Abstract
Signaling pathways engaged by angiogenic factors bFGF and VEGF in tumor angiogenesis are not fully understood. The current study identifies cytoplasmic tyrosine kinase c-Abl as a key factor differentially mediating bFGF- and VEGF-induced angiogenesis in microvascular endothelial cells. STI571, a c-Abl kinase inhibitor, only inhibited bFGF- but not VEGF-induced angiogenesis. bFGF induced membrane receptor cooperation between integrin β3 and FGF receptor, and triggered a downstream cascade including FAK, c-Abl, and MAPK. This signaling pathway is different from one utilized by VEGF that includes integrin β5, VEGF receptor-2, Src, FAK, and MAPK. Ectopic expression of wild-type c-Abl sensitized angiogenic response to bFGF, but kinase dead mutant c-Abl abolished this activity. Furthermore, the wild-type c-Abl enhanced angiogenesis in both Matrigel implantation and tumor xenograft models. These data provide novel insights into c-Abl's differential functions in mediating bFGF- and VEGF-induced angiogenesis.
INTRODUCTION
Tumor angiogenesis, a process of new vasculature formation, is appreciated to be an integral part of solid tumor development (Hanahan and Folkman, 1996; Bussolino et al., 1997). The supply of new blood vessels in tumors not only fosters autonomous tumor growth but also helps remove accumulated waste and ameliorate burden metabolism. Microvascular endothelial cells, a major component of microvasculatures exert angiogenic function via increased cell adhesion, migration, and capillary network formation (Folkman, 1996; Bergers and Benjamin, 2003).
A number of angiogenic factors derived from tumor cells and tumor infiltrating inflammatory cells such as macrophages and mast cells can stimulate endothelial cell activation and initiate angiogenesis. Growth factors such as basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), and platelet-derived growth factor (PDGF), angiopoietins, and ephrins have been well characterized in tumor angiogenesis (Sato et al., 1995; Yancopoulos et al., 2000; Ferrari et al., 2006). For example, a plethora of evidence has demonstrated that elevated bFGF and VEGF production by cancer cells directly correlates with tumor angiogenesis and tumor development (Kim et al., 1993; Polnaszek et al., 2003). As one of the first angiogenic peptides discovered, bFGF is largely sequestered in extracellular matrix (ECM) of virtually all tissues and its angiogenic function appears to become prominent when tumors invade and degrade ECM to release the sequestered bFGF. There is evidence showing that blockade of bFGF production markedly restrained tumor angiogenesis and tumor growth in xenograft tumor models (Fahmy et al., 2003). Like bFGF, VEGF is considered the most potent angiogenic factor capable of inducing angiogenesis in multiple tumor models (Kim et al., 1993). Systemic administration of anti-VEGF antibody can dramatically inhibit tumor angiogenesis in xenograft mouse models, suggesting significant benefits for the therapeutic intervention in cancer patients. All of the evidence has highlighted the pathological importance of these two growth factors in the regulation of tumor angiogenesis.
VEGF exerts its angiogenic function through binding to two homologous tyrosine kinase receptors, termed VEGFR1 (also called flt-1) and VEGFR2 (named Flk-1/KDR) on the endothelial cell membrane. Accumulating evidence demonstrates that Flk-1/KDR functions as a positive regulator of endothelial cell proliferation and cell migration, whereas the functional role of flt-1 is still controversial, with a more likely negative nature in regulation of cell activities (Millauer et al., 1996; Neufeld et al., 1999; Prewett et al., 1999; Ferrari et al., 2006; Schumacher et al., 2007). Once activated by VEGF, Flk-1/KDR undergoes autophosphorylation on specific tyrosine residues followed by a connection of the Tyr(P) residues to adapter and signaling proteins that contain Src homology domain 2 (SH2; Guo et al., 1995). Subsequently, these mediators can activate multiple downstream effectors including focal adhesion kinase (FAK) and mitogen-activated protein kinase (MAPK) kinases (Erk, p38, and/or JNK kinase; Abedi and Zachary, 1997; Wheeler-Jones et al., 1997). Flk-1/KDR also has the ability to trigger the activation of other several signaling cascades including phospholipase C-γ (PLC γ) and PI3K-dependent Akt/PKB (Sun et al., 2005; Zeng et al., 2006). This Flk-1/KDR-mediated intracellular signaling appears to be similar to the pathway that bFGF engages; however, there is evidence suggesting that unlike VEGF, bFGF-induced angiogenesis is independent of Src kinase activity (Eliceiri et al., 1999, 2002). Furthermore, distinct upstream components of FAK may mediate VEGF- and bFGF-induced angiogenic activity, respectively.
Since the last decade, research has focused on characterization of interactions among multiple membrane-bound receptors, leading to the establishment of a paradigm that, instead of transmitting signals across the membrane individually, each membrane-anchored receptor usually associates and coordinates with other adjacent membrane-bound receptors to synergistically induce an array of intracellular signaling cascades (Schneller et al., 1997; Eliceiri, 2001; Weis et al., 2007). Such a typical example of the receptor interaction and cooperation has been illustrated in a model of cross-talk between α/β integrins and membrane-associated tyrosine kinase receptors, a synergistic event responsible for transmitting signals for both ligands (Senger et al., 1996; Soldi et al., 1999). The cytoplasmic tail of the β subunit integrin participates in a physical interaction between integrins and the cytoplasmic domain of growth factor receptors to achieve the coordination of both signaling pathways (Blystone et al., 1996; Jones et al., 1997). By this mechanism, growth factors that activate receptor tyrosine kinases can modulate integrin-mediated functions such as cell adhesion, spreading, and migration via alterations in integrin activation and localization. Conversely, signals from integrins can regulate the full activation of growth factor–induced signaling cascades. For example, engagement of integrin αvβ3 with activated Flk-1/KDR in human umbilical vein endothelial cells (HUVECs) is required for cell migration and adhesion in response to VEGF (Senger et al., 1996; Soldi et al., 1999). Activated EGFR is required to cooperate with integrin αvβ5 in the same manner for vitronectin-induced carcinoma cell migration. Furthermore, blockade of integrin αvβ3 activity with antibody (LM 609) prevents blood vessel formation induced by bFGF in a chick chorioallantoic membrane assay (Brooks et al., 1994a). Likewise, anti-integrin αvβ5 antibody (P1F6) inhibits VEGF-dependent angiogenesis (Friedlander et al., 1995). Taken together, the data indicate that the convergence of signaling cascades from integrins and tyrosine kinase receptors is essential for angiogenic activity.
The proto-oncoprotein c-Abl is a family member of nonreceptor tyrosine kinases with a molecular mass of 140 kDa and contains multiple functional domains including an SH2, SH3, kinase, and DNA-binding and actin-binding domains. The kinase activity of c-Abl is putatively inactive in most cells and is largely regulated by SH3 domain–binding protein and ATP binding of lysine at position 290 (Echarri and Pendergast, 2001; Le Bras et al., 2007). A point mutation of lysine substituted with arginine (K290R) leads to kinase inhibition, whereas deletion or mutation of the SH3 domain results in kinase activation. Interestingly, evidence that aberrant expression of c-Abl is present in microvasculature of a variety of human cancers, including infiltrating breast carcinoma, gastric adenocarcinoma, and liposarcoma, suggests c-Abl to be intimately associated with tumor angiogenesis (O'Neill et al., 1997). The current study is to determine whether c-Abl is a key intracellular molecule–mediating angiogenesis induced by bFGF and/or VEGF.
MATERIALS AND METHODS
Generation of Cells Stably Expressing Different Versions of c-Abl
Full-length of c-Abl wild-type (WT) or mutant cDNA in a retroviral pCMV-neo-vector was transduced to human microvascular endothelial cells (HMVECs) by a retroviral infection system. 293T retroviral packaging cells were transfected with the c-Abl constructs or vector control in the presence of pCL 10A1 vector using Fugene 6 as the delivery vehicle. Forty-eight hours after transfection, the supernatant was harvested and filtered through a 0.45-μm pore size filter, and the virus-containing medium was used to infect HMVECs. Selection with 400 μg/ml G418 was started 48 h after infection, and the drug-resistant cell populations were used for subsequent studies.
