Abstract
Active vaccination of elderly Alzheimer's disease (AD) patients with fibrillar amyloid-β peptide (Aβ42), even in the presence of a potent Th1 adjuvant, induced generally low titers of antibodies in a small fraction (∼20% responders) of those that received the AN-1792 vaccine. To improve the immunogenicity and reduce the likelihood of inducing adverse autoreactive T-cells specific for Aβ42, we previously tested in wild-type mice an alternative approach for active immunization: an epitope vaccine that selectively initiate B cell responses toward an immunogenic self-epitope of Aβ in the absence of anti-Aβ T cell responses. Here, we describe a second generation epitope vaccine composed of two copies of Aβ1–11 fused with the promiscuous nonself T cell epitope, PADRE (pan human leukocyte antigen DR-binding peptide) that completely eliminates the autoreactive T cell responses and induces humoral immune responses in amyloid precursor protein transgenic 2576 mice with pre-existing AD-like pathology. Based on the titers of anti-Aβ1–11 antibody experimental mice were divided into low, moderate and high responders, and for the first time we report a positive correlation between the concentration of anti-Aβ1–11 antibody and a reduction of insoluble, cerebral Aβ plaques. The reduction of insoluble Aβ deposition was not associated with adverse events, such as CNS T cell or macrophage infiltration or microhemorrhages. Surprisingly, vaccination did not alter the levels of soluble Aβ. Alternatively, early protective immunization before substantial neuropathology, neuronal loss and cognitive deficits have become firmly established may be more beneficial and safer for potential patients, especially if they can be identified in a preclinical stage by the development of antecedent biomarkers of AD.
Keywords: immunotherapy, Alzheimer's disease, epitope vaccine, antibody, PADRE, β-amyloid
Introduction
The age-related accumulation of amyloid-β (Aβ) in the CNS has been hypothesized to play a central role in a cascade of events that eventually induces neuronal loss in affected brain regions in Alzheimer's disease (AD) (Selkoe, 1991, 1994; Hardy and Higgins, 1992; Price and Sisodia, 1994; Esler and Wolfe, 2001; Hardy and Selkoe, 2002). Previously, major support for the amyloid cascade hypothesis emerged from Aβ-immunotherapy studies demonstrated that anti-Aβ antibodies were capable of reducing AD-like pathology (Schenk et al., 1999) and improving behavior in amyloid precursor protein (APP) transgenic (Tg) mice (Chen et al., 2000; Janus et al., 2000; Morgan et al., 2000). First, immunotherapy clinical trial in AD patients using the AN-1792 vaccine containing B and T cell “self epitopes” of Aβ42 was halted during Phase IIa, when ∼6% of the participants developed aseptic meningoencephalitis (Schenk, 2002; Weiner and Selkoe, 2002; Hock et al., 2003; Nicoll et al., 2003; Orgogozo et al., 2003; Ferrer et al., 2004; Bayer et al., 2005; Fox et al., 2005; Gilman et al., 2005; Masliah et al., 2005; Patton et al., 2006). Importantly, only vaccinated participants (n = 18) developed meningoencephalitis, whereas none of the control patients (n = 72) injected with placebo developed adverse events (Orgogozo et al., 2003). Data from these trials suggest that the aseptic meningoencephalitis may have been caused by a T cell-mediated autoimmune response (Nicoll et al., 2003; Ferrer et al., 2004), and that anti-Aβ antibodies were not responsible for the observed adverse effects after active vaccination. In fact, low/moderate titers of anti-Aβ antibodies generated in a small subset of immunized patients (19.7%) were capable of reducing parenchymal amyloid pathology (Nicoll et al., 2003, 2006; Ferrer et al., 2004; Masliah et al., 2005; Patton et al., 2006; Boche et al., 2007; Nitsch and Hock, 2007) and diminishing progressive cognitive decline associated with the disease (Hock et al., 2003; Gilman et al., 2005). However, ∼80% of the immunized subjects failed to develop anti-Aβ antibody titers (“nonresponders”), indicating that the Aβ self-antigen in the AN-1792 vaccine was not a strong immunogen, thus suggesting that alternative immunotherapeutic strategies should be pursued.
Based on the results generated in mouse models of AD (Bard et al., 2000; DeMattos et al., 2001; Dodart et al., 2002), a new clinical trial, AAB-001 (Elan and Wyeth Pharmaceuticals, http://elan.com/investorrelations/events/elanwyethsymposium_adpd.asp), has been initiated by using passive transfer of a humanized monoclonal anti-Aβ antibody (bapineuzumab) in an attempt to avoid the problems associated with active immunization of elderly AD patients. However, the design of this new trial is associated with additional challenges such as multiple injections of high concentrations of anti-Aβ antibody every 13 weeks, the high cost of this monoclonal humanized antibody as well as possible side effects of passive vaccination, including microhemorrhages observed in passively immunized very old APP Tg mice (Pfeifer et al., 2002; Wilcock et al., 2004; Racke et al., 2005). This suggests that development of safe active immunotherapeutic strategies may still be desirable.
