Abstract
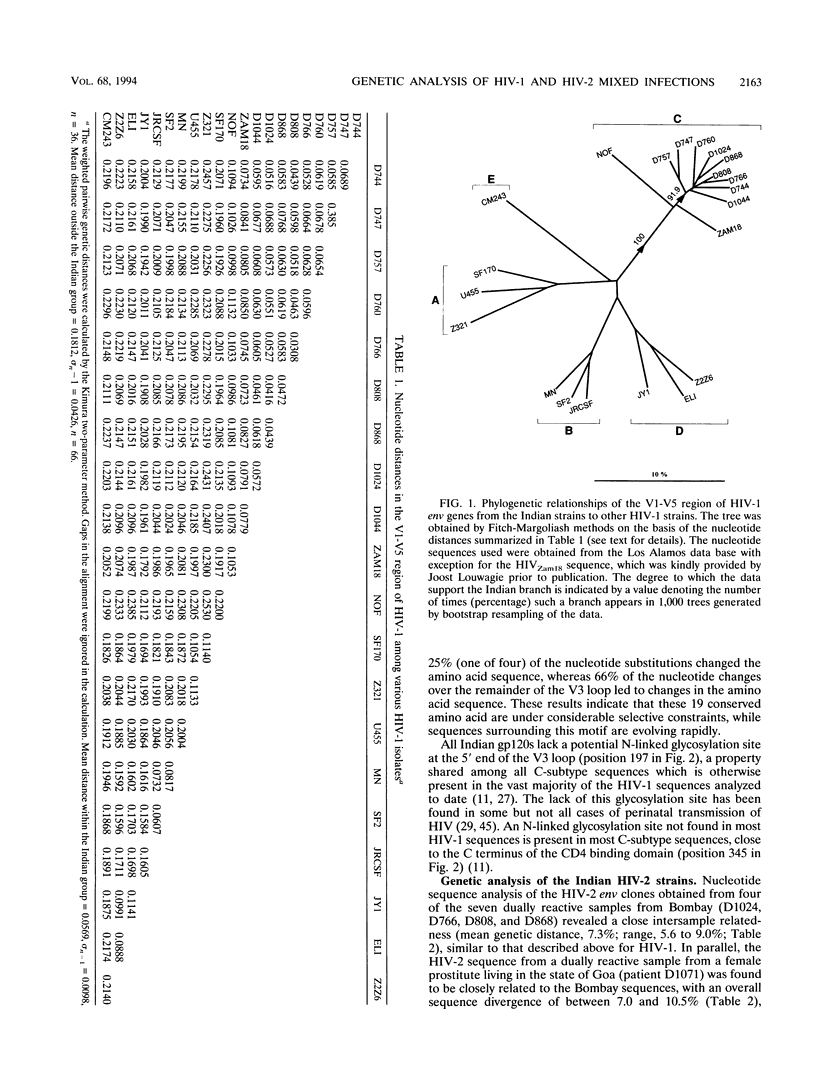
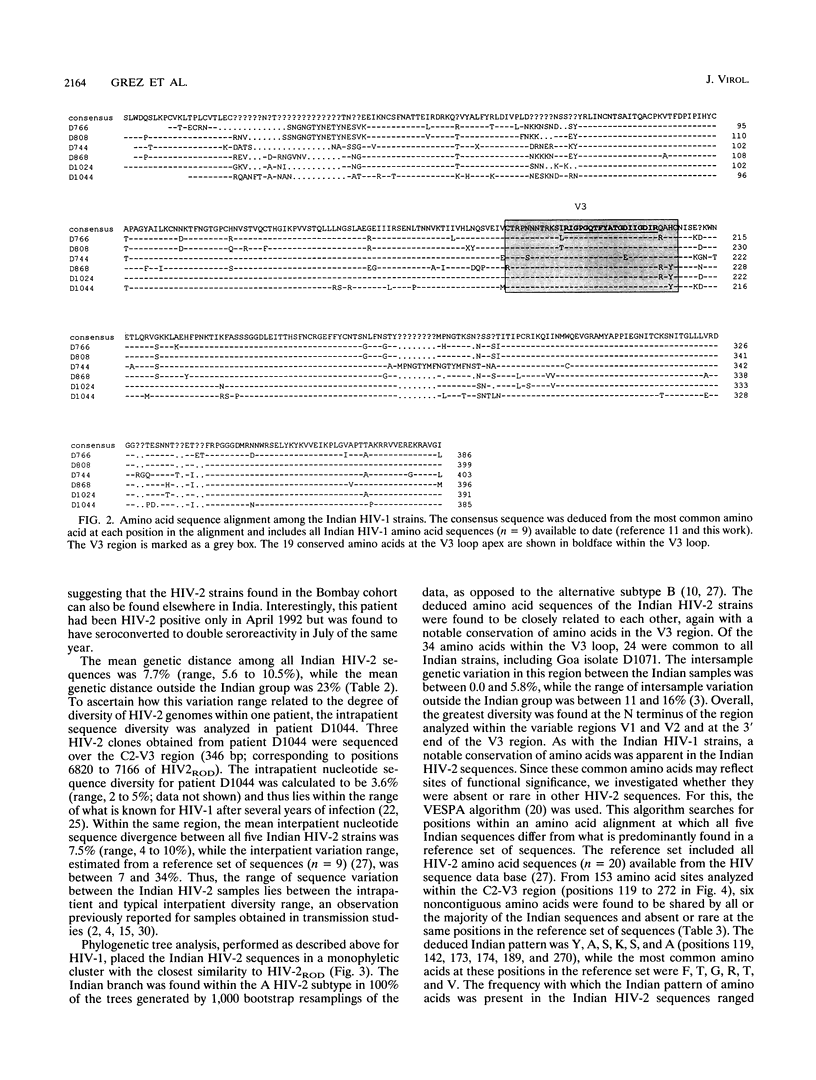
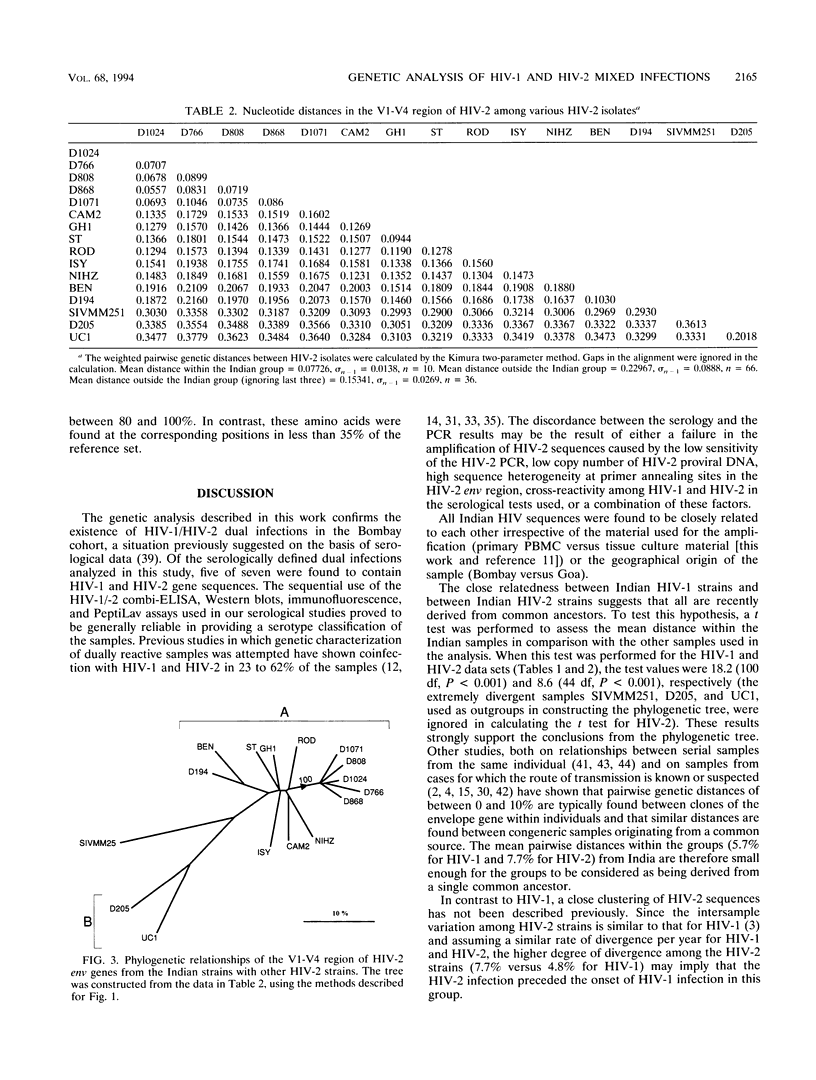
DNA sequences encoding the surface envelope glycoproteins of human immunodeficiency virus type 1 (HIV-1) and type 2 (HIV-2) were amplified by PCR from uncultured peripheral blood mononuclear cells obtained from patients with serologically defined HIV-1/HIV-2 mixed infections from Bombay, India. HIV-1-specific PCR products were obtained in seven of seven randomly chosen doubly reactive cases, while HIV-2-specific sequences were detected in five of seven cases (71%). DNA sequence analysis showed that the HIV-1 gp120 coding sequences were closely related to each other (nucleotide sequence divergence of between 3.1 and 6.8%). Phylogenetic tree analysis placed the Indian strains within the C subtype of HIV-1, being most similar to sequences previously found in East and South Africa. The HIV-2 sequences were also closely related to each other, with an overall sequence divergence of between 5.6 and 10.5%. The low level of nucleotide divergence among Indian HIV-1 and HIV-2 sequences suggests a fairly recent introduction of each virus into this population from a single point of entry in each case. The HIV-2 sequences reported here represent the first analysis of Asian HIV-2 strains and confirm the serological pattern previously detected in India. These data show that a substantial spread of HIV-2, together with HIV-1, has appeared outside Africa in a population hitherto unexposed to HIV. These findings imply that further spread of HIV-2 worldwide is to be expected and have important implications for future vaccine and therapy development.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alizon M., Wain-Hobson S., Montagnier L., Sonigo P. Genetic variability of the AIDS virus: nucleotide sequence analysis of two isolates from African patients. Cell. 1986 Jul 4;46(1):63–74. doi: 10.1016/0092-8674(86)90860-3. [DOI] [PubMed] [Google Scholar]
