Abstract
Reciprocal cooperative signaling by integrins and growth factor receptors at G1 phase during cell cycle progression is well documented. By contrast, little is known about the cross-talk between integrin and transforming growth factor (TGF)-β signaling. Here, we show that integrin signaling counteracts the inhibitory effects of TGF-β on cell growth and that this effect is mediated by p130Cas (Crk-associated substrate, 130 kDa). Adhesion to fibronectin or laminin reduces TGF-β–induced Smad3 phosphorylation and thus inhibits TGF-β–mediated growth arrest; loss of p130Cas abrogates these effects. Loss and gain of function studies demonstrated that, once tyrosine-phosphorylated via integrin signaling, p130Cas binds to Smad3 and reduces phosphorylation of Smad3. That in turn leads to inhibition of p15 and p21 expression and facilitation of cell cycle progression. Thus, p130Cas-mediated control of TGF-β/Smad signaling may provide an additional clue to the mechanism underlying resistance to TGF-β–induced growth inhibition.
INTRODUCTION
Transforming growth factor (TGF)-β is a multifunctional cytokine that regulates a wide range of cellular functions, including proliferation, apoptosis and differentiation (Ten Dijke et al., 2002; Siegel and Massague, 2003). In most cell types (e.g., normal epithelial, endothelial, and hematopoietic cells and primary fibroblasts of embryonic origin), TGF-β inhibits growth by causing arrest at G1 phase of the cell cycle (Massague, 1990; Wolfraim et al., 2004). This anti-proliferative effect of TGF-β is attributable to induction of cyclin-dependent kinase (CDK) inhibitors such as p15 and p21 and repression of growth-promoting transcription factors. Thus, induction of p15 and p21 represent key events in TGF-β–induced growth arrest (Siegel and Massague, 2003).
TGF-β signals are mainly transduced by a cell surface complex composed of TGF-β receptor type I (TβRI) and II (TβRII) and Smad intracellular effector proteins. The binding of a ligand to the TβRI/II complex activates the serine kinase activity of TβRI, which then phosphorylates the C-terminal serine residues (SXS) of the receptor-activated Smad proteins (R-Smad) Smad2 and Smad3 (Shi and Massague, 2003). Phosphorylation of Smad2/3 induces subsequent complex formation with Smad4, after which this heteromeric Smad complex translocates into the nucleus (Di Guglielmo et al., 2003). Within the nucleus, the Smad complex regulates the transcription of target genes by associating with DNA binding partners that act as transcriptional coactivators or corepressors (Feng and Derynck, 2005).
Because of its critical role in determining cell fate, the intensity or duration of TGF-β signaling is tightly controlled by a web of positive and negative regulator proteins. Negative regulation of TGF-β signaling is accomplished by the rapid attenuation or even cancellation of TβRI/II and/or Smad activities (Di Guglielmo et al., 2003; Feng and Derynck, 2005), which often occurs through a negative feedback loop. An example of a molecule that down-regulates TGF-β signaling through a negative feedback loop is Smad7, which diminishes TGF-β signaling by competitively inhibiting the binding of Smad3 to activated TβRI, and by targeting the receptor for degradation. TGF-β and Smad3, themselves, induce Smad7 expression, thereby mediating an inhibitory feedback loop through Smad7 expression (Nakao et al., 1997; Di Guglielmo et al., 2003).
TGF-β signaling is also regulated through cross-talk with other signal transduction pathways. For example, it is now known that signaling in the extracellular signal-regulated kinase pathway can lead to direct phosphorylation of specific serine residues in the linker domain of R-Smads, which blocks their nuclear translocation and transcriptional output (Hayashida et al., 2003). The c-Jun NH2-terminal kinase pathway also inhibits Smad2-dependent transcription by enhancing complex formation by Smad2 and its corepressor protein TGIF (Seo et al., 2006). In addition, recent reports suggest that integrin signaling also may be involved in negatively regulating TGF-β signaling, because adhesion reduces TGF-β-induced Smad2 phosphorylation and transcriptional activity (Thannickal et al., 2003), and β1-integrin–mediated adhesion to extracellular matrix (ECM) suppresses TGF-β–induced apoptosis and growth inhibition (Zhang et al., 2004).
Integrins are the major receptors that connect cells to the ECM (Giancotti and Ruoslahti, 1999). Integrin-mediated adhesion and the binding of growth factors to their receptors, which are often receptor tyrosine kinases (RTKs), exert effects that synergistically promote cell cycle progression (Comoglio et al., 2003; Giancotti and Tarone, 2003; Guo and Giancotti, 2004). That is, during the cell cycle collaborative signaling between integrins and RTKs induces the progression of G1 phase and the initiation of S phase through activation of G1 cyclin-dependent kinases (CDKs) and the down-regulation of CDK inhibitors (Schwartz and Ginsberg, 2002; Giancotti and Tarone, 2003). Moreover, both integrins and RTKs share multiple cytoplasmic signaling pathways that involve a variety of integrin-associated signaling proteins, including p130Cas (Bouton et al., 2001; Defilippi et al., 2006), which is extensively tyrosine phosphorylated in response to ECM adhesion or growth factor stimulation. For example, cross-talk between integrins and epidermal growth factor receptors is crucially dependent on c-Src and p130Cas (Moro et al., 2002); moreover, p130Cas plays a pivotal role in signaling via other receptor systems, including G protein-coupled receptors and vascular endothelial growth factor receptors (Casamassima and Rozengurt, 1997; Avraham et al., 2003; Defilippi et al., 2006).
p130Cas is an adapter protein that contains multiple protein–protein interaction domains, including an Src homology 3 (SH3) domain, an Src-binding (SBD) domain, a large “tyrosine kinase substrate“ (SD) domain, and a helix-loop-helix domain (HLH). The SD domain is characterized by 15 tyrosine-Xaa-Xaa-proline (YXXP) motifs that are regarded as the major site of p130Cas phosphorylation (Sakai et al., 1994; Chodniewicz and Klemke, 2004). ECM-integrin coupling induces phosphorylation of the p130Cas SD domain, after which p130Cas is translocated from the cytosol to the membrane, where it serves as a mediator for integrin signaling mainly promoting cell migration, survival, and proliferation (Cary et al., 1998; Shin et al., 2004).
Interestingly, human enhancer of filamentation 1 (HEF1), which is an adaptor protein belonging to the p130Cas protein family, reportedly interacts with Smad3, thereby inhibiting Smad3-mediated gene expression (Liu et al., 2000). Bearing that in mind, we wondered whether p130Cas also plays a role in determining the cellular responsiveness to TGF-β and focused on Smad3 as a possible target of inhibition by p130Cas. In this study, we show that, after tyrosine phosphorylation of p130Cas mediated by integrin signaling, the phosphorylated p130Cas is able to interact with phosphorylated Smad3 and in turn prevent transcriptional activation by Smad3. This inhibitory effect is mediated via a pathway that is dependent upon the association of p130Cas with Smad3, and it provides evidence for cross-talk between the TGF-β and integrin pathways and for a mechanism by which TGF-β signaling is silenced by p130Cas.
MATERIALS AND METHODS
DNA Constructs and Reagents
Myc-Smad2, Myc-Smad3 and the Smad3-responsive (CAGA)12-luc reporter were gifts from Aristidis Moustakas (Ludwig Institute for Cancer Research, Uppsala, Sweden). Flag-Smad3 was from J. Massagué (Sloan-Kettering Institute, New York). Myc-Smad3 deletion mutants were polymerase chain reaction (PCR)-amplified from Myc-Smad3 and cloned into pcDNA3.0-6Myc vector. Myc-Smad3 phosphorylation mutants (S3A and S3D) were generated from pcDNA3.0-6Myc-Smad3 by using a QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). Serine residues at positions 464, 465, and 467 of Smad3 (WT) were replaced with alanines (S3A) or aspartates (S3D). After PCR amplification, Myc-p130Cas deletion mutants were cloned into pcDNA3.0-6Myc vector. TGF-β1 was purchased from R&D Systems (Minneapolis, MN). Human fibronectin (FN) and mouse laminin (LN) were obtained from Invitrogen (Carlsbad, CA); synthetic poly-l-lysine (PLL) was from Sigma-Aldrich (St. Louis, MO).
