Abstract
Golgi-specific DHHC (Asp-His-His-Cys) zinc finger protein (GODZ) is a DHHC family palmitoyl acyltransferase that is implicated in palmitoylation and regulated trafficking of diverse substrates that function either at inhibitory or excitatory synapses. Of particular interest is the γ2 subunit of GABAA receptors, which is required for targeting these receptors to inhibitory synapses. Here, we report that GODZ and, to a lesser extent, its close paralog sertoli cell gene with a zinc finger domain-β (SERZ-β) are the main members of the DHHC family of enzymes that are able to palmitoylate the γ2 subunit in heterologous cells. Yeast two-hybrid and colocalization assays in human embryonic kidney 293T (HEK293T) cells indicate that GODZ and SERZ-β show indistinguishable palmitoylation-dependent interaction with the γ2 subunit. After coexpression in HEK293T cells, they form homomultimers and heteromultimers, as shown by coimmunoprecipitation and in vivo cross-linking experiments. Analyses in neurons transfected with dominant-negative GODZ (GODZC157S) or plasmid-based GODZ-specific RNAi indicate that GODZ is required for normal accumulation of GABAA receptors at synapses, for normal whole-cell and synaptic GABAergic inhibitory function and, indirectly, for GABAergic innervation. Unexpectedly, GODZ was found to be dispensable for normal postsynaptic AMPA receptor-mediated glutamatergic transmission. We conclude that GODZ-mediated palmitoylation of GABAA receptors and possibly other substrates contributes selectively to the formation and normal function of GABAergic inhibitory synapses.
Keywords: trafficking, synaptogenesis, inhibitory synapses, palmitoylation, DHHC-CRD proteins, GABAA receptors
Introduction
GABAA receptors are the principal mediators of neural inhibition by the neurotransmitter GABA. Structurally, they represent heteropentameric ligand-gated chloride channels composed of subunits from seven homologous subclasses (α1–6, β1–3, γ1–3, δ, ε, π, and θ), with the majority of receptors containing α, β, and γ2 subunits (Sieghart and Sperk, 2002). Of particular interest is the γ2 subunit, which is essential for the accumulation and function of a major subset of these receptors at inhibitory synapses (Essrich et al., 1998; Schweizer et al., 2003; Li et al., 2005).
Reversible posttranslational modifications of GABAA receptors are implicated in dynamic regulation of cell surface expression and function of these receptors and in modulating GABAergic inhibitory transmission (Kittler and Moss, 2003; Luscher and Keller, 2004). Specifically, the addition of the 16-carbon saturated fatty acid palmitate to cysteine residues has emerged as a reversible posttranslational protein modification involved in regulated trafficking and functional modulation of diverse membrane proteins and membrane-associated signaling factors, especially in neurons (El-Husseini and Bredt, 2002; Bijlmakers and Marsh, 2003). The γ2 subunit of GABAA receptors is subject to palmitoylation on multiple cysteine residues that are conserved in the major cytoplasmic loop region of all three γ subunits but absent in other GABAA receptor subunits, and this mechanism is required for normal expression of GABAA receptors at the cell surface of neurons (Keller et al., 2004; Rathenberg et al., 2004). Candidate proteins implicated in palmitoylation of the γ2 subunit in vivo include the Golgi-specific DHHC (Asp-His-His-Cys) zinc finger protein, GODZ [also known as DHHC3 (Uemura et al., 2002; Keller et al., 2004)], as well as other members of the family of DHHC-cysteine-rich domain (DHHC-CRD)-containing putative palmitoyl-acyltransferases (PATs). GODZ interacts selectively with the major cytoplasmic loop region of γ1–3 subunits of GABAA receptors and palmitoylates the γ2 subunit after cotransfection into human embryonic kidney 293 T (HEK 293T) cells (Keller et al., 2004). In addition to the α2 subunit, other in vitro substrates of GODZ include the Gs-protein α subunit, soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein 25b (SNAP25b), postsynaptic density-95 (PSD-95) (Fukata et al., 2004), and the AMPA receptor glutamate receptor 1–4 (GluR1–4) subunits (Hayashi et al., 2005).
Here, we addressed further which of the known DHHC proteins might be able to palmitoylate the γ2 subunit, and whether GODZ plays a role in trafficking GABAA receptors in neurons and in modulating synaptic transmission. GODZ and its most closely related paralog sertoli cell gene with a zinc finger domain-β (SERZ-β) (Chaudhary and Skinner, 2002) (also known as DHHC7) were the only members of the DHHC family of putative PATs that could efficiently palmitoylate the γ2 subunit on cotransfection into HEK293T cells. Furthermore, GODZ and SERZ-β formed homomultimers and heteromultimers after coexpression in heterologous cells. Dominant-negative GODZ (GODZC157S) and GODZ-specific short hairpin RNA (shRNA) constructs transfected into neurons revealed that GODZ was required for normal trafficking of γ2 subunit-containing GABAA receptors to synapses and for normal GABAergic inhibitory transmission. Indirectly, GODZ-mediated palmitoylation was required for GABAergic innervation. GODZ therefore contributes to presynaptic and postsynaptic assembly and normal function of inhibitory synapses. In contrast, GODZ was found to be dispensable for AMPA receptor-mediated glutamatergic transmission.
Materials and Methods
Protein–protein interaction assays and plasmid constructs.
Interactions between the γ2 subunit cytoplasmic loop domain and GODZ or SERZ-β, respectively, were tested using the SOS recruitment/Cytotrap yeast two-hybrid assay system as described previously (Keller et al., 2004). The point mutant yeast two-hybrid prey and bait constructs were generated by PCR-based site-directed mutagenesis from the corresponding wild-type constructs in pSOS and pMyr, respectively (Keller et al., 2004). The expression vectors for the myc-tagged γ2 subunit contained the mouse γ2 subunit cDNA in pRK5 with the myc 9E10 epitope inserted between the fourth and the fifth amino acid of the mature polypeptide (Keller et al., 2004). The expression vectors for FLAG-tagged GODZC157S (FL-GODZC157S) and green fluorescent protein (GFP)-GODZC157S were generated by PCR-based site-directed mutagenesis from the corresponding wild-type constructs containing the mouse cDNAs in pCMV-Tag 2B (Stratagene, La Jolla, CA) (Keller et al., 2004). The mammalian expression vectors for HA-tagged DHHC1–23 proteins including GFP-GODZ (DHHC3) and GFP-SERZ-β (DHHC7) have been described previously (Fukata et al., 2004; Keller et al., 2004). The shRNA constructs for knock-down of GODZ were derivatives of pSIREN-DNR-DsRed (Clontech, Mountain View, CA) and contained the oligonucleotides 5′-gggca tagaa caatt gaaaT GAAGC TTGAt ttcaa ttgtt ctatg cccTT TT-3′ (GODZ shRNA 1, cDNA-derived target sequences are shown in lower case) and 5′-gaaat gccac taaag agTGA AGCTT GActc tttag tggca tttcT TTTT-3′ (GODZ shRNA2), respectively, cloned between BamHI and EcoRI sites of pSIREN-DNR-DsRed. Basic local alignment search tool searches of the mouse EST database confirmed that these target sequences are specific for GODZ. The control shRNA construct contained the insert 5′-gggca tatta caatt gaaaT GAAGC TTGAt ttcaa ttgta atatg cccTT TTT-3′ with an inverted repeat sequence that differed from that of GODZ shRNA1 by two nucleotides (in italics).
Cell culture and transfection.
HEK293T cells were obtained from ATCC (Manassas, VA) and maintained in DMEM (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum. For colocalization assay, cells were seeded onto poly-l-lysine-coated coverslips and transfected by means of calcium phosphate coprecipitation with mixtures of plasmids (1–2 μg each per 3 cm dish containing neurons or HEK293T cells on coverslips) and fixed and analyzed 40–44 h later as described previously (Alldred et al., 2005). For treatment of cells with 2-BrP, 50 μm 2-BrP was added to the culture medium as a 10 mm stock solution in ethanol, and the cells were fixed and analyzed 16 h later. Cultures of cortical neurons were generated from embryonic day 14.5 (E14.5) mouse embryos, as described previously (Alldred et al., 2005). They were transfected at 18 d in vitro (DIV) and used for immunofluorescent analyses 2 d later as described previously (Alldred et al., 2005). Hypothalamic cultures were generated from E15–E16 rat embryos. The cells were dissociated by treatment of the tissue with 0.05% trypsin-EDTA followed by mechanical trituration. They were plated at 2.8 × 104 cells/cm2 onto poly-l-lysine-coated glass coverslips containing a monolayer of astrocytes in Minimal Essential Medium (Invitrogen) containing 5% fetal bovine serum, 2% B27 (Invitrogen), 0.02% NaHCO3, 20 mm d-glucose, 0.5 mm l-glutamine, and 25 U/ml penicillin/streptomycin. The cells were transfected on 10–13 DIV as described previously (Alldred et al., 2005) and used for electrophysiological recordings 24–48 h later.
Cross-linking and immunoblotting.