- Balfe P., Simmonds P., Ludlam C. A., Bishop J. O., Brown A. J. Concurrent evolution of human immunodeficiency virus type 1 in patients infected from the same source: rate of sequence change and low frequency of inactivating mutations. J Virol. 1990 Dec;64(12):6221–6233. doi: 10.1128/jvi.64.12.6221-6233.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeri E., Giri A., Lillo F., Ferrari G., Varnier O. E., Ferro A., Sabbatani S., Saxinger W. C., Franchini G. In vivo genetic variability of the human immunodeficiency virus type 2 V3 region. J Virol. 1992 Jul;66(7):4546–4550. doi: 10.1128/jvi.66.7.4546-4550.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger H., Weiser B., Flaherty K., Gulla J., Nguyen P. N., Gibbs R. A. Evolution of human immunodeficiency virus type 1 nucleotide sequence diversity among close contacts. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11236–11240. doi: 10.1073/pnas.88.24.11236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K., Nishio J., Perryman S. Macrophage-tropic human immunodeficiency virus isolates from different patients exhibit unusual V3 envelope sequence homogeneity in comparison with T-cell-tropic isolates: definition of critical amino acids involved in cell tropism. J Virol. 1992 Nov;66(11):6547–6554. doi: 10.1128/jvi.66.11.6547-6554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daar E. S., Moudgil T., Meyer R. D., Ho D. D. Transient high levels of viremia in patients with primary human immunodeficiency virus type 1 infection. N Engl J Med. 1991 Apr 4;324(14):961–964. doi: 10.1056/NEJM199104043241405. [DOI] [PubMed] [Google Scholar]
- De Cock K. M., Adjorlolo G., Ekpini E., Sibailly T., Kouadio J., Maran M., Brattegaard K., Vetter K. M., Doorly R., Gayle H. D. Epidemiology and transmission of HIV-2. Why there is no HIV-2 pandemic. JAMA. 1993 Nov 3;270(17):2083–2086. doi: 10.1001/jama.270.17.2083. [DOI] [PubMed] [Google Scholar]
- Delwart E. L., Shpaer E. G., Louwagie J., McCutchan F. E., Grez M., Rübsamen-Waigmann H., Mullins J. I. Genetic relationships determined by a DNA heteroduplex mobility assay: analysis of HIV-1 env genes. Science. 1993 Nov 19;262(5137):1257–1261. doi: 10.1126/science.8235655. [DOI] [PubMed] [Google Scholar]
- Dietrich U., Adamski M., Kreutz R., Seipp A., Kühnel H., Rübsamen-Waigmann H. A highly divergent HIV-2-related isolate. Nature. 1989 Dec 21;342(6252):948–950. doi: 10.1038/342948a0. [DOI] [PubMed] [Google Scholar]
- Dietrich U., Grez M., von Briesen H., Panhans B., Geissendörfer M., Kühnel H., Maniar J., Mahambre G., Becker W. B., Becker M. L. HIV-1 strains from India are highly divergent from prototypic African and US/European strains, but are linked to a South African isolate. AIDS. 1993 Jan;7(1):23–27. doi: 10.1097/00002030-199301000-00003. [DOI] [PubMed] [Google Scholar]