Migration Assays
HMVECs (2 × 105) were preincubated with serum-free medium for 12 h and transferred onto transwells (24-well plates) precoated with collagen IV (100 μg/ml) or fibronectin (50 μg/ml). The lower chamber of transwells included bFGF or VEGF, with or without the c-Abl kinase inhibitor STI571. After 4 h of incubation, cells in the top chamber of the transwells membrane were removed using a Q-tip. The membrane-trapped cells were fixed and stained with hematoxylin. Average cell numbers were calculated from five different fields in each sample.
Tube Formation Assays
HMVECs were preincubated with serum-free medium for 12 h. The cells (0.1 × 105) treated with bFGF, VEGF, or STI571 were transferred onto 96-well Matrigel (Becton Dickson Laboratories, Bedford, MA). After 12 h of incubation, tube-forming structures were fixed with 10% Formalin and stained with SolII of Diff-Quick stain set (Dade Behring, Newark, DE).
[3H]thymidine Incorporation
Cells were grown in 6- or 12-well plates to subconfluence, and then culture medium was changed to serum-free medium supplemented with 0.2% BSA in the presence of bFGF, VEGF, or STI571 for 24 h. [3H]thymidine, 1–2 μCi, was introduced to each well for 6 h. After extensive washes with PBS, the cells were scraped and precipitated in 200 μl of 10% trichloroacetic acid and then dissolved in 0.3 ml of 0.3 M NaOH. Radioactivity was quantitated by a liquid scintillation counter.
Cell Survival Assay
Cells (5 × 105) were planted on the 35-mm plates and starved with serum-free medium for 5 d in the presence of bFGF, VEGF, or STI571. Cells were washed with PBS and then survival cells were fixed, stained, and counted.
Immunocytochemistry
Cells were transferred onto glass slides to culture for 2 d. After treatment of the cells with bFGF or VEGF in the absence or presence of STI571, the cells were fixed with 4% paraformaldehyde and incubated with rhodamine phalloidin (Molecular Probes, Eugene, OR) for 1 h. Finally, the florescence was evaluated under a microscope.
Immunoprecipitation and Western Blot
Cells were lysed with lysis buffer (pH 7.4) containing 0.25 mM HEPES, 14.9 mM NaCl, 10 mM NaF, 2 mM MgCl2, 0.5% NP-40, 0.1 mM PMSF, 20 μM pepstatin A, and 20 μM leupeptin. The lysates were centrifuged at 1000 × g for 10 min, and the resulting supernatant was collected for immunoblotting. For immunoprecipitation, cells were lysed with lysis buffer containing 10 mM Tris, pH 7.4, 1% triton, 0.5% NP-40, 150 mM NaCl, 20 mM NaF, 0.2 mM Na3VO4, 1 mM EDTA, 1 mM EGTA, and 0.2 mM PMSF. The samples were then incubated with anti-integrin β3, β5 (Chemicon, Temecula, CA), Flk-1/KDR (Santa Cruz Biotechnology, Santa Cruz, CA), FGFR (Cell Signaling, Beverly, MA), or c-Abl antibody at 4°C for overnight, followed by incubation with protein A Sepharose beads at 4°C for 2 h. The immunocomplex was extensively washed, and the samples were subjected to immunoblots. The primary antibodies pY20 (ICN, Costa Mesa, CA), Erk 1 and Erk 2 (Santa Cruz), EGFR, Flk-1/KDR, FAK, Src, β3, β5, and c-Abl pY245 (Biosource, Camarillo, CA) were used to examine protein expressions. Specific signals were detected using an ECL kit (Pierce VWR, Rockford, IL).
Angiogenesis In Vivo
HMVECs (1 × 106) were mixed with Matrigel (0.5 ml at 10 mg/ml, in liquid form at 4°C) and 64 U/ml heparin in the presence of bFGF or VEGF (1 μg). The samples were injected into the dorsal subcutaneous tissues of 4-wk-old female SCID/Beige mice (Charles River, Wilmington, MA). Some animals were treated with STI571 (150 mg/kg/d) or vehicle by oral gavage every other day for 10 d. Once the mice were killed, the recovered Matrigel plugs were fixed in 10% Formalin.
Induction of Tumor Xenografts in Mice
SCID/Beige mice were subcutaneously injected with MDA-MB-231 cells (1 × 106) and control or c-Abl–producing HMVECs (1 × 105) in 0.2 ml of Hanks' balance buffer without calcium and magnesium. The growth of solid tumors from the injected cells was monitored daily for up to 12 wk before the animals were killed to remove tumors for analysis. The tumors were measured and calculated as follows: volume = length × width2× 0.52.
Immunohistochemistry
For paraffin-embedded Matrigel plug, vessels formed by HMVECs were analyzed by examining CD31 immunohistochemistry staining. In brief, the samples were blocked with 3% H2O2 to block endogenous peroxidase activity at room temperature for 30 min followed by incubation with blocking buffer (Vector Laboratories, Burlingame, CA) containing 10% goat serum for 1 h. Then, mouse anti-CD31 antibody (1: 500, Dako) was incubated at room temperature for 2 h and goat anti-rat secondary antibody (1: 100) conjugated with HRP were added at room temperature for 30 min. Finally, DAB substrate (Dako, Carpinteria, CA) was introduced for several minutes, and after washing, methyl green was used for counterstaining. For tumor angiogenesis, the frozen tumor tissues were cut to 6–10-μm thickness and processed for the staining of CD31 (Becton Dickinson Pharmagen, Mountain View, CA).
Statistics
Data are expressed as mean ± SE, and n refers to the numbers of individual experiments performed. Differences among groups were determined using one-way analysis of variance followed by the Newman-Keuls produce. The 0.05 level of probability was used as the criterion of significance.
RESULTS
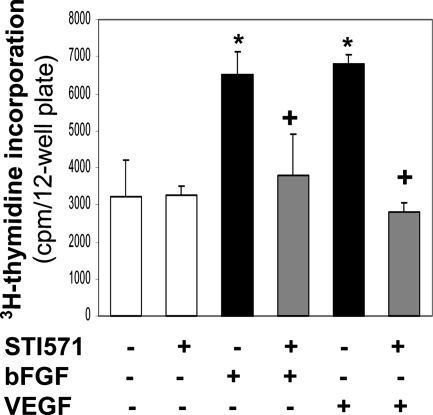
To characterize the angiogenic activity of growth factors bFGF and VEGF in vitro, we used HMVECs, a model preferential for the study of angiogenesis (Shao and Guo, 2004). The cells were immortalized by the stable expression of telomerase catalytic protein gene in primary endothelial cells. As potent mitogens, both bFGF and VEGF stimulated HMVEC proliferation (Figure 1). To determine whether this growth factor–induced cell proliferation requires activity of the nonmembrane receptor tyrosine kinase c-Abl, we added a c-Abl kinase inhibitor STI571 (also named imatinib mesylate) into the cells. As shown in Figure 1, treatment with STI571 abolished the responses of cell division to both bFGF and VEGF, suggesting that c-Abl activity is essential for cell proliferation induced by bFGF or VEGF.
Figure 1.
STI571 inhibits HMVEC proliferation induced by bFGF and VEGF. HMVECs were pretreated with bFGF (5 ng/ml) or VEGF (10 ng/ml) in the absence or presence of STI571 (10 μM) for 24 h. [3H]thymidine (1 μCi) was added for 6 h, and radioactivity was quantified. *p < 0.05 compared with control cells; +p < 0.05 compared with the cells treated with bFGF or VEGF. n = 6.