Cell Culture, Transfection, and Luciferase Assays
Smad3−/− mouse embryonic fibroblasts (MEFs), HaCaT human keratinocytes, and human embryonic kidney (HEK)293T cells were maintained in DMEM supplemented with 10% fetal bovine serum (FBS) (Invitrogen). Cas+/+ and Cas−/− MEFs have been described previously (Honda et al., 1998). Cas−/− MEFs engineered to stably express either wild-type p130Cas (CasWT) or Cas15FxxP (Cas15F), in which all 15 YxxP tyrosines were replaced with phenylalanine, have been described previously (Fonseca et al., 2004), and they were cultured in DMEM supplemented with 10% FBS. Cell culture plates were coated with 15 μg/ml LN, 15 μg/ml FN, or 75 μg/ml PLL. All transfections were carried out using Lipofectamine Plus (Invitrogen) according to the manufacturer's instructions. HaCaT cell clones stably expressing p130Cas were obtained by transfection with pcDNA3.0-p130Cas followed by selection for 1–2 wk in Geneticin (G418; Invitrogen). After transfection, luciferase activity was determined using a luciferase assay system (Promega, Madison, WI) and normalized to β-galactosidase activity to correct for differences in transfection efficiency. To analyze the interaction of endogenous p130Cas and Smad3 during the cell cycle, HaCaT cells were synchronized at G0/G1 phase by contact inhibition and serum starvation as described previously (Matsuura et al., 2004). The cells were then detached and replated on culture dishes coated with FN or PLL, and then they were incubated in fresh complete medium.
Generation of an Antibody against the N Terminus of Smad3
To generate a corresponding antibody (Ab), a cDNA (Smad3N) encoding the N terminus of Smad3 (amino acids 1–183) was amplified by PCR and subcloned, in-frame, into pGEX4T vector for expression in a glutathione transferase (GST) fusion protein. The resultant GST-Smad3N fusion protein was then overexpressed in bacteria and purified using glutathione-agarose 4B beads, after which the purified protein was used as an immunogen for Ab production. After the fourth injection into rabbits, the serum specificity was tested by immunoprecipitation analysis.
In Vitro Protein–Protein Interaction Assays
GST or GST-Smad3 was expressed in Escherichia coli and purified through adsorption onto glutathione-Sepharose 4B beads (GE Healthcare, Chalfont St. Giles, United Kingdom). 35S-labeled p130Cas was generated by in vitro transcription and translation using a TNT kit (Promega). The radiolabeled translation mixture was incubated with 10 μg of the bound GST or GST-Smad3 in binding buffer (50 mM HEPES, pH 7.5, 150 mM NaCl, 0.1% Tween 20, 1 mM EDTA, 2.5 mM EGTA, 10% glycerol, 1 mM dithiothreitol [DTT], 0.1 mM NaVO4, 1 mM phenylmethylsulfonyl fluoride [PMSF], and protease inhibitors), after which the beads were extensively washed in the same buffer, and the adsorbed proteins were separated by SDS-polyacrylamide gel electrophoresis (PAGE) and visualized by autoradiography.
Immunoprecipitation and Immunoblotting
MEFs or HaCaT cells incubated with or without 2 ng/ml TGF-β1 were initially lysed in modified radioimmunoprecipitation assay buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 0.25% sodium deoxycholate, 10 mM NaF, 1 mM PMSF, 1 mM sodium ortho-vanadate, 10 μM leupeptin, 1.5 μM pepstatin, and 10 μg/ml aprotinin). For analysis of endogenous Smad3-Smad4, Smad3-p130Cas, and p130Cas-TβRI interactions, the lysates were immunoprecipitated with anti-Smad2/3 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-Smad3N or anti-TβRI Ab, followed by immunoblotting with anti-Smad4 monoclonal antibody (mAb) (Santa Cruz Biotechnology) or anti-p130Cas mAb (BD Biosciences, San Jose, CA). Tyrosine phosphorylation of p130Cas was analyzed by immunoprecipitation with anti-Cas2 Ab (Sakai et al., 1994), followed by immunoblotting with anti-phosphotyrosine mAb (4G10; Upstate Biotechnology, Lake Placid, NY) or with anti-pY165-Cas Ab (Cell Signaling Technology, Beverly, MA), which specifically recognizes phosphorylated YxxP tyrosines (Fonseca et al., 2004). For analysis of exogenous Smad3-p130Cas interactions, HEK293T cells were transfected with Flag-Smad3, Myc-Smad2/3, or one of its substitution mutants, or Myc-p130Cas or one of its deletion mutants. Cell lysates were subjected to immunoprecipitation with anti-Cas2 Ab or anti-p130Cas mAb (BD Biosciences), followed by immunoblotting with anti-Myc (9E10; Cell Signaling Technology) or anti-Flag (Sigma-Aldrich) mAb. Immunoblotting was carried out using anti-Smad3 Ab (Zymed Laboratories, South San Francisco, CA), anti-phospho-Smad3 Ab (a kind gift of Edward B. Leof, Mayo Clinic Cancer Center, MN), anti-tubulin mAb (Sigma-Aldrich), anti-TβRI Ab (Santa Cruz Biotechnology), anti-p15 Ab (Santa Cruz Biotechnology), or anti-p21 mAb (BD Biosciences).
Immunofluorescence
To detect endogenous p130Cas, Smad2/3, phospho-Smad3, or p15 in MEFs, the cells were treated as described in the figure legends, fixed, permeabilized, blocked, incubated with the appropriate primary Ab, and stained with Alexa Fluor-conjugated secondary Ab (Invitrogen). Immunohistochemical detection of transfected proteins was carried out using green fluorescent protein (GFP) or anti-Myc mAb (9E10; Cell Signaling Technology) as the primary Ab. Nuclei were labeled with Hoechst dye 33258 (Sigma-Aldrich). Images were obtained using a Leica fluorescence microscope (Leica Microsystems, Wetzlar, Germany) and a CoolSNAPfx charge-coupled device camera driven by MetaMorph imaging software (Molecular Devices, Sunnyvale, CA). Fluorescence intensities within nuclei were measured using MetaMorph 6.3r6 image analysis software (Molecular Devices).
Silencing Endogenous p130Cas by Using Small Interfering RNA (siRNA)
HaCaT and MCF10A cells were depleted of p130Cas by using siRNA corresponding to nucleotides 2366-2384 (CCCACAAGCUGGUGUUACU dT dT) of human p130Cas (Bioneer, Daejeon, Korea). Cells were transiently transfected with control (nonsilencing fluorescein-labeled siRNA duplex; Bioneer) or anti-p130Cas siRNA in Opti-MEM I medium (Invitrogen) by using Lipofectamine 2000 reagent (Invitrogen) following the manufacturer's instructions. Six hours later, the Opti-MEM I medium was replaced with fresh culture medium. Effects of down-regulating p130Cas were assessed 24–60 h after transfection; TGF-β was added to the cultures 24–36 h after the transfection. The transfection efficiency for the siRNAs, as analyzed with fluorescein-labeled siRNA duplex, was consistently >95%.
In Vitro Kinase Assays
HaCaT cells transfected with control or p130Cas siRNAs were incubated with 2 ng/ml TGF-β, lysed in lysis buffer (1% NP-40, 20 mM HEPES, pH 7.4, 135 mM NaCl, 5 mM EDTA, 10% glycerol, 0.5 mM NaVO4, and protease inhibitors), and immunoprecipitated with anti-TβRI Ab. The resultant TβRI immune complexes were washed three times with lysis buffer and once with kinase buffer (20 mM HEPES, pH 7.4, 1 mM DTT, 20 mM MgCl2, 0.1 mM NaVO4, 2 mM EGTA, and protease inhibitors), and then they were resuspended in kinase buffer containing 25 μM ATP and 5 μCi of [γ-32P]ATP. The reaction mixtures were incubated with GST or GST-Smad3 (Santa Cruz Biotechnology) for 30 min at 30°C, after which the reaction was terminated by addition of SDS sample buffer. Samples were then analyzed by 10% SDS-PAGE and autoradiography. The gels also were stained with Coomassie Blue to detect levels of substrates before exposure to x-ray film. The levels of p130Cas and TβRI in lysates were determined by immunoblotting with anti-p130Cas mAb and anti-TβRI Ab.
[3H]Thymidine Incorporation Assays
MEFs or HaCaT cells were seeded to a density of 2 × 105 cells/well in six-well plates coated as described in the figure legends and then incubated for 24 h with or without TGF-β1; 4 μCi of [3H]thymidine were added for the last 3 h of the incubation. The cells were then fixed in 10% trichloroacetic acid and lysed in 0.2 M NaOH. [3H]thymidine incorporation into the DNA was measured using a scintillation counter.
