Abstract
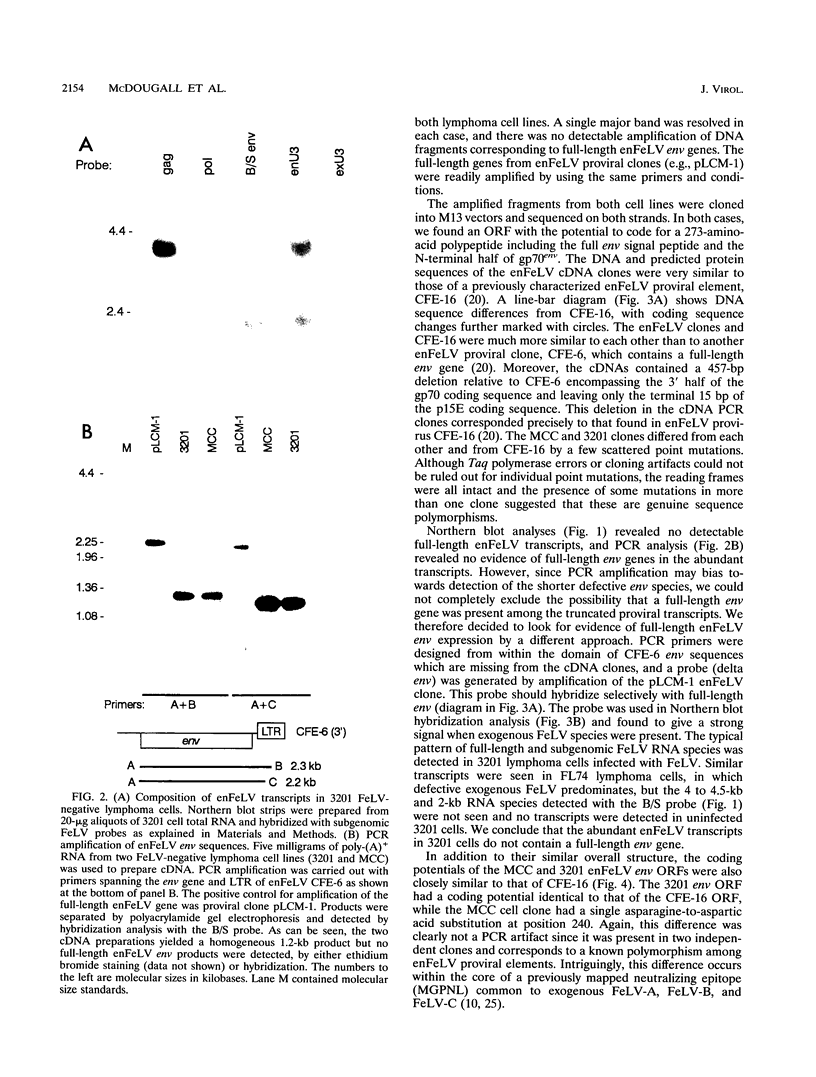
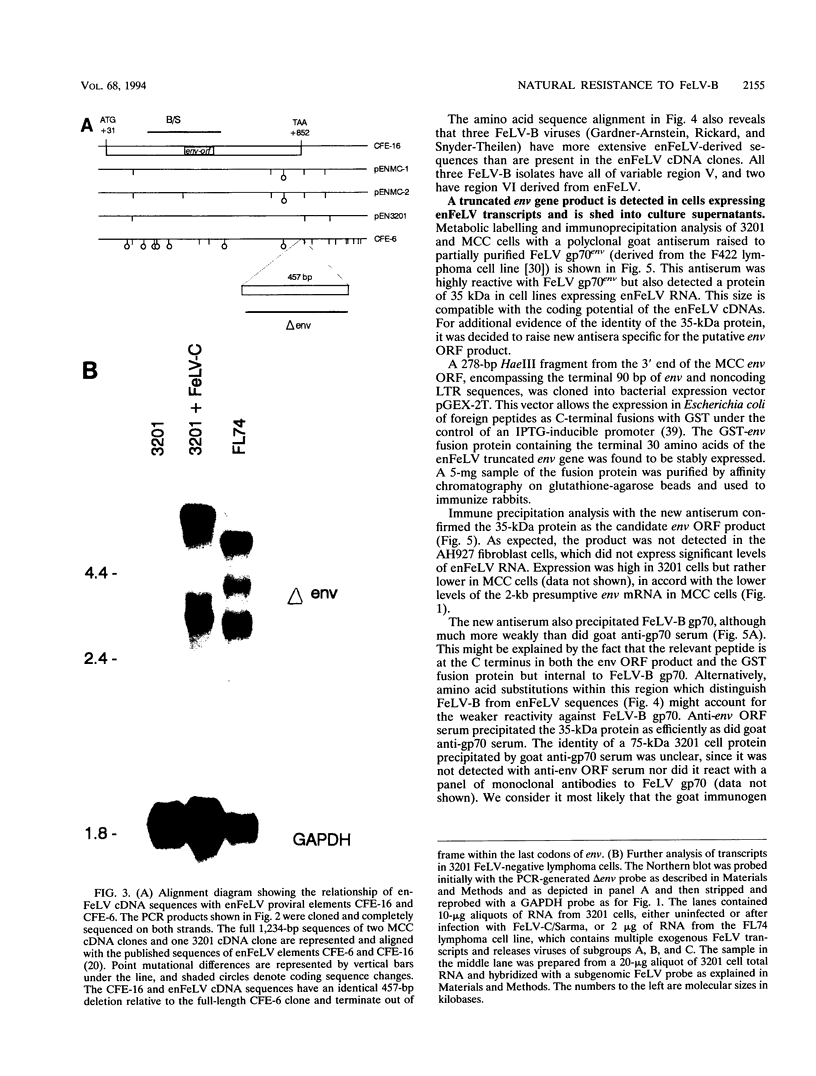
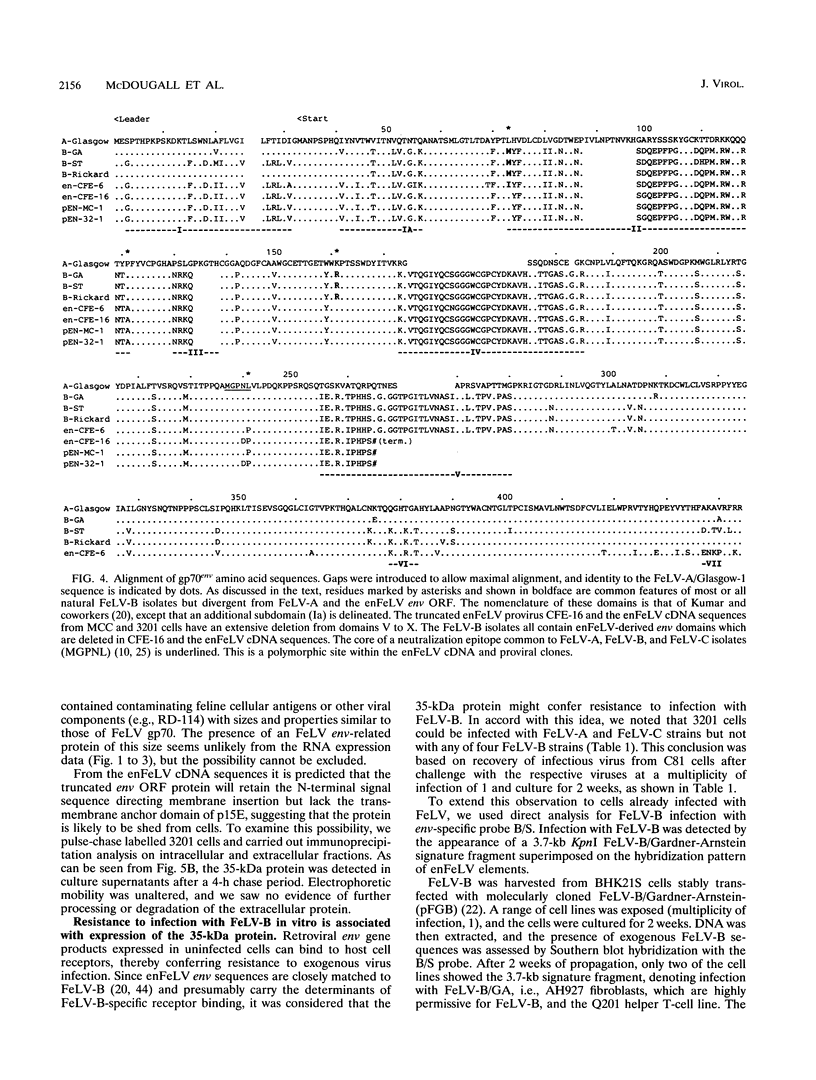
Endogenous feline leukemia virus (FeLV)-related sequences (enFeLV) are a family of proviral elements found in domestic cats and their close relatives. These elements can recombine with exogenous, infectious FeLVs of subgroup A (FeLV-A), giving rise to host range variants of FeLV-B. We found that a subset of defective enFeLV proviruses is highly expressed in lymphoma cell lines and in a variety of primary tissues, including lymphoid tissues from healthy specific-pathogen-free cats. At least two RNA species were detected, a 4.5-kb RNA containing gag, env, and long terminal repeat sequences and a 2-kb RNA containing env and long terminal repeat sequences. Cloning of enFeLV cDNA from two FeLV-free lymphoma cell lines (3201 and MCC) revealed a long open reading frame (ORF) encoding a truncated env gene product corresponding to the N-terminal portion of gp70env. Interestingly, all of three natural FeLV-B isolates include 3' env sequences which are missing from the highly transcribed subset and hence must be derived from other enFeLV elements. The enFeLV env ORF cDNA clones were closely similar to a previously characterized enFeLV provirus, CFE-16, but were polymorphic at a site corresponding to an exogenous FeLV neutralization epitope. Site-specific antiserum raised to a C-terminal 30-amino-acid peptide of the enFeLV env ORF detected an intracellular product of 35 kDa which was also shed from cells in stable form. Expression of the 35-kDa protein correlated with enFeLV RNA levels and was negatively correlated with susceptibility to infection with FeLV-B. Cell culture supernatant containing the 35-kDa protein specifically blocked infection of permissive fibroblast cells with FeLV-B isolates. We suggest that the truncated env protein mediates resistance by receptor blockade and that this form of enFeLV expression mediates the natural resistance of cats to infection with FeLV-B in the absence of FeLV-A.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balmain A., Krumlauf R., Vass J. K., Birnie G. D. Cloning and characterisation of the abundant cytoplasmic 7S RNA from mouse cells. Nucleic Acids Res. 1982 Jul 24;10(14):4259–4277. doi: 10.1093/nar/10.14.4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Sherr C. J., Todaro G. J. Evolution of type C viral genes: origin of feline leukemia virus. Science. 1975 Nov 28;190(4217):886–888. doi: 10.1126/science.52892. