 with that of an ERGIC marker, ERGIC-53, we used HeLa cells because our anti-ERGIC-53 antibody does not cross-react with simian ERGIC-53. As shown in Figure 1E, juxtanuclear ERGIC-53–positive structures were substantially disrupted by BFA treatment, whereas Bap31 accumulation was not. These results suggest that the area where Bap31 accumulated upon proteasome inhibitor treatment is different from the Golgi or an intermediate compartment containing ERGIC-53.
Figure 1.
Bap31 is a component of ER quality control compartments. (A) Localization of Bap31 in intact COS-7 cells. Arrows indicate the prominent juxtanuclear accumulation of Bap31. (B) COS-7 cells were treated with dimethyl sulfoxide or 25 μM lactacystin for 5 h (top and second rows) or with 5 μM MG132 for 16 h (bottom row), and stained for Bap31, BiP, or calnexin. (C) COS-7 cells expressing GFP-CFTRΔF508 were incubated with 5 μM MG132 for 8 h, and then they were stained for Bap31. (D and E) COS-7 cells (D) or HeLa cells (E) were incubated with 10 μM lactacystin for 6 h (Lactacystin), for 5.5 h and then plus 10 μM BFA for 0.5 h (Lactacystin→BFA), or for 3 h and then plus 5 μg/ml Noc for 3 h (Lactacystin→Noc), and stained for Bap31 (D) or double stained for Bap31 and ERGIC-53 (E). (F) Control COS-7 cells (left) or cells expressing FLAG-dynamitin (middle and right) were incubated with lactacystin for 6 h, and stained for Bap31 (left) or double stained for Bap31 (middle) and FLAG (right). Bars, 10 μm.
As intact microtubules are required for maintaining juxtanuclear accumulation of Bap31 in lactacystin-treated cells (Figure 1D, right), we asked whether the microtubule minus-end–directed motor dynein-dynactin is involved in Bap31 redistribution. We overexpressed dynamitin, which is known to disrupt the function of the minus-end–directed motor dynein-dynactin (Burkhardt et al., 1997), in HeLa cells, and then the cells were incubated with lactacystin for 6 h. When dynamitin was overexpressed, the number of cells exhibiting the juxtanuclear accumulation of Bap31 was decreased by approximately threefold. Figure 1F shows typical distribution patterns of Bap31 in lactacystin-treated cells without (left) and with dynamitin overexpression (middle).
Quality Control Compartments Contain Components of the Retrotranslocation Machinery
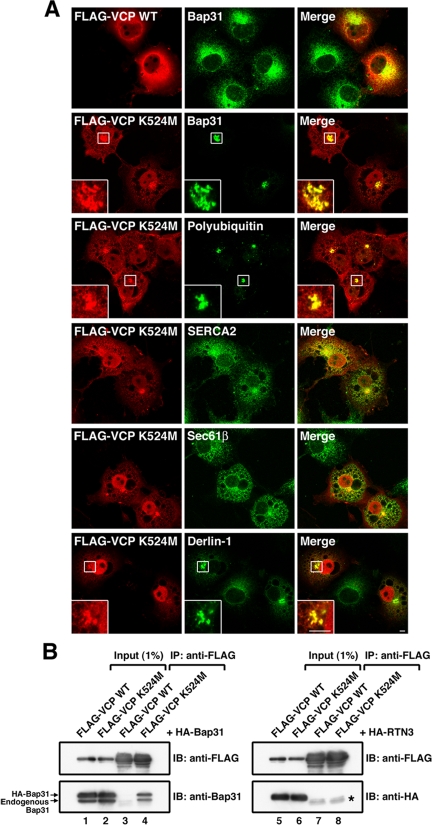
ERAD substrates are retrotranslocated from the ER through a retrotranslocation channel, ubiquitinated, and degraded by the proteasome system (for review, see Ellgaard and Helenius, 2003; Meusser et al., 2005; Römisch, 2005). If juxtanuclear accumulation of Bap31 indeed occurs as a consequence of inhibition of ERAD, blocking of retrotranslocation, as well as inhibition of proteasomal activity by chemicals, must induce juxtanuclear accumulation of Bap31. To test this idea, we overexpressed a VCP mutant in which Lys-524 in D2 domain was replaced by Met. VCP is an ATPase that mediates retrotranslocation of ERAD substrates (Ye et al., 2001), and this type of mutants can exert a dominant-negative effect on retrotranslocation (Ye et al., 2003). When FLAG-VCP K524M was expressed in COS-7 cells, Bap31 as well as the expressed VCP mutant accumulated at the juxtanuclear region (Figure 2A, second row). Polyubiquitin also accumulated at the same area (third row). In contrast, no prominent accumulation of Bap31 was observed when FLAG-VCP WT was expressed (top row). Although an ER marker, SERCA2, also exhibited some concentration in the vicinity of the nucleus (fourth row), perhaps due to a consequence of the formation of large vacuoles in cells (Hirabayashi et al., 2001), the accumulation of Bap31 at the juxtanuclear region was much more conspicuous compared with SERCA2, and very faint reticular staining was observed for Bap31 (second row). These results confirm that redistribution of Bap31 to the juxtanuclear region is a consequence of inhibition of ERAD and that Bap31 is a component of the ER quality control compartment. Notably, Derlin-1 accumulated at the juxtanuclear region upon expression of the VCP mutant (bottom row). Derlin-1 seems to be part of a retrotranslocation channel that is associated with both the polyubiquitination machinery and the VCP complex containing VCP-interacting membrane protein (Ye et al., 2003, 2004, 2005; Lilley and Ploegh, 2004). The presence of Derlin-1 in the quality control compartment suggests that this compartment represents the site for retrotranslocation of ERAD substrates from the ER. In contrast to Derlin-1, Sec61β, a subunit of the Sec61 translocon that was originally assumed to be a channel for ERAD substrates (Wiertz et al., 1996), did not markedly accumulate at the juxtanuclear region (Figure 2, fifth row).
Figure 2.
Expression of a dominant-negative VCP mutant induces juxtanuclear accumulation of Bap31 and the retrotranslocation machinery. (A) A dominant-negative FLAG-VCP mutant (K524M) or FLAG-VCP WT was expressed in COS-7 cells, and the cells were stained for the indicated proteins. The boxed areas are enlarged in the insets. Bars, 5 μm. (B) Lysates of 293T cells expressing FLAG-VCP K524M or FLAG-VCP WT with HA-Bap31 (left) or HA-reticulon 3 (RTN3) (right) were subjected to immunoprecipitation with anti-FLAG, and then they were immunoblotted with the indicated antibodies. The star denotes immunoglobulin light chain.
To explore the possible link between Bap31 and VCP in quality control compartments, FLAG-VCP WT or FLAG-VCP K524M was coexpressed with HA-tagged Bap31, and then it was immunoprecipitated using anti-FLAG. As shown in Figure 2B, Bap31 was coprecipitated with the VCP mutant (lane 4), but not with the wild-type protein (lane 3). A control ER protein, reticulon 3 was not coprecipitated with VCP WT (lane 7) or the mutant (lane 8). These results may suggest that Bap31 interacts with VCP in quality control compartments.
