Abstract
The major tea polyphenol, (-)-epigallocatechin-3-gallate (EGCG), inhibits carcinogenesis in many in vivo models. Many potential mechanisms of action have been proposed based on cell line studies, including pro-oxidant activity. In the present study, we studied the effect of N-acetylcysteine (NAC) on the inhibitory effects of EGCG on lung cancer cell growth. We found that NAC (0 – 2 mM) dose-dependently enhanced the growth inhibitory activity of EGCG against murine and human lung cancer cells. The combination of NAC and EGCG caused an 8.8-fold increase in apoptosis in CL13 mouse and H1299 human lung cancer cells compared to treatment with either agent alone. Addition of 2 mM NAC increased the stability of EGCG in the presence of CL13 cells (t1/2 = 8.5 h vs. 22.7 h). Intracellular levels of EGCG were increased 5.5-fold by the addition of 2 mM NAC. HPLC and LC-MS analyses of cell culture medium from CL13 cells treated with EGCG and NAC for 24 h revealed that EGCG-2′-NAC was time-dependently formed. This adduct was not formed in the absence of NAC. The present results show that under cell culture conditions, EGCG and NAC interact to form a previously unreported adduct, EGCG-2′-NAC, which may contribute to enhancement of EGCG-mediated cell killing.
Keywords: (-)-epigallocatechin-3-gallate, N-acetylcysteine, lung cancer, apoptosis
Introduction
Green tea (Camellia sinensis, Theaceae) and its major catechin component, (-)-epigallocatechin-3-gallate (EGCG, Fig. 1), have been shown to inhibit tumorigenesis in a number of animal models [1, 2]. Numerous mechanisms of action have been proposed, including inhibition of growth factor signaling, inhibition of kinases, inhibition of DNA methyltransferase, and others [3, 4]. EGCG undergoes auto-oxidation resulting in oligomerization and the formation of reactive oxygen species (ROS) under cell culture conditions [5, 6]. An increasing number of reports have implicated EGCG-produced ROS as key mediators for EGCG anticancer activity in vitro [7–9].
Figure 1.
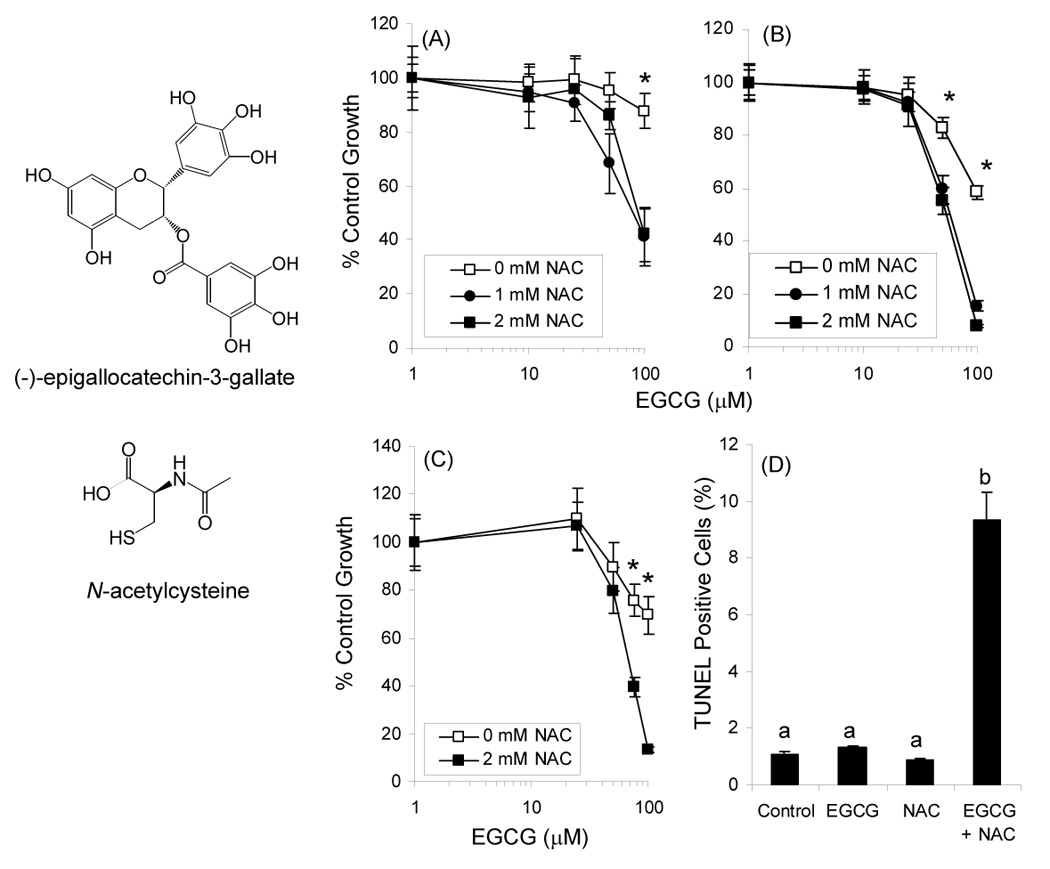
Structure of EGCG and NAC. Growth inhibitory effects of EGCG alone and in combination with NAC against CL13 mouse lung adenocarcinoma cells after 24h (A) or 48 h (B) were determined by the MTT assay. Growth inhibition was also assessed in H1299 human lung adenocarcinoma cells (C). Induction of apoptosis in CL13 cells following treatment for 48 h with EGCG and NAC was determined using the TUNEL assay (D). Each point represents n = 12 – 18 for growth inhibition and n = 3 for apoptosis. Cells were treated in the presence of 5 U/mL SOD, 30 U/mL catalase, and 10% FBS. Error bars represent the SD. * = p < 0.05 by two-way ANOVA with Bonferroni post-test. Different superscripted letters indicate statistically signficant differences by one-way ANOVA with Tukey’s post-test.