Transfected HEK293T cells were washed and resuspended in PBS, and aliquots were either mock-treated or treated with 2 mm dithiobis(succinimidyl propionate) (DSP) in PBS for 15 min on ice. The reaction was stopped by addition of 50 mm Tris-HCl, pH 7.5, followed by extraction of the cells with DOC buffer (10 mm Tris-HCl, pH 7.5, 150 mm NaCl, 0.5% deoxycholate, 0.05% phosphatidyl choline, 1 mm benzamidine, 100 μg/ml bacitracin, 1 μg/ml antipain, 1 μg/ml pepstatin A, 1 μg/ml leupeptin, and 0.5 mm PMSF). Aliquots of the samples were diluted with gel loading buffer (100 mm Tris-HCl, pH 6.8, 2% SDS, 20% glycerol, 0.2% bromophenol blue) with or without 200 mm DTT and analyzed by SDS-PAGE and Western blotting using monoclonal antibody (mAb) anti-Flag (1:1000; Sigma, St. Louis, MO).
Palmitoylation assays.
Metabolic labeling of HEK293T cells was done as described previously (Fukata et al., 2004; Keller et al., 2004). Briefly, cultures in standard six-well plates were transfected with mixtures of expression vectors for the mouse 9E10γ2 subunit and DHHC proteins as indicated in Figure 1 using Lipofectamine Plus (Invitrogen), following the protocol provided by Invitrogen. The transfected cells were washed with PBS, preincubated for 30 min in DMEM with fatty acid-free bovine serum albumin (10 mg/ml; Sigma) and then incubated with 0.5 mCi/ml 3H-palmitic acid (PerkinElmer, Boston, MA) for 4 h. Cells were scraped directly into sample loading buffer (62.5 mm Tris-HCl, pH 6.8, 10% glycerol, 2% SDS, and 0.001% bromophenol blue, 10 mm DTT) and the samples processed by SDS-PAGE. Aliquots of protein samples were processed in parallel on duplicate gels and used for fluorography and Western blot analysis as a loading control.
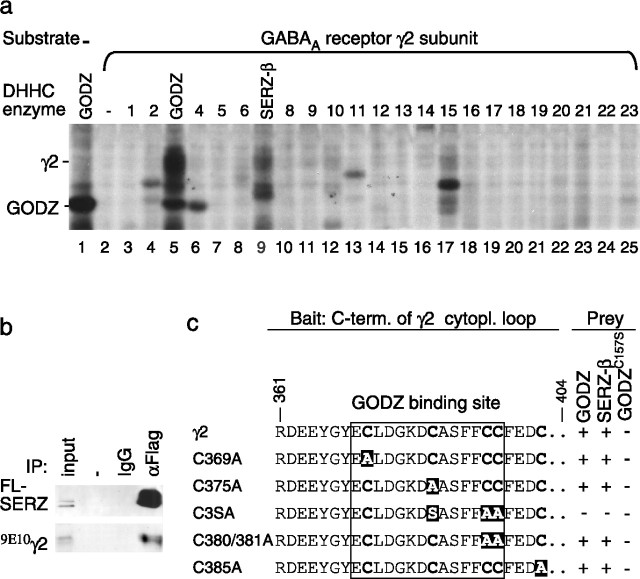
Figure 1.
GODZ and SERZ-β palmitoylate the γ2 subunit in vitro and interact with indistinguishable sequence specificity. a, Individual DHHC clones (1–23, with DHHC3 and 7 indicated as GODZ and SERZ-β, respectively) were cotransfected with (9E10)γ2 into HEK293T cells. The cells were metabolically labeled with 3H-palmitate and the proteins separated by SDS-PAGE and analyzed by fluorography. Note the selective labeling of the γ2 subunit in cells expressing GODZ (lane 5) or SERZ-β (lane 9), with a trace of labeling also apparent with DHHC15 (lane 17). No labeling above background was evident in the absence of transfected γ2 subunit (lane 1) or in the presence of any of the other DHHC proteins (lanes 2–4, 6–8, 10–25). b, HEK293T cells were cotransfected with the γ2 subunit and FL-SERZ-β and tested for interaction by coimmunoprecipitation and Western blot analysis. Precipitation of FL-SERZ-β was confirmed with mAb anti-FLAG. FLAG rabbit antiserum, but not IgG, resulted in efficient coimmunoprecipitation of the (9E10)γ2 subunit analogous to results previously shown for GODZ (Keller et al., 2004). c, Yeast two-hybrid assays using a γ2 subunit fragment (amino acids 361–404) or the indicated mutant derivatives as bait were used to test for interaction with full-length GODZ, SERZ-β, or GODZC157S as prey. The GODZ binding site (Keller et al., 2004) is boxed with the mutated cysteines highlighted in white on a black background. Note the identical sequence specificity of GODZ and SERZ-β for interaction with the γ2 subunit. Enzymatically inactive GODZC157S failed to interact with the γ2 constructs.
Immunofluorescence analyses of cultured cells.
Transfected HEK293T cells and cortical neurons were processed for immunofluorescence analyses under permeabilized conditions as described previously (Alldred et al., 2005). The following primary antibodies were used: guinea pig anti-γ2 (1:1500; kindly provided by J. M. Fritschy, University of Zurich, Zurich, Switzerland), mouse anti-glutamic acid decarboxylase-6 (GAD-6; 1:75; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), rabbit anti-vesicular inhibitory amino acid transporter (VIAAT) (Dumoulin et al., 1999) (1:5000; kindly provided by B. Gasnier, Centre Nationale de la Recherche Scientific, Paris, France), rabbit anti-myc (1:500; MBL, Woburn, MA), mAb anti-FLAG (1:1000; Sigma), and mAb 7a specific for gephyrin (1:500, gift from H. Betz, Max-Plank-Institute for Brain Research, Frankfurt, Germany). For detection of primary antibodies, Alexa Fluor 488-conjugated goat anti-guinea pig (Molecular Probes, Eugene, OR), Alexa Fluor 488-conjugated goat anti-rabbit, Cy3 donkey anti-mouse, and Cy3 donkey anti-guinea pig antibodies (Jackson ImmunoResearch, West Grove, PA) or Alexa Fluor 647-conjugated goat anti rabbit (Invitrogen) were used as appropriate. Fluorescent images were captured from a Zeiss Axiophot 2 microscope equipped with a 40 × 1.3 numerical aperture objective and an ORCA-100 video camera linked to an OpenLab imaging system (Improvision, Lexington, MA), or with a confocal laser-scanning microscope (Olympus FlowView FV300). Images were adjusted for contrast using OpenLab or FlowView software, respectively, and assembled into figure palettes using Adobe Photoshop (Adobe Systems, San Jose, CA).
Quantitation of RNAi knock-down by flow cytometry.
Analyses were performed on an XL-MCL flow cytometer using EXPO software (Beckman-Coulter, Miami-Lakes, FL). Cells were cotransfected with DsRed-encoding GODZ shRNA 1 or 2 or control shRNA vector, together with GFP-GODZ or GFP-SERZ-β, respectively. Cells transfected with single vectors and nontransfected cells were used to set the optimal fluorescence threshold for detection of DsRed- and GFP-positive cells. The rela-tive GFP expression values for each of the shRNA(DsRed)-GFP vector combinations were defined as the percentage of GFP-positive cells among 5000 DsRed-positive cells, multiplied by their mean green fluorescence intensity. The values obtained for GODZ shRNAs were then normalized to values determined in parallel with control shRNA, which were set at 100%.
Quantitation of immunofluorescent staining.
For semiquantitative analyses of immunofluorescently labeled GABAA receptor clusters, digitized microscopic images were recorded, and two dendritic segments of 40 μm each in length were selected from each transfected neuron from three or more independent experiments (Alldred et al., 2005). Immunoreactive puncta stained for the γ2 subunit were automatically selected using OpenLab, applying a fluorescence intensity threshold corresponding to twice the diffuse fluorescence measured on the shaft of the same dendrite and a target size range of 0.2–2 μm in diameter. For semiquantitative analysis of the degree of GABAergic innervation of shRNA-transfected neurons, we imaged GABAergic innervation selectively of dsRed-positive GAD-negative neurons. VIAAT-positive puncta were quantified as described for punctate γ2 subunit immunoreactivity. The experimenter performing the quantitation was blind to the type of shRNA vector transfected. Statistical comparisons were performed using Student's t test.
Electrophysiology.
Conventional whole-cell recordings were performed using a Multiclamp 700A patch-clamp amplifier (Molecular Devices Corporation, Sunnyvale, CA). Patch pipettes were pulled from borosilicate glass and fire-polished (4–6 MΩ). The recording chamber was continuously perfused with a bath solution containing 128 mm NaCl, 30 mm glucose, 25 mm HEPES, 5 mm KCl, 2 mm CaCl2, and 1 mm MgCl2, adjusted to pH 7.3 with NaOH. Patch pipettes were filled with 147 mm KCl, 5 mm Na2-phosphocreatine, 2 mm EGTA, 10 mm HEPES, 2 mm MgATP, and 0.3 mm Na2GTP, adjusted to pH 7.3 with KOH. The series resistance was typically 10–20 MΩ and compensated by 40–60%. Data were acquired using pClamp 9 software (Molecular Devices Corporation), sampled at 10 kHz, and filtered at 1 kHz. To assess whole-cell currents, pulses (5 s) of GABA (20 μm) or AMPA (10 μm) were applied through a glass pipette with the pipette tip close to the cell soma, and evoked currents were recorded with the membrane potential clamped at −70 mV. Current peak amplitudes were measured using Clampfit 9 software (Molecular Devices Corporation) and divided by the cell membrane capacitances to obtain GABAA or AMPA receptor-mediated current densities (pA/pF). Miniature IPSCs (mIPSCs) and mEPSCs were recorded in the presence of 0.5 μm tetrodotoxin (TTX) and either 10 μm 6-cyano-7-nitoquinoxaline-2,3-dione (CNQX) (for mIPSCs) or 20 μm bicucullin (for mEPSCs), respectively, and analyzed by MiniAnalysis software (Synaptosoft, Decatur, GA). Extrasynaptic GABAA receptor currents were assessed using acute application of the extrasynaptic GABAA receptor selective agonist 4,5,6,7-tetrahydroisoxazolo[5,4-c]-pyridin-3-ol (THIP) in the continuous presence of TTX (0.5 μm) and CNQX (10 μm) as described previously (Lindquist et al., 2003; Drasbek and Jensen, 2006).