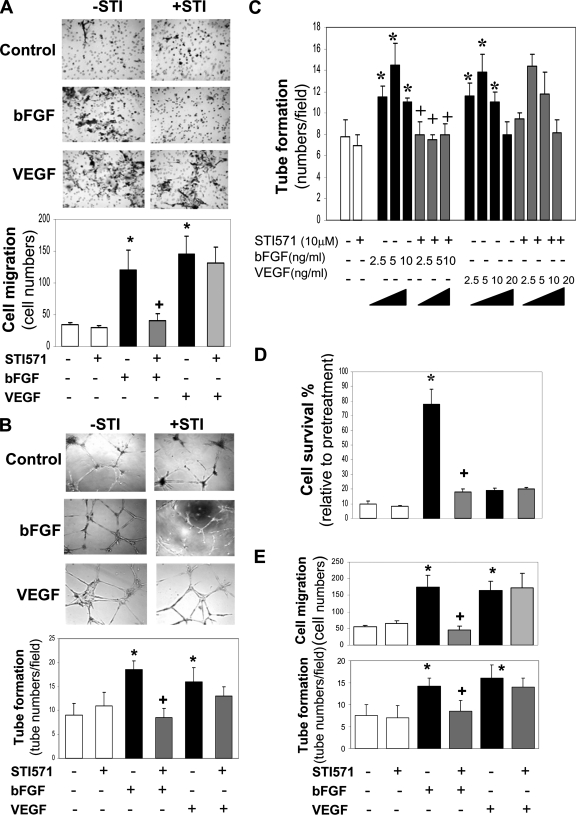
To test whether c-Abl activity is also required for bFGF- and VEGF-induced angiogenesis, we evaluated cell angiogenic activity as measured by cell migration and tube formation. As expected, both growth factors enhanced cell migration that was threefold higher than the control (Figure 2A). Surprisingly, STI571 specifically inhibited cell migration induced by bFGF but not VEGF. The result was confirmed by monitoring cell motility using a wound healing assay (data not shown). In the tube formation assay, bFGF and VEGF caused an increase in the appearance of vasculature-like tubes in comparison to the controls (Figure 2B). Likewise, STI571 inhibited bFGF-induced tube formation but not VEGF-induced activity. STI571 blocked bFGF-induced dose-dependent tube formation (Figure 2C), suggesting that c-Abl mediates bFGF- but not VEGF-induced angiogenesis.
Figure 2.
Divergent effects of STI571 on bFGF- and VEGF-induced angiogenesis. (A) STI571 inhibits bFGF- but not VEGF-induced cell migration. HMVECs were loaded into the upper chamber of transwells, and bFGF (5 ng/ml) or VEGF (10 ng/ml) was added to the low chamber in the absence or presence of STI571 (10 μM) for 4 h. Cells migrated to the membrane were quantitated. n = 5. (B) STI571 blocks bFGF- but not VEGF-induced tube formation. The cells were loaded onto the Matrigel and incubated for 12 h in the presence of bFGF (5 ng/ml), VEGF (10 ng/ml), or STI571 (10 μM). The tubes density was quantitated. n = 4. (C) Dose-dependent effects of bFGF and VEGF on tube formation. The indicated different doses of bFGF and VEGF in the absence or presence of STI571 were added to the cells, and the extent of tube formation was quantitatively analyzed. n = 4. (D) STI571 blocks bFGF- but not VEGF-induced cell survival activity. The cells were treated with serum-free medium for 5 d in the presence of bFGF (5 ng/ml), VEGF (10 ng/ml), or STI571 (10 μM). The survival cells were quantified. n = 3. (E) STI571 inhibits cell migration induced by bFGF but not VEGF in HMEC-1. The cell migration and tube formation were measured similarly as in A, except HMEC-1 instead of HMVEC. n = 4. *p < 0.05 compared with control; +p < 0.05 compared with bFGF-treated cells.
In an attempt to test whether c-Abl also mediates cell survival activity, the cells were challenged to serum-free medium for 5 d. As shown in Figure 2D, bFGF prevented 80% of cells from death induced by depletion of serum and growth factors, whereas VEGF lacked this protection. STI571 treatment resulted in the loss of the protective effect of bFGF similar to its anti-angiogenic effects demonstrated earlier. To determine whether these divergent angiogenic functions mediated by c-Abl are unique in this particular endothelial cell line immortalized with hTERT, we explored the functional role of c-Abl in another human microvascular endothelial cell line HMEC-1 established by the introduction of SV40 large T antigen. Agreed with the above distinct effects on the motility of HMVEC, STI571 abrogated cell migration and tube formation induced by bFGF but not VEGF in HMEC-1 (Figure 2E). Taken together, the data strongly implicate a key role of c-Abl in mediating bFGF-induced angiogenesis.
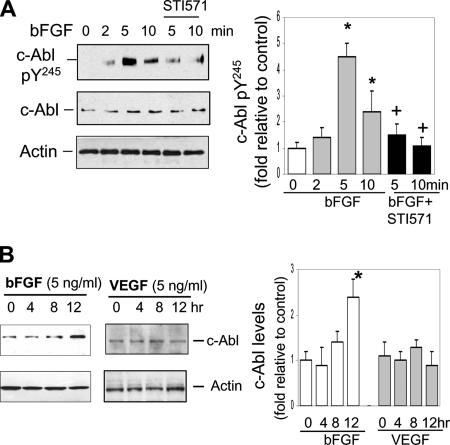
Next, to determine if bFGF directly activates c-Abl, we examined its effect on the level of c-Abl tyrosine phosphorylation on pY245 as an indicator of c-Abl activation. As shown in Figure 3A, c-Abl pY245 was detected at five- and twofold higher levels after stimulation of cells with bFGF for 5 and 10 min, respectively, relative to the basal level, and these effects were significantly reduced by STI571. It is known that STI571 also has the ability to inhibit PDGF receptor (PDGFR) and c-kit kinase in addition to c-Abl (Hagerstrand et al., 2006; Price et al., 2007). To test the possibility that PDGFR and/or c-kit kinase may also participate in bFGF-induced angiogenesis, we exposed the cells to bFGF and then examined potential changes in the levels of activated forms of PDGFR and c-kit. Neither PDGFR nor c-kit tyrosine phosphorylated form was up-regulated by bFGF stimulation in the absence or presence of STI571 (data not shown). To further test whether bFGF regulates c-Abl protein expression by which bFGF-induced angiogenesis is enhanced in a positive feedback manner, the cells were exposed to bFGF for up to 12 h (Figure 3B). Stimulation with bFGF resulted in an increase in c-Abl protein expression ∼2.5-fold greater than the basal level. The data demonstrate that bFGF directly increases c-Abl activation as well as protein expression.
Figure 3.
bFGF induces c-Abl activation and protein expression. (A) After treatment with serum-free medium for 12 h, the cells were stimulated with bFGF (5 ng/ml) for 2, 5, and 10 min in the absence or presence of STI571 (10 μM). The cell lysates were subjected for immunoblotting using antibodies against c-Abl pY245, total c-Abl, and actin. c-Abl pY245 level was evaluated by densitometry analyses followed by normalizing with actin. (B) The cells were treated with bFGF or VEGF (5 ng/ml) for 12 h. The cell lysates were used for immunoblotting using anti-c-Abl antibody. c-Abl level was evaluated by normalizing with actin. *p < 0.05 compared with control at the zero time; +p < 0.05 compared with corresponding time cells treated with bFGF alone. n = 3–4.
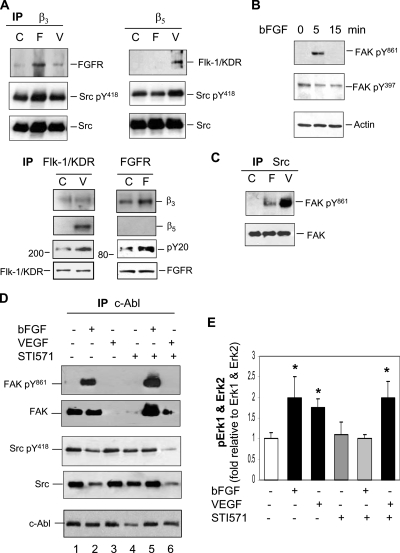
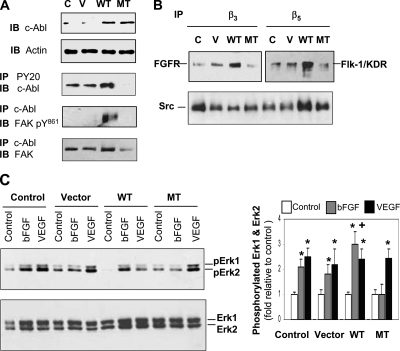
To monitor cross-talk between integrins and growth factor receptors that may initiate angiogenic signaling, we examined their physical interaction using coimmunoprecipitation and immunoblotting approach. After stimulation of the cells with bFGF or VEGF, integrin β3- and β5-associated proteins were coimmunoprecipitated using anti-β3 or β5 antibody followed by immunoblotting (Figure 4A). bFGF receptor 1 (FGFR) associated with integrin β3 was increased in the cells treated with bFGF but not VEGF. In contrast, the physical interaction between integrin β5 and Flk-1/KDR was detectable only by stimulation with VEGF. A reciprocal experiment exhibited a pattern similar to these receptor interactions that activated FGFR enhanced its binding with integrin β3, whereas activated Flk-1/KDR was associated with integrin β5 (Figure 4A). These results suggest that angiogenic signaling induced by each growth factor requires specific integrins and corresponding receptors for their collaboration. Both active and inactive Src were found to associate with integrin β3 and β5 in the absence or presence of either growth factor (Figure 4A); however under no circumstance did c-Abl directly interact with either integrin (data not shown), suggesting that the two nonreceptor kinases may play distinct roles in downstream of the integrins. Stimulation of cells with VEGF increased the association of tyrosine phosphorylated-form of Src (pY418) with integrin β5, implicating that increased interaction between Src and β5 may be involved in VEGF-induced angiogenic signaling.