Bap31 Accumulates at the Juxtanuclear Region by H89 Treatment
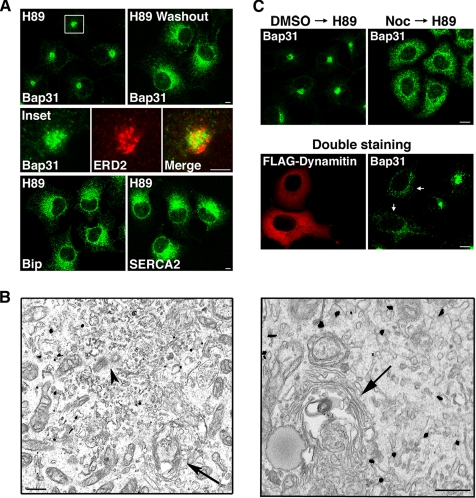
Which feature of Bap31 allows it to accumulate at the juxtanuclear region of cells when ERAD is inhibited? Given that the formation of the ER quality control compartment containing Bap31 at the juxtanuclear region is dependent on microtubules (Figure 1D), one possibility is that Bap31 in normal cells may move within the ER or between the peripheral ER and a juxtanuclear ER-related compartment in a microtubule-dependent manner. To explore this possibility, we investigated whether chemicals that perturb microtubule-dependent membrane transport from the ER affect Bap31 localization. H89 blocks protein export out of the ER (Jamora et al., 1999) perhaps by mildly inhibiting the binding of COPII proteins to ER exit sites (Aridor and Balch, 2000; Lee and Linstedt, 2000), where COPII-coated vesicles involved in ER-to-Golgi transport are formed (Gurkan et al., 2006). It also blocks Golgi disassembly occurring during mitosis, likely by keeping Arf1, a small GTPase that regulates the recruitment of COPI to membranes (Lippincott-Schwartz and Liu, 2006), in an active state (Altan-Bonnet et al., 2003). We found that H89 induces a rapid (within 15 min) and reversible accumulation of Bap31 at the juxtanuclear region (Figure 3A, top row). Although the region where Bap31 accumulated was very close to the Golgi apparatus, represented by the KDEL receptor ERD2, at the level of light microscopy (Figure 3A, middle row), immunoelectron microscopic analysis showed that accumulated Bap31 is not localized at the Golgi apparatus, but in an area of the ER or ER-related compartment close to the centrosome (Figure 3B). The effect of H89 on Bap31 is specific because H89 had no effect on the localization of BiP and SERCA2 (Figure 3A, bottom row) and other ER proteins (data not shown). The H89-induced accumulation of Bap31 at the juxtanuclear region was blocked by Noc (Figure 3C, top row) or overexpression of dynamitin (bottom row), indicating the involvement of microtubules in this transport process.
Figure 3.
Bap31 moves to the juxtanuclear region along the microtubule track by H89 treatment. (A) COS-7 cells were incubated with 50 μM H89 for 15 min, and then they were stained for Bap31 (top left), BiP, or SERCA2 (bottom row). The middle row shows enlarged images of the boxed area double stained for Bap31 and ERD2. To demonstrate the reversibility of the effect of H89, H89-treated cells were incubated for 3 h without H89 and stained for Bap31 (top right). Bars, 5 μm. (B) Electron microscopic analysis of H89-treated COS-7 cells. Gold-enhanced immunogold-labeled Bap31 is located close to the centrosome (arrowhead), but not in the Golgi apparatus (arrows). Bar, 500 nm. (C) Preincubation of COS-7 cells with 5 μg/ml Noc for 3 h (top row) or overexpression of FLAG-dynamitin (bottom row) blocks H89-induced juxtanuclear accumulation of Bap31. Arrows indicate cells expressing FLAG-dynamitin. Bars, 10 μm.
To investigate the correlation between H89 activity on Bap31 redistribution and COPII localization, we performed a titration experiment. As shown in Supplemental Figure S1, H89, at concentrations up to 75 μM, did not induce marked change in the distribution of Sec31A, a COPII subunit, or β-COP, a COPI subunit (Bonifacino and Glick, 2004; Lee et al., 2004), whereas Bap31 redistribution occurred in many cells upon incubation with H89 at a concentration as low as 50 μM. At 100 μM H89, some background staining was seen for Sec31A, which may reflect dissociation of COPII from membranes. We could not test H89 at concentrations higher than 100 μM because COS-7 cells were detached from coverslips, even those coated with poly-l-lysine. It seemed that the effect of H89 on COPII localization is weaker in COS-7 cells than HeLa cells (Lee and Linstedt, 2000; Puri and Linstedt, 2003; Jiang et al., 2006).
Next, we examined whether Bap31, upon H89 treatment, moves to the juxtanuclear region through ER exit sites, as in the case of secretory cargos. To this end, cells were incubated with Noc to depolymerize microtubules. In Noc-treated cells, cargo proteins that are transported through the conventional pathway can exit the peripheral ER and accumulate at ER exit sites. Simultaneously, proteins in the ERGIC and the Golgi apparatus are redistributed to ER exit sites (Cole et al., 1996; Storrie et al., 1998; Hammond and Glick, 2000). As shown in Supplemental Figure S2, in Noc-treated cells, Bap31 did not accumulate at ER exit sites defined by Sec31A (third row), but remained within the ER where SERCA2 was present (second row) upon H89 treatment. H89 treatment did not affect the exit site localization of β-COP (fourth row) or a Golgi marker, GM130 (Nakamura et al., 1995) (bottom row), in Noc-treated cells. These results support the idea that the transport of Bap31 occurs not through the conventional transport pathway.
Bap31 and Derlin-1 Accumulate at the Juxtanuclear Region in Active Arf1-expressing Cells
To further characterize H89-induced Bap31 movement to the juxtanuclear region, we overexpressed the constitutively active and inactive forms of Sar1p and Arf1, which are small guanosine triphosphatases (GTPases) responsible for the formation of COPII- and COPI-coated vesicles, respectively (Bonifacino and Glick, 2004; Lee et al., 2004). Expression of these mutants in cells block ER–Golgi transport at specific stages in conventional transport processes (Ward et al., 2001; Altan-Bonnet et al., 2003).
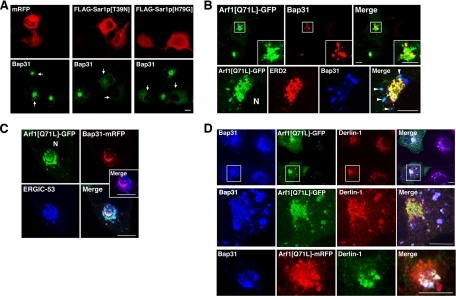
When inactive Sar1p (Sar1p[T39N]) or the active form (Sar1p[H79G]) was expressed in COS-7 cells, H89-induced juxtanuclear accumulation of Bap31 was blocked (Figure 4A). In contrast, when the active form of Arf1 (Arf1[Q71L]-GFP) was expressed, Bap31 accumulated at the juxtanuclear region without H89 treatment, and it was substantially colocalized with Arf1[Q71L]-GFP (Figure 4B, top row). Triple staining revealed that some Arf1[Q71L]-GFP-, Bap31-positive structures are negative for the Golgi marker ERD2 (Figure 4B, bottom row, arrowheads). Similarly, some Arf1[Q71L]-GFP-, Bap31-mRFP-positive structures were negative for the ERGIC marker ERGIC-53 (Figure 4C). These results may imply that Arf1 binds not only to ERGIC and Golgi membranes but also to ER membranes to some extent.
Figure 4.