Results
Identification of GABAA receptor γ2 subunit palmitoyltransferases
To determine systematically which of the known DHHC-CRD-containing putative palmitoyltransferases could palmitoylate the γ2 subunit, we tested 23 known members of the mouse DHHC-family (DHHC1–23) of proteins (Fukata et al., 2004) for palmitoylation of the γ2 subunit using metabolic labeling of transfected HEK293T cells. GODZ and, to a lesser extent, its most closely related paralog SERZ-β (60.9% amino acid identity with GODZ) showed significant PAT activity in this assay. A trace of activity was also seen with the distant GODZ paralog DHHC15 (17.7% amino acid identity with GODZ) (Fig. 1a). However, none of the other DHHC proteins were able to use the γ2 subunit as a substrate.
Expression patterns and sequence specificity of GODZ and SERZ-β
To assess further whether GODZ and SERZ-β might be functionally redundant, we compared their mRNA expression patterns in brain and other tissues. In situ hybridization of brain sections revealed that SERZ-β mRNA was expressed throughout the brain very similar to GODZ mRNA, with the signal being most intense in brain regions of high neuronal cell density (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Northern blot analyses showed similar tissue specificity. Together with previous data showing pan-neural expression of GODZ immunoreactivity in brain (Keller et al., 2004), the data suggest neuronal-specific expression in brain also for SERZ-β. Previous yeast two-hybrid assays showed that both GODZ and its paralog SERZ-β can interact with the γ2 subunit of GABAA receptors (Keller et al., 2004). To determine whether SERZ-β can form stable complexes with the γ2 subunit in mammalian cells similar to GODZ, FL-SERZ-β and the myc-tagged γ2S subunit [(9E10)γ2] were cotransfected into HEK293T cells, and solubilized extracts were immunoprecipitated with a rabbit antiserum against the FLAG epitope of FL-SERZ-β (Fig. 1b). Immunoprecipitation of FL-SERZ-β was confirmed by immunoblot using mAb anti-FLAG. Coimmunoprecipitated (9E10)γ2 subunit was readily detected with an antibody against the myc epitope, confirming that SERZ-β can form stable complexes with the γ2 subunit in transfected HEK293T cells.
We previously mapped the GODZ and SERZ-β interaction domain in the γ2 subunit to a Cys-rich 14 aa peptide in the C-terminal half of the intracellular loop [γ2(ICL)] that is highly conserved in γ1–3 subunits (Keller et al., 2004). To compare the specificities of interaction of GODZ and SERZ-β, we generated a series of γ2 subunit bait constructs with single, double, or triple Cys substitutions in the 14 aa GODZ-binding domain and tested these in yeast two-hybrid interaction assays. GODZ and SERZ-β were indistinguishable in that they both interacted with single and double Cys-substituted γ2 subunit bait constructs but failed to interact with a triple Cys-substituted construct (Fig. 1c). An enzymatically inactive point mutant of GODZ (GODZC157S) (Hayashi et al., 2005) failed to interact with all γ subunit constructs. Thus, although GODZ and SERZ-β are likely to have multiple substrates in vivo, they appear to be the only PATs that might play a role in modulating the trafficking of γ2 subunit-containing GABAA receptors. The data suggest that GODZ and SERZ-β exhibit similar substrate specificity and expression patterns, suggesting possible functional redundancy in vivo.
Palmitoylation-dependent interaction between GODZ/SERZ-β and the γ2 subunit
Given that interactions between the γ2 subunit and GODZ or SERZ-β require Cys residues, we hypothesized that association of the two types of proteins might be stabilized by palmitoylation. We used colocalization assays in transfected HEK293T cells to test this idea. The C-terminal half of the γ2 subunit major intracellular loop region (amino acids 361–404) was tagged with YFP [YFPγ2(ICL)] and cotransfected with FL-GODZ into HEK293T cells. On expression alone, YFPγ2(ICL) was diffusely expressed throughout the cytoplasm and nucleus, whereas FL-GODZ was highly restricted to the Golgi complex (Keller et al., 2004). On coexpression of the two proteins, YFPγ2(ICL) was excluded from the nucleus and trapped in the Golgi complex where it colocalized with GODZ, confirming interaction of the two proteins (Fig. 2a-a″). In contrast, in YFPγ2(ICL)- and FL-GODZ-cotransfected cells that were treated with the palmitoylation inhibitor 2-bromopalmitate (2-BrP), YFPγ2(ICL) remained diffusely expressed and failed to accumulate in the Golgi (Fig. 2b-b″). Notably, GODZ remained highly restricted to the Golgi complex of 2-BrP-treated cells, suggesting that its association with this organelle is mostly independent of palmitoylation. A YFPγ2(ICL) construct in which three of four Cys residues were mutated to Ala or Ser, respectively, [YFPγ2(ICLC3SA)] failed to relocalize to the Golgi on cotransfection with FL-GODZ (Fig. 2c-c″), consistent with results from yeast two-hybrid assays and with palmitoylation-dependent interaction. Analogously, YFPγ2(ICL) remained diffusely expressed when coexpressed with enzymatically inactive GODZC157S (Fig. 2d-d″). FL-SERZ-β was indistinguishable from FL-GODZ in that it relocalized YFPγ2(ICL) to the Golgi complex (Fig. 2e-e″), and this effect was absent when cells were treated with 2-BrP, as expected (Fig. 2f-f″). Palmitoylation-dependent interaction supports the idea that palmitate is transferred from GODZ or SERZ-β as the palmitate donor to the γ2 subunit substrate.
Figure 2.
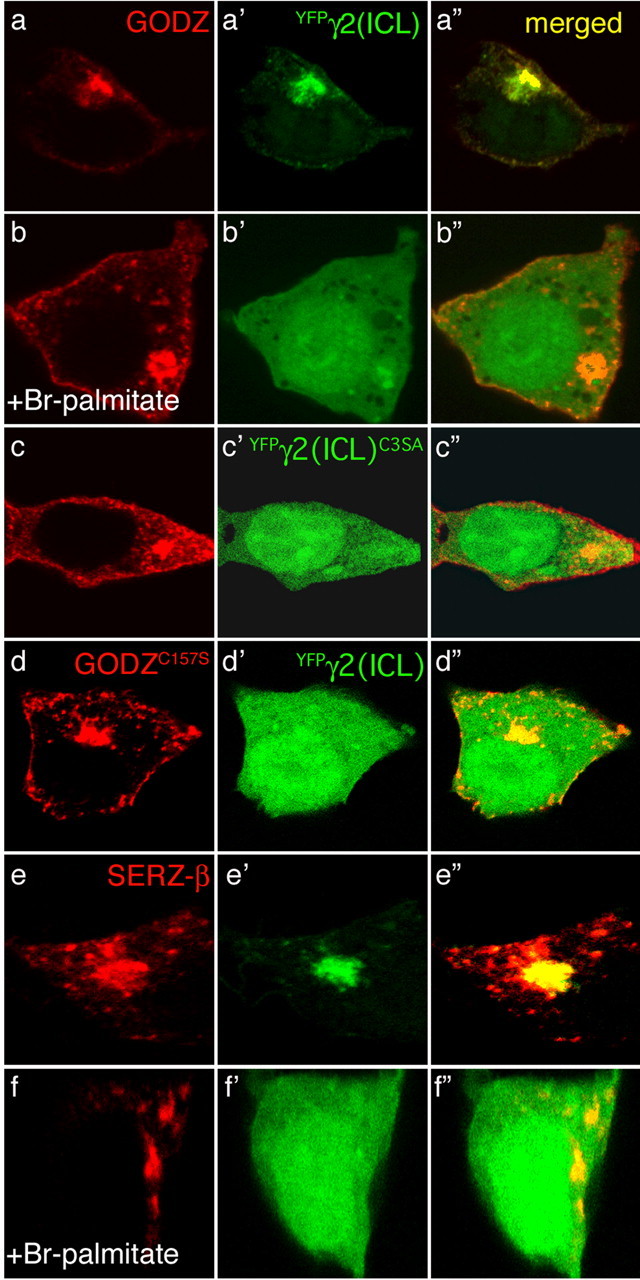
Palmitoylation-dependent interaction of GODZ and SERZ-β with the γ2 subunit. The DHHC proteins FL-GODZ (a–c), FL-GODZC157S (d), or FL-SERZ-β (e, f) were cotransfected with YFPγ2(ICL) (a′, b′, d′–f′) or its mutant derivative YFPγ2(ICL)C3SA (c′) and tested for colocalization after treatment of transfected cells with ethanol (a–a″, e–e″) or 2-BrP in ethanol (b–b″, f–f″), or without additional treatment (c–c″, d–d″). Immunofluorescently labeled FL-GODZ and FL-SERZ-β are shown in red (a–f), the YFP-tagged γ2 constructs are shown in green (a′–f′), and colocalization of FL-GODZ or FL-SERZ-β with YFPγ2(ICL) is shown in yellow (a″–f″). Restriction of YFPγ2(ICL) to the Golgi is indicative of interaction with GODZ or SERZ-β. Note the diffuse distribution of YFP-tagged γ2 constructs in experiments involving transfection of GODZC157S (d) or YFPγ2(ICL)C3SA (c) or treatment with 2-BrP (b, f), respectively, indicating that palmitoylation is required for interaction between substrate and PATs.