We have reported that treatment of human esophageal cancer cells with EGCG results in dose-dependent decreases in the levels of phosphorylated and non-phosphorylated epidermal growth factor receptor (EGFR). These effects are diminished by inclusion of superoxide dismutase (SOD) which stablizes EGCG, and apparently prevents oxidative damage of EGFR [10]. Similarly, inclusion of SOD and/or catalase reduces the growth inhibitory and pro-apoptotic activity of EGCG in many in vitro systems. For example, our laboratory has shown that inclusion of catalase delayed induction of apoptosis in 21BES transformed human bronchial epithelial cells [11]. Inclusion of catalase or SOD and catalase also decreased growth inhibition and apoptosis induced by EGCG in H661 and H1299 human lung cancer cells, respectively [12, 13]. These studies were conducted in the presence of fetal bovine serum (FBS). By contrast, inclusion of SOD enhanced growth inhibition in KYSE150 human esophageal cancer cells treated with EGCG under serum-free conditions, possibly by increasing the stability of EGCG [13].
Under certain experimental conditions, the pro-oxidant activities of EGCG have been observed in vivo. For example, we have observed that daily treatment of xenograft tumor-bearing athymic nude mice with intraperitoneal EGCG results in increased expression of γ-histone 2AX and metallothionein in the tumor and liver [13]. We have also observed that treatment of mice with toxic doses of EGCG results in the excretion of EGCG-2′-cysteine and EGCG-2″-cysteine in the urine [14]. These metabolites are hypothesized to arise from cysteine conjugation to the activated carbon in EGCG quinone. We and others have previously reported such reactions between EGCG and thiol-containing compounds such as cysteine and glutathione in cell culture and in vitro systems [14, 15].
If generating ROS is a key mechanism for the induction of apoptosis by EGCG, then addition of the thiol antioxidant, N-acetylcysteine (NAC, Fig. 1) to the cell culture medium would reduce such activity by further quenching EGCG-mediated ROS production. In the present report we examined the effect of NAC on the stability, cell uptake, and growth inhibitory activity of EGCG against lung cancer cell lines. Herein, we report the results of these studies.
Materials and Methods
Chemicals
EGCG, epigallocatechin (EGC), epicatechin-3-gallate (ECG), and epicatechin (EC) was provided by Mitsui Norin Co. Ltd. (Tokyo, Japan). NAC was purchased from Aldrich Chemical Co. (Milwaukee, WI, USA). Stock solutions (100 mM) of EGCG and NAC (100 mM) were prepared in DMSO and water, respectively. Standard solutions of EGCG, EGC, ECG and EC (10 µg/mL) for HPLC were made in 0.2% ascorbic acid-0.005% EDTA solution and stored at −80°C. All other reagents and HPLC solvents were of the highest grade available.
Cell Culture
CL13 mouse lung adenocarcinoma cells were generously provided by Dr. Steven A. Belinsky (Lovelace Respiratory Research Institute, Albuquerque, NM). H1299 human lung adenocarcinoma cells were obtained from American Type Tissue Collection (Rockville, MD). Both cell lines were maintained in log-phase growth in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 0.1 mg/mL streptomycin at 37°C in 95% humidity and 5% CO2.
Growth Inhibition and Apoptosis
To determine the growth inhibitory activity of EGCG in combination with NAC, CL13 or H1299 cells were plated in 96-well plates (5 × 103 cells per well) and allowed to attach for 24 h. The medium was replaced with fresh, serum-complete medium containing 0 – 100 µM of EGCG in the presence or absence of 0 – 2 mM NAC. Cells were incubated for 24 – 48 h at 37°C. The medium was removed, the cells were washed once with medium containing no EGCG or NAC, and the number of viable cells were determined using the 3,[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay [16]. Treatments were conducted in the presence of 5 U/mL SOD and 30 U/mL catalase to stabilize EGCG and quench EGCG-derived ROS.