Effects of Sar1p and Arf1 mutants on the localization of Bap31. (A) COS-7 cells expressing mRFP, as a control, or the indicated FLAG-Sar1p constructs were subjected to H89 treatment, and then they were double stained for FLAG and Bap31. Arrows indicate cells expressing mRFP or the FLAG-Sar1p constructs. (B) Arf1[Q71L]-GFP–expressing COS-7 cells were stained for Bap31 alone (top row) or for both Bap31 and ERD2 (bottom row). The boxed areas are enlarged in the insets. Arrowheads indicate the structures where Bap31 is colocalized with Arf1[Q71L]-GFP, but not with ERD2. N denotes the nucleus. (C) Tet-on HeLa cells expressing both Bap31-mRFP (top right) and Arf1[Q71L]-GFP (top left) were stained for ERGIC-53 (bottom left). N, nucleus. (D) COS-7 cells expressing Arf1[Q71L]-GFP (top two rows) and Arf1[Q71L]-mRFP (bottom row) were double stained for Bap31 and Derlin-1. The middle row shows enlarged images of the boxed areas in the top row. Bars, 10 μm.
To assess whether active-Arf1-induced juxtanuclear Bap31 accumulation is related to the quality control compartment, we compared the localization of Bap31 with that of the putative retrotranslocation channel Derlin-1. As shown in Figure 4D, Derlin-1 and Bap31 accumulated at the juxtanuclear region and substantially colocalized with expressed Arf1[Q71L]-GFP (top two rows) and Arf1[Q71L]-mRFP (bottom row). Obviously, the tags did not significantly affect redistribution of Bap31 and Derlin-1 induced by Arf1[Q71L].
Bap31 Movement and Golgi Reassembly Occur Independently during Recovery from BFA Treatment
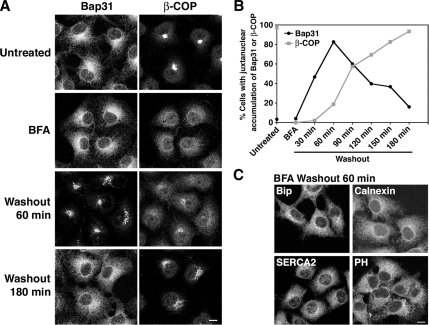
We next tested the effect of BFA on the localization of Bap31. Contrary to H89 (Altan-Bonnet et al., 2003), BFA prevents the activation of Arf1, which leads to Golgi disassembly and redistribution of Golgi components to the ER (Lippincott-Schwartz and Liu, 2006). BFA treatment had no apparent effect on Bap31 localization, but during recovery from BFA treatment, Bap31 was found to move to the juxtanuclear region and then back to the peripheral region (Figure 5A, left column). No such marked redistribution took place for other ER proteins (Figure 5C). Because Golgi reassembly occurs after BFA washout, we followed the time courses of Bap31 movement and reassembly of the Golgi apparatus. As a Golgi marker, we first used β-COP because it exhibited an entirely diffuse pattern upon BFA treatment and accumulated at a juxtanuclear region much earlier than Golgi enzymes, such as mannosidase II, after BFA washout (data not shown). As shown in Figure 5, A and B, juxtanuclear accumulation of Bap31 preceded β-COP reassembly. Next, we compared the distribution of GM130 with that of Bap31 during recovery from BFA treatment. GM130 is one of the fast-moving proteins during BFA recovery (Jiang et al., 2006). As reported previously (Nakamura et al., 1995; Seemann et al., 2000; Ward et al., 2001), GM130 was distributed in punctate structures in BFA-treated cells (Supplemental Figure S3, second row). Although previous studies showed that combined treatment of normal rat kidney or HeLa cells with BFA followed by BFA plus H89 causes ER localization of GM130 (Puri and Linstedt, 2003; Jiang et al., 2006), such redistribution was not observed in COS-7 cells when 50 μM H89 in addition to 10 μM BFA was used (data not shown). This is perhaps due to a relatively weak action of H89 on COS-7 cells, as described above. At 60 min after BFA washout, the time when Bap31 accumulated at the juxtanuclear region in most cells, GM130 accumulated and clustered at the juxtanuclear region, but it did not exhibit a compact, ribbon-like pattern (Supplemental Figure S3, fourth row). A compact Golgi pattern was observed in many cells at 120 min or later after BFA washout. At these time points, Bap31 exhibited a diffuse pattern in many cells due to recycling to the peripheral ER. The time course of GM130 reassembly seemed similar to that observed for β-COP (Figure 5, A and B). These results suggest that Bap31 movement and Golgi reassembly occur independently.
Figure 5.
Bap31 cycles between the cell periphery and the juxtanuclear region during recovery from BFA treatment. (A) COS-7 cells were incubated with 10 μM BFA for 30 min, and then they were washed to remove BFA. Change in the localization of Bap31 (left column) and β-COP (right column). (B) Quantitation of cells with the juxtanuclear accumulation of Bap31 and β-COP. (C) Distributions of BiP, calnexin, SERCA2, and prolyl-4-hydroxylase β-subunit (PH) at 60 min after BFA washout. Bars, 10 μm.
To analyze the movement of Bap31 during recovery from BFA treatment in living cells, we constructed a plasmid encoding Bap31-mRFP. Bap31-mRFP and the Golgi marker NAGFP were transiently expressed in Tet-on HeLa cells, and their behavior was compared during BFA recovery using live cell imaging. The data show that the two proteins independently move to the juxtanuclear region (Supplemental Figure S4 and Supplemental Movie S1).
Comparison of the Behaviors of Bap31 and ERGIC-53 during Recovery from BFA Treatment
ERGIC-53 cycles principally between the ERGIC and the ER (Appenzeller-Herzog and Hauri, 2006). Because the behavior of Bap31 during recovery from BFA treatment seemed similar to that of ERGIC-53, we compared the behaviors of the two proteins in detail. As described above, because our anti-ERGIC-53 antibody does not recognize simian ERGIC-53, we used HeLa cells. In HeLa cells, Bap31 cycled between the juxtanuclear and peripheral regions more slowly compared with COS-7 cells; the ratio of HeLa cells with juxtanuclear Bap31 accumulation to total cells was maximum at 120 min after BFA washout (Supplemental Figure S5). As shown previously (Nakamura et al., 1995; Ward et al., 2001) and in this study (Figure 1E), BFA treatment caused some dispersion of ERGIC-53–positive clusters at the juxtanuclear region without changing punctate ERGIC-53 staining at the peripheral region. When BFA was washed out, ERGIC-53 seemed to become concentrated at the juxtanuclear region (Supplemental Figure S6). At 120–180 min after BFA washout, extensive tubular structures were emitted from centrally concentrated ERGIC-53–positive clusters. Perhaps, these tubules represent ERGIC-53 in the process of recycling back to the ER, as seen in the process of recovery from low temperature treatment (Klumperman et al., 1998). Importantly, Bap31 was not included in ERGIC-53–positive tubular structures.
Expression of Bap31 Induces Comigration of ER Proteins
The time course of the redistribution of transiently expressed Bap31-mRFP after BFA washout seemed to be considerably faster than that of endogenous Bap31. To minimize the effect of overexpression, Tet-on HeLa cells stably expressing Bap31-mRFP were established. As shown in Supplemental Figures S5, stably expressed Bap31-mRFP behaved quite similarly to the endogenous protein during recovery from BFA treatment.