GODZ and SERZ-β form heteromeric multimers on overexpression in heterologous cells
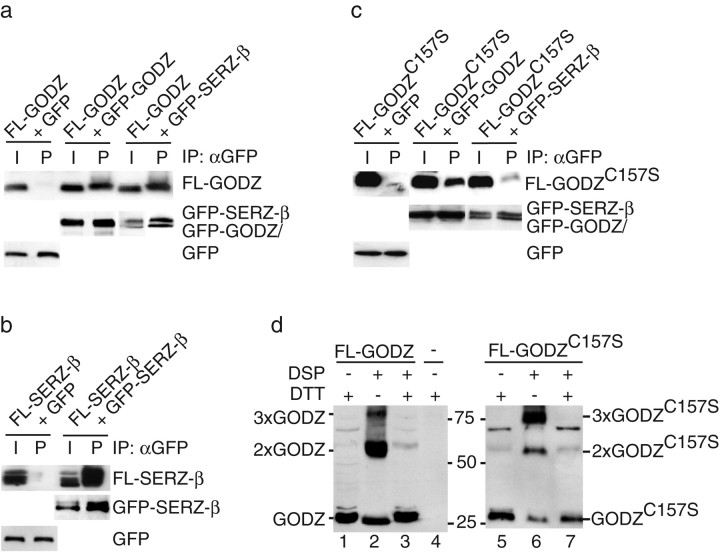
Transfection of a DHHC15 construct in which the conserved DHHC sequence had been mutated to DHHS has been shown to inhibit the function of DHHC15 but not GODZ or SERZ-β (Fukata et al., 2004) and suggested to us that perhaps DHHC-CRD proteins could act as multimers. To test this idea directly, we performed coimmunoprecipitation experiments using GFP- and FLAG-tagged GODZ constructs coexpressed in HEK293T cells. Immunoprecipitation of GFP-GODZ (but not GFP) resulted in efficient coimmunoprecipitation of FL-GODZ (Fig. 3a). Analogously, FL-SERZ-β coimmunoprecipitated with GFP-SERZ-β (Fig. 3b). Furthermore, coexpression of FL-GODZ and GFP-SERZ-β allowed coimmunoprecipitation of FL-GODZ with GFP-SERZ-β, indicating that SERZ-β and GODZ can form heterodimers or multimers. Enzymatically inactive FL-GODZC157S coimmunoprecipitated with GFP-GODZ and GFP-SERZ-β in a manner similar to FL-GODZ and FL-SERZ-β (Fig. 3c). To determine further whether GODZ could dimerize in intact cells, we used cross-linking of proteins with the reversible cross-linker DSP. Analysis of extracts from DSP-treated FL-GODZ-transfected cells by Western blot revealed GODZ cross-linking products that showed a relative mobility in gel electrophoresis corresponding to dimers and trimers, respectively (Fig. 3d). Additional slower migrating FL-GODZ-containing cross-linking products were seen that could, however, not be attributed to discrete molecular species (data not shown). Cross-linking was reversible by treatment of extracts with reducing agent, as expected. Similar cross-linking products were detected after cross-linking of cells that had been transfected with FL-GODZC157S, indicating efficient multimerization of this enzymatically inactive mutant protein. Thus, GODZ, SERZ-β, and GODZC157S form homomeric and heteromeric multimers, which is consistent with overexpressed GODZC157S acting as a dominant-negative protein for both GODZ and SERZ-β.
Figure 3.
GODZ and SERZ-β form homomultimers and heteromultimers. a, FL-GODZ was cotransfected with GFP, GFP-GODZ, or GFP-SERZ-β into HEK293T cells. Aliquots of cell extracts (I, input) and αGFP immunoprecipitates (P) were analyzed by Western blot for coimmunoprecipitation of FL-GODZ with αFlag antiserum. The expression and immunoprecipitation of GFP proteins by αGFP antibody was verified on the same blot with rabbit αGFP antiserum. b, FL-SERZ-β was analyzed for coimmunoprecipitation with GFP-SERZ-β, as indicated in a. c, FL-GODZC157S was analyzed for coimmunoprecipitation with GFP-GODZ or GFP-SERZ-β, respectively, as indicated in a. d, FL-GODZ (left) or GODZC157S (right) were transfected into HEK293T cells and aliquots of the intact cells mock-treated or cross-linked with DSP. The cell extracts were processed for SDS-PAGE using loading buffer containing or lacking DTT (200 mm) and analyzed by Western blot using αFLAG antibody. Untransfected cells were processed as a control (lane 4). Note the DTT-sensitive cross-linking products corresponding in size to dimers and trimers of GODZ (lane 2, 3) and GODZC157S (lane 6, 7), respectively.
GODZ-dependent accumulation of GABAA receptors at synapses
GODZ-mediated palmitoylation of the γ2 subunit is implicated in trafficking and postsynaptic accumulation of γ2 subunit-containing GABAA receptors (Keller et al., 2004; Rathenberg et al., 2004). To test whether GODZC157S could interfere with postsynaptic accumulation of GABAA receptors, we analyzed the punctate γ2 subunit immunoreactivity of cultured cortical neurons (20 DIV) that had been transfected with either GFP-GODZ or GFP-GODZC157S 2 d earlier. Punctate γ2 subunit immunoreactivity in these cultures is representative of postsynaptic GABAA receptor clusters (Essrich et al., 1998; Baer et al., 1999; Alldred et al., 2005). There was a dramatic reduction in the number of γ2 subunit-immunoreactive puncta (Fig. 4a-b″, red) visible in dendrites of cells transfected with GFP-GODZC157S (green) compared with GFP-GODZ (green). Quantitation revealed that the number of γ2 subunit immunoreactive puncta observed per 40 μm dendritic segment of cortical neurons transfected with GFP-GODZC157S (4.3 ± 0.2 puncta per segment; n = 26 cells) was greatly reduced compared with control neurons transfected with GFP-GODZ (10.2 ± 1.0; n = 12; p < 0.001).
Figure 4.
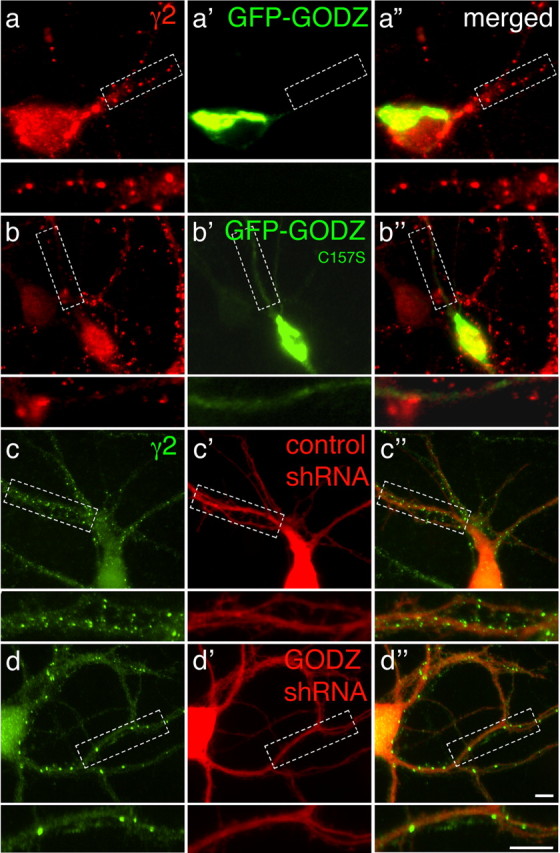
Overexpression of dominant-negative GODZ and GODZ-specific shRNA interferes with postsynaptic accumulation of GABAA receptors. a, b, Low-density cultures of cortical neurons (18 DIV) were transfected with GFP-GODZ (a–a″) or GFP-GODZC157S (b–b″) and subjected to immunofluorescent staining for the γ2 subunit 2 d later. Note the prominent punctate γ2 subunit staining in the GFP-GODZ-transfected neuron (a, red) and the highly restricted localization of GFP-GODZ to the Golgi complex (a′, green). In contrast, punctate immunoreactivity for the γ2 subunit was significantly reduced in the GFP-GODZC157S-transfected neuron (b). GFP-GODZC157S (b′) is concentrated in the Golgi complex and, unlike GFP-GODZ, also evident in dendrites. c, d, Cortical neurons were transfected with plasmid vectors encoding dsRed (red) and either control shRNA or GODZ-specific shRNA and processed for immunofluorescent analysis of the γ2 subunit as above. Note the significant reduction in punctate staining for the γ2 subunit (green) in the GODZ-shRNA-transfected neuron (d), compared with the neuron transfected with control shRNA (c). Merged images are shown in a″–d″, with boxed dendritic segments shown enlarged in separate panels below each image. Scale bars, 5 μm.