Induction of apoptosis was determined by measuring Terminal Deoxynucleotidyl Transferase Mediated dUTP Nick End Labeling (TUNEL) positivity. In brief, CL13 cells (2 × 105 cells/ 10 cm dish) were treated for 48 h with 75 µM EGCG, 2 mM NAC, or both. All treatments were in the presence 5 U/mL SOD and 30 U/mL catalase. Floating and attached cells were collected, washed with PBS, fixed with 5% buffered formalin, and permeabilized with 0.1% Triton X-100 in 0.1% sodium citrate. Cells were then labeled according to the manufacturer’s protocol (Roche Diagnostics, Indianapolis, IN) and analyzed by fluorescence-activated cell-sorting analysis with CellQuest software (BD Biosciences). Flow cytometric analysis was carried out at the Environmental and Occupational Health Sciences Institute (EOHSI) at Rutgers University.
Intracellular Concentration
Cytosolic levels of EGCG in the presence or absence of NAC were determined as previously reported [17]. In brief, cells were allowed to grow to 70 – 90% confluency in 12 – well plates. The medium was replaced with fresh medium containing 100 µM EGCG with or without 2 mM NAC. Medium also contained 10% FBS and 5 U/mL SOD and 30 U/mL catalase. The cells were incubated for 24 h at 37°C, after which the medium was removed, the cells were washed 2 times with cold PBS, 2% ascorbic acid was added to each well, and the cells were scraped and sonicated. The resulting solution was centrifuged at 16,000g for 20 min. The supernatant was combined with an equal volume of ice-cold methanol and centrifuged for 20 min at 16,000g to precipitate the protein. The resulting supernatant was analyzed by HPLC. Cytosolic EGCG was normalized to cytosolic protein concentration.
Stability of EGCG
The effect of NAC on the stability of EGCG under cell culture conditions was determined in the presence of CL13 cells. In brief, cells were allowed to grow to 70 – 90% confluency in 6 – well plates. The medium was replaced with fresh medium containing 100 µM EGCG with or without 2 mM NAC in the presence of 5 U/mL SOD and 30 U/mL catalase. Samples of medium were collected at 0, 0.25, 0.5, 2, 4, and 24 h, and combined with an equal volume of 0.2% ascorbic acid. Samples were diluted 1:10 with 10% methanol and analyzed by HPLC.
HPLC Analysis
EGCG levels were analyzed using an HPLC system consisting of two ESA model 580 dual-piston pumps (Chelmsford, MA), a Waters Model 717plus refrigerated autosampler (Milford, MA), and an ESA 5500 coulochem electrode array system (CEAS). The potentials of the CEAS were set at −100, 100, 300, and 500 mV. Separation was achieved using previously described methods [18].
LC/ESI-MS Analysis
LC-MS analysis was carried out with a Finnigan Spectra System which consisted of a Surveyour MS pump plus, a Surveyou refrigerated autosampler plus, and a LTQ linear ion trap mass detector (ThermoFinigan, San Jose, CA) equipped with an electrospray ionization (ESI) interface. A 50 × 2.0 mm i.d., 3 µm Gemini C18 column (Phenomenex) was used for separation with a flow rate of 0.2 mL/min. The column 8 elution started with 3-min isocratic phase of 100% solvent A (5% aqueous methanol with 0.2% acetic acid), followed by progressive, linear increases in B (95% aqueous methanol with 0.2% acetic acid) to 40% at 43 min and 100% at 50 min. The mobile phase was then re-equilibrated to 100% A at 51 min for 10 min. The LC elute was introduced into the ESI interface. The negative ion polarity mode was set for ESI ion source with the voltage on the ESI interface maintained at approximately 5 kV. Nitrogen gas was used as the sheath gas at a flow rate of 30 arb unites and the auxiliary gas at 5 arb units, respectively. The structural information of all the test compounds was obtained by tandem mass spectrometry (MS2) through collision-induced dissociation (CID) with a relative collision energy setting of 35%.
Statistical analysis
All experiments were performed at least three times. Experimental data were analyzed by two-tailed Student’s t-test, one-way ANOVA with Tukey’s multiple comparison tests, or two-way ANOVA with Bonferroni post-test as appropriate. A P-value of less than 0.05 was considered statistically significant.
Results and Discussion
Treatment of CL13 mouse lung adenocarcinoma cells with EGCG (0 – 100 µM) resulted in a dose-dependent decrease in the number of viable cells as measured by the MTT assay (Fig. 1). EGCG at 100 µM inhibited CL13 cell growth by 14% and 42% after 24 h and 48 h, respectively. Concurrent treatment of cells with EGCG and NAC (1 or 2 mM) resulted in increased growth inhibition with IC50 = 80 or 75 µM and 61 or 55 µM after 24 and 48 h treatment, respectively. These effects were also confirmed using H1299 human lung adenocarcinoma cells (Fig. 1C) NAC alone had no significant effect on the number of viable CL13 or H1299 cells.