RESULTS
Identification of p130Cas as a Smad3-interacting Protein
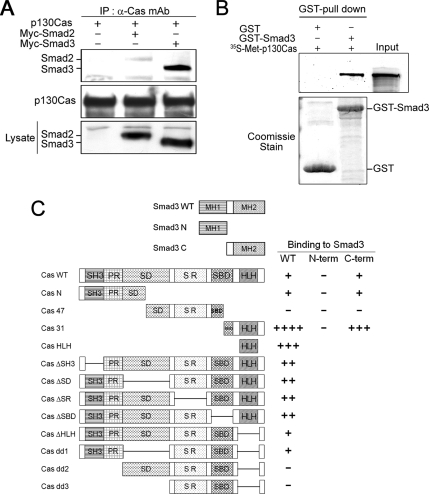
Using coimmunoprecipitation assays to search for components that may modulate Smad3 signaling, we readily identified p130Cas as a new partner that interacts with Smad3. Further analysis with inclusion of Smad2 and Smad3 revealed that p130Cas interacts with exogenous Smad3 and, to a lesser extent, with Smad2 (Figure 1A). We then tested whether the Smad3-p130Cas interaction was direct. Recombinant GST-Smad3 fusion protein was incubated with 35S-labeled p130Cas generated by in vitro transcription and translation. We found that GST-Smad3 can pull down p130Cas from the in vitro translation mixture (Figure 1B). By contrast, p130Cas did not bind to the GST protein, which is indicative of a direct and specific interaction of p130Cas with Smad3.
Figure 1.
p130Cas interacts directly with Smad3. (A) HEK293T cells were transfected with the indicated plasmids, after which their lysates were immunoprecipitated with anti-p130Cas mAb and immunoblotted with anti-Myc mAb. In this and all other experiments, the expression of proteins was determined by direct immunoblotting. (B) GST-Smad3 and GST were tested for their ability to directly interact with in vitro translated 35S-Met– labeled p130Cas. Equal amounts of GST or GST-Smad3 were used, as determined by Coomassie Blue staining (bottom). (C) Schematic representation of p130Cas and Smad3 deletion mutants, and the results of domain-mapping studies: Cas WT, wild-type p130Cas; Cas N, p130Cas N terminus; Cas 47, 47-kDa fragment of p130Cas; Cas 31, 31-kDa fragment p130Cas (Kook et al., 2000); Cas HLH, helix-loop-helix domain (Kim et al., 2004); ΔSH3, deletion of the SH3 domain; ΔSD, deletion of the substrate domain; ΔSR, deletion of the serine rich region; ΔSBD, deletion of the Src-binding domain; ΔHLH, deletion of the helix-loop-helix domain; dd1, deletion of the SD and HLH domain; dd2, deletion of the SH3, PR, and HLH domains; dd3, deletion of SH3, PR, SD, and HLH domains; Smad3 N, Smad3 N terminus; Smad3 C, linker region and C terminus of Smad3; − indicates a lack of detectable interaction; ++++ indicates a very strong interaction.
We next determined the p130Cas domains that mediate the interaction with Smad3 by constructing a set of p130Cas deletion mutants and assessing their abilities to interact with Smad3 in coimmunoprecipitation assays coupled to immunoblotting (Supplemental Figure 1). As shown in Figure 1C, both the CasN and Cas31 mutants retained the ability to interact with Smad3. However, deletion of the N terminus and HLH domains abolished the ability of p130Cas to interact with Smad3, indicating that the Smad3–p130Cas interaction is mediated by the N terminus and C-terminal HLH domain of p130Cas. We also used coimmunoprecipitation analysis to map the domains in Smad3 that interact with p130Cas and found that p130Cas primarily bound to the C terminus of Smad3, which contains a linker and Mad homology 2 domain (Figure 1C).
p130Cas Forms a Heteromeric Complex with Smad3 and TβRI in the Presence of TGF-β
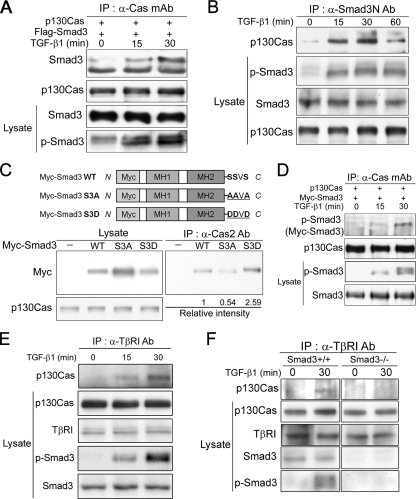
To study the interaction of Smad3 and p130Cas in vivo, we cotransfected HEK293 cells with pCDNA-p130Cas and Flag-Smad3, incubated the transfectants with or without TGF-β, and then subjected the cell extracts to coimmunoprecipitation coupled to immunoblotting. We found that, in the presence of TGF-β, immunoprecipitation of p130Cas by using anti-Cas mAb also brought down Flag-tagged Smad3 (Figure 2A). This Smad3–p130Cas interaction occurred only minimally in the absence of TGF-β stimulation. Using an anti-Smad3N polyclonal Ab raised against the N terminus of Smad3, we then tested whether endogenous Smad3–p130Cas interactions are also regulated by TGF-β. We found that endogenous p130Cas and Smad3 were significantly associated after 15 or 30 min of TGF-β treatment, but after 60 min the levels of Smad3-bound p130Cas began to decline (Figure 2B). Simultaneously, TGF-β induced Smad3 phosphorylation that was well correlated with the Smad3–p130Cas interaction.
Figure 2.
TGF-β induces Smad3-dependent association of p130Cas and TβRI. (A) HEK293T cells transfected with the indicated plasmids were incubated with or without 2 ng/ml TGF-β, after which their lysates were immunoprecipitated with anti-p130Cas mAb and immunoblotted with anti-Flag mAb. (B) HaCaT cells were incubated with or without 2 ng/ml TGF-β, after which their lysates were immunoprecipitated with anti-Smad3N Ab and immunoblotted with anti-p130Cas mAb. (C) HEK293T cells transfected with Myc-Smad3 (WT) or a phosphorylation mutant (S3A or S3D), after which their lysates were immunoprecipitated with anti-Cas2 Ab and immunoblotted with anti-Myc mAb. Relative band intensities quantified by densitometry are indicated numerically below. (D) HEK293T cells were transfected with Myc-Smad3 and incubated with or without TGF-β for the indicated times. Cell lysates were immunoprecipitated with anti-Cas mAb and immunoblotted with anti-phospho-Smad3 Ab to detect p130Cas-bound phospho-Smad3. (E) Cas+/+ MEFs were incubated with or without 2 ng/ml TGF-β, after which their lysates were immunoprecipitated with anti-TβRI Ab and immunoblotted with anti-p130Cas mAb. (F) Smad3+/+ and Smad3−/− MEFs treated with 2 ng/ml TGF-β were subjected to immunoprecipitation as described in D.
Exposure to TGF-β rapidly induces receptor-mediated Smad3 phosphorylation at C-terminal serine residues (Shi and Massague, 2003). Given that the Smad3–p130Cas interaction is enhanced by TGF-β, we tested whether this interaction is positively regulated by TGF-β–induced Smad3 phosphorylation. Consistent with that idea, we found 1) that a Smad3 mutant that mimics the structural and electrostatic properties of phospho-Smad3 (S3D, S464SVS467-D464DVD467) bound to p130Cas as well as, if not better than, wild-type Smad3 (WT) (Figure 2C); 2) that a mutant lacking phosphorylation sites (S3A, S464SVS467-A464AVA467) exhibited reduced binding to p130Cas (Figure 2C); and 3) that a substantial amount of phospho-Smad3 was present within the exogenous Smad3–p130Cas complex in the presence of TGF-β (Figure 2D). Together, these results suggest that the p130Cas–Smad3 interaction is enhanced by Smad3 phosphorylation mediated by TGF-β under physiological conditions.
TGF-β signaling through Smad3 is initiated when TβRI, activated by TβRII-mediated phosphorylation, directly phosphorylates the C-terminal serine residues of Smad3 (Shi and Massague, 2003; Siegel and Massague, 2003). We therefore tested whether p130Cas can interact with TβRI and whether or not their binding is affected by TGF-β stimulation. Wild-type p130cas (Cas+/+) MEFs were subjected to immunoprecipitation with anti-TβRI Ab coupled to immunoblotting. This analysis revealed that there is little or no association of p130Cas with TβRI in the absence of TGF-β stimulation, but that this association is significantly increased by incubating the cells with TGF-β for 15 or 30 min (Figure 2E). Smad3 phosphorylation in response to TGF-β was also well correlated with the association of p130Cas and TβRI. Moreover, this association was lost in Smad3-null (Smad3−/−) MEFs (Figure 2F). That phosphorylation of Smad3 induced its interaction with p130Cas (Figure 2, B–D) suggests p130Cas interacts with TβRI through the intermediation of Smad3 in the presence of TGF-β.