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Segregation of RD-114 AND FeL-V-related sequences in crosses between domestic cat and leopard cat. Nature. 1975 Oct 9;257(5526):506–508. doi: 10.1038/257506a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry B. T., Ghosh A. K., Kumar D. V., Spodick D. A., Roy-Burman P. Structure and function of endogenous feline leukemia virus long terminal repeats and adjoining regions. J Virol. 1988 Oct;62(10):3631–3641. doi: 10.1128/jvi.62.10.3631-3641.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller R. S., Ahmed A., Portis J. L. Identification of two forms of an endogenous murine retroviral env gene linked to the Rmcf locus. J Virol. 1987 Jan;61(1):29–34. doi: 10.1128/jvi.61.1.29-34.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch M. P., Devi B. G., Soe L. H., Perbal B., Baluda M. A., Roy-Burman P. Characterization of the expression of cellular retrovirus genes and oncogenes in feline cells. Hematol Oncol. 1983 Jan-Mar;1(1):61–75. doi: 10.1002/hon.2900010108. [DOI] [PubMed] [Google Scholar]
- Cheney C. M., Rojko J. L., Kociba G. J., Wellman M. L., Di Bartola S. P., Rezanka L. J., Forman L., Mathes L. E. A feline large granular lymphoma and its derived cell line. In Vitro Cell Dev Biol. 1990 May;26(5):455–463. doi: 10.1007/BF02624087. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Donahue P. R., Hoover E. A., Beltz G. A., Riedel N., Hirsch V. M., Overbaugh J., Mullins J. I. Strong sequence conservation among horizontally transmissible, minimally pathogenic feline leukemia viruses. J Virol. 1988 Mar;62(3):722–731. doi: 10.1128/jvi.62.3.722-731.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., McGee J. S., Munson M., Houghten R. A., Kloetzer W., Bittle J. L., Grant C. K. Localization of neutralizing regions of the envelope gene of feline leukemia virus by using anti-synthetic peptide antibodies. J Virol. 1987 Jan;61(1):8–15. doi: 10.1128/jvi.61.1.8-15.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischinger P. J., Blevins C. S., Nomura S. Simple, quantitative assay for both xenotropic murine leukemia and ecotropic feline leukemia viruses. J Virol. 1974 Jul;14(1):177–179. doi: 10.1128/jvi.14.1.177-179.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Yetter R. A., Morse H. C., 3rd A mouse gene on chromosome 5 that restricts infectivity of mink cell focus-forming recombinant murine leukemia viruses. J Exp Med. 1983 Jul 1;158(1):16–24. doi: 10.1084/jem.158.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard J. M., Danos O. An amino-terminal fragment of the Friend murine leukemia virus envelope glycoprotein binds the ecotropic receptor. J Virol. 