Apoptosis induction was determined by measuring TUNEL positive cells with flow cytometry. Neither 75 µM EGCG nor 2 mM NAC siginificantly induced apoptosis in CL13 cells (Fig. 1D). The combination of EGCG and NAC, however, resulted in an 8.8-fold increase in apoptosis compared to vehicle-treated cells.
In the presence of 2 mM NAC the cellular uptake of EGCG (100 µM) increased by 2.5-fold (Fig. 2A). This increase in cytosolic levels appears to be due to increased stability of EGCG in the presence of NAC. HPLC analysis revealed that addition of 2 mM NAC increased the level of EGCG in the cell culture medium of CL13 cells by 5.5-fold compared after 24 h incubation (Fig 2B). The t1/2 of EGCG was 8.5 h and 22.7 h in the absence and presence of 2 mM NAC, respectively.
Figure 2.
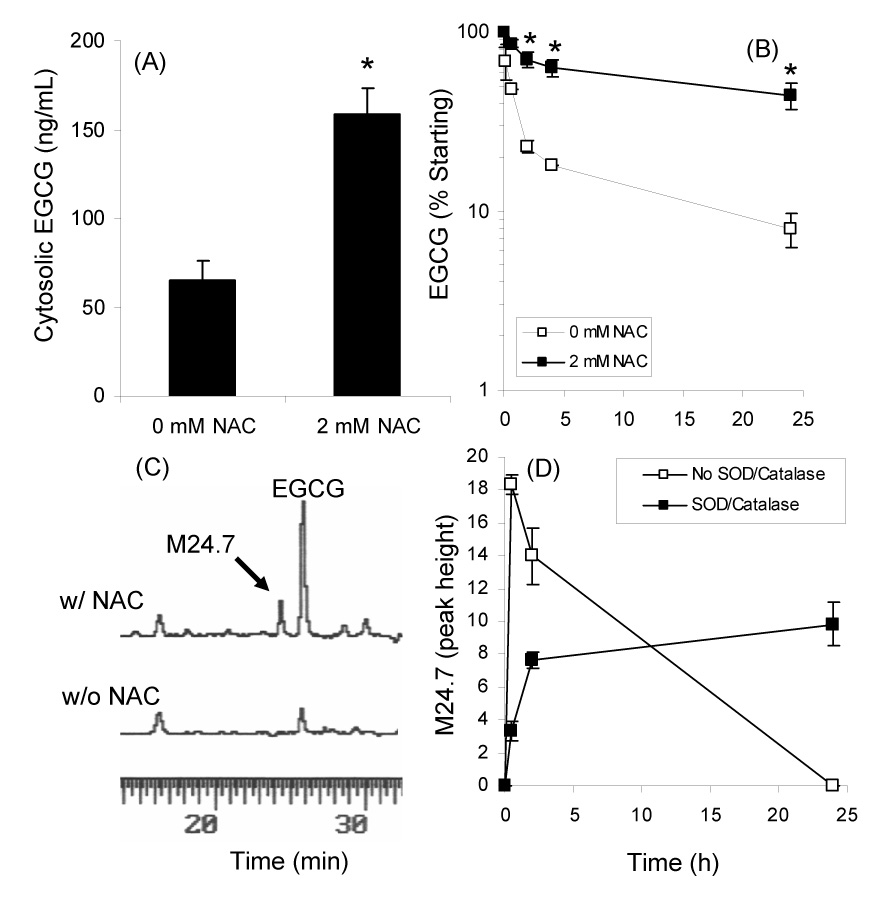
Effect of 2 mM NAC on the cytosolic levels (A) and stability (B) of EGCG in the presence of CL13 mouse lung adenocarcinoma cells. (C) HPLC analysis of medium from CL13 cells treated with 100 µM EGCG alone or in combination with 2 mM NAC. (D) Formation of novel peak M24.7 under cell culture conditions in the presence of 100 µM EGCG, 2 mM NAC, and CL13 cells. Each point represents n = 3. Error bars represent the SD. * = p < 0.05 by two-tailed Students’ t-test
To study the possible interactions between NAC and EGCG, RPMI 1640 medium was collected after CL13 cells were incubated with EGCG (100 µM) alone or its combination with NAC (2 mM) in the presence of SOD and catalase (5 and 30 U/mL, respectively) and 10% FBS. HPLC-ECD analysis revealed the presence of several new peaks in the medium from the EGCG/NAC treated cells that were not present in the EGCG only treated cells (Fig. 2C). These peaks were not present at the 0 h time point and increased as a function of incubation time. The kinetics of formation of the major peak M24.7 are shown in Figure 2D. In the absence of SOD and catalase, M24.7 formed rapidly and then disappeared as a function of time, whereas in the presence of SOD (5 U/mL) and catalase (30 U/mL), M24.7 formed more slowly and was stable. This behavior was similar to what we and others have previously observed for EGCG under cell culture conditions [5, 10].