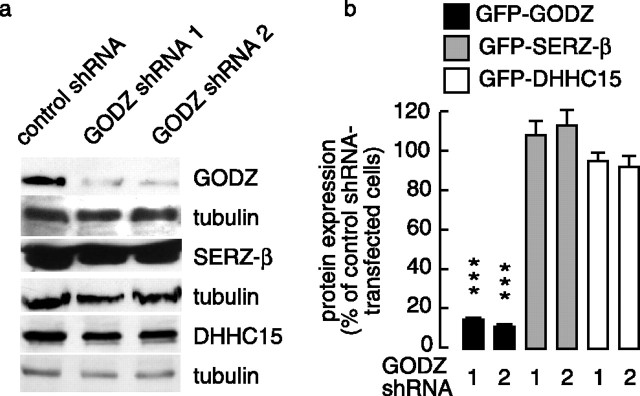
Coimmunoprecipitation experiments have indicated that overexpressed GODZC157S can interact similarly with both GODZ and SERZ-β. The observed GFP-GODZC157S-induced reduction in clustering of γ2 subunit-containing GABAA receptors therefore might indicate a reduction in the activity of both GODZ and SERZ-β. As an independent approach to address specifically the function of GODZ, we designed two GODZ-specific shRNA expression vectors (GODZ shRNA1 and 2) that, on cotransfection with GODZ or SERZ-β into HEK293T cells, selectively knocked down the expression of GODZ but not SERZ-β or DHHC15 (Fig. 5a,b) (see Material and Methods). These shRNA vectors include a separate transcription unit for DsRed to allow identification of transfected cells by fluorescence. Transfection of an shRNA construct that encoded a mutated GODZ target sequence did not affect expression of GODZ, SERZ-β, and DHHC15 (Fig. 5a,b) and was used as a control. Immunofluorescent analyses of cortical neurons transfected with a 1:1 mixture of the two GODZ-specific shRNA vectors revealed a marked reduction of punctate γ2 subunit immunoreactivity compared with neurons transfected with control shRNA (Fig. 4c,d, green). Quantitation revealed that the number of γ2 subunit-immunoreactive puncta per 40 μm dendritic segment of GODZ shRNA-transfected neurons (4.8 ± 1.4; n = 47 transfected cells) was greatly reduced compared with values of control shRNA-transfected neurons (11.0 ± 0.3; n = 26; p < 0.001).
Figure 5.
Selective knock-down of GODZ by GODZ-specific shRNA. a, Analysis of the target specificity of shRNA constructs. A control vector encoding a mutated GODZ shRNA target sequence or one of two GODZ-specific shRNA constructs (GODZ shRNA 1, 2) were cotransfected with either FL-GODZ or FL-SERZ-β or FL-DHHC15 into HEK293T cells, and the cell extracts were analyzed by Western blot using mAb anti-FLAG. The blots were stripped and reprobed with anti-tubulin antibody as a loading control. Note the dramatic reduction in GODZ in cells cotransfected with GODZ shRNA 1 or 2, whereas SERZ-β and DHHC15 expression was unaffected under the same conditions. No effect on GODZ, SERZ-β, or DHHC15 expression was observed on cotransfection with the mutant shRNA control vector. b, Quantitation of GODZ shRNA-mediated effects on GODZ, SERZ-β, and DHHC15 expression in transfected HEK293T cells by flow cytometry (see Materials and Methods). The cells were transfected with GFP-GODZ, GFP-SERZ-β, or GFP-DHHC15 together with GODZ shRNA 1, GODZ shRNA 2, or control shRNA vector. Note the drastic reduction in GFP-GODZ expression by cotransfected GODZ shRNA1 (15.15 ± 0.91% of control shRNA; n = 6; p < 0.001) or GODZ shRNA2 (11.48 ± 1.48% of control; n = 6; p < 0.001). In contrast, neither of the two GODZ shRNA constructs had an effect on expression of GFP-SERZ-β (GODZ shRNA1, 109 ± 8.3% of control shRNA, n = 12, p > 0.05; GODZ shRNA2, 113 ± 8.7%, n = 12, p > 0.05) or GFP-DHHC15 (GODZ shRNA1, 95 ± 3.7% of control shRNA, n = 5, p > 0.05; GODZ shRNA2, 92 ± 5.0%, n = 6, p > 0.05).
GODZ is essential for GABAergic but not glutamatergic transmission
To assess the function of GODZ in GABAergic transmission, we analyzed mIPSCs and GABA-induced whole-cell currents of hypothalamic neurons transfected in parallel cultures with either GFP-GODZ or dominant-negative GFP-GODZC157S. Both of these proteins are highly concentrated in the Golgi complex. Compared with cortical cultures, hypothalamic cultures contain a larger portion of GABAergic neurons (∼40%) and consistently high levels of spontaneous GABAergic activity already at 10 DIV (Chen et al., 1996). The transfection efficiency was between 1 and 5%, and synaptic inputs therefore originated generally from nontransfected cells. Interestingly, both the mIPSC amplitude and frequency of neurons transfected with GFP-GODZC157S were markedly reduced compared with GFP-GODZ-transfected neurons (Fig. 6a). In addition to the γ2 subunit, potential in vivo substrates for palmitoylation by GODZ include PSD-95 and the AMPA receptor subunits (Fukata et al., 2004; Hayashi et al., 2005). However, GFP-GODZC157S-transfected neurons were indistinguishable from GFP-GODZ-transfected neurons with respect to amplitude or frequency of mEPSCs (Fig. 6b). The data indicate that GODZ- and/or SERZ-β-mediated palmitoylation is limiting for the function of GABAergic synapses but dispensable for glutamatergic synapses.
Figure 6.
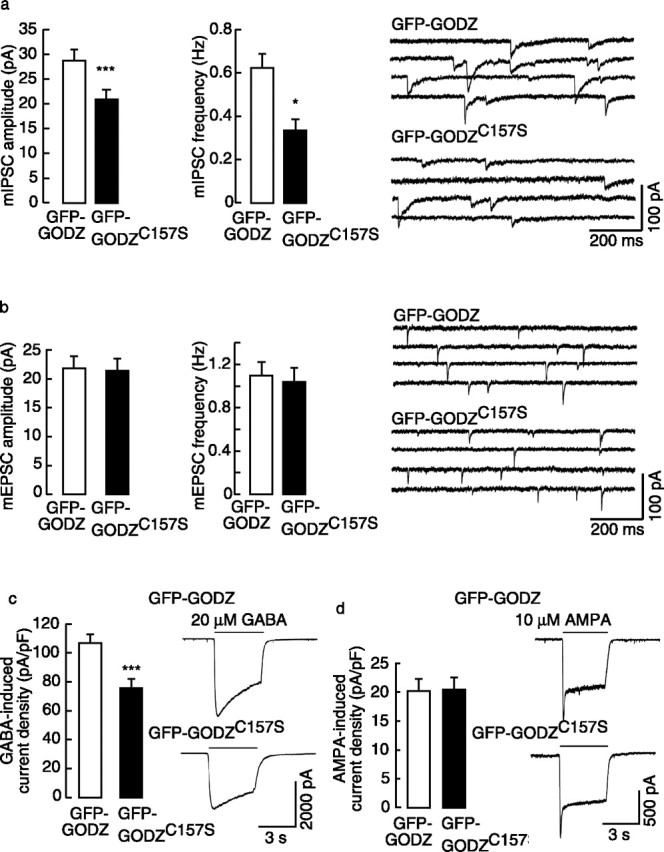
Analyses of functional consequences of dominant-negative GODZ on GABAergic and glutamatergic transmission. GABAergic and glutamatergic miniature postsynaptic currents as well as GABA- and AMPA-induced whole-cell currents were recorded from transfected hypothalamic neurons (12–15 DIV). a, Analyses of mIPSCs of neurons transfected with dominant-negative GFP-GODZC157S revealed a significant reduction in both the amplitude (20.3 ± 1.4 pA) and frequency (0.31 ± 0.08 Hz; n = 26) of mIPSCs compared with matched GFP-GODZ-transfected controls (29.7 ± 2.0 pA, p < 0.001; 0.63 ± 0.10 Hz, n = 25, p < 0.05). b, In contrast, the mEPSC amplitude (21.9 ± 0.8 pA) and frequency (1.00 ± 0.24 Hz; n = 18) of GFP-GODZC157S-transfected neurons were indistinguishable from GFP-GODZ controls (22.4 ± 1.8 pA, 1.13 ± 0.24 Hz; n = 15; p > 0.05). c, The GABA (20 μm)-induced whole-cell current density in GODZC157S-transfected neurons (75.7 ± 4.4 pA/pF; n = 17) was significantly reduced compared with GFP-GODZ controls (107.0 ± 5.1 pA/pF; n = 14; p < 0.001). d, The AMPA (10 μm)-induced current density was unchanged in GFP-GODZC157S transfected neurons (19.1 ± 2.2 pA/pF; n = 15) compared with GFP-GODZ controls (19.8 ± 2.4 pA/F; n = 17; p > 0.05). Representative current traces are shown on the right of the respective bar diagrams. Data represent means ± SE (*p < 0.05, **p < 0.01, ***p < 0.001, Student's t test).