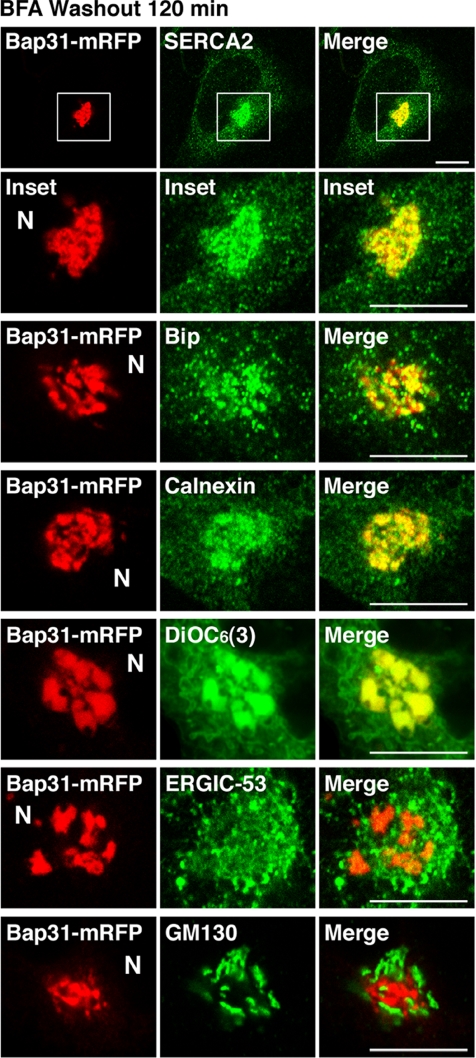
Interestingly, some ER proteins, such as SERCA2 (Figure 6, top and second rows), BiP (third row), and calnexin (fourth row), comigrated with Bap31-mRFP to the juxtanuclear region during recovery from BFA treatment. This is likely not due to the nonspecific aggregation of proteins because stably expressed Bap31-mRFP exhibited a reticular ER pattern and behaved similarly to endogenous Bap31. Similar comigration was observed for FLAG-tagged Bap31 (data not shown), ruling out the trivial possibility that the ER proteins comigrate with Bap31-mRFP by binding to the mRFP moiety. Given that Bap31 interacts with transmembrane proteins through mutual TMDs (Adachi et al., 1996; Annaert et al., 1997; Spiliotis et al., 2000; Ladasky et al., 2006; Szczesna-Skorupa and Kemper, 2006), it is tempting to speculate that expression of Bap31 may shift the Bap31/interacting proteins equilibrium toward complex formation, leading to comigration of Bap31 and its interacting proteins. It should be noted that juxtanuclear Bap31-mRFP–positive structures could be stained with an ER/mitochondria-staining probe, 3,3′-dihexyloxacarbocyanine iodide [DiOC6(3)] (Figure 6, fifth row), confirming that Bap31-mRFP at the juxtanuclear region is associated with ER or ER-related membranes. Colocalization of Bap31-mRFP with an ERGIC marker, ERGIC-53 (sixth row), or a Golgi marker, GM130 (bottom row), was not conspicuous compared with ER proteins.
Figure 6.
Expression of Bap31-mRFP induces comigration of ER proteins to the juxtanuclear region during recovery from BFA treatment. At 120 min after BFA washout, Tet-on HeLa cells stably expressing Bap31-mRFP were fixed and stained for SERCA2, BiP, calnexin, ERGIC-53, or GM130, or labeled with DiOC6(3). The second row shows enlarged images of the boxed areas in the top row. N, nucleus. Bars, 10 μm.
TMD2 Is Responsible for Bap31 Recycling from the Juxtanuclear Region
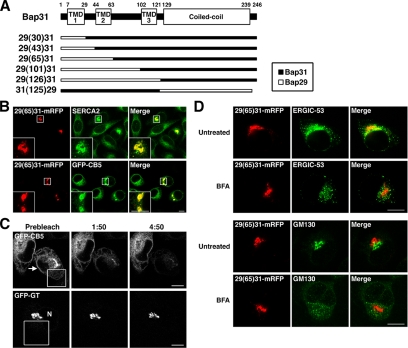
To gain insight into the mechanism underlying Bap31 cycling, we determined the regions of Bap31 responsible for cycling. Bap31 possesses three putative TMDs (TMD1-3) and short N-terminal and long C-terminal cytoplasmic tails with a C-terminal dilysine motif (Figure 7A). The Bap31 homologue Bap29 shares 47% amino acid identity with Bap31 and possesses essentially the same hydropathy profile. Nevertheless, Bap29-mRFP was found not to efficiently cycle between the peripheral and juxtanuclear regions (Table 1A). We therefore constructed chimeras in which the N-terminal or C-terminal region of Bap31 was replaced with the corresponding region of Bap29 to determine the domains required for cycling (Figure 7A). We reasoned if domains responsible for recycling from the juxtanuclear region are replaced, the resultant mutants must show juxtanuclear localization without BFA/washout treatment. In contrast, if domains that participate in the movement to the juxtanuclear region are replaced, the resultant mutants would not change their localization during recovery from BFA treatment. The results of chimera analysis are shown in Supplemental Figure S7 and Table 1B. When regions comprising TMD2 were replaced by the corresponding Bap29 regions, the resultant chimeras [29(65)31-mRFP and 29(101)31-mRFP] showed a juxtanuclear distribution without BFA/washout treatment (Supplemental Figure S7, third and fourth rows and Table 1B, untreated), although some dot-like structures were occasionally observed around the juxtanuclear region. These chimeras accumulated at the juxtanuclear region were dispersed by Noc or overexpression of dynamitin (data not shown), suggesting that their distribution is dependent on active transport along microtubules. In contrast, 29(43)31-mRFP, which possesses a Bap29 region encompassing from the N-terminal tail to the cytoplasmic loop between TMD1 and TMD2, displayed a reticular distribution. These results suggest that TMD2 is required for recycling from the juxtanuclear region to the cell periphery.
Figure 7.
Juxtanuclear structures of a Bap31 mutant are associated with the ER. (A) Schematic representation of Bap31 WT and chimeras in which the N- or C-terminal region of Bap31 is replaced with corresponding region of Bap29. (B) At 3 h after transfection of the plasmid for 29(65)31-mRFP, parental Tet-on HeLa cells (top row) or those stably expressing GFP-CB5 (bottom row) were incubated with 1 μg/ml doxycycline for 40 h. The cells were stained for SERCA2 (top row). The boxed areas are enlarged in the insets. (C) Fluorescence loss in photobleaching. The boxed areas were bleached as described in Materials and Methods. Fluorescence of GFP-CB5 cells (top row) and GFP-galactosyltransferase (GFP-GT) (bottom row). Arrow indicates a cell expressing 29(65)31-mRFP. N denotes the nucleus. Time is indicated in minutes:seconds. (D) Tet-on HeLa cells expressing 29(65)31-mRFP were incubated in the absence (top and third rows) or presence (second and bottom rows) of 10 μM BFA for 30 min. Bars, 10 μm.
Table 1.
Quantitation of cells with juxtanuclear accumulation of Bap31, Bap29, and chimeras
| Construct | Untreated | BFA washout 120 min | BFA washout 360 min |
|---|---|---|---|
| A | |||
| Bap31-mRFP | 2% | 72% | 10% |
| Bap29-mRFP | 3% | 16% | 4 |
| B | |||
| 29(30)31-mRFP | 0% | 71.3 ± 7.6% | 2.7 ± 3.1% |
| 29(43)31-mRFP | 0.7 ± 1.2 | 33.3 ± 3.1 | 0.7 ± 1.2 |
| 29(65)31-mRFP | 89.3 ± 5.0 | 98.7 ± 2.3 | 94.7 ± 1.2 |
| 29(101)31-mRFP | 91.3 ± 3.1 | 96.0 ± 3.5 | 92.7 ± 1.2 |
| 29(126)31-mRFP | 1.3 ± 2.3 | 19.3 ± 4.2 | 8.7 ± 2.3 |
| 31(125)29-mRFP | 0.7 ± 1.2 | 54.0 ± 8.7 | 3.3 ± 3.1 |
In each experiment, 50 cells expressing mRFP-fused chimeric proteins were analyzed. The average values of the three independent experiments are shown. In A, ∼200 cells expressing mRFP-fused proteins were analyzed.