Figure 4.
c-Abl mediates bFGF-induced signaling cascade, whereas Src mediates VEGF-induced signaling activation. (A) bFGF induces association of integrin β3 with FGFR, and VEGF induces association of integrin β5 with Flk-1/KDR. Cells were stimulated with bFGF (5 ng/ml) or VEGF (10 ng/ml) for 5 min, and cell lysates were immunoprecipitated with anti-integrin β3, β5, Flk-1/KDR, or FGFR antibody, followed by immunoblotting using anti-FGFR, Flk-1/KDR, pY20, Src, Src pY418, β3, or β5 antibody. C, F, and V indicate control, bFGF, and VEGF, respectively. (B) bFGF stimulates FAK activation. The cells were stimulated with bFGF (5 ng/ml) for 5 and 15 min and then subjected to immunoblotting using anti-FAK pY397 and pY861 antibody. (C) VEGF but not bFGF induces association of FAK pY861 with Src. The cells were stimulated with bFGF (5 ng/ml) or VEGF (10 ng/ml) for 5 min, and cell lysates were immunoprecipitated with anti-Src antibody followed by immunoblotting using anti-FAK and FAK pY861 antibody. (D) bFGF promotes association of c-Abl with FAK pY861 but dissociation from Src. Five minutes after treatment with bFGF (5 ng/ml) or VEGF (10 ng/ml) in the absence or presence of STI571 (10 μM), the cells were harvested, and cell lysates were immunoprecipitated with anti-c-Abl antibody followed by immunoblotting using anti-c-Abl, FAK, FAK pY861, Src, or Src pY418 antibody. (E) STI571 only inhibits bFGF-induced Erk 1 and Erk 2 activation. Cells were stimulated with bFGF (5 ng/ml) or VEGF (10 ng/ml) in the absence or presence of STI571 (10 μM) for 5 min before cell lysis for immunoblotting against Erk 1, Erk 2, pErk 1, or pErk 2. The phosphorylated Erk 1 and Erk 2 were quantitated by normalizing with nonphosphorylated Erk 1 and Erk 2. *p < 0.05 compared with control. n = 3.
To define a role of FAK in mediating downstream signaling, we examined potential changes in the status of activated form of FAK by exposure of cells to bFGF. Consistent with previous results that an activated form of FAK pY861 mediates VEGF-induced angiogenesis (Abu-Ghazaleh et al., 2001), bFGF increased the level of FAK pY861 but not that of FAK pY397 (Figure 4B). However, the level of FAK pY861 induced by bFGF did not significantly increase in association with Src (Figure 4C). Conversely, the association of FAK pY861 with Src was noticeably augmented by stimulation with VEGF (Figure 4C), suggesting that Src-FAK interaction primarily mediates VEGF-induced intracellular signaling. To determine whether c-Abl interacts with FAK to mediate bFGF signaling, we performed coimmunoprecipitation using anti-c-Abl antibody followed by immunoblotting against Src or FAK (Figure 4D). Once the cells were treated with bFGF, association of c-Abl with both Src and Src pY418 was reduced; in contrast, its association with FAK pY861 was apparently increased (Fig. 4D, lane 2), implying that c-Abl disassociates from membrane-associated Src to increase association with FAK pY861 promoting intracellular signaling. Treatment of the cells with STI571 alone resulted in decreased interaction between c-Abl and inactive FAK (cf. lane 4 with lane 1), suggesting that their association is partially dependent on basal c-Abl activity in the absence of growth factors. When the cells were treated with bFGF in the presence of STI571, the interaction between c-Abl and FAK/FAK pY861 recapitulated the pattern of bFGF treatment alone (cf. lane 5 with lane 2), indicating that the association of c-Abl with FAK/FAK pY861 is independent of c-Abl activity. This combined treatment also restored the association of c-Abl with Src/Src pY418 (cf. lane 5 with lanes 1 and 2), suggesting that c-Abl increases the interaction with Src that is associated with membrane-anchored receptors, and terminates intracellular signaling. When the cells were treated with VEGF alone (lane 3), FAK disassociated from c-Abl and presumably formed association with Src to participate in VEGF signaling (Figure 4C). The combined treatment of cells with VEGF and STI571 (lane 6) led to c-Abl disassociation from a protein complex containing Src and FAK, both of which are involved in mediating VEGF signaling (Figure 4C), independently of c-Abl.
To further identify downstream effectors of FAK, we investigated MAP kinase Erk 1 and Erk 2 activation in the cells. As the quantification of immunoblotting assay shown in Figure 4E, both bFGF and VEGF augmented Erk 1 and Erk 2 tyrosine phosphorylation, and STI571 abrogated bFGF effects but failed to inhibit VEGF effects. Collectively, the data suggest that bFGF induces the coordination of FGFR and integrin β3 to trigger downstream the FAK-c-Abl-Erk signaling cascade, whereas VEGF induces coupling of integrin β5 to Flk-1/KDR, resulting in downstream signaling Src-FAK-Erk activation.
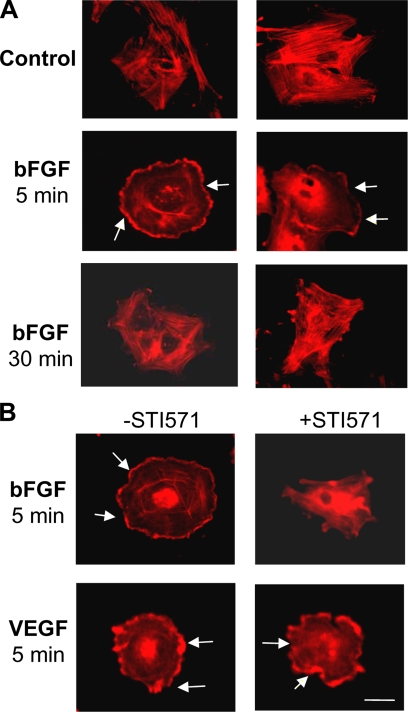
Because c-Abl harbors an actin-binding domain at the C-terminus, it may have a direct impact on cell motile function through interaction with and/or reorganization of cytoskeleton protein actin. To test this, we used immunocytochemistry staining of cell cytoskeleton with rhodamine phalloidin to observe actin reorganization/relocalization as indicative of cell motility changed. As shown in Figure 5A, cytoplasmic membrane ruffling containing dorsal ruffles was prominent in the cells exposed to bFGF for 5 min and diminished at 30 min. Consistent with the results of functional assays demonstrated earlier, STI571 only inhibited bFGF- but not VEGF-induced cytoskeleton redistribution (Figure 5B).
Figure 5.
STI571 blocks bFGF-induced cell cytoskeleton reorganization. (A) Cells were treated with bFGF (5 ng/ml) or VEGF (10 ng/ml) for 5 and 30 min. Then, the cells were immunostained using rhodamine phalloidin. (B) The cells were stimulated with bFGF (5 ng/ml) or VEGF (10 ng/ml) in the absence or presence of STI571 (10 μM) for 5 min followed by cytoskeleton staining. Arrows indicate the ruffling of plasma membrane. The images show one representative of three individual experiments. Bars, 10 μm.