In order to determine whether EGCG reacts with NAC in a manner analogous to its reaction with cysteine, medium was collected from CL13 cells that were treated with EGCG (100 µM) alone or in combination with NAC (2 mM) for 24 h in the presence of 5 U/mL SOD and 30 U/mL catalase and 10% FBS. LC/ESI-MS analysis of the medium samples revealed a peak with a molecular ion m/z [M−H]− = 618 that was present only in the samples from cells treated with EGCG and NAC. This corresponded with the predicted molecular weight of EGCG-NAC conjugate (Fig. 3). MS2 produced the major fragment ions of m/z = 448 and 489 correponding to loss of gallate and cleavage of the C – S bond in NAC, respectively (Fig. 3). In order to determine where the NAC-conjugate was located on the EGCG molecule, the MS3 spectra of the m/z = 489 ion of the putative EGCG-NAC conjugate was compared to that of EGCG-2′-cys (Fig. 3). These spectra were identical confirming that the NAC is linked via a C – S bond at the 2′-position of EGCG.
Figure 3.
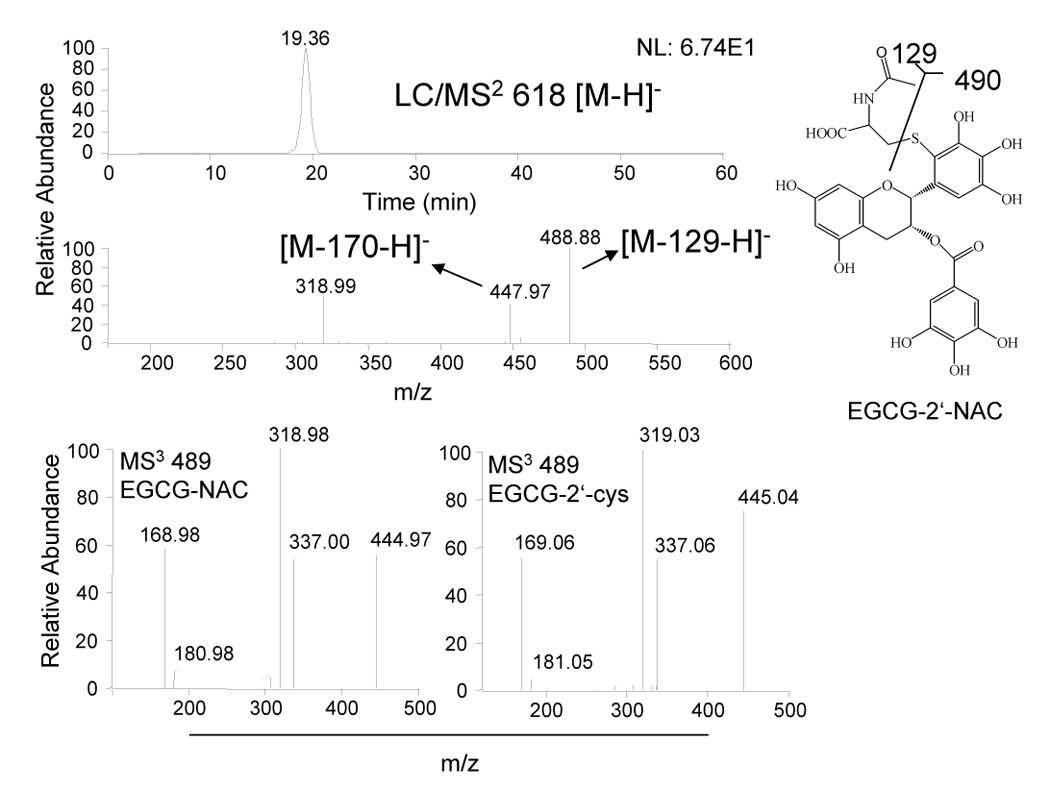
LC-MS analysis of medium from CL13 cells treated with 100 µM EGCG alone or in combination with 2 mM NAC. Medium and cytosol were collected after 24 h treatment of CL13 cells in the presence of 5 U/mL SOD, 30 U/mL catalase, and 10% FBS. Structure of EGCG-NAC conjugate as determined by LC-MS.
We propose, based on our previous work, that EGCG-2′-NAC forms through the oxidation of EGCG by some ROS such as superoxide anion or enzymatically to form either a quinone or semiquinone (Fig. 4). The resulting activated 2′-carbon then reacts with the thiol group of NAC. Since our experimental conditions include SOD and catalase, which are not able to enter the cells, we propose that the ROS which drive the reaction are formed intracellularly, and that the reaction between EGCG and NAC may also occur within the cells.
Figure 4.