The conditions chosen for transfection of neurons generally result in a low transfection efficiency of <5%, indicating that presynaptic cells innervating the transfected postsynaptic cells were in general untransfected and that deficits in mIPSCs recorded from GFP-GODZC157S transfected neurons can be attributed selectively to postsynaptic deficits. Consistent with a GFP-GODZC157S-induced postsynaptic GABAA receptor deficit, the GABA-induced whole-cell current density in GFP-GODZC157S transfected neurons was reduced compared with controls (Fig. 6c). In addition to being a critically important constituent of postsynaptic GABAA receptors, the γ2 subunit can contribute to tonic currents mediated by extrasynaptic GABAA receptors, as demonstrated for α3 or α5 subunit-containing receptors in neurons of the inferior olivary nucleus and hippocampal pyramidal cells, respectively (Devor et al., 2001; Caraiscos et al., 2004). Furthermore, prototypical postsynaptic GABAA receptor subtypes may, in low concentrations, also occur at extrasynaptic sites as suggested by the diffuse staining of corresponding receptor subunits in cortical and hippocampal neurons (Essrich et al., 1998; Brunig et al., 2002; Christie et al., 2005). We therefore wondered whether the reduced GABA-evoked whole-cell currents recorded from GFP-GODZC157S-transfected hypothalamic neurons might reflect reduced expression or function of extrasynaptic GABAA receptors in addition to postsynaptic GABAA receptor deficits. Interestingly, the current response to acute application of the extrasynaptic GABAA receptor-selective agonist THIP was unchanged in GFP-GODZC157S transfected (14.8 ± 4.4 pA; n = 7) compared with GFP-GODZ-transfected (11.3 ± 2.6 pA; n = 8; p = 0.5) controls. The data suggest that GODZC157S-induced deficits in GABA-evoked whole-cell currents of hypothalamic neurons are attributable to selective deficits in synaptic GABAA receptors. In contrast to the effect of GODZC157S on GABA-evoked whole-cell currents and consistent with unchanged mEPSCs, the AMPA-induced current density in GFP-GODZC157S-transfected neurons was indistinguishable from GFP-GODZ-transfected controls (Fig. 6d).
In an independent approach designed to test specifically the function of GODZ, we next analyzed synaptic currents of cultured hypothalamic neurons transfected with GODZ-specific shRNA using neurons transfected with the mutant shRNA construct as a control. Transfected neurons were identified by the DsRed fluorescence encoded by the shRNA expression vector(s). Similar to results obtained with dominant-negative GODZ, the amplitude and frequency of mIPSCs recorded from GODZ shRNA-transfected neurons (24.4 ± 1.2 pA; 1.36 ± 0.24 Hz; n = 23) were significantly reduced compared with mIPSCs recorded from control shRNA vector-transfected neurons (30.1 ± 1.7 pA, p < 0.01; 2.69 ± 0.38 Hz, n = 21, p < 0.01). In further agreement with results obtained with dominant-negative GODZ, mEPSCs remained unaffected by RNAi-mediated knock-down of GODZ (GODZ shRNA, 21.8 ± 1.6 pA, 3.97 ± 0.64 Hz, n = 21; control shRNA, 22.9 ± 14 pA, p > 0.05, 4.20 ± 0.59 Hz, n = 17, p > 0.05). Similar GODZ-specific RNAi effects were apparent in GODZ shRNA-transfected cortical neurons (data not shown).
GODZ-specific RNAi had virtually identical effects on γ2 subunit localization and mIPSCs as overexpression of dominant-negative GODZ, although the latter is predicted to inhibit both GODZ and SERZ-β. Furthermore, the activity of recombinant SERZ-β in γ2 subunit palmitoylation assays (Fig. 1) and its expression assessed by Northern blot and in situ hybridization (supplemental Fig. 1, available at www.jneurosci.org as supplemental material) was consistently lower than that of GODZ. Together, these observations suggest that GODZ, rather than SERZ-β, is the major enzyme that controls the trafficking of GABAA receptors and function of inhibitory synapses.
GODZ-mediated palmitoylation in postsynaptic neurons regulates GABAergic innervation
The reduced mIPSC frequency observed in GODZC157S- and GODZ shRNA-transfected neurons cannot readily be explained by postsynaptic GABAA receptor deficits alone. Instead, the reduced mIPSC frequency points to possible presynaptic deficits, such as indirect loss of GABAergic innervation associated with postsynaptic deficits in γ2 subunit-containing GABAA receptors. Such deficits have recently been described following RNAi knock-down of the γ2 subunit in single neurons (Li et al., 2005). To address whether downregulation of GODZ in postsynaptic cells might affect presynaptic GABAergic innervation by untransfected cells, we double stained control shRNA- and GODZ shRNA-transfected cortical neurons for the presynaptic GABAergic markers VIAAT and GAD or postsynaptic gephyrin. Control shRNA-transfected neurons showed extensive GABAergic innervation, illustrated by the punctate staining for GAD and VIAAT-positive axonal varicosities along dendrites of transfected cells (Fig. 7a-a″). In contrast, GODZ-shRNA-transfected neurons were mostly deprived of GABAergic innervation, with GAD/VIAAT-positive axons of neighboring untransfected neurons either passing by or growing across rather than along dendrites of GODZ-shRNA-transfected cells (Fig. 7b-b″). GAD remained perfectly colocalized with VIAAT under all conditions, indicating that loss of innervation was not simply a result of loss of expression of one or the other presynaptic marker proteins. Nearby untransfected cells in the same cultures were innervated similarly to control shRNA-transfected neurons.
Figure 7.
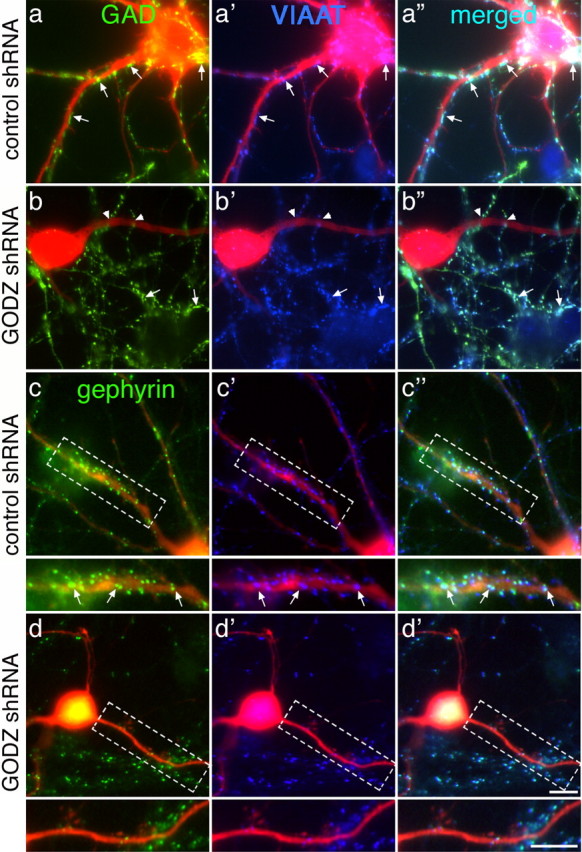
GODZ shRNA expression in postsynaptic neurons interferes with GABAergic innervation. Cortical neurons were transfected with either control shRNA (a–a″, c–c″) or GODZ shRNA (b–b″, d–d″) as described in Figure 5 and double-immunostained for GAD (a, b, green) or gephyrin (c, d, green) together with VIAAT (a′, b′, c′, d′, blue), with merged images shown in a″, b″, c″, and d″. Transfected neurons and their major processes are identified by the red fluorescence of shRNA vector-encoded dsRed (red). Note the GAD- and VIAAT-positive GABAergic varicosities (arrows) that are evident along most of the dendrites of a representative control shRNA-transfected neuron (a–a″). In contrast, the GODZ shRNA-transfected neuron (b–b″) is essentially devoid of contact with GABAergic axons, which, if present, appear to grow across (arrowheads) rather than along dendrites. A nearby, untransfected neuron in the same image (b–b″) is highly innervated (arrows), similar to the control shRNA-transfected neuron shown in a–a″. The punctate staining for gephyrin in a control shRNA transfected neuron (c) is perfectly colocalized with immunofluorescent staining of presynaptic VIAAT (c′, c″, arrows), and staining for both of these marker proteins is greatly reduced in a GODZ shRNA-transfected neuron (d, d′, d″). Note the untransfected pair of dendrites in the same image that is decorated with gephyrin clusters and strongly innervated by GABAergic axons. Scale bars, 5 μm.
Double labeling of neurons for presynaptic VIAAT and postsynaptic gephyrin (Fig. 7c,d) revealed that loss of postsynaptic gephyrin staining in GODZ shRNA-transfected neurons (−56.4 ± 3.1% compared with control shRNA-transfected neurons; n = 24–25; p < 0.001) was paralleled with an equal loss of punctate VIAAT staining (−51.3 ± 4.5%; n = 9–13; p < 0.001). Moreover, colocalization of punctate gephyrin and VIAAT staining remained unaffected in GODZ shRNA-transfected neurons (87.3 ± 2.7% of punctate gephyrin staining colocalized with VIAAT immunoreactivity) compared with controls (84.9 ± 3.8%) (Fig. 7c,d). The data confirm that loss of innervation was tightly linked to loss of postsynaptic GABAA receptors and gephyrin. Reduced GABAergic innervation is consistent with the reduced mIPSC frequency observed in GODZC157S-transfected (Fig. 6) and GODZ shRNA-transfected neurons. In summary, the data indicate that GODZ-mediated palmitoylation is essential for normal accumulation and function of γ2 subunit-containing GABAA receptors at the plasma membrane and at synapses, a mechanism that in turn is limiting for GABAergic innervation.