Integrin-mediated Tyrosine Phosphorylation of p130Cas Increases Its Interaction with Smad3
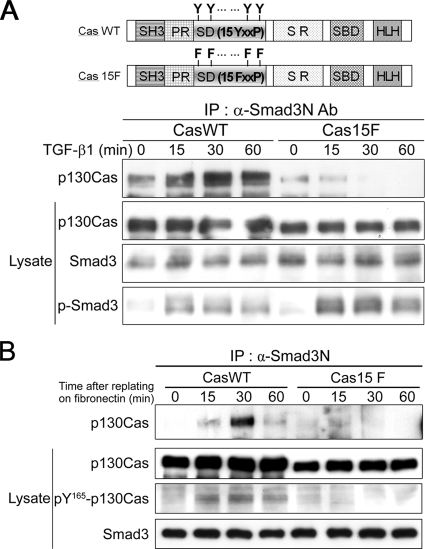
p130Cas is extensively tyrosine phosphorylated in response to integrin-mediated adhesion to ECM and to growth factor stimulation (Sakai et al., 1994; Yamada and Even-Ram, 2002; Defilippi et al., 2006). The hyperphosphorylated p130Cas is active, and it provides multiple protein-interaction motifs, enabling it to participate in a number of cellular processes (Bouton et al., 2001; Kim et al., 2004; Shin et al., 2004). We therefore tested whether the interaction of Smad3 and p130Cas is also regulated by tyrosine phosphorylation of p130Cas mediated by integrin signaling. The SD of p130Cas is composed of 15 Tyr-Xaa-Xaa-Pro (YxxP) motifs, with the YxxP tyrosines representing the major sites of p130Cas phosphorylation (Sakai et al., 1994; Shin et al., 2004). p130Cas-null (Cas−/−) MEFs were engineered to stably express wild-type p130Cas (CasWT cells) or a p130Cas mutant (Cas15F cells) in which all of the tyrosine residues in the 15 YxxP motifs in the SD were substituted with phenylalanine (15FxxP) (Shin et al., 2004). After incubating these cell lines with or without TGF-β, we subjected the cell extracts to coimmunoprecipitation coupled to immunoblotting. As shown in Figure 3A, wild-type p130Cas interacted with Smad3, and this interaction was enhanced by TGF-β stimulation. In contrast, SD mutations that blocked integrin-mediated tyrosine phosphorylation weakened this interaction, both in the absence and presence of TGF-β. In addition, Cas15F cells showed higher levels of TGF-β–induced Smad3 phosphorylation than CasWT cells, suggesting that p130Cas may reduce the level of phospho-Smad3.
Figure 3.
The interaction of Smad3 and p130Cas is enhanced by tyrosine phosphorylation of p130Cas mediated by integrin signaling. (A) Cas−/− MEFs stably expressing either wild-type p130Cas (CasWT) or a p130Cas mutant in which all 15 YxxP tyrosines were replaced with phenylalanines (Cas15F) were incubated with or without 2 ng/ml TGF-β, after which immunoprecipitation was carried out as described in Figure 2B. (B) Lysates from CasWT and Cas15F cells plated on FN were immunoprecipitated with anti-Smad3N Ab and immunoblotted with anti-p130Cas mAb. Levels of p130Cas SD phosphorylation in lysates were determined by immunoblot analysis with anti-pY165-Cas Ab, which specifically recognizes phosphorylated YxxP tyrosines.
To further confirm that the Smad3–p130Cas interaction is directly regulated by integrin signaling, both CasWT and Cas15F cells were plated on FN-coated dishes and then subjected to immunoprecipitation analysis. Adhesion to FN by CasWT cells induced phosphorylation of the p130Cas SD and promoted endogenous interaction of Smad3 and p130Cas, so that a significant amount of phospho-p130Cas was detected within the Smad3–p130Cas complex (Figure 3B). In contrast, both the phosphorylation of p130Cas and its interaction with Smad3 were eliminated in Cas15F cells adhering to FN. Together, these data suggest that the Smad3–p130Cas interaction is positively regulated by integrin-mediated tyrosine phosphorylation of p130Cas.
p130Cas Reduces Both TβRI-mediated Phosphorylation and Nuclear Translocation of Smad3
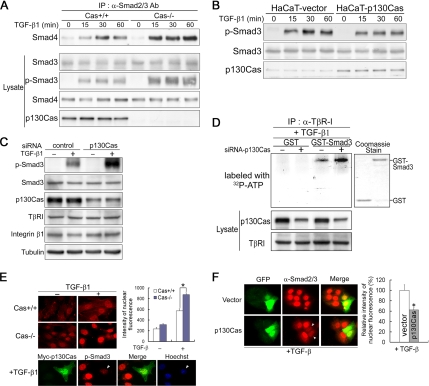
Given that the Smad3–p130Cas interaction is enhanced after integrin activation or TGF-β stimulation, and Cas15F cells showed higher levels of TGF-β–induced Smad3 phosphorylation than CasWT cells (Figure 3A), we set out to determine the functional role of p130Cas in TGF-β signaling. We found that p130Cas deficiency leads to increases in TGF-β-induced Smad3 phosphorylation and Smad3–Smad4 association (Figure 4A). Conversely, stable overexpression of p130Cas in HaCaT cells markedly reduced TGF-β–induced Smad3 phosphorylation (Figure 4B). To investigate the inhibitory effect of p130Cas under physiological conditions, we analyzed HaCaT cells expressing siRNA targeting p130Cas. The transfection efficiency for siRNAs, as analyzed with fluorescein-labeled siRNA duplex, was consistently >95%. We observed that knocking down p130Cas by using siRNA, which did not affect expression of β1 integrin or TβRI, led to increased TGF-β–induced Smad3 phosphorylation (Figure 4C).
Figure 4.
p130Cas reduces TGF-β–induced phosphorylation and nuclear translocation of Smad3. (A) Cas+/+ and Cas−/− MEFs were treated with 2 ng/ml TGF-β, after which their lysates were immunoprecipitated with anti-Smad2/3 Ab and immunoblotted with anti-Smad4 mAb and anti-Smad3 Ab. (B) HaCaT cells stably transfected with empty vector (HaCaT-vector) or p130Cas (HaCaT-p130Cas) were treated with 2 ng/ml TGF-β for the indicated time period, and then they were subjected to immunoblot analysis with anti-phospho-Smad3 Ab, anti-smad3 Ab, or anti-p130Cas mAb. (C) HaCaT cells transfected with control or p130Cas siRNA were treated with 2 ng/ml TGF-β, and then they were subjected to immunoblot analysis with anti-phospho-Smad3 Ab and anti-p130Cas mAb. (D) HaCaT cells transfected with control or p130Cas siRNA were incubated with 2 ng/ml TGF-β, after which their lysates were immunoprecipitated with anti-TβRI Ab. The precipitated immune complex was incubated with GST or GST-Smad3, after which in vitro kinase reactions were carried out using [γ-32P]ATP. The levels of p130Cas and TβRI in the lysates were determined by immunoblot analysis with anti-p130Cas mAb and anti-TβRI Ab. Equal amounts of GST and GST-Smad3 were used, as determined by Coomassie Blue staining (right). (E) Cas−/− and Cas+/+ MEFs were incubated with or without 2 ng/ml TGF-β, fixed, and stained with anti-phospho-Smad3 Ab. Fluorescence intensities within nuclei are indicated by the bar graph on the right. The data are means ± SD (n > 50 different cells; *p < 0.01). Bottom, Cas−/− MEFs transfected with Myc-p130Cas for 24 h were stained with anti-Myc mAb and anti-phospho-Smad3 Ab. Hoechst staining was used as a nuclear marker. (F) HaCaT cells transfected with GFP or GFP-p130Cas for 24 h were incubated with or without 2 ng/ml TGF-β, fixed, and stained with anti-Smad2/3 Ab. Quantification of the nuclear translocation of Smad2/3 is shown on the right. Data are means ± SD (n > 5 transfected cells). *p < 0.05 versus GFP-vector–transfected cells.
Because activated TβRI directly phosphorylates Smad3 on its C-terminal serine residues, we tested whether p130Cas interferes with the phosphorylation of Smad3 by TβRI. Using in vitro kinase assays with purified recombinant GST-Smad3 and TβRI immune complexes, we found that Smad3 was phosphorylated by TβRI immune complexes obtained from HaCaT cells (Figure 4D), which is consistent with previous reports (Shi and Massague, 2003). Moreover, TβRI immune complexes from p130Cas-knockdown HaCaT cells induced a greater increase of Smad3 phosphorylation than those from p130Cas-positive HaCaT cells (Figure 4D). This suggests that, in response to TGF-β, p130Cas may form Smad3-dependent complexes with TβRI and reduce the amount of Smad3 phosphorylation mediated by TβRI in intact cells.