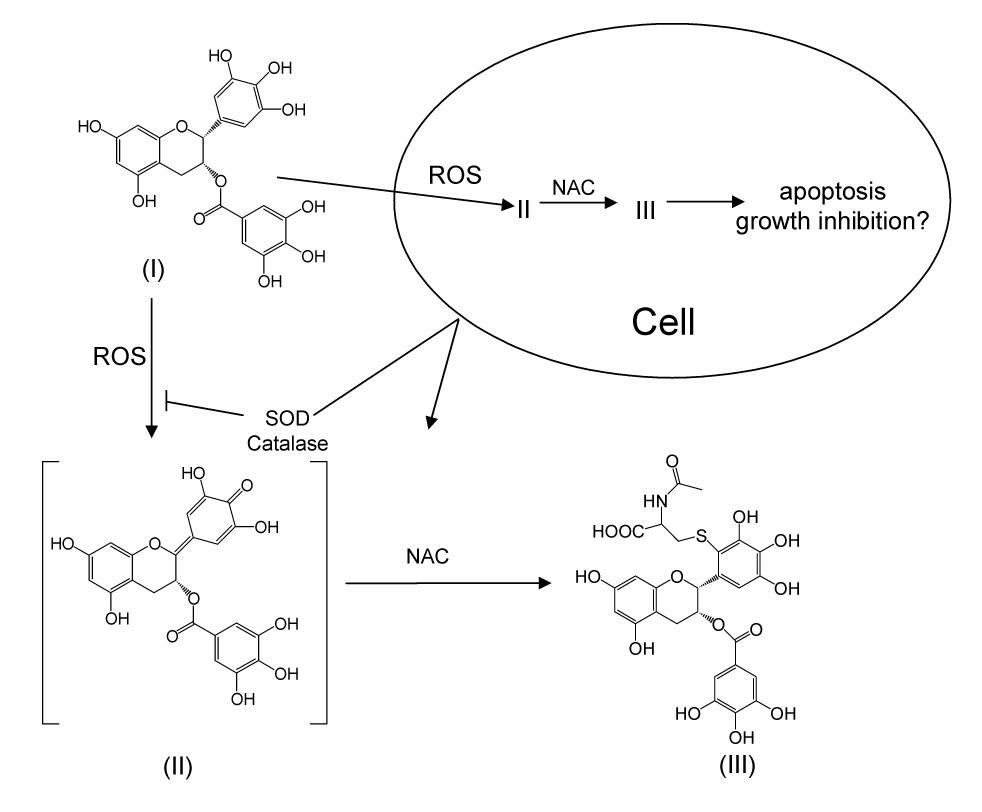
Proposed mechanism for the formation of EGCG-2′-NAC under cell culture conditions. I = EGCG, II = EGCG quinone, III = EGCG-2′-NAC.
These enhanced growth inhibitory and pro-apoptotic effects of the combination of EGCG and NAC appear to partially correlate with NAC-mediated increases in EGCG stability and EGCG cell-uptake. Previously, however, we and others have reported that addition of SOD and catalase decreased EGCG-mediated formation of ROS and decreased cell apoptosis and growth inhibition [13, 19]. These effects were observed in several cell lines, including H1299 cells, and were particularly pronounced in the presence of FBS, which binds tightly to EGCG and prevents its movement from the medium into the cells.
The results in this current study seem contradictory and suggest that the increase in growth inhibitory activity observed using the combination of EGCG and NAC maybe due to the activity of EGCG-2′-NAC. Such a hypothesis is supported by previous work on other catechol-containing compounds. For example, the catechol metabolites of 3,4 methylenedioxymethamphetamine undergo oxidation to form a quinone intermediate that then reacts with glutathione. The resulting thiolquinone is highly redox active and cytotoxic [20, 21]. We have observed similar results with the EGCG-2′-cysteine and EGCG-2″-cysteine conjugates. Incubation of these compounds under cell culture conditions results in the formation of H2O2 at a more rapid rate than incubation of equimolar concentrations of EGCG (Lambert, unpublished). Similarly, these compounds retain the growth inhibitory activity of EGCG (Lambert, unpublished). These data would suggest that the EGCG-2′-NAC conjugate is biologically active and may be more redox active than EGCG. We are attempting to synthesize larger quantities of EGCG-2′-NAC to directly test this hypothesis. Direct assessment of the activity of this compound will improve our understanding of the interaction between EGCG and NAC, and may represent an interesting lead compound for further study.
It is also interesting to note that the cysteine and glutathione conjugates of EGCG are readily formed in a horseradish peroxidase/H2O2 catalyzed reaction, but the NAC conjugate is produced in only small quantities [14]. Further studies are needed to determine what factors in the present cell culture conditions facilitate the formation of this compound. This information will be useful in scaling up the synthesis of EGCG-2′- NAC for future biological studies. Further studies are also needed to determine if this new metabolite of EGCG is formed in vivo and if so under what conditions it forms.