To determine whether manipulation of c-Abl gene expression in the cells can lead to altered interaction and/or activities of signaling molecules, we ectopically expressed WT and kinase dead K290R mutant (MT) c-Abl in the cells by a retroviral infection system (Figure 6A). The level of active c-Abl was elevated in the cells expressing WT c-Abl but substantially suppressed in the cells expressing MT c-Abl (Figure 6A). The level of FAK pY861 associated with c-Abl became detectable in the cells expressing WT c-Abl, suggesting that FAK-mediated signaling is activated in the cells. The association of c-Abl with FAK was decreased in the cells ectopically expressing MT c-Abl (Figure 6A), consistent with the results showing inhibitory effects of STI571 on their interaction (Figure 4D, lane 4). To determine if the introduction of different versions of c-Abl subsequently alter membrane-bound receptor interaction that initiates angiogenic signaling, we utilized the same coimmunoprecipitation approach as described earlier. As shown in Figure 6B, enforced expression of WT c-Abl resulted in dramatic increases in the associations of integrin β3 with FGFR, and integrin β5 with Flk-1/KDR. Interestingly, the interaction between integrin β3 and Src was reduced; conversely, the interaction between β5 and Src was enhanced in the cells overexpressing WT c-Abl, suggesting that Src preferentially integrates into the membrane-bound receptor complex consisting of integrin β5 and Flk-1/KDR, which might facilitate VEGF-induced signaling cascade in WT c-Abl–expressing cells. Association of integrin β5 with Src in MT c-Abl cells was also enhanced, implying that overexpression of MT c-Abl may result in the same effect as WT c-Abl on Src, favoring VEGF signaling. To further examine the downstream signaling pathways, we assessed the status of Erk activation. As expected, the parental and vector-control cells responded similarly to bFGF and VEGF in increasing the levels of pErk 1 and pErk 2 (Figure 6C). In WT c-Abl–expressing cells, bFGF displayed a stronger effect on the increase in pErk 1 and pErk 2 levels than VEGF. However, in MT c-Abl–expressing cells, only bFGF- but not VEGF-induced increases in pErk 1 and pErk 2 were blocked. This specific inhibition of bFGF activity by STI571 supports our hypothesis that c-Abl mediates bFGF-induced angiogenic signaling.
Figure 6.
Overexpression of WT and MT c-Abl results in altered protein interaction and activation. (A) Ectopic expression of WT c-Abl increases both active c-Abl level and its association with FAK pY861. Cell lysates from cells expressing vector control, WT, or MT c-Abl were used for immunoblotting to determine c-Abl expression or immunoprecipitation with anti-pY20 or c-Abl antibody before immunoblotting using anti-c-Abl, FAK, or FAK pY861 antibody. C, parental cells as control; V, cells engineered with an empty retroviral vector. (B) Cells expressing WT c-Abl exhibit changes in protein interaction. Cells ectopically expressing different versions of c-Abl were harvested, and cell lysates were used for immunoprecipitation using anti-integrin β3 or β5 antibody, followed by immunoblotting using antibodies against FGFR, Flk-1/KDR, or Src. (C) WT c-Abl enhances Erk 1 and Erk 2 activation in response to bFGF, but MT c-Abl blocks this activity. Cells ectopically expressing different versions of c-Abl were stimulated with bFGF (5 ng/ml) or VEGF (10 ng/ml) for 5 min, and then the lysates were used for immunoblotting to examine phosphorylated or nonphosphorylated Erk 1 and Erk 2. The phosphorylated Erk 1 and Erk 2 were quantitated by normalizing with nonphosphorylated Erk 1 and Erk 2. *p < 0.05 compared with respective control in each group; +p < 0.05 compared with the levels in either parental or vector control group treated with bFGF. n = 3.
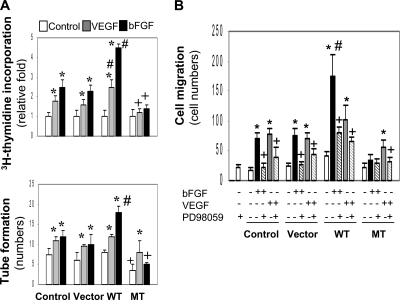
To test whether ectopic expression of the different versions of c-Abl may alter cell angiogenic functions, we measured cell activities including proliferation, migration, and tube formation. Both bFGF and VEGF stimulated the proliferation of the parental and vector control cells, which was further enhanced with the expression of WT c-Abl (Figure 7A, top panel). However, the presence of MT c-Abl blocked cell proliferation induced by both growth factors, a result consistent with the inhibition of STI571 on cell growth (Figure 1), suggesting that the signaling pathway involved in the cell division is divergent from the one engaged in angiogenesis. In the tube formation assay, whereas both VEGF and bFGF increased tube formation in comparison to the control, ectopic expression of the WT c-Abl sensitized cellular response only to bFGF (Figure 7A). Similar results were obtained in the migration assay (Figure 7B). These differential cellular responses to bFGF and VEGF may be attributed to the different levels of pErk 1 and pErk 2 activation (Figure 6C). To confirm the role of these downstream effectors in mediating angiogenic activities, the cells were treated with PD98059, a specific Erk 1 and Erk 2 inhibitor. PD98059 inhibited both bFGF- and VEGF-induced cell migration regardless of ectopic expression of different versions of c-Abl (Figure 7B), demonstrating that the downstream effectors Erk 1 and Erk 2 mediate both growth factor–induced angiogenesis. Taken together, the results indicate that ectopic expression of WT c-Abl enhances bFGF- but not VEGF-induced angiogenic activities; in contrast, MT c-Abl abrogates the enhanced angiogenic responses.
Figure 7.
Divergent effects of WT and MT c-Abl on angiogenesis in vitro. After treatment with bFGF (5 ng/ml) or VEGF (10 ng/ml), cells expressing different versions of c-Abl were measured for cell proliferation and tube formation (A). *p < 0.05 compared with respective control in each group; +,#p < 0.05 compared with cells treated with respective growth factor in the parental and vector control groups. After treatment with bFGF or VEGF combined with PD98059 (10 μM), the cells were examined for cell migration (B). *p < 0.05 compared with respective control in each group, +p < 0.05 compared with the cells treated with growth factors in the absence of PD98059; #p < 0.05 compared with the cells treated with bFGF in the parental and vector control groups. n = 4–6.
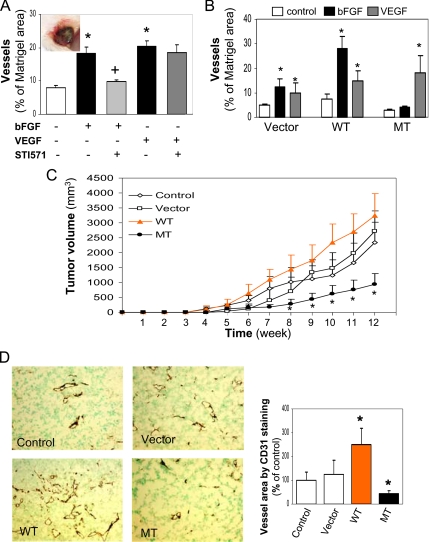
To determine if c-Abl mediates bFGF-induced angiogenesis in vivo, we used a Matrigel implantation model in mice. Matrigel was premixed with HMVEC in the presence of bFGF or VEGF and subcutaneously injected into the mice. The animals were treated with vehicle or STI571 by oral gavage every other day for 10 d. The removed Matrigel plugs were subjected to an analysis of blood vessel density using anti-CD31 antibody that specifically recognizes human vascular endothelial cells. Consistent with the angiogenic results observed in vitro (Figure 2), STI571 selectively blocked the effects of bFGF but not VEGF on induction of blood vessels (Figure 8A), indicating that the ability of bFGF to induce angiogenesis is dependent on c-Abl activity. To further test whether mutant c-Abl has the same ability to inhibit bFGF-induced angiogenesis, we implanted the mice with Matrigel premixed with HMVECs expressing different versions of c-Abl in the presence of bFGF or VEGF. Ten days later, blood vessels formed in Matrigel were analyzed as we did earlier. With the vector control cells, bFGF and VEGF stimulated vessel formation (Figure 8B). With the cells expressing WT c-Abl, bFGF exhibited a substantially increased activity over that of VEGF in promoting vessel formation. Conversely, the cells expressing MT c-Abl failed to respond to bFGF in vessel development, but their sensitivity to VEGF remained at levels similar to those observed in either vector control or WT c-Abl–expressing cells, consistent with the results obtained from the in vitro assays (Figure 7). These in vivo data demonstrate that c-Abl plays a central role in angiogenesis induced by bFGF.