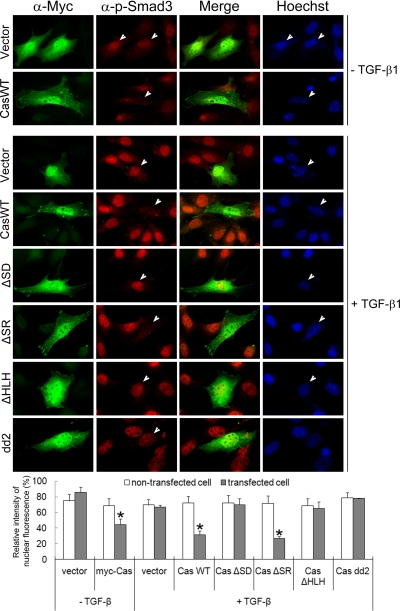
Consistent with those results, Cas−/− MEFs showed a much higher degree of nuclear translocation of phospho-Smad3 than did Cas+/+ MEFs, both in the absence and presence of TGF-β, and introduction of Myc-p130Cas into Cas−/− MEFs inhibited the nuclear translocation of phospho-Smad3 (Figure 4E). That p130Cas inhibits nuclear translocation of Smad3 was also confirmed by immunofluorescence analysis of HaCaT cells transiently transfected with GFP or GFP-p130Cas. Introduction of GFP-p130Cas into HaCaT cells led to a 40% reduction in the nuclear translocation of Smad3, compared with GFP-transfected cells (Figure 4F). Furthermore, using a set of p130Cas deletion mutants, we determined that deletion of its SD or HLH domain eliminated the ability of p130Cas to inhibit nuclear translocation of phospho-Smad3 (Figure 5). The Myc-p130Cas-negative cells that served as internal controls in these assays showed extensive nuclear phospho-Smad3 staining. We conclude from these experiments that p130Cas inhibits TGF-β-induced nuclear accumulation of Smad3 by reducing both its phosphorylation and its association with Smad4.
Figure 5.
Determination of the p130Cas regions required for their functionality. Cas−/− MEFs transfected for 24 h with Myc-p130Cas or one of its mutants were incubated with or without 2 ng/ml TGF-β, fixed, stained with anti-Myc mAb and anti-phospho-Smad3 Ab, and examined under a fluorescence microscope. Nuclei were stained with Hoechst dye. The maximum intensity of nuclear phospho-Smad3 fluorescence was set to 100% in each group. The data are means ± SD (n = ∼100 different nontransfected cells and ∼15 transfected cells). *p < 0.01 versus nontransfected control.
p130Cas Inhibits TGF-β–induced Transcriptional Activity and Growth Arrest
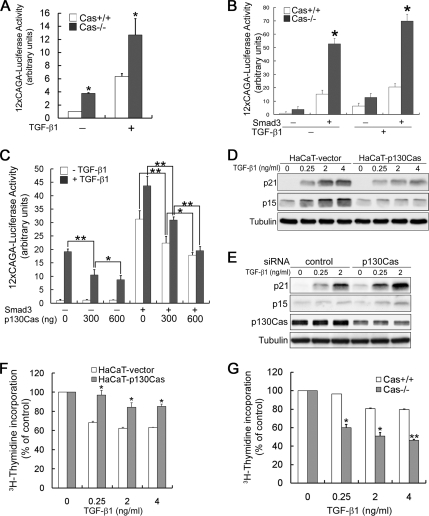
That p130Cas inhibits nuclear translocation of phospho-Smad3 prompted us to test its effect on TGF-β–induced transcriptional activity. For this analysis, we carried out reporter gene assays by using a Smad3-responsive (CAGA)12-luciferase reporter known to be activated by TGF-β through direct binding of Smad3 to the CAGA sequences upstream of the luciferase promoter. We found that this reporter showed greater activity when transfected into Cas−/− MEFs than when transfected into Cas+/+ MEFs (Figure 6A). As shown previously, overexpression of Smad3 activated transcription both in the absence and presence of TGF-β, and Cas−/− MEFs showed a higher degree of Smad3-dependent transcriptional activity than Cas+/+ MEFs (Figure 6B). This was further confirmed by the finding that overexpression of p130Cas in HEK293T cells dose-dependently suppressed Smad3-dependent transcriptional activation (Figure 6C). These results suggest that p130Cas may selectively suppress Smad3-dependent transcriptional activation by TGF-β signaling.
Figure 6.
p130Cas inhibits TGF-β–mediated transcription and growth arrest. (A) MEFs transfected with the Smad3-responsive reporter (CAGA)12-luc were incubated with or without 2 ng/ml TGF-β. Luciferase activity was measured 36 h later and normalized to the β-galactosidase activity. Luciferase activity is expressed as the mean ± SD of triplicates from a representative experiment performed at least three times. *p < 0.01 versus Cas+/+ MEFs. (B) MEFs transfected with the indicated plasmids were incubated with or without 2 ng/ml TGF-β, after which luciferase activity was measured as described in A. Data are representative of at least three independent experiments, and they are expressed as the mean ± SD. *p < 0.01 versus Cas+/+ MEFs. (C) HEK293T cells were cotransfected with a Smad3-responsive luciferase reporter, Smad3, and/or p130Cas, as indicated, after which luciferase activity was measured as described in A. Luciferase activity is expressed as the mean ± SD of triplicates from a representative experiment performed at least three times. *p < 0.05 and **p < 0.01. (D) HaCaT cells treated with the indicated concentration of TGF-β were subjected to immunoblot analysis with anti-p21 mAb and anti-p15 Ab. (E) HaCaT cells transfected with control or p130Cas siRNA were treated with 2 ng/ml TGF-β, and then they were subjected to immunoblot analysis with anti-p21 mAb and anti-p15 Ab. (F) HaCaT cells, (G) Cas+/+ and Cas−/− MEFs were incubated with or without the indicated concentration of TGF-β, after which [3H]thymidine incorporation was assayed as described in Materials and Methods. [3H]thymidine incorporation by cells incubated without TGF-β was defined as 100%. Data are representative of four independent experiments, and they are expressed as means ± SD. *p < 0.05 and **p < 0.01 versus HaCaT-vector or Cas+/+ MEFs.
It should be noted that one physiological function of TGF-β is to induce growth arrest at G1 phase, whereas a physiological function of integrin-coupled p130Cas is to promote cell cycle progression. In that context, we next asked whether the antiproliferative activity of TGF-β is negatively regulated by p130Cas. To address this question, we first tested the effect of p130Cas on TGF-β-induced p15 and p21 expression and growth arrest. As shown in Figure 6D, stable overexpression of p130Cas suppressed TGF-β-induced expression of both p15 and p21. Alternatively, silencing p130Cas using siRNA in HaCaT cells led to elevated expression of both p15 and p21 (Figure 6E).
To examine the effect of p130Cas on TGF-β–induced inhibition of cell proliferation, we carried out [3H]thymidine incorporation assays in HaCaT cells and MEFs. We found that HaCaT cells exhibited normal TGF-β dose-dependent inhibition of growth ([3H]thymidine incorporation; Figure 6F), and stable overexpression of p130Cas suppressed the TGF-β–induced growth inhibition. Consistent with these results, TGF-β–induced inhibition of growth was enhanced in Cas−/− MEFs, compared with Cas+/+ MEFs (Figure 6G). Thus, p130Cas seems to inhibit the antiproliferative effects of TGF-β.