Finally, it is unclear what the implications of the present study are in terms of lung cancer prevention in vivo. Certainly, enhancement of biological activity through the use of combinations of agents is interesting and is the subject of considerable research [22–24]. In the absence of strong in vivo data, it would be unwise for us to speculate on whether the current combination will prove useful in the prevention of cancer. Given that NAC and EGCG are both widely used as dietary supplements, and have been studied individually for their anticancer and cancer preventive activities, we believe that further studies of the present combination are warranted [2, 25–28].
Acknowledgements
This work was supported by American Institute for Cancer Research Grant 05A047 (to JDL), National Cancer Institute Grant CA125780 (to JDL), and National Cancer Institute Grant CA121390 (to SS). The authors wish to acknowledge technical assistance from Dr. Hang Xiao and Ms. Joan DuBois.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lambert JD, Yang CS. Cancer chemopreventive activity and bioavailability of tea and tea polyphenols. Mutat Res. 2003;523–524:201–208. doi: 10.1016/s0027-5107(02)00336-6. [DOI] [PubMed] [Google Scholar]
- 2.Lambert JD, Hong J, Yang GY, Liao J, Yang CS. Inhibition of carcinogenesis by polyphenols: evidence from laboratory investigations. Am J Clin Nutr. 2005;81:284S–291S. doi: 10.1093/ajcn/81.1.284S. [DOI] [PubMed] [Google Scholar]
- 3.Hou Z, Lambert JD, Chin KV, Yang CS. Effects of tea polyphenols on signal transduction pathways related to cancer chemoprevention. Mutat Res. 2004;555:3–19. doi: 10.1016/j.mrfmmm.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 4.Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003;43:89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- 5.Hong J, Lu H, Meng X, Ryu JH, Hara Y, Yang CS. Stability, cellular uptake, biotransformation, and efflux of tea polyphenol (-)-epigallocatechin-3- gallate in HT-29 human colon adenocarcinoma cells. Cancer Res. 2002;62:7241–7246. [PubMed] [Google Scholar]
- 6.Sang S, Lee MJ, Hou Z, Ho CT, Yang CS. Stability of Tea Polyphenol (-)-Epigallocatechin-3-gallate and Formation of Dimers and Epimers under Common Experimental Conditions. J Agric Food Chem. 2005;53:9478–9484. doi: 10.1021/jf0519055. [DOI] [PubMed] [Google Scholar]
- 7.Yang CS, Hong J, Hou Z, Sang S. Green tea polyphenols: antioxidative and prooxidative effects. J Nutr. 2004;134:3181S. doi: 10.1093/jn/134.11.3181S. [DOI] [PubMed] [Google Scholar]
- 8.Yang CS, Sang S, Lambert JD, Hou Z, Ju J, Lu G. Possible mechanisms of the cancer-preventive activities of green tea. Mol Nutr Food Res. 2006;50:170–175. doi: 10.1002/mnfr.200500105. [DOI] [PubMed] [Google Scholar]
- 9.Chan MM, Soprano KJ, Weinstein K, Fong D. Epigallocatechin-3-gallate delivers hydrogen peroxide to induce death of ovarian cancer cells and enhances their cisplatin susceptibility. J Cell Physiol. 2006;207:389–396. doi: 10.1002/jcp.20569. [DOI] [PubMed] [Google Scholar]
- 10.Hou Z, Sang S, You H, Lee MJ, Hong J, Chin KV, Yang CS. Mechanism of Action of (-)-Epigallocatechin-3-Gallate: Auto-oxidation- Dependent Inactivation of Epidermal Growth Factor Receptor and Direct Effects on Growth Inhibition in Human Esophageal Cancer KYSE 150 Cells. Cancer Res. 2005;65:8049–8056. doi: 10.1158/0008-5472.CAN-05-0480. [DOI] [PubMed] [Google Scholar]
- 11.Vittal R, Selvanayagam ZE, Sun Y, Hong J, Liu F, Chin KV, Yang CS. Gene expression changes induced by green tea polyphenol (-)-epigallocatechin- 3-gallate in human bronchial epithelial 21BES cells analyzed by DNA microarray. Mol Cancer Ther. 2004;3:1091–1099. [PubMed] [Google Scholar]
- 12.Yang GY, Liao J, Kim K, Yurkow EJ, Yang CS. Inhibition of growth and induction of apoptosis in human cancer cell lines by tea polyphenols. Carcinogenesis. 1998;19:611–616. doi: 10.1093/carcin/19.4.611. [DOI] [PubMed] [Google Scholar]
- 13.Hou Z, Xiao H, Lambert J, You H, Yang CS. Green tea polyphenol, (-)-epigallocatechin-3-gallate, induces oxidative stress and DNA damage in cancer cell lines, xenograft tumors, and mouse liver. Proc Amer Assoc Cancer Res. 2006 [Google Scholar]
