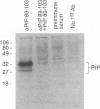
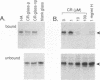
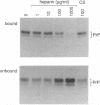
Abstract
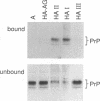
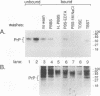
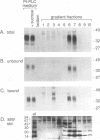
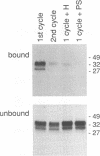
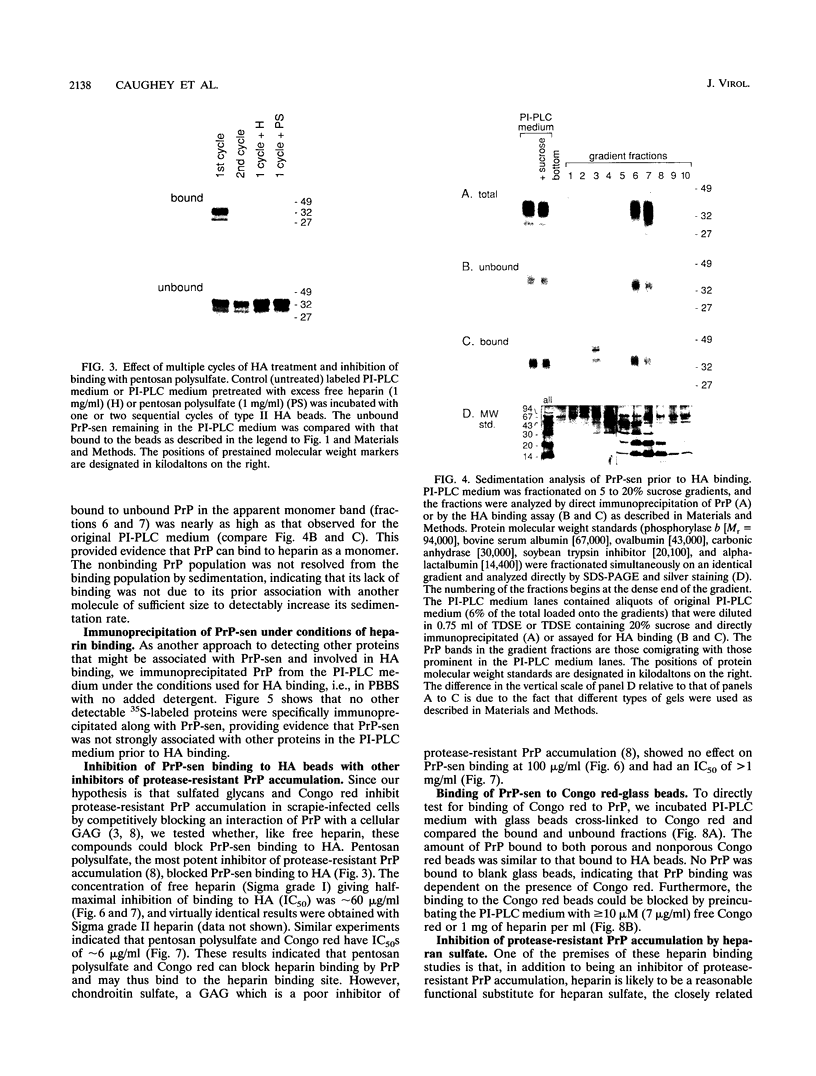
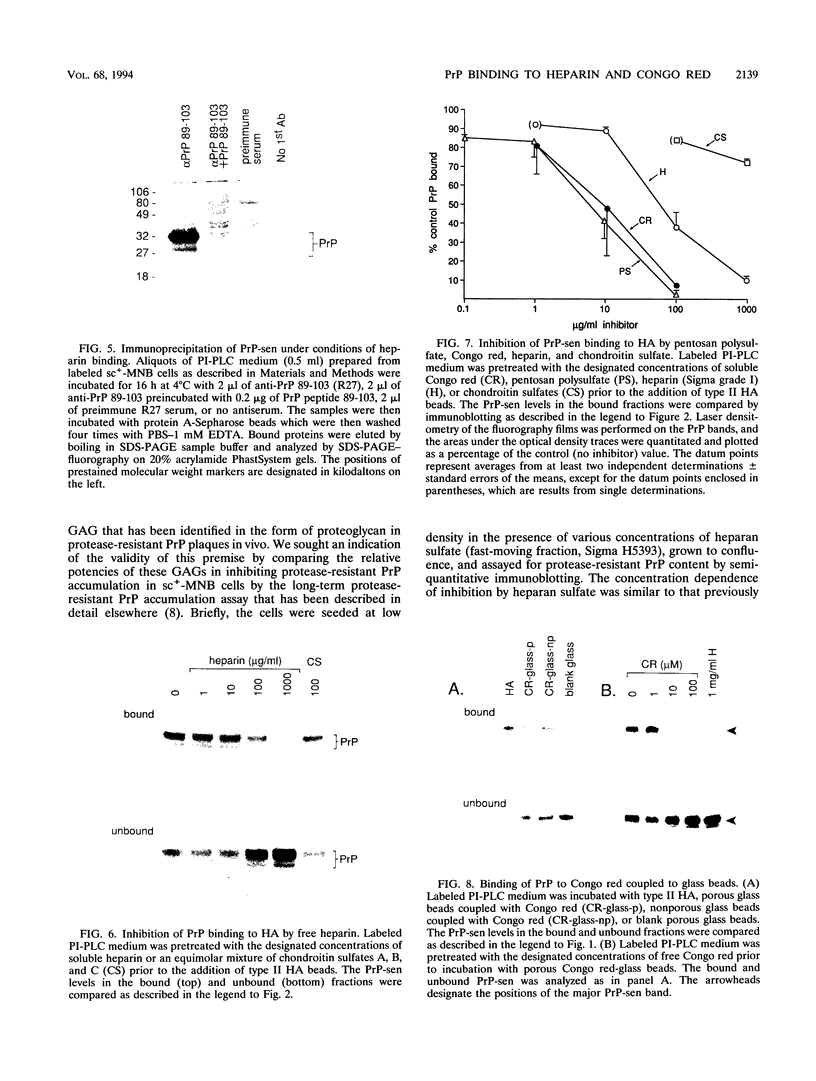
Congo red and certain sulfated glycans are potent inhibitors of protease-resistant PrP accumulation in scrapie-infected cells. One hypothesis is that these inhibitors act by blocking the association between protease-resistant PrP and sulfated glycosaminoglycans or proteoglycans (e.g., heparan sulfate proteoglycan) that is observed in amyloid plaques of scrapie-infected brain tissue. Accordingly, we have investigated whether the apparent precursor of protease-resistant PrP, protease-sensitive PrP, binds to Congo red and heparin, a highly sulfated glycosaminoglycan with an inhibitory potency like that of heparan sulfate. Protease-sensitive PrP released from the surface of mouse neuroblastoma cells bound to heparin-agarose and Congo red-glass beads. Sucrose density gradient fractionation provided evidence that at least some of the PrP capable of binding heparin-agarose was monomeric. Free Congo red blocked PrP binding to heparin and vice versa, suggesting that these ligands share a common binding site. The relative efficacies of pentosan polysulfate, Congo red, heparin, and chondroitin sulfate in blocking PrP binding to heparin-agarose corresponded with their previously demonstrated potencies in inhibiting protease-resistant PrP accumulation. These results are consistent with the idea that sulfated glycans and Congo red inhibit protease-resistant PrP accumulation by interfering with the interaction of PrP with an endogenous glycosaminoglycan or proteoglycan.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendheim P. E., Barry R. A., DeArmond S. J., Stites D. P., Prusiner S. B. Antibodies to a scrapie prion protein. Nature. 1984 Aug 2;310(5976):418–421. doi: 10.1038/310418a0. [DOI] [PubMed] [Google Scholar]
- Borchelt D. R., Scott M., Taraboulos A., Stahl N., Prusiner S. B. Scrapie and cellular prion proteins differ in their kinetics of synthesis and topology in cultured cells. J Cell Biol. 1990 Mar;110(3):743–752. doi: 10.1083/jcb.110.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughey B., Ernst D., Race R. E. Congo red inhibition of scrapie agent replication. J Virol. 1993 Oct;67(10):6270–6272. doi: 10.1128/jvi.67.10.6270-6272.