- Evans L. A., Moreau J., Odehouri K., Seto D., Thomson-Honnebier G., Legg H., Barboza A., Cheng-Mayer C., Levy J. A. Simultaneous isolation of HIV-1 and HIV-2 from an AIDS patient. Lancet. 1988 Dec 17;2(8625):1389–1391. doi: 10.1016/s0140-6736(88)90586-7. [DOI] [PubMed] [Google Scholar]
- George J. R., Ou C. Y., Parekh B., Brattegaard K., Brown V., Boateng E., De Cock K. M. Prevalence of HIV-1 and HIV-2 mixed infections in Côte d'Ivoire. Lancet. 1992 Aug 8;340(8815):337–339. doi: 10.1016/0140-6736(92)91406-x. [DOI] [PubMed] [Google Scholar]
- Goodenow M., Huet T., Saurin W., Kwok S., Sninsky J., Wain-Hobson S. HIV-1 isolates are rapidly evolving quasispecies: evidence for viral mixtures and preferred nucleotide substitutions. J Acquir Immune Defic Syndr. 1989;2(4):344–352. [PubMed] [Google Scholar]
- Guyader M., Emerman M., Sonigo P., Clavel F., Montagnier L., Alizon M. Genome organization and transactivation of the human immunodeficiency virus type 2. Nature. 1987 Apr 16;326(6114):662–669. doi: 10.1038/326662a0. [DOI] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. Fast and sensitive multiple sequence alignments on a microcomputer. Comput Appl Biosci. 1989 Apr;5(2):151–153. doi: 10.1093/bioinformatics/5.2.151. [DOI] [PubMed] [Google Scholar]
- Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980 Dec;16(2):111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Korber B., Myers G. Signature pattern analysis: a method for assessing viral sequence relatedness. AIDS Res Hum Retroviruses. 1992 Sep;8(9):1549–1560. doi: 10.1089/aid.1992.8.1549. [DOI] [PubMed] [Google Scholar]
- Kusumi K., Conway B., Cunningham S., Berson A., Evans C., Iversen A. K., Colvin D., Gallo M. V., Coutre S., Shpaer E. G. Human immunodeficiency virus type 1 envelope gene structure and diversity in vivo and after cocultivation in vitro. J Virol. 1992 Feb;66(2):875–885. doi: 10.1128/jvi.66.2.875-885.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühnel H., von Briesen H., Dietrich U., Adamski M., Mix D., Biesert L., Kreutz R., Immelmann A., Henco K., Meichsner C. Molecular cloning of two west African human immunodeficiency virus type 2 isolates that replicate well in macrophages: a Gambian isolate, from a patient with neurologic acquired immunodeficiency syndrome, and a highly divergent Ghanian isolate. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2383–2387. doi: 10.1073/pnas.86.7.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlink R. G., Ricard D., M'Boup S., Kanki P. J., Romet-Lemonne J. L., N'Doye I., Diop K., Simpson M. A., Greco F., Chou M. J. Clinical, hematologic, and immunologic cross-sectional evaluation of individuals exposed to human immunodeficiency virus type-2 (HIV-2). AIDS Res Hum Retroviruses. 1988 Apr;4(2):137–148. doi: 10.1089/aid.1988.4.137. [DOI] [PubMed] [Google Scholar]
- McCutchan F. E., Hegerich P. A., Brennan T. P., Phanuphak P., Singharaj P., Jugsudee A., Berman P. W., Gray A. M., Fowler A. K., Burke D. S. Genetic variants of HIV-1 in Thailand. AIDS Res Hum Retroviruses. 1992 Nov;8(11):1887–1895. doi: 10.1089/aid.1992.8.1887. [DOI] [PubMed] [Google Scholar]
- Meyerhans A., Cheynier R., Albert J., Seth M., Kwok S., Sninsky J., Morfeldt-Månson L., Asjö B., Wain-Hobson S. Temporal fluctuations in HIV quasispecies in vivo are not reflected by sequential HIV isolations. Cell. 1989 Sep 8;58(5):901–910. doi: 10.1016/0092-8674(89)90942-2. [DOI] [PubMed] [Google Scholar]
- Nei M., Gojobori T. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol Biol Evol. 1986 Sep;3(5):418–426. doi: 10.1093/oxfordjournals.molbev.a040410. [DOI] [PubMed] [Google Scholar]
- Orloff G. M., Kalish M. L., Chiphangwi J., Potts K. E., Ou C. Y., Schochetman G., Dallabetta G., Saah A. I., Miotti P. G. V3 loops of HIV-1 specimens from pregnant women in Malawi uniformly lack a potential N-linked glycosylation site. AIDS Res Hum Retroviruses. 1993 Jul;9(7):705–706. doi: 10.1089/aid.1993.9.705. [DOI] [PubMed] [Google Scholar]
- Ou C. Y., Ciesielski C. A., Myers G., Bandea C. I., Luo C. C., Korber B. T., Mullins J. I., Schochetman G., Berkelman R. L., Economou A. N. Molecular epidemiology of HIV transmission in a dental practice. Science. 1992 May 22;256(5060):1165–1171. doi: 10.1126/science.256.5060.1165. [DOI] [PubMed] [Google Scholar]
- Peeters M., Gershy-Damet G. M., Fransen K., Koffi K., Coulibaly M., Delaporte E., Piot P., van der Groen G. Virological and polymerase chain reaction studies of HIV-1/HIV-2 dual infection in Côte d'Ivoire. Lancet. 1992 Aug 8;340(8815):339–340. doi: 10.1016/0140-6736(92)91407-y. [DOI] [PubMed] [Google Scholar]