- 14.Sang S, Lambert JD, Hong J, Tian S, Lee MJ, Stark RE, Ho CT, Yang CS. Synthesis and structure identification of thiol conjugates of (-)-epigallocatechin gallate and their urinary levels in mice. Chem Res Toxicol. 2005;18:1762–1769. doi: 10.1021/tx050151l. [DOI] [PubMed] [Google Scholar]
- 15.Galati G, Lin A, Sultan AM, O'Brien PJ. Cellular and in vivo hepatotoxicity caused by green tea phenolic acids and catechins. Free Radic Biol Med. 2006;40:570–580. doi: 10.1016/j.freeradbiomed.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 16.Bold RJ, Virudachalam S, McConkey DJ. Chemosensitization of pancreatic cancer by inhibition of the 26S proteasome. J Surg Res. 2001;100:11–17. doi: 10.1006/jsre.2001.6194. [DOI] [PubMed] [Google Scholar]
- 17.Hong J, Lambert JD, Lee SH, Sinko PJ, Yang CS. Involvement of multidrug resistance-associated proteins in regulating cellular levels of (-)-epigallocatechin-3-gallate and its methyl metabolites. Biochem Biophys Res Commun. 2003;310:222–227. doi: 10.1016/j.bbrc.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Lee MJ, Prabhu S, Meng X, Li C, Yang CS. An improved method for the determination of green and black tea polyphenols in biomatrices by high-performance liquid chromatography with coulometric array detection. Anal Biochem. 2000;279:164–169. doi: 10.1006/abio.2000.4487. [DOI] [PubMed] [Google Scholar]
- 19.Yang GY, Liao J, Li C, Chung J, Yurkow EJ, Ho CT, Yang CS. Effect of black and green tea polyphenols on c-jun phosphorylation and H(2)O(2) production in transformed and non-transformed human bronchial cell lines: possible mechanisms of cell growth inhibition and apoptosis induction. Carcinogenesis. 2000;21:2035–2039. doi: 10.1093/carcin/21.11.2035. [DOI] [PubMed] [Google Scholar]
- 20.Monks TJ, Jones DC, Bai F, Lau SS. The role of metabolism in 3,4-(+)-methylenedioxyamphetamine and 3,4-(+)-methylenedioxymethamphetamine (ecstasy) toxicity. Ther Drug Monit. 2004;26:132–136. doi: 10.1097/00007691-200404000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Bai F, Lau SS, Monks TJ. Glutathione and N-acetylcysteine conjugates of alpha-methyldopamine produce serotonergic neurotoxicity: possible role in methylenedioxyamphetamine-mediated neurotoxicity. Chem Res Toxicol. 1999;12:1150–1157. doi: 10.1021/tx990084t. [DOI] [PubMed] [Google Scholar]
- 22.Reddy BS, Wang CX, Kong AN, Khor TO, Zheng X, Steele VE, Kopelovich L, Rao CV. Prevention of azoxymethane-induced colon cancer by combination of low doses of atorvastatin, aspirin, and celecoxib in F 344 rats. Cancer Res. 2006;66:4542–4546. doi: 10.1158/0008-5472.CAN-05-4428. [DOI] [PubMed] [Google Scholar]
- 23.Chauhan D, Li G, Podar K, Hideshima T, Shringarpure R, Catley L, Mitsiades C, Munshi N, Tai YT, Suh N, Gribble GW, Honda T, Schlossman R, Richardson P, Sporn MB, Anderson KC. The bortezomib/proteasome inhibitor PS-341 and triterpenoid CDDO-Im induce synergistic anti-multiple myeloma (MM) activity and overcome bortezomib resistance. Blood. 2004;103:3158–3166. doi: 10.1182/blood-2003-08-2873. [DOI] [PubMed] [Google Scholar]
- 24.Stadheim TA, Suh N, Ganju N, Sporn MB, Eastman A. The novel triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid (CDDO) potently enhances apoptosis induced by tumor necrosis factor in human leukemia cells. J Biol Chem. 2002;277:16448–16455. doi: 10.1074/jbc.M108974200. [DOI] [PubMed] [Google Scholar]
- 25.Izzotti A, Bagnasco M, Cartiglia C, Longobardi M, Balansky RM, Merello A, Lubet RA, De Flora S. Chemoprevention of genome, transcriptome, and proteome alterations induced by cigarette smoke in rat lung. Eur J Cancer. 2005;41:1864–1874. doi: 10.1016/j.ejca.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 26.Vanisree AJ, Shyamaladevi CS. The effect of N-acetylcysteine in combination with vitamin C on the activity of ornithine decarboxylase of lung carcinoma cells- -In vitro. Life Sci. 2006;79:654–659. doi: 10.1016/j.lfs.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Thanopolou E, Baltayiannis N, Lykogianni V. Nutritional aspects regarding lung cancer chemoprevention. J Buon. 2006;11:7–20. [PubMed] [Google Scholar]
- 28.Lambert JD, Yang CS. Mechanisms of cancer prevention by tea constituents. J Nutr. 2003;133:3262S–3262S. doi: 10.1093/jn/133.10.3262S. [DOI] [PubMed] [Google Scholar]