1991 Aug;65(8):4026–4032. doi: 10.1128/jvi.65.8.4026-4032.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson I. C., Lieber M. M., Todaro G. J. Mink cell line Mv 1 Lu (CCL 64). Focus formation and the generation of "nonproducer" transformed cell lines with murine and feline sarcoma viruses. Virology. 1974 Jul;60(1):282–287. doi: 10.1016/0042-6822(74)90386-9. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Laigret F., Martin M. A., Repaske R. Characterization of a molecularly cloned retroviral sequence associated with Fv-4 resistance. J Virol. 1985 Sep;55(3):768–777. doi: 10.1128/jvi.55.3.768-777.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett O., Hardy W. D., Jr, Golder M. C., Hay D. The frequency of occurrence of feline leukaemia virus subgroups in cats. Int J Cancer. 1978 Mar 15;21(3):334–337. doi: 10.1002/ijc.2910210314. [DOI] [PubMed] [Google Scholar]
- Jarrett O., Russell P. H. Differential growth and transmission in cats of feline leukaemia viruses of subgroups A and B. Int J Cancer. 1978 Apr 15;21(4):466–472. doi: 10.1002/ijc.2910210411. [DOI] [PubMed] [Google Scholar]
- Kumar D. V., Berry B. T., Roy-Burman P. Nucleotide sequence and distinctive characteristics of the env gene of endogenous feline leukemia provirus. J Virol. 1989 May;63(5):2379–2384. doi: 10.1128/jvi.63.5.2379-2384.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins J. I., Brody D. S., Binari R. C., Jr, Cotter S. M. Viral transduction of c-myc gene in naturally occurring feline leukaemias. 1984 Apr 26-May 2Nature. 308(5962):856–858. doi: 10.1038/308856a0. [DOI] [PubMed] [Google Scholar]
- Mullins J. I., Casey J. W., Nicolson M. O., Burck K. B., Davidson N. Sequence arrangement and biological activity of cloned feline leukemia virus proviruses from a virus-productive human cell line. J Virol. 1981 May;38(2):688–703. doi: 10.1128/jvi.38.2.688-703.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil J. C., Fulton R., Rigby M., Stewart M. Feline leukaemia virus: generation of pathogenic and oncogenic variants. Curr Top Microbiol Immunol. 1991;171:67–93. doi: 10.1007/978-3-642-76524-7_4. [DOI] [PubMed] [Google Scholar]
- Niman H. L., Stephenson J. R., Gardner M. B., Roy-Burman P. RD-114 and feline leukaemia virus genome expression in natural lymphomas of domestic cats. Nature. 1977 Mar 24;266(5600):357–360. doi: 10.1038/266357a0. [DOI] [PubMed] [Google Scholar]