- Pfützner A., Dietrich U., von Eichel U., von Briesen H., Brede H. D., Maniar J. K., Rübsamen-Waigmann H. HIV-1 and HIV-2 infections in a high-risk population in Bombay, India: evidence for the spread of HIV-2 and presence of a divergent HIV-1 subtype. J Acquir Immune Defic Syndr. 1992 Oct;5(10):972–977. [PubMed] [Google Scholar]
- Pieniazek D., Peralta J. M., Ferreira J. A., Krebs J. W., Owen S. M., Sion F. S., Filho C. F., Sereno A. B., de Sa C. A., Weniger B. G. Identification of mixed HIV-1/HIV-2 infections in Brazil by polymerase chain reaction. AIDS. 1991 Nov;5(11):1293–1299. doi: 10.1097/00002030-199111000-00002. [DOI] [PubMed] [Google Scholar]
- Poulsen A. G., Kvinesdal B., Aaby P., Mølbak K., Frederiksen K., Dias F., Lauritzen E. Prevalence of and mortality from human immunodeficiency virus type 2 in Bissau, West Africa. Lancet. 1989 Apr 15;1(8642):827–831. doi: 10.1016/s0140-6736(89)92281-2. [DOI] [PubMed] [Google Scholar]
- Rayfield M., De Cock K., Heyward W., Goldstein L., Krebs J., Kwok S., Lee S., McCormick J., Moreau J. M., Odehouri K. Mixed human immunodeficiency virus (HIV) infection in an individual: demonstration of both HIV type 1 and type 2 proviral sequences by using polymerase chain reaction. J Infect Dis. 1988 Dec;158(6):1170–1176. doi: 10.1093/infdis/158.6.1170. [DOI] [PubMed] [Google Scholar]
- Rey F., Salaun D., Lesbordes J. L., Gadelle S., Ollivier-Henry F., Barré-Sinoussi F., Chermann J. C., Georges A. J. HIV-I and HIV-II double infection in Central African Republic. Lancet. 1986 Dec 13;2(8520):1391–1392. doi: 10.1016/s0140-6736(86)92027-1. [DOI] [PubMed] [Google Scholar]
- Roos M. T., Lange J. M., de Goede R. E., Coutinho R. A., Schellekens P. T., Miedema F., Tersmette M. Viral phenotype and immune response in primary human immunodeficiency virus type 1 infection. J Infect Dis. 1992 Mar;165(3):427–432. doi: 10.1093/infdis/165.3.427. [DOI] [PubMed] [Google Scholar]
- Rübsamen-Waigmann H., Briesen H. V., Maniar J. K., Rao P. K., Scholz C., Pfützner A. Spread of HIV-2 in India. Lancet. 1991 Mar 2;337(8740):550–551. doi: 10.1016/0140-6736(91)91333-p. [DOI] [PubMed] [Google Scholar]