Discussion
The work presented here provides biochemical, immunohistochemical, and functional evidence that the palmitoyltransferase GODZ plays an important and selective role in assembly and function of GABAergic inhibitory synapses. Our evidence is threefold. First, GODZ is the principal enzyme that interacts with and palmitoylates the γ2 subunit of GABAA receptors in vitro. Second, the γ2 subunit is one of the most important substrates of GODZ in vivo, because interference with its activity or expression results in a dramatic and specific functional deficit of inhibitory but not excitatory synapses. Third, GODZ-mediated palmitoylation of the γ2 subunit regulates presynaptic innervation of GABAergic synapses.
Using RNAi knock-down of the γ2 subunit, Li et al. (2005) have shown recently that γ2 subunit-containing GABAA receptors are essential for normal GABAergic innervation. Our experiments extend these findings and show that knock-down of GODZ results in a similar deficit, suggesting that postsynaptic GODZ-mediated palmitoylation of the γ2 subunit is capable of controlling presynaptic GABAergic innervation. Interestingly, in contrast to shRNA plasmid-mediated knock-down of GODZ [or γ2 (Li et al. 2005)] in single neurons, no such presynaptic deficit is evident in neuron cultures and brain sections of GABAA receptor γ2 subunit knock-out mice, which exhibit a uniform deficit in postsynaptic GABAA receptors in all neurons (Essrich et al., 1998; Schweizer et al., 2003). The data suggest that GABAergic axons can effectively discriminate among target neurons that differ in the level of expression of γ2 subunit-containing GABAA receptors and that they preferentially innervate neurons with higher concentrations of these receptors at the cell surface.
GODZ and SERZ-β appear to be functionally redundant with respect to palmitoylation of the γ2 subunit in heterologous cells. However, comparison of results obtained in neurons with either dominant-negative GODZ or GODZ-specific shRNA suggest that SERZ-β contributes little if anything to modulation of inhibitory synapses in vivo. Moreover, multiple potential substrates have been identified that can be palmitoylated by cotransfected GODZ in heterologous cells. These include the Gs-protein α subunit, SNAP25b, PSD-95 (Fukata et al., 2004), and the GluR1–4 subunits of AMPA receptors (Hayashi et al., 2005). Among these, PSD-95 and AMPA receptors are of interest because dynamic changes in their palmitoylation states are implicated in activity-dependent plasticity of excitatory synapses (deSouza and Ziff, 2002; El-Husseini et al., 2002; Fukata et al., 2004; Hayashi et al., 2005). However, the analyses in cultured neurons presented here indicate remarkable selectivity of GODZ in modulating inhibitory synapses and suggest that GODZ is dispensable for normal function of AMPA receptor-mediated synaptic and whole-cell currents. Indeed, it is not known whether GODZ exerts activity toward these substrates in vivo. Furthermore, whereas GODZ and SERZ-β are the only DHHC proteins that exhibit significant PAT activity toward the γ2 subunit in vitro, multiple candidate enzymes exist for PSD-95 (Fukata et al., 2004; Huang et al., 2004) and AMPA receptors (Hayashi et al., 2005), suggesting far greater functional redundancy of different PATs for substrates acting at excitatory than at inhibitory synapses. Lack of an effect of GODZ on excitatory transmission suggests that other PATs may be more important than GODZ for palmitoylation of substrates that are targeted to glutamatergic synapses.
The exact mechanism by which palmitoylation exerts its effect on trafficking of GABAA receptors remains unclear. The restricted localization of GODZ to the Golgi (Keller et al., 2004) suggests that palmitoylation might facilitate release of GABAA receptors from the endoplasmatic reticulum, as suggested for Pfa4-mediated palmitoylation of the chitin synthase Chs3 in yeast (Lam et al., 2006). Alternatively, palmitoylation might affect post-Golgi sorting of GABAA receptor-containing vesicles or affect receptor levels by modulating the stability or endocytic recycling in the plasma membrane. Consistent with all of these ideas, global blockade of palmitoylation with 2-BrP or substitution of palmitoylation sites in the γ2 subunit results in reduced surface expression of GABAA receptors (Rathenberg et al., 2004). Independent evidence indicates that newly synthesized postsynaptic GABAA receptors are inserted into the plasma membrane at extrasynaptic membrane sites, from where they are recruited to synaptic sites (Bogdanov et al., 2006). Based on this mechanism, one might predict that a deficit in exocytosis of postsynaptic GABAA receptor subtypes should be accompanied by a reduction in tonic GABAA receptor currents. However, the function of extrasynaptic GABAA receptors in GODZC157S-transfected hypothalamic neurons was not different from controls. The primary role of GODZ therefore is not to regulate extrasynaptic versus postsynaptic localization of γ2 subunit-containing GABAA receptors that have already been inserted into the plasma membrane. Consistent with unaltered extrasynaptic GABAA receptor function, the apposition of presynaptic and postsynaptic markers was unchanged in GODZ shRNA-transfected neurons. Future experiments will have to address whether γ2 subunit-containing GABAARs at the cell surface remain palmitoylated, and whether their endocytic stability in the plasma membrane is dependent on GODZ-mediated palmitoylation.
As part of our analyses, we demonstrated that overexpressed, enzymatically inactive GODZC157S can multimerize and interact with GODZ and its paralog SERZ-β. This finding provides a rationale for dominant-negative activity of Cys-Ala-substituted DHHC proteins. However, we have so far been unable to detect multimeric GODZ cross-linking products in brain extracts, and the relevance of this finding for the function of native GODZ is currently unclear.
Coexpression of GODZ with GABAA or AMPA receptors in heterologous cells results in intracellular trapping and reduced surface expression of these receptors (Uemura et al., 2002; Keller et al., 2004; Hayashi et al., 2005), which has led to the suggestion that GODZ might negatively regulate the surface expression of AMPA receptors (Hayashi et al., 2005). However, our results obtained in neurons are incompatible with such a mechanism. Whereas GODZ readily forms a stable complex on overexpression with the γ2 subunit or AMPA receptor subunits in heterologous cells, no such complex could be detected in brain extracts (Uemura et al., 2002; Keller et al., 2004). We have now shown that stable interaction of GODZ with the γ2 subunit is palmitoylation dependent. Such stable complexes might represent long-lived enzymatic reaction intermediates resulting from low catalytic processivity of GODZ in heterologous cells. Interestingly, the yeast effector of Ras function, Erf2p, is a member of the DHHC family of proteins that is distantly related to GODZ and exists as a complex with another structurally unrelated protein, Erf4p, both of which are essential for palmitoylation of Ras in yeast (Lobo et al., 2002). By extension, stable interactions between GODZ and its substrate(s) in heterologous cells and suboptimal enzymatic processivity of GODZ might be alleviated in neurons by a functional neuron-specific analog of Erf4p that contributes to GODZ-mediated palmitoylation.
Palmitoylation has been reported recently to negatively regulate the ubiquitination of substrate proteins in yeast (Valdez-Taubas and Pelham, 2005). Preliminary evidence suggests that GABAA receptors are subject to ubiquitin-mediated degradation by the proteasome (Bedford et al., 2001; Luscher and Keller, 2001), allowing speculation that GODZ-mediated palmitoylation could negatively regulate degradation of γ2 subunit-containing GABAA receptors. Furthermore, palmitoylated proteins preferentially localize to plasma membrane microdomains that are enriched in cholesterol and glycosphingolipids (lipid rafts) (Patterson, 2002; Resh, 2004), a mechanism that is implicated in targeting of AMPA and GABAA receptors to postsynaptic membranes (Hering et al., 2003). The observed deficits in mIPSCs following knock-down of GODZ or treatment with dominant-negative GODZ therefore might be a consequence of reduced exocytosis or stability of GABAA receptor expression at the cell surface or, alternatively, point to a direct role of palmitoylation in targeting of GABAA receptors to synapses.
The presynaptic deficit described here is induced by knock-down of GODZ expression selectively in postsynaptic cells. However, GODZ might independently contribute to synaptic plasticity by modulating the trafficking and function of presynaptic proteins of the neurotransmitter release machinery, many of which are know to be palmitoylated (El-Husseini and Bredt, 2002). Future experiments will need to address whether GODZ regulates the trafficking and function of presynaptic proteins and whether such an independent mechanism would affect inhibitory or excitatory synaptic transmission or both.
Footnotes
This work was supported by National Institute of Mental Health Grants MH62391 and MH60989 (B.L.) and National Science Foundation Grant 0236429 (G.C.). We are grateful to Michelle Martin, Denis Diloreto, and Sue Lingenfelter for expert technical assistance. We are indebted to Laura Snyder and Melissa Alldred for help with plasmid construction and to J. M. Fritschy, H. Betz, and B. Gasnier for generous gifts of antisera. Finally, we thank June Liu, Randen Patterson, and the members of the Luscher laboratory for critical comments on this manuscript.