- Nunberg J. H., Rodgers G., Gilbert J. H., Snead R. M. Method to map antigenic determinants recognized by monoclonal antibodies: localization of a determinant of virus neutralization on the feline leukemia virus envelope protein gp70. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3675–3679. doi: 10.1073/pnas.81.12.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onions D., Lees G., Forrest D., Neil J. Recombinant feline viruses containing the myc gene rapidly produce clonal tumours expressing T-cell antigen receptor gene transcripts. Int J Cancer. 1987 Jul 15;40(1):40–45. doi: 10.1002/ijc.2910400108. [DOI] [PubMed] [Google Scholar]
- Overbaugh J., Riedel N., Hoover E. A., Mullins J. I. Transduction of endogenous envelope genes by feline leukaemia virus in vitro. Nature. 1988 Apr 21;332(6166):731–734. doi: 10.1038/332731a0. [DOI] [PubMed] [Google Scholar]
- Pandey R., Ghosh A. K., Kumar D. V., Bachman B. A., Shibata D., Roy-Burman P. Recombination between feline leukemia virus subgroup B or C and endogenous env elements alters the in vitro biological activities of the viruses. J Virol. 1991 Dec;65(12):6495–6508. doi: 10.1128/jvi.65.12.6495-6508.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickard C. G., Post J. E., Noronha F., Barr L. M. A transmissible virus-induced lymphocytic leukemia of the cat. J Natl Cancer Inst. 1969 Jun;42(6):987–1014. [PubMed] [Google Scholar]
- Riedel N., Hoover E. A., Gasper P. W., Nicolson M. O., Mullins J. I. Molecular analysis and pathogenesis of the feline aplastic anemia retrovirus, feline leukemia virus C-Sarma. J Virol. 1986 Oct;60(1):242–250. doi: 10.1128/jvi.60.1.242-250.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby M. A., Rojko J. L., Stewart M. A., Kociba G. J., Cheney C. M., Rezanka L. J., Mathes L. E., Hartke J. R., Jarrett O., Neil J. C. Partial dissociation of subgroup C phenotype and in vivo behaviour in feline leukaemia viruses with chimeric envelope genes. J Gen Virol. 1992 Nov;73(Pt 11):2839–2847. doi: 10.1099/0022-1317-73-11-2839. [DOI] [PubMed] [Google Scholar]
- Rojko J. L., Hoover E. A., Finn B. L., Olsen R. G. Determinants of susceptibility and resistance to feline leukemia virus infection. II. Susceptibility of feline lymphocytes to productive feline leukemia virus infection. J Natl Cancer Inst. 1981 Oct;67(4):899–910. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma P. S., Log T. Subgroup classification of feline leukemia and sarcoma viruses by viral interference and neutralization tests. Virology. 1973 Jul;54(1):160–169. doi: 10.1016/0042-6822(73)90125-6. [DOI] [PubMed] [Google Scholar]