To verify that juxtanuclear 29(65)31-mRFP–positive structures are associated with ER membranes, but not with Golgi membranes, we performed fluorescence loss in photobleaching experiments. To this end, the plasmid for 29(65)31-mRFP was transfected into cells stably expressing cytochrome b5 fused with GFP (GFP-CB5). When 29(65)31-mRFP was expressed, GFP-CB5 (Figure 7B, bottom row), as well as endogenous SERCA2 (top row), displayed a juxtanuclear accentuation, in addition to a reticular distribution. The repeated bleaching of a peripheral area of a 29(65)31-mRFP–expressing cell resulted in a marked decrease in the intensity of juxtanuclear fluorescence derived from GFP-CB5 (Figure 7C, top row). In contrast, no decrease in the intensity of juxtanuclear fluorescence derived from Golgi-localized GFP-galactosyltransferase was observed in an experiment using a similar protocol (Figure 7C, bottom row). These results are consistent with the idea that the juxtanuclear 29(65)31-mRFP–positive structures are continuous with ER or ER-related membranes.
To show that 29(65)31-mRFP is not localized in an intermediate compartment containing ERGIC-53, we performed two experiments. First, we treated 29(65)31-mRFP–expressing HeLa cells with BFA. As shown in Figure 7D, juxtanuclear 29(65)31-mRFP was not dispersed by BFA treatment, whereas the dispersion of the ERGIC (ERGIC-53) (second row) and the Golgi apparatus (GM130) (bottom row) occurred. Next, we examined whether the distribution of 29(65)31-mRFP is changed at 15°C, as seen for ERGIC-53 (Klumperman et al., 1998). On incubation of 29(65)31-mRFP-expressing HeLa cells at 15°C for 2 h, ERGIC-53 was redistributed to larger punctate structures, whereas the distribution of 29(65)31-mRFP was not significantly changed (Supplemental Figure S8).
TMD3 Is Important for the Movement of Bap31 to the Juxtanuclear Region
To determine the domains responsible for the movement to the juxtanuclear region, chimera-expressing cells were subjected to BFA/washout treatment. Although a chimera [29(126)31-mRFP], like wild-type Bap31-mRFP, exhibited a reticular pattern, it did not efficiently move to the juxtanuclear region during recovery from BFA treatment (Supplemental Figure S7, fifth row; and Table 1B, BFA washout 120 min), suggesting that TMD3 is a principal determinant for the movement to the juxtanuclear region. However, we do not exclude the possibility that other regions may contribute to this movement. For example, 29(43)31-mRFP moved less efficiently to the juxtanuclear region compared with 29(30)31-mRFP (Table 1B). This result may suggest some contribution of the cytoplasmic loop between TMD1 and TMD2 for the movement to the juxtanuclear region.
In accordance with the view that the TMDs of Bap31 are responsible for Bap31 cycling, 31(125)29-mRFP in which the C-terminal long cytoplasmic tail of Bap31 was replaced with that of Bap29 and a mutant lacking the C-terminal cytoplasmic domain including a dilysine motif efficiently cycled between the periphery and the juxtanuclear region during recovery from BFA treatment (Supplemental Figure S7 and Table 1B; and data not shown). It should be noted the di-lysine motif of Bap31 is not required for Bap31 cycling.
DISCUSSION
Bap31 is a putative cargo receptor for ER export of several transmembrane proteins (Ladasky et al., 2006, and references therein). In the present study, we disclosed two important features of Bap31. One is that Bap31 is a component of the ER quality control compartment, and the other is that Bap31 cycles between the peripheral ER and a juxtanuclear ER or ER-associated compartment distinct from the conventional ER–Golgi intermediate compartment in a microtubule-dependent manner.
Bap31 Is a Component of the ER Quality Control Compartment
Several previous studies reported the presence of ER quality control compartments in mammals (Kamhi-Nesher et al., 2001; Spiliotis et al., 2002) and yeast (Kiser et al., 2001; Zhang et al., 2001; Huyer et al., 2004). Although detection of the mammalian ER quality control compartment in previous studies was principally dependent on the use of proteasome inhibitors (Kamhi-Nesher et al., 2001; Spiliotis et al., 2002), our present results showed that the ATPase-defective VCP mutant is also usable for this purpose. Furthermore, our results obtained using the VCP mutant unequivocally demonstrated that the formation of discernible juxtanuclear quality control compartments is due to inhibition of ERAD. In cells where proteasomal activity is inhibited and those where retrotranslocation is blocked, Bap31 accumulates in the juxtanuclear quality control compartment, implying that Bap31, which is not an ERAD substrate, is a component of this compartment. In addition to Bap31, we identified another component of the quality control compartment, i.e., Derlin-1. Because Derlin-1 seemingly constitutes a retrotranslocation channel (Lilley and Ploegh, 2004; Ye et al., 2004, 2005), it is likely that the quality control compartment represents the site from which retrotranslocation substrates exit the ER into the cytosol. Although our results suggest that Sec61β, a subunit of the Sec61 translocon that was originally assumed to be a retrotranslocation channel (Wiertz et al., 1996), is not a bona fide component of the quality control compartment, it may be incorporated into this compartment through the interaction with ERAD substrates. Indeed, Kamhi-Nesher et al. (2001) reported when human asialoglycoprotein receptor H2a, an ERAD substrate that binds Sec61β, is ectopically expressed, Sec61β is observed in the quality control compartment.
Cycling of Bap31 between the Peripheral ER and a Juxtanuclear ER or ER-related Compartment
Although initial evidence for Bap31 cycling was obtained by using chemicals (H89 and BFA) that perturb cycling, two lines of evidence suggest that this cycling occurs under normal conditions. First, expression of the constitutively active Arf1 caused the accumulation of Bap31 at the juxtanuclear region. Second, some Bap31/Bap29 chimeras localize to the juxtanuclear region in a microtubule-dependent manner without any artificial treatment. Why Bap31 cycling has not been discovered? Presumably, mixed movement of Bap31, i.e., microtubule-dependent cycling and lateral diffusion, has hampered the identification of this cycling pathway. Obviously, the rate of lateral diffusion is faster than that of Bap31 cycling. Therefore, use of chemicals that perturb Bap31 cycling might be inevitable for us to notice this novel cycling pathway.
Several lines of evidence clearly indicate that the juxtanuclear area where Bap31 accumulates is not the Golgi apparatus or an intermediate compartment containing ERGIC-53. First, at the electron microscopic level, Bap31 was not observed in the Golgi apparatus (Figure 3B). Second, during BFA recovery Bap31 movement to the juxtanuclear region preceded Golgi reassembly (Figure 5) and Bap31 was not included in ERGIC-53–positive tubules (Supplemental Figure S6). Third, Bap31-mRFP–posivie structures could be stained with an ER probe, DiOC6(3) (Figure 6). Fourth, juxtanuclear fluorescence of GFP-CB5 in 29(65)31-mRFP-expressing cells decreased by repeated bleaching of a peripheral area (Figure 7C). Last, in contrast to the sensitivity of the ERGIC to BFA and low temperature treatment, the distribution of juxtanuclear 29(65)31-mRFP was not changed by either treatment (Figure 7D and Supplemental Figure S8). Although we prefer the idea that the juxtanuclear area where Bap31 accumulates represents the ER, our data do not exclude the possibility that the Bap31-accumulated area is a recycling compartment that does not contain ERGIC-53. If this possibility is correct, Bap31 cycling between the ER and the recycling compartment is expected to occur by a novel nonvesicular mechanism that may be dependent on COPII and COPI. Our results also do not exclude the possibility that some Bap31 is transported to the ERGIC and/or the Golgi apparatus in certain cells, as reported previously (Bell et al., 2001). It is possible that some Bap31 escapes the cycling pathway and that it is incorporated into transport vesicles destined for the Golgi apparatus in some cells and/or under certain circumstances.