- Rübsamen-Waigmann H., Grez M., von Briesen H., Dietrich U., Shroff H. J., Kaaya E. E., Biberfeld P. Kaposi's sarcoma in an Indian woman infected with HIV-1 and HIV-2. AIDS Res Hum Retroviruses. 1993 Jun;9(6):573–577. doi: 10.1089/aid.1993.9.573. [DOI] [PubMed] [Google Scholar]
- Salminen M., Nykänen A., Brummer-Korvenkontio H., Kantanen M. L., Liitsola K., Leinikki P. Molecular epidemiology of HIV-1 based on phylogenetic analysis of in vivo gag p7/p9 direct sequences. Virology. 1993 Jul;195(1):185–194. doi: 10.1006/viro.1993.1359. [DOI] [PubMed] [Google Scholar]
- Simmonds P., Balfe P., Ludlam C. A., Bishop J. O., Brown A. J. Analysis of sequence diversity in hypervariable regions of the external glycoprotein of human immunodeficiency virus type 1. J Virol. 1990 Dec;64(12):5840–5850. doi: 10.1128/jvi.64.12.5840-5850.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds P., Zhang L. Q., McOmish F., Balfe P., Ludlam C. A., Brown A. J. Discontinuous sequence change of human immunodeficiency virus (HIV) type 1 env sequences in plasma viral and lymphocyte-associated proviral populations in vivo: implications for models of HIV pathogenesis. J Virol. 1991 Nov;65(11):6266–6276. doi: 10.1128/jvi.65.11.6266-6276.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfs T. F., Zwart G., Bakker M., Goudsmit J. HIV-1 genomic RNA diversification following sexual and parenteral virus transmission. Virology. 1992 Jul;189(1):103–110. doi: 10.1016/0042-6822(92)90685-i. [DOI] [PubMed] [Google Scholar]
- Wolinsky S. M., Wike C. M., Korber B. T., Hutto C., Parks W. P., Rosenblum L. L., Kunstman K. J., Furtado M. R., Muñoz J. L. Selective transmission of human immunodeficiency virus type-1 variants from mothers to infants. Science. 1992 Feb 28;255(5048):1134–1137. doi: 10.1126/science.1546316. [DOI] [PubMed] [Google Scholar]
- Zhang L. Q., MacKenzie P., Cleland A., Holmes E. C., Brown A. J., Simmonds P. Selection for specific sequences in the external envelope protein of human immunodeficiency virus type 1 upon primary infection. J Virol. 1993 Jun;67(6):3345–3356. doi: 10.1128/jvi.67.6.3345-3356.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wolf F., Meloen R. H., Bakker M., Barin F., Goudsmit J. Characterization of human antibody-binding sites on the external envelope of human immunodeficiency virus type 2. J Gen Virol. 1991 Jun;72(Pt 6):1261–1267. doi: 10.1099/0022-1317-72-6-1261. [DOI] [PubMed] [Google Scholar]