Integrin Signaling Counteracts the Inhibitory Effects of TGF-β on Cell Growth
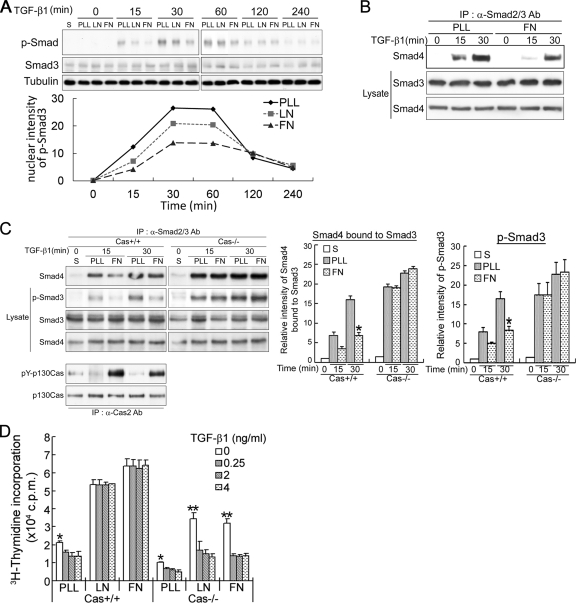
Studies have shown that adhesion reduces TGF-β–induced Smad2 phosphorylation and transcriptional activity (Thannickal et al., 2003) and that β1-integrin–mediated adhesion to ECM suppresses TGF-β–induced apoptosis and growth inhibition (Zhang et al., 2004). Based on these findings, we hypothesized that integrin signaling counteracts the inhibitory effects of TGF-β on growth by modulating the cellular responsiveness to TGF-β. To test that idea, we assessed the extent to which activation of integrin signaling affects the TGF-β signaling pathway leading to Smad3 phosphorylation. For this analysis, HaCaT cells were attached to LN-, FN- or PLL-coated plates, after which the effects of TGF-β on Smad3 phosphorylation and Smad3-Smad4 association were examined. We found that TGF-β–induced Smad3 phosphorylation was markedly reduced in cells plated on FN or LN, compared with PLL (Figure 7A), and that adhesion to FN also reduced Smad3–Smad4 association (Figure 7B), suggesting integrin-mediated adhesion to ECM may reduce the intensity of TGF-β signaling.
Figure 7.
p130Cas is essential for integrin-specific control of TGF-β–induced Smad3 phosphorylation and growth arrest. (A) HaCaT cells were plated on PLL-, LN- or FN-coated dishes for 10 min, and then they were incubated with 2 ng/ml TGF-β for up to 240 min, after which they were subjected to immunoblot analysis with anti-phospho-Smad3 Ab, anti-Smad3 Ab, or anti-tubulin mAb. The graphical presentations show the levels of phospho-Smad3 quantified by densitometry. S, suspension. (B) Lysates of HaCaT cells treated as described in A were immunoprecipitated with anti-Smad2/3 Ab and immunoblotted with anti-Smad4 mAb. (C) Cas+/+ and Cas−/− MEFs growing on PLL- or FN-coated dishes were incubated with 2 ng/ml TGF-β and immunoprecipitated with anti-Smad2/3 Ab to assess levels of Smad3-bound Smad4 and phosphorylation of Smad3. Levels of p130Cas and pY-p130Cas were determined by immunoprecipitation by using anti-Cas2 Ab, followed by immunoblot analysis with anti-p130Cas mAb or anti-phosphotyrosine Ab (4G10). The intensities of the bands corresponding to the immunoprecipitated Smad4 and phosphorylated Smad3 were quantified using LAS-3000 (Fujifilm, Tokyo, Japan). S, suspension. The data are expressed as means ± SD of triplicates. *p < 0.05 versus PLL-treated cells. (D) MEFs plated on PLL, LN, or FN were incubated for 24 h with or without TGF-β before labeling with [3H]thymidine, after which they were harvested and [3H]thymidine incorporation was assayed. Data are expressed as means ± SD of triplicates from a representative experiment performed at least three times. *p < 0.05 and **p < 0.01 versus TGF-β–treated cells.
Next, we examined whether p130Cas is essential for integrin-specific control of TGF-β–induced Smad3 phosphorylation. To address this question, TGF-β–induced Smad3 phosphorylation and Smad3–Smad4 association were examined in Cas +/+ and Cas−/− MEFs plated on FN or PLL. In Cas+/+ MEFs, TGF-β–induced Smad3 phosphorylation and Smad3–Smad4 association were both attenuated to a greater degree by adhesion to FN than to PLL, which is consistent with the results summarized in Figure 7, A and B. These effects were completely lost in Cas−/− MEFs (Figure 7C), so that levels of Smad3 phosphorylation and Smad3–Smad4 association were substantially higher in Cas−/− than Cas+/+ MEFs. Tyrosine phosphorylation of p130Cas in Cas+/+ MEFs was strongly induced by adhesion to FN, but not PLL, and it served as a marker of activation of integrin signaling by FN.
We then tested more directly whether p130Cas mediates the integrin-specific control of TGF-β–induced growth arrest. For this analysis, [3H]thymidine incorporation was assayed after allowing the cells to adhere to ECM and then treating them with TGF-β. As shown in Figure 7D, TGF-β functioned as a powerful inhibitor of [3H]thymidine incorporation (cell growth) in both Cas+/+ and, to a greater extent, Cas−/− MEFs plated on PLL, whereas the inhibitory effect of TGF-β on growth was largely lost in Cas+/+ MEFs, but not Cas−/− MEFs, plated on FN or LN. Thus, in the absence of p130Cas, TGF-β was capable of inhibiting growth, even in cells adhering to FN or LN. These data therefore suggest that adhesion to FN or LN reduces TGF-β–induced Smad3 phosphorylation, which in turn inhibits TGF-β growth arrest, and that p130Cas is an essential mediator of those effects.
The Cell Cycle Promotion by ECM–Integrin Coupling Induces p130–Smad3 Interaction
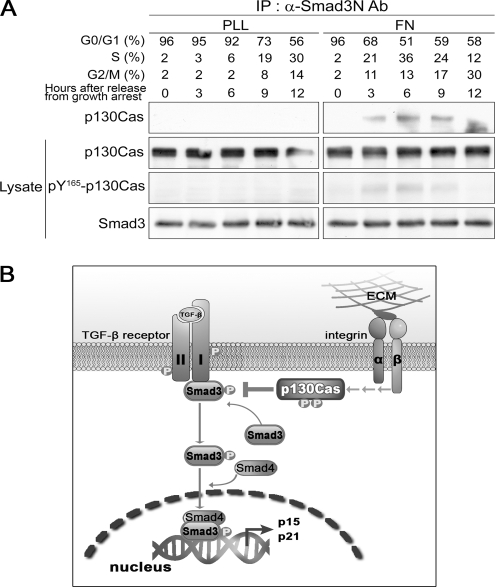
Our findings indicate that integrin signaling counteracts the inhibitory effects of TGF-β on cell cycle progression and that this effect is mediated by p130Cas. In addition, p130Cas reduced TGF-β–induced phosphorylation of Smad3 by direct interaction and this interaction was regulated by tyrosine phosphorylation of p130Cas mediated by integrin signaling. The cell cycle promotion by ECM–integrin coupling induces and requires tyrosine phosphorylation of p130Cas (Bouton et al., 2001; Chodniewicz and Klemke, 2004). Finally, therefore, we investigated whether the integrin-mediated interaction of p130Cas and Smad3 occurs during cell cycle progression from G1 to S phase. To address this question, HaCaT cells were initially synchronized at G0/G1 phase by contact inhibition and serum starvation, after which they were released from growth arrest by plating on FN- or PLL-coated dishes containing complete medium. At selected times thereafter, some cells were lysed and immunoprecipitated with anti-Smad3N Ab to assess Smad3-p130Cas interaction, whereas others were prepared in parallel and analyzed by flow cytometry to determine the cell cycle distribution at each time point. In cells plated on FN, maximal levels of Smad3–p130Cas interaction and tyrosine phosphorylation of p130Cas were detected at the G1/S junction (Figure 8A), whereas these phenomena were completely absent in cells plated on PLL. It thus seems likely that the Smad3–p130Cas interaction at G1/S phase is involved in cell cycle progression.
Figure 8.
Interaction Smad3 and p130Cas and the tyrosine phosphorylation of p130Cas oscillate in a cell cycle-dependent manner. (A) HaCaT cells were synchronized by contact inhibition and serum starvation. The resultant synchronized cells were plated on PLL- or FN-coated dishes containing fresh complete medium, after which the lysates were immunoprecipitated with anti-Smad3N Ab and immunoblotted with anti-p130Cas mAb, anti-Smad3 Ab, or anti-pY165-Cas Ab. (B) Model illustrating a possible mechanism by which p130Cas regulates TGF-β–mediated cell cycle arrest.
DISCUSSION
We provide evidence that p130Cas can interact with Smad3, a key mediator of TGF-β signaling, and as a consequence of this interaction, integrin signaling counteracts the inhibitory effects of TGF-β on cell growth. Using coimmunoprecipitation and GST-pull down assays with lysates from p130Cas- or Smad3-overexpressing cells, we examined the interaction between p130Cas and Smad3. Although some endogenous basal p130Cas–Smad3 interaction was detected, upon integrin-ECM coupling or TGF-β stimulation, p130Cas or Smad3 was phosphorylated, and their interaction increased considerably (Figures 2 and 3). Under conditions favorable for this interaction, activation of Smad3 and its translocation into the nucleus were inhibited, thereby reducing its transcriptional activity. That in turn led to inhibition of p15 and p21 expression and facilitation of cell cycle progression from G1 to S phase. The molecular mechanism by which p130Cas inhibits the receptor-mediated phosphorylation of Smad3, its interaction with Smad4 and nuclear accumulation likely involves the association of p130Cas with Smad3, suggesting Smad3 serves as a target of inhibition by p130Cas.