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughey B., Neary K., Buller R., Ernst D., Perry L. L., Chesebro B., Race R. E. Normal and scrapie-associated forms of prion protein differ in their sensitivities to phospholipase and proteases in intact neuroblastoma cells. J Virol. 1990 Mar;64(3):1093–1101. doi: 10.1128/jvi.64.3.1093-1101.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughey B., Race R. E. Potent inhibition of scrapie-associated PrP accumulation by congo red. J Neurochem. 1992 Aug;59(2):768–771. doi: 10.1111/j.1471-4159.1992.tb09437.x. [DOI] [PubMed] [Google Scholar]
- Caughey B., Raymond G. J., Ernst D., Race R. E. N-terminal truncation of the scrapie-associated form of PrP by lysosomal protease(s): implications regarding the site of conversion of PrP to the protease-resistant state. J Virol. 1991 Dec;65(12):6597–6603. doi: 10.1128/jvi.65.12.6597-6603.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughey B., Raymond G. J. Sulfated polyanion inhibition of scrapie-associated PrP accumulation in cultured cells. J Virol. 1993 Feb;67(2):643–650. doi: 10.1128/jvi.67.2.643-650.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughey B., Raymond G. J. The scrapie-associated form of PrP is made from a cell surface precursor that is both protease- and phospholipase-sensitive. J Biol Chem. 1991 Sep 25;266(27):18217–18223. [PubMed] [Google Scholar]
- Caughey B. Scrapie associated PrP accumulation and its prevention: insights from cell culture. Br Med Bull. 1993 Oct;49(4):860–872. doi: 10.1093/oxfordjournals.bmb.a072651. [DOI] [PubMed] [Google Scholar]
- Diringer H., Ehlers B. Chemoprophylaxis of scrapie in mice. J Gen Virol. 1991 Feb;72(Pt 2):457–460. doi: 10.1099/0022-1317-72-2-457. [DOI] [PubMed] [Google Scholar]
- Diringer H., Gelderblom H., Hilmert H., Ozel M., Edelbluth C., Kimberlin R. H. Scrapie infectivity, fibrils and low molecular weight protein. Nature. 1983 Dec 1;306(5942):476–478. doi: 10.1038/306476a0. [DOI] [PubMed] [Google Scholar]
- Ehlers B., Diringer H. Dextran sulphate 500 delays and prevents mouse scrapie by impairment of agent replication in spleen. J Gen Virol. 1984 Aug;65(Pt 8):1325–1330. doi: 10.1099/0022-1317-65-8-1325. [DOI] [PubMed] [Google Scholar]
- Ehlers B., Rudolph R., Diringer H. The reticuloendothelial system in scrapie pathogenesis. J Gen Virol. 1984 Feb;65(Pt 2):423–428. doi: 10.1099/0022-1317-65-2-423. [DOI] [PubMed] [Google Scholar]