Figure 8.
Distinct effects of c-Abl on angiogenesis in vivo. (A) STI571 inhibits angiogenesis induced by bFGF in Matrigel implantation model. HMVECs premixed with Matrigel in the presence of bFGF or VEGF (1 μg) were subcutaneously injected into mice (three mice in each group). After the mice were treated with vehicle alone as control or STI571 for 10 d, Matrigel plugs were extracted for the analysis of CD31 staining as described in Materials and Methods. An insert represents a Matrigel plug. Blood vessel areas were quantified with an image-scanning program developed by the National Institutes of Health. *p < 0.05 compared with the control group; +p < 0.05 compared with the bFGF-treated group. (B) WT and MT c-Abl exhibit opposite effects on bFGF-induced angiogenesis. HMVECs ectopically expressing vector control, WT, or MT c-Abl were mixed with Matrigel in the presence of bFGF or VEGF as above. On day 10, the Matrigel plugs were excised for immunohistochemistry staining of CD31. *p < 0.05 compared with respective untreated control group. (C and D) WT and MT c-Abl exert opposite effects on tumor growth and angiogenesis in xenograft animal model. MDA-MB-231 cells and HMVECs expressing control or different versions of c-Abl were coinjected into immunodeficient mice as described in Materials and Methods. Tumor sizes were measured weekly for 12 wk (C, n = 6). Removed tumor tissues were stained with an anti-CD31 antibody, and a representative tumor section for each condition is shown. CD31-positive endothelial cells with brown staining indicated vasculature network, and vessel area was analyzed by quantifying CD31-positive staining. *p < 0.05 compared with the control group (D, n = 6).
Finally, to explore the angiogenic role for c-Abl in tumor development, we used a cotransplantation model that was established to evaluate tumor angiogenesis induced by interaction between exogenous endothelial cells and tumor cells in animals (Tei et al., 2002). We subcutaneously coinjected both a breast cancer line MDA-MB-231 and HMVECs expressing different versions of c-Abl into immunodeficient mice. As shown in Figure 8C, mice receiving MDA-MB-231 cells and HMVECs expressing WT c-Abl formed tumors faster than the mice inoculated with MDA-MB-231 cells and parental or vector-control HMVECs. However, tumor growth was significantly suppressed in the mice injected with tumor cells and HMVECs expressing MT c-Abl, as measured by tumor volume. Consistent with the differences in tumor size and growth rate, the vasculature density in the tumors carrying tumor cells and HMVECs expressing WT c-Abl was significantly greater than that in the control tumors (Figure 8D); however, blood vessels in the tumors containing tumor cells and HMVECs overexpressing MT c-Abl were dramatically reduced. These data strongly support our hypothesis that c-Abl plays a vital role in tumor angiogenesis.
DISCUSSION
Although bFGF- and VEGF-induced angiogenesis was extensively investigated, we still have an incomplete model that describes intracellular signaling pathways in responses to each growth factor–induced angiogenesis. For example, a study on cell motility has shown that bFGF-induced cell migration is chemokinetic, whereas VEGF's effect is dependent on chemotaxis (Yoshida et al., 1996). Alavi et al. (2003) have demonstrated that distinct intracellular signaling molecules are required for each growth factor–induced cell survival activity. Both Raf-1 (through serine phosphorylation at Ser-338 and Ser-339) and PAK-1 activation mediate cell survival activity induced by bFGF, whereas other active forms of Raf-1 (tyrosine phosphorylation at Tyr-340 and Tyr-341) and MEK1 activation are essential for VEGF-induced survival. Furthermore, in vivo experimental evidence has revealed that bFGF-induced angiogenesis requires integrin αvβ3 engagement, whereas VEGF-induced angiogenesis depends on integrin αvβ5 activity (Brooks et al., 1994a; Friedlander et al., 1995). To date, however, the intracellular molecules distinctly mediating bFGF- and VEGF-induced angiogenesis have not been systemically illustrated both in vitro and in vivo. Our current work demonstrates for the first time that c-Abl divergently mediates bFGF- and VEGF-induced angiogenic responses in microvascular endothelial cells. The functional and mechanistic characterization for c-Abl in cultured cells and in vivo angiogenic models of Matrigel implantation as well as xenograft tumors, substantially underscores a vital role for c-Abl in bFGF-induced angiogenesis possibly as a downstream effector of FAK. Additional evidence that VEGF-induced angiogenesis utilizes Src, FAK, and MAPK confirms the specificity of divergent signaling cascades engaged by individual growth factors.
A variety of cell types express the cytoplasmic tyrosine kinase Abl proto-oncoproteins that consists of c-Abl (also named Abl-1) and Arg (called Abl-2). In microvascular endothelial cells, c-Abl is expressed at a relatively higher level than Arg (data not shown). Although the active level of endogenous c-Abl is quite low, it can be induced by bFGF to a level sufficient to mediate angiogenesis, implicating its angiogenic activity in the development of tumors that contain a variety of elevated growth factors including bFGF. STI571 inhibited angiogenic responses specifically to bFGF both in vitro and in vivo, consistent with the angiogenic suppression by a mutant form of c-Abl, supporting the functional role of c-Abl in bFGF-induced angiogenesis.
Extensive research efforts have been made to achieve a better understanding of selective coordination of inputs from growth factor receptors and integrins for the synergic execution of growth factor and extracelllar matrix protein functions (Soldi et al., 1999; Byzova et al., 2000; Weis et al., 2007). Vascular endothelial cells predominantly express integrin αvβ3 and αvβ5, and elevated activities of these integrins are known to be intimately associated with tumor angiogenesis (Brooks et al., 1994a,b; Friedlander et al., 1995). Our results, as with other findings, have indicated that bFGF-induced angiogenesis needs the coupling of integrin αvβ3 with FGFR, whereas VEGF requires the cooperation between Flk-1/KDR and integrin αvβ5.
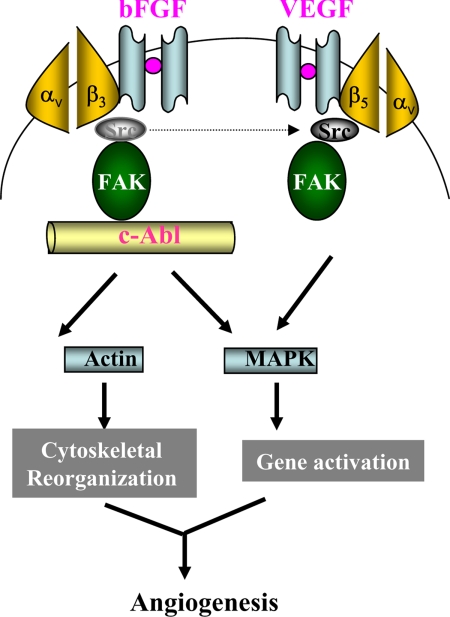
The N terminus of FAK harbors an SH2 domain to which Src-family kinases bind, whereas its C terminus indirectly interacts with integrins in the presence of paxillin and/or talin adaptors (Sieg et al., 2000; Hauck et al., 2002). Based on the molecular structure of FAK, it is conceivable that FAK acts as a key factor in the coordination of intracellular signaling of both integrins and growth factor receptors. Consistent with previous findings (Abu-Ghazaleh et al., 2001), FAK activation through tyrosine phosphorylation at residue 861 was identified to mediate endothelial cell angiogenic activity. bFGF induces association of activated FAK pY861 with c-Abl but not Src. In contrast, VEGF primarily promotes interaction of FAK pY861 with Src. The results demonstrate that the interaction of activated FAK with either c-Abl or Src depends on the presence of individual growth factor. To summarize the data we have generated through this study, we proposed a model for the differential signaling pathways in which c-Abl is a downstream molecule of FAK in bFGF-induced signaling cascade, whereas in VEGF signaling, Src but not c-Abl is the essential factor to activate the intracellular cascade (Figure 9).
Figure 9.