References
- Alldred MJ, Mulder-Rosi J, Lingenfelter SE, Chen G, Luscher B. Distinct γ2 subunit domains mediate clustering and synaptic function of postsynaptic GABAA receptors and gephyrin. J Neurosci. 2005;25:594–603. doi: 10.1523/JNEUROSCI.4011-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer K, Essrich C, Benson JA, Benke D, Bluethmann H, Fritschy J-M, Luscher B. Postsynaptic clustering of GABAA receptors by the γ3 subunit in vivo. Proc Natl Acad Sci USA. 1999;96:12860–12865. doi: 10.1073/pnas.96.22.12860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford FK, Kittler JT, Muller E, Thomas P, Uren JM, Merlo D, Wisden W, Triller A, Smart TG, Moss SJ. GABAA receptor cell surface number and subunit stability are regulated by the ubiquitin-like protein Plic-1. Nat Neurosci. 2001;4:908–916. doi: 10.1038/nn0901-908. [DOI] [PubMed] [Google Scholar]
- Bijlmakers MJ, Marsh M. The on-off story of protein palmitoylation. Trends Cell Biol. 2003;13:32–42. doi: 10.1016/s0962-8924(02)00008-9. [DOI] [PubMed] [Google Scholar]
- Bogdanov Y, Michels G, Armstrong-Gold C, Haydon PG, Lindstrom J, Pangalos M, Moss SJ. Synaptic GABA(A) receptors are directly recruited from their extrasynaptic counterparts. EMBO J. 2006;25:4381–4389. doi: 10.1038/sj.emboj.7601309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunig I, Scotti E, Sidler C, Fritschy JM. Intact sorting, targeting, and clustering of gamma-aminobutyric acid A receptor subtypes in hippocampal neurons in vitro. J Comp Neurol. 2002;443:43–55. doi: 10.1002/cne.10102. [DOI] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, MacDonald JF, Orser BA. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha5 subunit-containing gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci USA. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary J, Skinner MK. Identification of a novel gene product, Sertoli cell gene with a zinc finger domain, that is important for FSH activation of testicular Sertoli cells. Endocrinology. 2002;143:426–435. doi: 10.1210/endo.143.2.8618. [DOI] [PubMed] [Google Scholar]
- Chen G, Trombley PQ, van den Pol AN. Excitatory actions of GABA in developing rat hypothalamic neurones. J Physiol (Lond) 1996;494:451–464. doi: 10.1113/jphysiol.1996.sp021505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie SB, Li RW, Miralles CP, Yang BY, De Blas AL. Clustered and non-clustered GABA(A) receptors in cultured hippocampal neurons. Mol Cell Neurosci. 2005;31:1–14. doi: 10.1016/j.mcn.2005.08.014. [DOI] [PubMed] [Google Scholar]
- deSouza S, Ziff EB. AMPA receptors do the electric slide. Sci STKE. 2002;2002:PE45. doi: 10.1126/stke.2002.156.pe45. [DOI] [PubMed] [Google Scholar]
- Devor A, Fritschy JM, Yarom Y. Spatial distribution and subunit composition of GABA(A) receptors in the inferior olivary nucleus. J Neurophysiol. 2001;85:1686–1696. doi: 10.1152/jn.2001.85.4.1686. [DOI] [PubMed] [Google Scholar]
- Drasbek KR, Jensen K. THIP, a hypnotic and antinociceptive drug, enhances an extrasynaptic GABAA receptor-mediated conductance in mouse neocortex. Cereb Cortex. 2006;16:1134–1141. doi: 10.1093/cercor/bhj055. [DOI] [PubMed] [Google Scholar]
- Dumoulin A, Rostaing P, Bedet C, Levi S, Isambert MF, Henry JP, Triller A, Gasnier B. Presence of the vesicular inhibitory amino acid transporter in GABAergic and glycinergic synaptic terminal boutons. J Cell Sci. 1999;112:811–823. doi: 10.1242/jcs.112.6.811. [DOI] [PubMed] [Google Scholar]
- El-Husseini AD, Bredt DS. Protein palmitoylation: a regulator of neuronal development and function. Nat Rev Neurosci. 2002;3:791–802. doi: 10.1038/nrn940. [DOI] [PubMed] [Google Scholar]
- El-Husseini AD, Schnell E, Dakoji S, Sweeney N, Zhou Q, Prange O, Gauthier-Campbell C, Aguilera-Moreno A, Nicoll RA, Bredt DS. Synaptic strength regulated by palmitate cycling on PSD-95. Cell. 2002;108:849–863. doi: 10.1016/s0092-8674(02)00683-9. [DOI] [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson J, Fritschy J-M, Luscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the γ2 subunit and gephyrin. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- Fukata M, Fukata Y, Adesnik H, Nicoll RA, Bredt DS. Identification of PSD-95 palmitoylating enzymes. Neuron. 2004;44:987–996. doi: 10.1016/j.neuron.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Rumbaugh G, Huganir RL. Differential regulation of AMPA receptor subunit trafficking by palmitoylation of two distinct sites. Neuron. 2005;47:709–723. doi: 10.1016/j.neuron.2005.06.035. [DOI] [PubMed] [Google Scholar]
- Hering H, Lin CC, Sheng M. Lipid rafts in the maintenance of synapses, dendritic spines, and surface AMPA receptor stability. J Neurosci. 2003;23:3262–3271. doi: 10.1523/JNEUROSCI.23-08-03262.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K, Yanai A, Kang R, Arstikaitis P, Singaraja RR, Metzler M, Mullard A, Haigh B, Gauthier-Campbell C, Gutekunst CA, Hayden MR, El-Husseini A. Huntingtin-interacting protein HIP14 is a palmitoyl transferase involved in palmitoylation and trafficking of multiple neuronal proteins. Neuron. 2004;44:977–986. doi: 10.1016/j.neuron.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Keller CA, Yuan X, Panzanelli P, Martin ML, Alldred MJ, Sassoè-Pognetto M, Luscher B. The γ2 subunit of GABAA receptors is a substrate for palmitoylation by GODZ. J Neurosci. 2004;24:5881–5891. doi: 10.1523/JNEUROSCI.1037-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Moss SJ. Modulation of GABA(A) receptor activity by phosphorylation and receptor trafficking: implications for the efficacy of synaptic inhibition. Curr Opin Neurobiol. 2003;13:341–347. doi: 10.1016/s0959-4388(03)00064-3. [DOI] [PubMed] [Google Scholar]
- Lam KK, Davey M, Sun B, Roth AF, Davis NG, Conibear E. Palmitoylation by the DHHC protein Pfa4 regulates the ER exit of Chs3. J Cell Biol. 2006;174:19–25. doi: 10.1083/jcb.200602049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RW, Yu W, Christie S, Miralles CP, Bai J, Loturco JJ, De Blas AL. Disruption of postsynaptic GABA receptor clusters leads to decreased GABAergic innervation of pyramidal neurons. J Neurochem. 2005;95:756–770. doi: 10.1111/j.1471-4159.2005.03426.x. [DOI] [PubMed] [Google Scholar]
- Lindquist CE, Ebert B, Birnir B. Extrasynaptic GABA(A) channels activated by THIP are modulated by diazepam in CA1 pyramidal neurons in the rat brain hippocampal slice. Mol Cell Neurosci. 2003;24:250–257. doi: 10.1016/s1044-7431(03)00128-3. [DOI] [PubMed] [Google Scholar]
- Lobo S, Greentree WK, Linder ME, Deschenes RJ. Identification of a Ras palmitoyltransferase in Saccharomyces cerevisiae. J Biol Chem. 2002;277:41268–41273. doi: 10.1074/jbc.M206573200. [DOI] [PubMed] [Google Scholar]
- Luscher B, Keller CA. Ubiquitination, proteasomes and GABA(A) receptors. Nat Cell Biol. 2001;3:E232–233. doi: 10.1038/ncb1001-e232. [DOI] [PubMed] [Google Scholar]
- Luscher B, Keller CA. Regulation of GABAA receptor trafficking and channel activity in functional plasticity of inhibitory synapses. Pharmacol Ther. 2004;102:195–221. doi: 10.1016/j.pharmthera.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Patterson SI. Posttranslational protein S-palmitoylation and the compartmentalization of signaling molecules in neurons. Biol Res. 2002;35:139–150. doi: 10.4067/s0716-97602002000200005. [DOI] [PubMed] [Google Scholar]
- Rathenberg J, Kittler JT, Moss SJ. Palmitoylation regulates the clustering and cell surface stability of GABAA receptors. Mol Cell Neurosci. 2004;26:251–257. doi: 10.1016/j.mcn.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Resh MD. Membrane targeting of lipid modified signal transduction proteins. Subcell Biochem. 2004;37:217–232. doi: 10.1007/978-1-4757-5806-1_6. [DOI] [PubMed] [Google Scholar]
- Schweizer C, Balsiger S, Bluethmann H, Mansuy M, Fritschy JM, Mohler H, Luscher B. The γ2 subunit of GABAA receptors is required for maintenance of receptors at mature synapses. Mol Cell Neurosci. 2003;24:442–450. doi: 10.1016/s1044-7431(03)00202-1. [DOI] [PubMed] [Google Scholar]
- Sieghart W, Sperk G. Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr Top Med Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- Uemura T, Mori H, Mishina M. Isolation and characterization of a Golgi apparatus-specific GODZ with the DHHC zinc finger domain. Biochem Biophys Res Commun. 2002;296:492–496. doi: 10.1016/s0006-291x(02)00900-2. [DOI] [PubMed] [Google Scholar]
- Valdez-Taubas J, Pelham H. Swf1-dependent palmitoylation of the SNARE Tlg1 prevents its ubiquitination and degradation. EMBO J. 2005;24:2524–2532. doi: 10.1038/sj.emboj.7600724. [DOI] [PMC free article] [PubMed] [Google Scholar]