But how does p130Cas inhibit the TGF-β–dependent phosphorylation of Smad3? One possible mechanism is illustrated in Figure 8B. TGF-β signaling is initiated when a ligand induces formation of a heteromeric complex of TβRII and TβRI (Massague and Chen, 2000; Shi and Massague, 2003). After receptor activation, Smad3 is recruited to the receptor complex, where TβRI phosphorylates its C-terminal SXS motif. This causes a conformational change in and activation of Smad3, as well as its dissociation from the complex (Shi and Massague, 2003). We showed herein that p130Cas can interact with TβRI through the mediation of Smad3 and that both the Smad3-p130Cas and p130Cas–TβRI interactions occurred in the presence of TGF-β, but it is not yet clear whether these proteins constitute a trimer or sequential dimers. Based on these findings, we hypothesize that p130Cas acts to block or attenuate the dissociation of phospho-Smad3 from TβRI via the complex formation. Through that action, p130Cas likely sets a threshold level for TGF-β–mediated responses, which depends on the level of expression or tyrosine-phosphorylation of p130Cas. Additional details of the molecular mechanism of p130Cas as a regulatory component in the TGF-β signaling pathway await further analysis.
We defined p130Cas as an inhibitor that functions at an early step in the TGF-β signaling pathway, preventing receptor-dependent phosphorylation of Smad3, its subsequent association with Smad4, and its nuclear accumulation. We therefore suggest that p130Cas acts as a general inhibitor of phospho-Smad3, as opposed to modulating subsets of Smad3-mediated responses, such as growth inhibition or transcription of target genes that include cell cycle regulators (p15 and p21). These conclusions are broadly consistent with all of the transcriptional assays and [3H]thymidine incorporation assays that we used to study activation of Smad3 in response to TGF-β signaling. In Smad3-responsive (CAGA)12-luciferase reporter assays, p130Cas dose-dependently suppressed the (CAGA)12-luciferase transcriptional response to TGF-β, in both MEFs and epithelial cells, suggesting that p130Cas selectively and directly suppresses Smad3-dependent transcriptional activation.
It is reasonable to suggest that integrin signaling might counteract the inhibitory effects of TGF-β on cell growth and that p130Cas is an essential mediator of this effect. In fact, we observed 1) that the interaction of Smad3 and p130Cas is positively regulated by tyrosine phosphorylation of p130Cas mediated by integrin signaling; 2) that the integrin-mediated interaction of p130Cas and Smad3 occurs during cell cycle progression from G1 to S phase; 3) that integrin-mediated adhesion to ECM such as FN and LN reduces phosphorylation of Smad3 and its association with Smad4, thereby inhibiting TGF-β-induced growth arrest in both MEFs and HaCaT cells; and 4) that knocking out p130Cas in [3H]thymidine incorporation assays not only reduced the rate of MEF proliferation but also attenuated the inhibitory effect of ECM on TGF-β signaling. Our results thus establish p130Cas as a negative modulator of the TGF-β signaling pathway and provide novel insight into the negative regulation of TGF-β signaling by the integrin signal pathway.
Members of the TGF-β superfamily are potent regulators of the growth and differentiation of a wide range of cell types (Massague, 1990). This makes spatial and temporal control of their activity critical to normal development and homeostasis (Siegel and Massague, 2003). A wide range of mediators exist to control the activity of TGF-β, including various intracellular antagonists such as C-Myc, Ras and CDKs, which bind to TGF-β receptors, and Smads, which prevent the receptors' activation (Kretzschmar et al., 1999; Massague and Chen, 2000; Feng and Derynck, 2005). Our studies also define a novel intracellular mechanism that can function to suppress TGF-β signaling. Perturbation of these negative regulators of TGF-β signaling could be linked to the pathogenesis of various clinical disorders, especially the initiation and progression of tumors (Kretzschmar, 2000; Pardali and Moustakas, 2007).
The loss of the antiproliferative function of TGF-β, sometimes as a result of mutations that directly inactivate components of the TGF-β/Smad signaling pathway, is a hallmark of many cancers (Wakefield and Roberts, 2002). Indeed, such mutations have been identified in various human cancers (e.g., breast cancer), making the cells refractory to TGF-β–induced growth inhibition (Kretzschmar, 2000; Siegel and Massague, 2003; Pardali and Moustakas, 2007). It is therefore noteworthy that our findings suggest that overexpression or hyperphosphorylation of p130Cas can enhance TGF-β resistance. In fact, we have early evidence that p130Cas is often overexpressed or hyperphosphorylated in human cancer cells, especially breast cancer and melanoma cells (data not shown) and that this is essential for induction of cell transformation and anchorage-independent growth (Kook et al., 2000; Guo and Giancotti, 2004; Wei et al., 2004). For example, although cells that have undergone neoplastic transformation are much less dependent on ECM adhesion for their survival, they often exhibit integrin expression that is critical for the initiation of tumorigenesis and maintenance of their proliferative capacity, but they tend to lose expression of integrins that exert an inhibitory effect on growth (Giancotti and Ruoslahti, 1999; Owens et al., 2003; Guo and Giancotti, 2004). Thus, integrin signaling via p130Cas may provide an important clue to the mechanism underlying the resistance to TGF-β–induced growth suppression seen in some cancers, and it will be important in the future to determine whether p130Cas-mediated control of TGF-β/Smad signaling contributes to the initiation and progression of human cancers that harbor an active integrin signal.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Edward B. Leof (Mayo Clinic College of Medicine) for anti-phospho-Smad3 antibody, Dr. Aristidis Moustakas (Ludwig Institute for Cancer Research) for Myc-Smad2/3 cDNAs and the Smad3-responsive (CAGA)12-luc reporter, Dr. J. Massagué (Sloan-Kettering Institute) for Flag-Smad3 cDNA, and Dr. Anita B. Roberts (National Institutes of Health) for Smad3−/− MEFs. This study was supported by grants from the Cell Dynamics Research Center (R11-2007-007-02001-0), the Bioimaging Research Center, the Research Program for New Drug Target Discovery (2006-02594, The Ministry of Science and Technology, South Korea), and the U.S. National Institutes of Health (grant No. GM049882 to S.K.H.).
Abbreviations used:
- Ab
antibody
- FN
fibronectin
- FxxP
phenylalanine-Xaa-Xaa-proline
- HLH
helix-loop-helix
- LN
laminin
- mAb
monoclonal antibody
- MEF
mouse embryonic fibroblast
- p130Cas
Crk-associated substrate, 130 kDa
- PLL
poly-l-lysine
- S3A
S464SVS467-A464AVA467 mutation
- S3D
S464SVS467-D464DVD467 mutation
- SD
substrate domain
- TGF
transforming growth factor
- TβRI
transforming growth factor-β type I receptor
- TβRII
transforming growth factor-βtype II receptor
- YxxP
tyrosine-Xaa-Xaa-proline.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-10-0991) on March 5, 2008.