A hypothetical model for the signaling pathways induced by bFGF and VEGF. Once endothelial cells are stimulated with bFGF, the cooperation of membrane-anchored receptors between integrin αvβ3 and FGFR leads to the “out-side in” signaling activation. c-Abl disassociates from Src that is connected to integrin β3 and increases in association with activated FAK, resulting in downstream MAPK activation. In addition, c-Abl may directly regulate reorganization of cytoskeleton actin to facilitate cell motility. VEGF engages a similar but c-Abl–independent angiogenic machinery in which Src acts as an upstream effector of FAK.
It has been well established that development of solid tumor exclusively depends on the activation of angiogenesis with the formation of new blood vasculatures generated from the pre-existing vascular capillaries of host tissues (Folkman, 1995; Hanahan and Folkman, 1996). Evidence that the coinoculation of mixed tumor cells and endothelial cells ectopically expressing WT or MT c-Abl resulted in opposite outcomes in tumor growth indicates the importance of angiogenesis acted by c-Abl–expressing cells in tumor development. Moreover, using an antibody specifically reacting with human vascular endothelial cells in immunohistochemistry study, we found altered blood vessel densities that corresponded to the presence of different functional versions of c-Abl. These results supported the model that exogenous endothelial cells ectopically expressing c-Abl mainly contribute to tumor angiogenesis. It is quite possible that other angiogenic factors such as PDGF (Plattner et al., 1999), besides bFGF, may also utilize c-Abl to mediate their angiogenic activities in vivo. Thus, the inhibition of basal activity of c-Abl by MT c-Abl might interfere with signaling pathways that are engaged by multiple angiogenic factors present in the milieu of in vivo tumors. As with a recent report that SKI-606, a Src/Abl inhibitor, can effectively block tumor growth and invasion (Jallal et al., 2007), our results have demonstrated that c-Abl acts as an important player in mediating tumor angiogenesis.
Substantial evidence has demonstrated that constitutively active mutant forms of Abl are expressed in variety of leukemia (Soverini et al., 2006; Jiang et al., 2007). As an effective inhibitor of Abl kinase, STI571 has been used in the clinic to treat cancer patients with certain types of leukemia (Swords et al., 2007). The present finding that STI571 can effectively block bFGF-induced angiogenesis would provide valuable information for its utility in the treatment of solid tumors, because bFGF similar to VEGF is overproduced in multiple types of human cancers.
ACKNOWLEDGMENTS
We thank Dr. Ann Marie Pendergast (Duke University) for kindly providing c-Abl DNA constructs, anti-c-Abl antibody, and partial STI571. This work was supported by Rays of Hope, breast cancer research, BHS; Collaborative Biomedical Research Program, Baystate Medical Center/University of Massachusetts at Amherst; startup funds from the Pioneer Valley Life Sciences Institute, Baystate Medical Center/University Massachusetts at Amherst; and in part by a Department of Defense grant (R.S.)
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-10-1068) on March 19, 2008.
REFERENCES
- Abedi H., Zachary I. Vascular endothelial growth factor stimulates tyrosine phosphorylation and recruitment to new focal adhesions of focal adhesion kinase and paxillin in endothelial cells. J. Biol. Chem. 1997;272:15442–15451. doi: 10.1074/jbc.272.24.15442. [DOI] [PubMed] [Google Scholar]
- Abu-Ghazaleh R., Kabir J., Jia H., Lobo M., Zachary I. Src mediates stimulation by vascular endothelial growth factor of the phosphorylation of focal adhesion kinase at tyrosine 861, and migration and anti-apoptosis in endothelial cells. Biochem. J. 2001;360:255–264. doi: 10.1042/0264-6021:3600255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alavi A., Hood J. D., Frausto R., Stupack D. G., Cheresh D. A. Role of Raf in vascular protection from distinct apoptotic stimuli. Science. 2003;301:94–96. doi: 10.1126/science.1082015. [DOI] [PubMed] [Google Scholar]
- Bergers G., Benjamin L. E. Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- Blystone S. D., Lindberg F. P., Williams M. P., McHugh K. P., Brown E. J. Inducible tyrosine phosphorylation of the beta3 integrin requires the alphaV integrin cytoplasmic tail. J. Biol. Chem. 1996;271:31458–31462. doi: 10.1074/jbc.271.49.31458. [DOI] [PubMed] [Google Scholar]
- Brooks P. C., Clark R. A., Cheresh D. A. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994a;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- Brooks P. C., Montgomery A. M., Rosenfeld M., Reisfeld R. A., Hu T., Klier G., Cheresh D. A. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994b;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Bussolino F., Mantovani A., Persico G. Molecular mechanisms of blood vessel formation. Trends Biochem. Sci. 1997;22:251–256. doi: 10.1016/s0968-0004(97)01074-8. [DOI] [PubMed] [Google Scholar]
- Byzova T. V., Goldman C. K., Pampori N., Thomas K. A., Bett A., Shattil S. J., Plow E. F. A mechanism for modulation of cellular responses to VEGF: activation of the integrins. Mol. Cell. 2000;6:851–860. [PubMed] [Google Scholar]
- Echarri A., Pendergast A. M. Activated c-Abl is degraded by the ubiquitin-dependent proteasome pathway. Curr. Biol. 2001;11:1759–1765. doi: 10.1016/s0960-9822(01)00538-3. [DOI] [PubMed] [Google Scholar]
- Eliceiri B. P. Integrin and growth factor receptor crosstalk. Circ. Res. 2001;89:1104–1110. doi: 10.1161/hh2401.101084. [DOI] [PubMed] [Google Scholar]
- Eliceiri B. P., Paul R., Schwartzberg P. L., Hood J. D., Leng J., Cheresh D. A. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol. Cell. 1999;4:915–924. doi: 10.1016/s1097-2765(00)80221-x. [DOI] [PubMed] [Google Scholar]
- Eliceiri B. P., Puente X. S., Hood J. D., Stupack D. G., Schlaepfer D. D., Huang X. Z., Sheppard D., Cheresh D. A. Src-mediated coupling of focal adhesion kinase to integrin alpha(v) beta5 in vascular endothelial growth factor signaling. J. Cell Biol. 2002;157:149–160. doi: 10.1083/jcb.200109079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahmy R. G., Dass C. R., Sun L. Q., Chesterman C. N., Khachigian L. M. Transcription factor Egr-1 supports FGF-dependent angiogenesis during neovascularization and tumor growth. Nat. Med. 2003;9:1026–1032. doi: 10.1038/nm905. [DOI] [PubMed] [Google Scholar]
- Ferrari G., Pintucci G., Seghezzi G., Hyman K., Galloway A. C., Mignatti P. VEGF, a prosurvival factor, acts in concert with TGF-beta1 to induce endothelial cell apoptosis. Proc. Natl. Acad. Sci. USA. 2006;103:17260–17265. doi: 10.1073/pnas.0605556103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis and tissue factor. Nat. Med. 1996;2:167–168. doi: 10.1038/nm0296-167. [DOI] [PubMed] [Google Scholar]
- Friedlander M., Brooks P. C., Shaffer R. W., Kincaid C. M., Varner J. A., Cheresh D. A. Definition of two angiogenic pathways by distinct alpha v integrins. Science. 1995;270:1500–1502. doi: 10.1126/science.270.5241.1500. [DOI] [PubMed] [Google Scholar]
- Guo D., Jia Q., Song H. Y., Warren R. S., Donner D. B. Vascular endothelial cell growth factor promotes tyrosine phosphorylation of mediators of signal transduction that contain SH2 domains. Association with endothelial cell proliferation. J. Biol. Chem. 1995;270:6729–6733. doi: 10.1074/jbc.270.12.6729. [DOI] [PubMed] [Google Scholar]