- Sheets R. L., Pandey R., Klement V., Grant C. K., Roy-Burman P. Biologically selected recombinants between feline leukemia virus (FeLV) subgroup A and an endogenous FeLV element. Virology. 1992 Oct;190(2):849–855. doi: 10.1016/0042-6822(92)90924-e. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Snyder H. W., Jr, Hardy W. D., Jr, Zuckerman E. E., Fleissner E. Characterisation of a tumour-specific antigen on the surface of feline lymphosarcoma cells. Nature. 1978 Oct 19;275(5681):656–658. doi: 10.1038/275656a0. [DOI] [PubMed] [Google Scholar]
- Snyder H. W., Jr, Singhal M. C., Zuckerman E. E., Jones F. R., Hardy W. D., Jr The feline oncornavirus-associated cell membrane antigen (FOCMA) is related to, but distinguishable from, FeLV-C gp70. Virology. 1983 Dec;131(2):315–327. doi: 10.1016/0042-6822(83)90500-7. [DOI] [PubMed] [Google Scholar]
- Soe L. H., Devi B. G., Mullins J. I., Roy-Burman P. Molecular cloning and characterization of endogenous feline leukemia virus sequences from a cat genomic library. J Virol. 1983 Jun;46(3):829–840. doi: 10.1128/jvi.46.3.829-840.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soe L. H., Shimizu R. W., Landolph J. R., Roy-Burman P. Molecular analysis of several classes of endogenous feline leukemia virus elements. J Virol. 1985 Dec;56(3):701–710. doi: 10.1128/jvi.56.3.701-710.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart M. A., Warnock M., Wheeler A., Wilkie N., Mullins J. I., Onions D. E., Neil J. C. Nucleotide sequences of a feline leukemia virus subgroup A envelope gene and long terminal repeat and evidence for the recombinational origin of subgroup B viruses. J Virol. 1986 Jun;58(3):825–834. doi: 10.1128/jvi.58.3.825-834.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theilen G. H., Dungworth D. L., Kawakami T. G., Munn R. J., Ward J. M., Harrold J. B. Experimental induction of lymphosarcoma in the cat with "C"-type virus. Cancer Res. 1970 Feb;30(2):401–408. [PubMed] [Google Scholar]
- Tzavaras T., Stewart M., McDougall A., Fulton R., Testa N., Onions D. E., Neil J. C. Molecular cloning and characterization of a defective recombinant feline leukaemia virus associated with myeloid leukaemia. J Gen Virol. 1990 Feb;71(Pt 2):343–354. doi: 10.1099/0022-1317-71-2-343. [DOI] [PubMed] [Google Scholar]
- Willett B. J., Hosie M. J., Dunsford T. H., Neil J. C., Jarrett O. Productive infection of T-helper lymphocytes with feline immunodeficiency virus is accompanied by reduced expression of CD4. AIDS. 1991 Dec;5(12):1469–1475. doi: 10.1097/00002030-199112000-00009. [DOI] [PubMed] [Google Scholar]