It is obvious that Bap31 cycling is intimately related with Arf1 function. H89 and BFA, both of which affect Bap31 cycling, are known to keep Arf1 in active and inactive states, respectively (Altan-Bonnet et al., 2003; Lippincott-Schwartz and Liu, 2006). Removal of BFA may rapidly and transiently increase the proportion of active Arf1 species and thereby induce discernible one round of Bap31 cycling. Expression of the constitutively active Arf1 resulted in the juxtanuclear accumulation of Bap31 (Figure 4, B and C). Importantly, the constitutively active Arf1 also induced the putative ER retrotranslocation channel Derlin-1 to redistribute to the juxtanuclear region (Figure 4D). This finding provides evidence for a link between the ER quality control compartment and protein cycling within the ER or ER-related compartments. It is possible that the vesicular transport machinery, independent of its membrane traffic function, plays a role in ERAD in mammalian cells, as reported in yeast cells (Fu and Sztul, 2003).
Possible Implication for Bap31 Cycling
Based on the present and other data with the assumption that Bap31 cycles within the ER, we can envisage the following model for the destiny of transmembrane proteins prone to ERAD (ERAD substrates) in the context of Bap31, although this model should be verified in future studies. As a cargo receptor, Bap31 moves within the ER with ERAD substrates, such as CFTR and class I MHC molecules. Bap31 may function to provide a milieu for efficient folding by recruiting some ER proteins including chaperons (Figure 6). Ladasky et al. (2006) recently reported that overexpression of Bap31 increases the cell surface level of class I MHC molecules, whereas Bap29 overexpression rather inhibits class I MHC molecule transport. This result may be relevant to the fact that Bap31 cycles between the juxtanuclear and peripheral regions, whereas Bap29 does not. If the folding of ERAD substrates takes place with the help of ER chaperones, the folded proteins may dissociate from Bap31 and be incorporated into COPII vesicles at peripheral ER exit sites (Ellgaard and Helenius, 2003). Perhaps because it takes some time for inefficiently folding proteins to be matured, they may move with Bap31 in a dynein–dynactin-dependent manner from the peripheral ER to the juxtanuclear region where ER exit sites are concentrated (Gurkan et al., 2006). When folding occurs, ERAD substrates exit the ER from the juxtanuclear exit sites. The vicinity of juxtanuclear ER exit sites to the Golgi apparatus may shorten the time required for ER-to-Golgi transport. When ERAD substrates do not fold correctly until Bap31–ERAD substrates reach quality control compartments at the juxtanuclear region, they may be retrotranslocated, ubiquitinated, and degraded by the proteasomal machinery that concentrates at the juxtanuclear region (Wigley et al., 1999), or deposited as aggresomes observed at the juxtanuclear region when misfolded proteins accumulate beyond the capacity of proteasomal activity (Johnston et al., 1998). Bap31, not an ERAD substrate, dissociates from immature proteins and recycles from quality control compartments back to the peripheral region. The inhibition of retrotranslocation by a dominant-negative VCP mutant may cause congestion of the retrotranslocation channel with Bap31–ERAD substrates, leading to some association of Bap31 with the VCP mutant, which was detectable by immunoprecipitation (Figure 2B). The fact that Bap31 accumulates at the juxtanuclear region in some COS-7 cells under basal conditions (Figure 1A) may reflect a high level of production of ERAD substrates that have occurred accidentally.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. Hans-Dieter Söling (Max-Planck-Institute, Göttingen, Germany), Roger Y. Tsien (University of California, San Diego, CA), Rainer Pepperkok (European Molecular Biology Laboratory, Heidelberg, Germany), Ron R. Kopito (Stanford University, Stanford, CA), Jennifer Lippincott-Schwartz (National Institutes of Health, Bethesda, MD), Masami Nagahama (Tokushima University, Tokushima, Japan), and Kohei Arasaki (this laboratory) for providing materials. This work was supported in part by Grants-in-Aid for Scientific Research 17048030, 18370081, 18050036, 18570186, and 18657044) from the Ministry of Education, Science, Sports and Culture of Japan.
Abbreviations used:
- BFA
brefeldin A
- CB5
cytochrome b5
- CFTR
cystic fibrosis transmembrane conductance regulator
- ER
endoplasmic reticulum
- ERAD
endoplasmic reticulum-associated degradation
- ERGIC
endoplasmic reticulum-Golgi intermediate compartment
- GFP
green fluorescent protein
- mRFP
monomeric red fluorescent protein
- Noc
nocodazole
- TMD
transmembrane domain
- WT
wild type.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-08-0781) on February 20, 2008.
REFERENCES
- Adachi T., Schamel W. W., Kim K. M., Watanabe T., Becker B., Nielsen P. J., Reth M. The specificity of association of the IgD molecule with the accessory proteins BAP31/BAP29 lies in the IgD transmembrane sequence. EMBO J. 1996;15:1534–1541. [PMC free article] [PubMed] [Google Scholar]
- Altan-Bonnet N., Phair R. D., Polishchuk R. S., Weigert R., Lippincott-Schwartz J. A role for Arf1 in mitotic Golgi disassembly, chromosome segregation, and cytokinesis. Proc. Natl. Acad. Sci. USA. 2003;100:13314–13319. doi: 10.1073/pnas.2234055100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annaert W. G., Becker B., Kistner U., Reth M., Jahn R. Export of cellubrevin from the endoplasmic reticulum is controlled by BAP31. J. Cell Biol. 1997;139:1397–1410. doi: 10.1083/jcb.139.6.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appenzeller-Herzog C., Hauri H. P. The ER-Golgi intermediate compartment (ERGIC): in search of its identity and function. J. Cell Sci. 2006;119:2173–2183. doi: 10.1242/jcs.03019. [DOI] [PubMed] [Google Scholar]
- Arasaki K., Taniguchi M., Tani K., Tagaya M. RINT-1 regulates the localization and entry of ZW10 to the syntaxin 18 complex. Mol. Biol. Cell. 2006;17:2780–2788. doi: 10.1091/mbc.E05-10-0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aridor M., Balch W. E. Kinase signaling initiates coat complex II (COPII) recruitment and export from the mammalian endoplasmic reticulum. J. Biol. Chem. 2000;275:35673–35676. doi: 10.1074/jbc.C000449200. [DOI] [PubMed] [Google Scholar]
- Bell A. W., et al. Proteomics characterization of abundant Golgi membrane proteins. J. Biol. Chem. 2001;276:5152–5165. doi: 10.1074/jbc.M006143200. [DOI] [PubMed] [Google Scholar]
- Bonifacino J. S., Glick B. S. The mechanism of vesicle budding and fusion. Cell. 2004;116:153–166. doi: 10.1016/s0092-8674(03)01079-1. [DOI] [PubMed] [Google Scholar]