REFERENCES
- Avraham H. K., Lee T. H., Koh Y., Kim T. A., Jiang S., Sussman M., Samarel A. M., Avraham S. Vascular endothelial growth factor regulates focal adhesion assembly in human brain microvascular endothelial cells through activation of the focal adhesion kinase and related adhesion focal tyrosine kinase. J. Biol. Chem. 2003;278:36661–36668. doi: 10.1074/jbc.M301253200. [DOI] [PubMed] [Google Scholar]
- Bouton A. H., Riggins R. B., Bruce-Staskal P. J. Functions of the adapter protein Cas: signal convergence and the determination of cellular responses. Oncogene. 2001;20:6448–6458. doi: 10.1038/sj.onc.1204785. [DOI] [PubMed] [Google Scholar]
- Cary L. A., Han D. C., Polte T. R., Hanks S. K., Guan J. L. Identification of p130Cas as a mediator of focal adhesion kinase-promoted cell migration. J. Cell Biol. 1998;140:211–221. doi: 10.1083/jcb.140.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casamassima A., Rozengurt E. Tyrosine phosphorylation of p130(cas) by bombesin, lysophosphatidic acid, phorbol esters, and platelet-derived growth factor. Signaling pathways and formation of a p130(cas)-Crk complex. J. Biol. Chem. 1997;272:9363–9370. doi: 10.1074/jbc.272.14.9363. [DOI] [PubMed] [Google Scholar]
- Chodniewicz D., Klemke R. L. Regulation of integrin-mediated cellular responses through assembly of a CAS/Crk scaffold. Biochim. Biophys. Acta. 2004;1692:63–76. doi: 10.1016/j.bbamcr.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Comoglio P. M., Boccaccio C., Trusolino L. Interactions between growth factor receptors and adhesion molecules: breaking the rules. Curr. Opin. Cell Biol. 2003;15:565–571. doi: 10.1016/s0955-0674(03)00096-6. [DOI] [PubMed] [Google Scholar]
- Defilippi P., Di Stefano P., Cabodi S. p130Cas: a versatile scaffold in signaling networks. Trends Cell Biol. 2006;16:257–263. doi: 10.1016/j.tcb.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Di Guglielmo G. M., Le Roy C., Goodfellow A. F., Wrana J. L. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat. Cell Biol. 2003;5:410–421. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- Feng X. H., Derynck R. Specificity and versatility in TGF-beta signaling through Smads. Annu. Rev. Cell Dev. Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- Fonseca P. M., Shin N. Y., Brabek J., Ryzhova L., Wu J., Hanks S. K. Regulation and localization of CAS substrate domain tyrosine phosphorylation. Cell Signal. 2004;16:621–629. doi: 10.1016/j.cellsig.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Giancotti F. G., Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Giancotti F. G., Tarone G. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu. Rev. Cell Dev. Biol. 2003;19:173–206. doi: 10.1146/annurev.cellbio.19.031103.133334. [DOI] [PubMed] [Google Scholar]
- Guo W., Giancotti F. G. Integrin signalling during tumour progression. Nat. Rev. Mol. Cell Biol. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- Hayashida T., Decaestecker M., Schnaper H. W. Cross-talk between ERK MAP kinase and Smad signaling pathways enhances TGF-beta-dependent responses in human mesangial cells. FASEB J. 2003;17:1576–1578. doi: 10.1096/fj.03-0037fje. [DOI] [PubMed] [Google Scholar]
- Honda H., et al. Cardiovascular anomaly, impaired actin bundling and resistance to Src-induced transformation in mice lacking p130Cas. Nat. Genet. 1998;19:361–365. doi: 10.1038/1246. [DOI] [PubMed] [Google Scholar]
- Kim W., Kook S., Kim D. J., Teodorof C., Song W. K. The 31-kDa caspase-generated cleavage product of p130cas functions as a transcriptional repressor of E2A in apoptotic cells. J. Biol. Chem. 2004;279:8333–8342. doi: 10.1074/jbc.M312026200. [DOI] [PubMed] [Google Scholar]
- Kook S., Shim S. R., Choi S. J., Ahnn J., Kim J. I., Eom S. H., Jung Y. K., Paik S. G., Song W. K. Caspase-mediated cleavage of p130cas in etoposide-induced apoptotic Rat-1 cells. Mol. Biol. Cell. 2000;11:929–939. doi: 10.1091/mbc.11.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar M. Transforming growth factor-beta and breast cancer: transforming growth factor-beta/SMAD signaling defects and cancer. Breast Cancer Res. 2000;2:107–115. doi: 10.1186/bcr42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar M., Doody J., Timokhina I., Massague J. A mechanism of repression of TGFbeta/ Smad signaling by oncogenic Ras. Genes Dev. 1999;13:804–816. doi: 10.1101/gad.13.7.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Elia A. E., Law S. F., Golemis E. A., Farley J., Wang T. A novel ability of Smad3 to regulate proteasomal degradation of a Cas family member HEF1. EMBO J. 2000;19:6759–6769. doi: 10.1093/emboj/19.24.6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J. The transforming growth factor-beta family. Annu. Rev. Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- Massague J., Chen Y. G. Controlling TGF-beta signaling. Genes Dev. 2000;14:627–644. [PubMed] [Google Scholar]
- Matsuura I., Denissova N. G., Wang G., He D., Long J., Liu F. Cyclin-dependent kinases regulate the antiproliferative function of Smads. Nature. 2004;430:226–231. doi: 10.1038/nature02650. [DOI] [PubMed] [Google Scholar]
- Moro L., et al. Integrin-induced epidermal growth factor (EGF) receptor activation requires c-Src and p130Cas and leads to phosphorylation of specific EGF receptor tyrosines. J. Biol. Chem. 2002;277:9405–9414. doi: 10.1074/jbc.M109101200. [DOI] [PubMed] [Google Scholar]
- Nakao A., et al. Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signalling. Nature. 1997;389:631–635. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- Owens D. M., Romero M. R., Gardner C., Watt F. M. Suprabasal alpha6beta4 integrin expression in epidermis results in enhanced tumourigenesis and disruption of TGFbeta signalling. J. Cell Sci. 2003;116:3783–3791. doi: 10.1242/jcs.00725. [DOI] [PubMed] [Google Scholar]
- Pardali K., Moustakas A. Actions of TGF-beta as tumor suppressor and pro-metastatic factor in human cancer. Biochim. Biophys. Acta. 2007;1775:21–62. doi: 10.1016/j.bbcan.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Sakai R., Iwamatsu A., Hirano N., Ogawa S., Tanaka T., Mano H., Yazaki Y., Hirai H. A novel signaling molecule, p130, forms stable complexes in vivo with v-Crk and v-Src in a tyrosine phosphorylation-dependent manner. EMBO J. 1994;13:3748–3756. doi: 10.1002/j.1460-2075.1994.tb06684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. A., Ginsberg M. H. Networks and crosstalk: integrin signalling spreads. Nat. Cell Biol. 2002;4:E65–68. doi: 10.1038/ncb0402-e65. [DOI] [PubMed] [Google Scholar]
- Seo S. R., Ferrand N., Faresse N., Prunier C., Abecassis L., Pessah M., Bourgeade M. F., Atfi A. Nuclear retention of the tumor suppressor cPML by the homeodomain protein TGIF restricts TGF-beta signaling. Mol. Cell. 2006;23:547–559. doi: 10.1016/j.molcel.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Shi Y., Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Shin N. Y., Dise R. S., Schneider-Mergener J., Ritchie M. D., Kilkenny D. M., Hanks S. K. Subsets of the major tyrosine phosphorylation sites in Crk-associated substrate (CAS) are sufficient to promote cell migration. J. Biol. Chem. 2004;279:38331–38337. doi: 10.1074/jbc.M404675200. [DOI] [PubMed] [Google Scholar]
- Siegel P. M., Massague J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat. Rev. Cancer. 2003;3:807–821. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- Ten Dijke P., Goumans M. J., Itoh F., Itoh S. Regulation of cell proliferation by Smad proteins. J. Cell. Physiol. 2002;191:1–16. doi: 10.1002/jcp.10066. [DOI] [PubMed] [Google Scholar]
- Thannickal V. J., Lee D. Y., White E. S., Cui Z., Larios J. M., Chacon R., Horowitz J. C., Day R. M., Thomas P. E. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J. Biol. Chem. 2003;278:12384–12389. doi: 10.1074/jbc.M208544200. [DOI] [PubMed] [Google Scholar]
- Wakefield L. M., Roberts A. B. TGF-beta signaling: positive and negative effects on tumorigenesis. Curr. Opin. Genet Dev. 2002;12:22–29. doi: 10.1016/s0959-437x(01)00259-3. [DOI] [PubMed] [Google Scholar]
- Wei L., Yang Y., Zhang X., Yu Q. Cleavage of p130Cas in anoikis. J. Cell. Biochem. 2004;91:325–335. doi: 10.1002/jcb.10760. [DOI] [PubMed] [Google Scholar]
- Wolfraim L. A., Walz T. M., James Z., Fernandez T., Letterio J. J. p21Cip1 and p27Kip1 act in synergy to alter the sensitivity of naive T cells to TGF-beta-mediated G1 arrest through modulation of IL-2 responsiveness. J. Immunol. 2004;173:3093–3102. doi: 10.4049/jimmunol.173.5.3093. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Even-Ram S. Integrin regulation of growth factor receptors. Nat. Cell Biol. 2002;4:E75–76. doi: 10.1038/ncb0402-e75. [DOI] [PubMed] [Google Scholar]
- Zhang H., Ozaki I., Mizuta T., Yoshimura T., Matsuhashi S., Eguchi Y., Yasutake T., Hisatomi A., Sakai T., Yamamoto K. Transforming growth factor-beta 1-induced apoptosis is blocked by beta 1-integrin-mediated mitogen-activated protein kinase activation in human hepatoma cells. Cancer Sci. 2004;95:878–886. doi: 10.1111/j.1349-7006.2004.tb02197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.