- Hagerstrand D., Hesselager G., Achterberg S., Wickenberg Bolin U., Kowanetz M., Kastemar M., Heldin C. H., Isaksson A., Nister M., Ostman A. Characterization of an imatinib-sensitive subset of high-grade human glioma cultures. Oncogene. 2006;25:4913–4922. doi: 10.1038/sj.onc.1209497. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Hauck C. R., Hsia D. A., Schlaepfer D. D. The focal adhesion kinase—a regulator of cell migration and invasion. IUBMB Life. 2002;53:115–119. doi: 10.1080/15216540211470. [DOI] [PubMed] [Google Scholar]
- Jallal H., Valentino M. L., Chen G., Boschelli F., Ali S., Rabbani S. A. A Src/Abl kinase inhibitor, SKI-606, blocks breast cancer invasion, growth, and metastasis in vitro and in vivo. Cancer Res. 2007;67:1580–1588. doi: 10.1158/0008-5472.CAN-06-2027. [DOI] [PubMed] [Google Scholar]
- Jiang X., Saw K. M., Eaves A., Eaves C. Instability of BCR-ABL gene in primary and cultured chronic myeloid leukemia stem cells.[see comment] J. Natl. Cancer Instit. 2007;99:680–693. doi: 10.1093/jnci/djk150. [DOI] [PubMed] [Google Scholar]
- Jones P. L., Crack J., Rabinovitch M. Regulation of tenascin-C, a vascular smooth muscle cell survival factor that interacts with the alpha v beta 3 integrin to promote epidermal growth factor receptor phosphorylation and growth. J. Cell Biol. 1997;139:279–293. doi: 10.1083/jcb.139.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. J., Li B., Winer J., Armanini M., Gillett N., Phillips H. S., Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- Le Bras S., Moon C., Foucault I., Breittmayer J. P., Deckert M. Abl-SH3 binding protein 2, 3BP2, interacts with CIN85 and HIP-55. FEBS Lett. 2007;581:967–974. doi: 10.1016/j.febslet.2007.01.084. [DOI] [PubMed] [Google Scholar]
- Millauer B., Longhi M. P., Plate K. H., Shawver L. K., Risau W., Ullrich A., Strawn L. M. Dominant-negative inhibition of Flk-1 suppresses the growth of many tumor types in vivo. Cancer Res. 1996;56:1615–1620. [PubMed] [Google Scholar]
- Neufeld G., Cohen T., Gengrinovitch S., Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- O'Neill A. J., Cotter T. G., Russell J. M., Gaffney E. F. Abl expression in human fetal and adult tissues, tumours, and tumour microvessels. J. Pathol. 1997;183:325–329. doi: 10.1002/(SICI)1096-9896(199711)183:3<325::AID-PATH941>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Plattner R., Kadlec L., DeMali K. A., Kazlauskas A., Pendergast A. M. c-Abl is activated by growth factors and Src family kinases and has a role in the cellular response to PDGF. Genes Dev. 1999;13:2400–2411. doi: 10.1101/gad.13.18.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polnaszek N., Kwabi-Addo B., Peterson L. E., Ozen M., Greenberg N. M., Ortega S., Basilico C., Ittmann M. Fibroblast growth factor 2 promotes tumor progression in an autochthonous mouse model of prostate cancer. Cancer Res. 2003;63:5754–5760. [PubMed] [Google Scholar]
- Prewett M., et al. Antivascular endothelial growth factor receptor (fetal liver kinase 1) monoclonal antibody inhibits tumor angiogenesis and growth of several mouse and human tumors. Cancer Res. 1999;59:5209–5218. [PubMed] [Google Scholar]
- Price N. D., Trent J., El-Naggar A. K., Cogdell D., Taylor E., Hunt K. K., Pollock R. E., Hood L., Shmulevich I., Zhang W. Highly accurate two-gene classifier for differentiating gastrointestinal stromal tumors and leiomyosarcomas. Proc. Natl. Acad. Sci. USA. 2007;104:3414–3419. doi: 10.1073/pnas.0611373104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T. N., Tozawa Y., Deutsch U., Wolburg-Buchholz K., Fujiwara Y., Gendron-Maguire M., Gridley T., Wolburg H., Risau W., Qin Y. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- Schneller M., Vuori K., Ruoslahti E. Alphavbeta3 integrin associates with activated insulin and PDGFbeta receptors and potentiates the biological activity of PDGF. EMBO J. 1997;16:5600–5607. doi: 10.1093/emboj/16.18.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher J. J., Dings R. P., Cosin J., Subramanian I. V., Auersperg N., Ramakrishnan S. Modulation of angiogenic phenotype alters tumorigenicity in rat ovarian epithelial cells. Cancer Res. 2007;67:3683–3690. doi: 10.1158/0008-5472.CAN-06-3608. [DOI] [PubMed] [Google Scholar]
- Senger D. R., Ledbetter S. R., Claffey K. P., Papadopoulos-Sergiou A., Peruzzi C. A., Detmar M. Stimulation of endothelial cell migration by vascular permeability factor/vascular endothelial growth factor through cooperative mechanisms involving the alphavbeta3 integrin, osteopontin, and thrombin. Am. J. Pathol. 1996;149:293–305. [PMC free article] [PubMed] [Google Scholar]
- Shao R., Guo X. Human microvascular endothelial cells immortalized with human telomerase catalytic protein: a model for the study of in vitro angiogenesis. Biochem. Biophys. Res. Commun. 2004;321:788–794. doi: 10.1016/j.bbrc.2004.07.033. [DOI] [PubMed] [Google Scholar]
- Sieg D. J., Hauck C. R., Ilic D., Klingbeil C. K., Schaefer E., Damsky C. H., Schlaepfer D. D. FAK integrates growth-factor and integrin signals to promote cell migration. Nat. Cell Biol. 2000;2:249–256. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- Soldi R., Mitola S., Strasly M., Defilippi P., Tarone G., Bussolino F. Role of alphavbeta3 integrin in the activation of vascular endothelial growth factor receptor-2. EMBO J. 1999;18:882–892. doi: 10.1093/emboj/18.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soverini S., et al. Contribution of ABL kinase domain mutations to imatinib resistance in different subsets of Philadelphia-positive patients: by the GIMEMA Working Party on Chronic Myeloid Leukemia. Clin. Cancer Res. 2006;12:7374–7379. doi: 10.1158/1078-0432.CCR-06-1516. [DOI] [PubMed] [Google Scholar]
- Sun J., et al. Inhibiting angiogenesis and tumorigenesis by a synthetic molecule that blocks binding of both VEGF and PDGF to their receptors. Oncogene. 2005;24:4701–4709. doi: 10.1038/sj.onc.1208391. [DOI] [PubMed] [Google Scholar]
- Swords R., Alvarado Y., Giles F. Novel Abl kinase inhibitors in chronic myeloid leukemia in blastic phase and Philadelphia chromosome-positive acute lymphoblastic leukemia. Clin. Lymphoma Myeloma. 2007;7(Suppl. 3):S113–S119. doi: 10.3816/clm.2007.s.011. [DOI] [PubMed] [Google Scholar]
- Tei K., Kawakami-Kimura N., Taguchi O., Kumamoto K., Higashiyama S., Taniguchi N., Toda K., Kawata R., Hisa Y., Kannagi R. Roles of cell adhesion molecules in tumor angiogenesis induced by cotransplantation of cancer and endothelial cells to nude rats. Cancer Res. 2002;62:6289–6296. [PubMed] [Google Scholar]
- Weis S. M., Lindquist J. N., Barnes L. A., Lutu-Fuga K. M., Cui J., Wood M. R., Cheresh D. A. Cooperation between VEGF and beta3 integrin during cardiac vascular development. Blood. 2007;109:1962–1970. doi: 10.1182/blood-2005-10-038893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler-Jones C., Abu-Ghazaleh R., Cospedal R., Houliston R. A., Martin J., Zachary I. Vascular endothelial growth factor stimulates prostacyclin production and activation of cytosolic phospholipase A2 in endothelial cells via p42/p44 mitogen-activated protein kinase. FEBS Lett. 1997;420:28–32. doi: 10.1016/s0014-5793(97)01481-6. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Davis S., Gale N. W., Rudge J. S., Wiegand S. J., Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- Yoshida A., Anand-Apte B., Zetter B. R. Differential endothelial migration and proliferation to basic fibroblast growth factor and vascular endothelial growth factor. Growth Factors. 1996;13:57–64. doi: 10.3109/08977199609034566. [DOI] [PubMed] [Google Scholar]
- Zeng L., Xiao Q., Margariti A., Zhang Z., Zampetaki A., Patel S., Capogrossi M. C., Hu Y., Xu Q. HDAC3 is crucial in shear- and VEGF-induced stem cell differentiation toward endothelial cells. J. Cell Biol. 2006;174:1059–1069. doi: 10.1083/jcb.200605113. [DOI] [PMC free article] [PubMed] [Google Scholar]