- Borgese N., Francolini M., Snapp E. Endoplasmic reticulum architecture: structures in flux. Curr. Opin. Cell Biol. 2006;18:358–364. doi: 10.1016/j.ceb.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckenridge D. G., Stojanovic M., Marcellus R. C., Shore G. C. Caspase cleavage product of BAP31 induces mitochondrial fission through endoplasmic reticulum calcium signals, enhancing cytochrome c release to the cytosol. J. Cell Biol. 2003;160:1115–1127. doi: 10.1083/jcb.200212059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt J. K., Echeverri C. J., Nilsson T., Vallee R. B. Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J. Cell Biol. 1997;139:469–484. doi: 10.1083/jcb.139.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell R. E., Tour O., Palmer A. E., Steinbach P. A., Baird G. S., Zacharias D. A., Tsien R. Y. A monomeric red fluorescent protein. Proc. Natl. Acad. Sci. USA. 2002;99:7877–7882. doi: 10.1073/pnas.082243699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole N. B., Sciaky N., Marotta A., Song J., Lippincott-Schwartz J. Golgi dispersal during microtubule disruption: regeneration of Golgi stacks at peripheral endoplasmic reticulum exit sites. Mol. Biol. Cell. 1996;7:631–650. doi: 10.1091/mbc.7.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosson P., Letourneur F. Coatomer interaction with di-lysine endoplasmic reticulum retention motifs. Science. 1994;263:1629–1631. doi: 10.1126/science.8128252. [DOI] [PubMed] [Google Scholar]
- Ellgaard L., Helenius A. Quality control in the endoplasmic reticulum. Nat. Rev. Mol. Cell Biol. 2003;4:181–191. doi: 10.1038/nrm1052. [DOI] [PubMed] [Google Scholar]
- Fu L., Sztul E. Traffic-independent function of the Sar1p/COPII machinery in proteasomal sorting of the cystic fibrosis transmembrane conductance regulator. J. Cell Biol. 2003;160:157–163. doi: 10.1083/jcb.200210086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurkan C., Stagg S. M., Lapointe P., Balch W. E. The COPII cage: unifying principles of vesicle coat assembly. Nat. Rev. Mol. Cell Biol. 2006;7:727–738. doi: 10.1038/nrm2025. [DOI] [PubMed] [Google Scholar]
- Hammond A. T., Glick B. S. Dynamics of transitional endoplasmic reticulum sites in vertebrate cells. Mol. Biol. Cell. 2000;11:3013–3030. doi: 10.1091/mbc.11.9.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi M., et al. VCP/p97 in abnormal protein aggregates, cytoplasmic vacuoles, and cell death, phenotypes relevant to neurodegeneration. Cell Death Differ. 2001;8:977–984. doi: 10.1038/sj.cdd.4400907. [DOI] [PubMed] [Google Scholar]
- Hirose H., Arasaki K., Dohmae N., Takio K., Hatsuzawa K., Nagahama M., Tani K., Yamamoto A., Tohyama M., Tagaya M. Implication of ZW10 in membrane trafficking between the endoplasmic reticulum and Golgi. EMBO J. 2004;23:1267–1278. doi: 10.1038/sj.emboj.7600135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyer G., Longsworth G. L., Mason D. L., Mallampalli M. P., McCaffery J. M., Wright R. L., Michaelis S. A striking quality control subcompartment in Saccharomyces cerevisiae: the endoplasmic reticulum-associated compartment. Mol. Biol. Cell. 2004;15:908–921. doi: 10.1091/mbc.E03-07-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamora C., Yamanouye N., Van Lint J., Laudenslager J., Vandenheede J. R., Faulkner D. J., Malhotra V. Gβγ-mediated regulation of Golgi organization is through the direct activation of protein kinase D. Cell. 1999;98:59–68. doi: 10.1016/S0092-8674(00)80606-6. [DOI] [PubMed] [Google Scholar]
- Jiang S., Rhee S. W., Gleeson P. A., Storrie B. Capacity of the Golgi apparatus for cargo transport prior to complete assembly. Mol. Biol. Cell. 2006;17:4105–4117. doi: 10.1091/mbc.E05-12-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston J. A., Ward C. L., Kopito R. R. Aggresomes: a cellular response to misfolded proteins. J. Cell Biol. 1998;143:1883–1898. doi: 10.1083/jcb.143.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamhi-Nesher S., Shenkman M., Tolchinsky S., Fromm S. V., Ehrlich R., Lederkremer G. Z. A novel quality control compartment derived from the endoplasmic reticulum. Mol. Biol. Cell. 2001;12:1711–1723. doi: 10.1091/mbc.12.6.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.-M., Adachi T., Nielsen P. J., Terashima M., Lamers M. C., Köhler G., Reth M. Two new proteins preferentially associated with membrane immunoglobulin D. EMBO J. 1994;13:3793–3800. doi: 10.1002/j.1460-2075.1994.tb06690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiser G. L., Gentzsch M., Kloser A. K., Balzi E., Wolf D. H., Goffeau A., Riordan J. R. Expression and degradation of the cystic fibrosis transmembrane conductance regulator in Saccharomyces cerevisiae. Arch. Biochem. Biophys. 2001;390:195–205. doi: 10.1006/abbi.2001.2385. [DOI] [PubMed] [Google Scholar]
- Klumperman J., Schweizer A., Clausen H., Tang B. L., Hong W., Oorschot V., Hauri H. P. The recycling pathway of protein ERGIC-53 and dynamics of the ER-Golgi intermediate compartment. J. Cell Sci. 1998;111:3411–3425. doi: 10.1242/jcs.111.22.3411. [DOI] [PubMed] [Google Scholar]
- Ladasky J. J., Boyle S., Seth M., Li H., Pentcheva T., Abe F., Steinberg S. J., Edidin M. Bap31 enhances the endoplasmic reticulum export and quality control of human class I MHC molecules. J. Immunol. 2006;177:6172–6181. doi: 10.4049/jimmunol.177.9.6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert G., Becker B., Schreiber R., Boucherot A., Reth M., Kunzelmann K. Control of cystic fibrosis transmembrane conductance regulator expression by BAP31. J. Biol. Chem. 2001;276:20340–20345. doi: 10.1074/jbc.M011209200. [DOI] [PubMed] [Google Scholar]
- Lee T. H., Linstedt A. D. Potential role for protein kinases in regulation of bidirectional endoplasmic reticulum-to-Golgi transport revealed by protein kinase inhibitor H89. Mol. Biol. Cell. 2000;11:2577–2590. doi: 10.1091/mbc.11.8.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. C., Miller E. A., Goldberg J., Orci L., Schekman R. Bi-directional protein transport between the ER and Golgi. Annu. Rev. Cell Dev. Biol. 2004;20:87–123. doi: 10.1146/annurev.cellbio.20.010403.105307. [DOI] [PubMed] [Google Scholar]
- Levine T., Rabouille C. Endoplasmic reticulum: one continuous network compartmentalized by extrinsic cues. Curr. Opin. Cell Biol. 2005;17:362–368. doi: 10.1016/j.ceb.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Lilley B. N., Ploegh H. L. A membrane protein required for dislocation of misfolded proteins from the ER. Nature. 2004;429:834–840. doi: 10.1038/nature02592. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Liu W. Insights into COPI coat assembly and function in living cells. Trends Cell Biol. 2006;16:e1–e4. doi: 10.1016/j.tcb.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Meusser B., Hirsch C., Jarosch E., Sommer T. ERAD: the long road to destruction. Nat. Cell Biol. 2005;7:766–772. doi: 10.1038/ncb0805-766. [DOI] [PubMed] [Google Scholar]