- Farquhar C. F., Dickinson A. G. Prolongation of scrapie incubation period by an injection of dextran sulphate 500 within the month before or after infection. J Gen Virol. 1986 Mar;67(Pt 3):463–473. doi: 10.1099/0022-1317-67-3-463. [DOI] [PubMed] [Google Scholar]
- Glenner G. G. Amyloid deposits and amyloidosis: the beta-fibrilloses (second of two parts). N Engl J Med. 1980 Jun 12;302(24):1333–1343. doi: 10.1056/NEJM198006123022403. [DOI] [PubMed] [Google Scholar]
- Griffith J. S. Self-replication and scrapie. Nature. 1967 Sep 2;215(5105):1043–1044. doi: 10.1038/2151043a0. [DOI] [PubMed] [Google Scholar]
- Guiroy D. C., Yanagihara R., Gajdusek D. C. Localization of amyloidogenic proteins and sulfated glycosaminoglycans in nontransmissible and transmissible cerebral amyloidoses. Acta Neuropathol. 1991;82(2):87–92. doi: 10.1007/BF00293949. [DOI] [PubMed] [Google Scholar]
- Kimberlin R. H., Walker C. A. Suppression of scrapie infection in mice by heteropolyanion 23, dextran sulfate, and some other polyanions. Antimicrob Agents Chemother. 1986 Sep;30(3):409–413. doi: 10.1128/aac.30.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladogana A., Casaccia P., Ingrosso L., Cibati M., Salvatore M., Xi Y. G., Masullo C., Pocchiari M. Sulphate polyanions prolong the incubation period of scrapie-infected hamsters. J Gen Virol. 1992 Mar;73(Pt 3):661–665. doi: 10.1099/0022-1317-73-3-661. [DOI] [PubMed] [Google Scholar]
- Merz P. A., Somerville R. A., Wisniewski H. M., Iqbal K. Abnormal fibrils from scrapie-infected brain. Acta Neuropathol. 1981;54(1):63–74. doi: 10.1007/BF00691333. [DOI] [PubMed] [Google Scholar]
- Narindrasorasak S., Lowery D., Gonzalez-DeWhitt P., Poorman R. A., Greenberg B., Kisilevsky R. High affinity interactions between the Alzheimer's beta-amyloid precursor proteins and the basement membrane form of heparan sulfate proteoglycan. J Biol Chem. 1991 Jul 15;266(20):12878–12883. [PubMed] [Google Scholar]
- Prusiner S. B., McKinley M. P., Bowman K. A., Bolton D. C., Bendheim P. E., Groth D. F., Glenner G. G. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell. 1983 Dec;35(2 Pt 1):349–358. doi: 10.1016/0092-8674(83)90168-x. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Novel proteinaceous infectious particles cause scrapie. Science. 1982 Apr 9;216(4542):136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- Snow A. D., Wight T. N., Nochlin D., Koike Y., Kimata K., DeArmond S. J., Prusiner S. B. Immunolocalization of heparan sulfate proteoglycans to the prion protein amyloid plaques of Gerstmann-Straussler syndrome, Creutzfeldt-Jakob disease and scrapie. Lab Invest. 1990 Nov;63(5):601–611. [PubMed] [Google Scholar]