- Nagahama M., Suzuki M., Hamada Y., Hatsuzawa K., Tani K., Yamamoto A., Tagaya M. SVIP is a novel VCP/p97-interacting protein whose expression causes cell vacuolation. Mol. Biol. Cell. 2003;14:262–273. doi: 10.1091/mbc.02-07-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K., Hirose H., Taniguchi M., Kurashina H., Arasaki K., Nagahama M., Tani K., Yamamoto A., Tagaya M. Involvement of BNIP1 in apoptosis and endoplasmic reticulum membrane fusion. EMBO J. 2004;23:3216–3226. doi: 10.1038/sj.emboj.7600333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N., Rabouille C., Watson R., Nilsson T., Hui N., Slusarewicz P., Kreis T. E., Warren G. Characterization of a cis-Golgi matrix protein, GM130. J. Cell Biol. 1995;131:1715–1726. doi: 10.1083/jcb.131.6.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquet M. E., Cohen-Doyle M., Shore G. C., Williams D. B. Bap29/31 influences the intracellular traffic of MHC class I molecules. J. Immunol. 2004;172:7548–7555. doi: 10.4049/jimmunol.172.12.7548. [DOI] [PubMed] [Google Scholar]
- Puri S., Linstedt A. D. Capacity of the Golgi apparatus for biogenesis from the endoplasmic reticulum. Mol. Biol. Cell. 2003;14:5011–5018. doi: 10.1091/mbc.E03-06-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römisch K. Endoplasmic reticulum-associated degradation. Annu. Rev. Cell Dev. Biol. 2005;21:435–456. doi: 10.1146/annurev.cellbio.21.012704.133250. [DOI] [PubMed] [Google Scholar]
- Schamel W. W., Kuppig S., Becker B., Gimborn K., Hauri H. P., Reth M. A high-molecular-weight complex of membrane proteins BAP29/BAP31 is involved in the retention of membrane-bound IgD in the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA. 2003;100:9861–9866. doi: 10.1073/pnas.1633363100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemann J., Jokitalo J., Pypaert M., Warren G. Matrix proteins can generate the higher order architecture of the Golgi apparatus. Nature. 2000;407:1022–1026. doi: 10.1038/35039538. [DOI] [PubMed] [Google Scholar]
- Shimoi W., Ezawa I., Nakamoto K., Uesaki S., Gabreski G., Aridor M., Yamamoto A., Nagahama M., Tagaya M., Tani K. p125 is localized in endoplasmic reticulum exit sites and involved in their organization. J. Biol. Chem. 2005;280:10141–10148. doi: 10.1074/jbc.M409673200. [DOI] [PubMed] [Google Scholar]
- Spiliotis E. T., Manley H., Osorio M., Zuniga M. C., Edidin M. Selective export of MHC class I molecules from the ER after their dissociation from TAP. Immunity. 2000;13:841–851. doi: 10.1016/s1074-7613(00)00081-9. [DOI] [PubMed] [Google Scholar]
- Spiliotis E. T., Pentcheva T., Edidin M. Probing for membrane domains in the endoplasmic reticulum: retention and degradation of unassembled MHC class I molecules. Mol. Biol. Cell. 2002;13:1566–1581. doi: 10.1091/mbc.01-07-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojanovic M., Germain M., Nguyen M., Shore G. C. BAP31 and its caspase cleavage product regulate cell surface expression of tetraspanins and integrin-mediated cell survival. J. Biol. Chem. 2005;280:30018–30024. doi: 10.1074/jbc.M501306200. [DOI] [PubMed] [Google Scholar]
- Storrie B., White J., Röttger S., Stelzer E. H., Suganuma T., Nilsson T. Recycling of Golgi-resident glycosyltransferases through the ER reveals a novel pathway and provides an explanation for nocodazole-induced Golgi scattering. J. Cell Biol. 1998;143:1505–1521. doi: 10.1083/jcb.143.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczesna-Skorupa E., Kemper B. BAP31 is involved in the retention of cytochrome P450 2C2 in the endoplasmic reticulum. J. Biol. Chem. 2006;281:4142–4148. doi: 10.1074/jbc.M509522200. [DOI] [PubMed] [Google Scholar]
- Tagaya M., Furuno A., Mizushima S. SNAP prevents Mg2+-ATP-induced release of N-ethylmaleimide-sensitive factor from the Golgi apparatus in digitonin-permeabilized PC12 cells. J. Biol. Chem. 1996;271:466–470. doi: 10.1074/jbc.271.1.466. [DOI] [PubMed] [Google Scholar]
- Vedrenne C., Hauri H. P. Morphogenesis of the endoplasmic reticulum: beyond active membrane expansion. Traffic. 2006;7:639–646. doi: 10.1111/j.1600-0854.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- Voeltz G. K., Rolls M. M., Rapoport T. A. Structural organization of the endoplasmic reticulum. EMBO Rep. 2002;3:944–950. doi: 10.1093/embo-reports/kvf202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana Y., Koyama S., Nakajima K., Hatsuzawa K., Nagahama M., Tani K., Hauri H.-P., Melancon P., Tagaya M. Reticulon 3 is involved in membrane trafficking between the endoplasmic reticulum and Golgi. Biochem. Biophys. Res. Commun. 2005;334:1198–1205. doi: 10.1016/j.bbrc.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Ward T. H., Polishchuk R. S., Caplan S., Hirschberg K., Lippincott-Schwartz J. Maintenance of Golgi structure and function depends on the integrity of ER export. J. Cell Biol. 2001;155:557–570. doi: 10.1083/jcb.200107045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiertz E. J., Tortorella D., Bogyo M., Yu J., Mothes W., Jones T. R., Rapoport T. A., Ploegh H. L. Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature. 1996;384:432–438. doi: 10.1038/384432a0. [DOI] [PubMed] [Google Scholar]
- Wigley W. C., Fabunmi R. P., Lee M. G., Marino C. R., Muallem S., DeMartino G. N., Thomas P. J. Dynamic association of proteasomal machinery with the centrosome. J. Cell Biol. 1999;145:481–490. doi: 10.1083/jcb.145.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y., Meyer H. H., Rapoprot T. A. The AAA ATPase Cdc48/p97 and its partners transport proteins from the ER into the cytosol. Nature. 2001;414:652–656. doi: 10.1038/414652a. [DOI] [PubMed] [Google Scholar]
- Ye Y., Meyer H. H., Rapoprot T. A. Function of the p97–Ufd1–Npl4 complex in retrotranslocation from the ER to the cytosol: dual recognition of nonubiquitinated polypeptide segments and polyubiquitin chains. J. Cell Biol. 2003;162:71–84. doi: 10.1083/jcb.200302169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y., Shibata Y., Yun C., Ron D., Rapoprot T. A. A membrane protein complex mediates retro-translocation from the ER lumen into the cytosol. Nature. 2004;429:841–847. doi: 10.1038/nature02656. [DOI] [PubMed] [Google Scholar]
- Ye Y., Shibata Y., Kikkert M., van Voorden S., Wiertz E., Rapoprot T. A. Recruitment of the p97 ATPase and ubiquitin ligases to the site of retrotranslocation at the endoplasmic reticulum membrane. Proc. Natl. Acad. Sci. USA. 2005;102:14132–14138. doi: 10.1073/pnas.0505006102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zen K., Utech M., Liu Y., Soto I., Nusrat A., Parkos C. A. Association of BAP31 with CD11b/CD18. Potential role in intracellular trafficking of CD11b/CD18 in neutrophils. J. Biol. Chem. 2004;279:44924–44930. doi: 10.1074/jbc.M402115200. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Nijbroek G., Sullivan M. L., McCracken A. A., Watkins S. C., Michaelis S., Brodsky J. L. Hsp70 molecular chaperone facilitates endoplasmic reticulum-associated protein degradation of cystic fibrosis transmembrane conductance regulator in yeast. Mol. Biol. Cell. 2001;12:1303–1314. doi: 10.1091/mbc.12.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.