Previously, we engineered and tested a first generation epitope vaccine in wild-type mice (Agadjanyan et al., 2005), and here we report the development and testing the safety and efficacy of therapeutic vaccination of APP Tg 2576 mice with pre-existing AD-like pathology with a second generation epitope vaccine composed of two copies of the B cell epitope, Aβ1–11 in tandem with pan human leukocyte antigen DR-binding peptide (PADRE), a synthetic, foreign promiscuous T cell epitope [pre-existing AD-like pathology implies the accumulation of soluble oligomeric forms of amyloid-beta peptide leading to the impairment of cognitive functions in ≥6-month-old APP Tg 2576 mice (Lesne et al., 2006)].
Materials and Methods
Mice, epitope vaccine, peptide immunogens, and experimental protocol.
Aged (∼9.4 months old) female APP Tg 2576 mice were bred and provided by the animal facility associated with the University of California at Irvine (UCI) Alzheimer's Disease Research Center. All animals were housed in a temperature and light-cycle controlled facility, and their care was under the guidelines of the National Institutes of Health and an approved Institutional Animal Care and Use Committee protocol at University of California at Irvine.
To engineer an epitope AD vaccine, we synthesized the N terminus of an immunodominant B cell epitope of Aβ1–11 (McLaurin et al., 2002; Bard et al., 2003; Cribbs et al., 2003) in tandem with a promiscuous foreign T cell epitope, so called pan-DR epitope, PADRE (Alexander et al., 1994). The peptide 2Aβ1–11-PADRE was synthesized as a multiple antigenic peptide (MAP), containing a core matrix of 4 branching lysines (Tam, 1988; Chai et al., 1992) to generate 2Aβ1–11-PADRE-MAP (Invitrogen, Carlsbad, CA). Aβ42 peptide was synthesized at the Peptide Core Facility at the Institute for Brain Aging and Dementia at UCI by solid-phase Fmoc amino acid substitution and purified by reverse-phase high-pressure liquid chromatography.
Mice were immunized with 2Aβ1–11-PADRE-MAP or fAβ42 as described previously (Cribbs et al., 2003; Petrushina et al., 2003; Agadjanyan et al., 2005). Briefly, 2Aβ1–11-PADRE-MAP (500 μg/ml) or the fibrillar (Schenk et al., 1999; Cribbs et al., 2003) Aβ42 peptide (500 μg/ml) were mixed with Quil A, a Th1-type conventional adjuvant, and mice were injected with 50 μg of antigen subcutaneously. Experimental mice at age 9.4 ± 0.9 months old were immunized with 2Aβ1–11-PADRE-MAP (n = 21) or fAβ42 (n = 12), whereas the control group of APP Tg 2576 mice (n = 11) was injected with an adjuvant only. All mice were boosted at monthly intervals. Cellular immune responses were analyzed in five mice from each group killed at 9 d after the fourth immunization. We continued to boost the remaining mice at monthly intervals and sera was collected at 8–10 d after the third, fourth, sixth and 10th immunizations and used for detection of anti-Aβ antibodies. At the end of the study, after 10 vaccinations when animals were 19.4 ± 0.9 months old, neuropathological changes were compared across groups in response to treatment. In these animals, cellular immune responses also were analyzed.
T cell proliferation.
The analysis of T cell proliferation was performed in splenocyte cultures from individual animals, as described previously (Cribbs et al., 2003; Agadjanyan et al., 2005). In addition, CD4+ T cell proliferation was assessed using FACS assay according to the manufacturer's instructions (BD Biosciences, San Jose, CA). Briefly, to detect antigen-specific proliferation of CD4+T cells, we stained splenocyte cultures by 1 μm succinimidyl ester of carboxyfluorescein diacetate (CFSE; Invitrogen) for 10 min at 37°C. After washing, the cells were incubated for 3 d in culture media alone or with PADRE (5 μm). After incubation, the cultures were stained with phycoerythrin (PE)-labeled rat anti-mouse CD4 monoclonal antibodies (BD Biosciences). Because dead cells might fluoresce nonspecifically, these cells were excluded from the assay using a nucleic acid dye (7-amino actinomycin D from BD PharMingen, San Diego, CA), and proliferation of viable cells was analyzed by FACScan flow cytometer (BD Biosciences) as described by the manufacturer. CD4+ population was separately analyzed using CellQuest software (BD Biosciences).
Production of cytokines by immune splenocytes.
The same splenocytes used to assess T cell proliferation were used for detection of Th1 (IFNγ) or Th2 (IL4) lymphokines by ELISPOT (BD PharMingen) as described previously (Cribbs et al., 2003; Agadjanyan et al., 2005). In addition, the FACS method was used for detection of specific cytokines by CD4+ T cells (Pala et al., 2000). Briefly, the cultures of splenocytes from experimental and control animals were restimulated for 3–4 d with PADRE peptide (5 μm), and then for 4–6 h with PMA (phorbol 12-myristate 12-acetate) and ionophore (ionomycin) (both from Sigma, St. Louis, MO). In addition, we used Brefeldin A (BD Biosciences) to block cytokine secretion, which increases intracellular accumulation. Surface staining was performed using FITC-labeled anti-mouse CD4 monoclonal antibodies (MoAb; BD PharMingen). Cells were washed, fixed, permeabilized, and CD4+T cell subset producing IL-4 or IFNγ was detected using the appropriate PE-labeled anti-mouse cytokine antibodies (BD PharMingen).
Detection of anti-Aβ antibodies by ELISA.
Total anti-Aβ42 antibodies were detected as described previously (Cribbs et al., 2003; Petrushina et al., 2003) with small modifications: to develop the color reaction we used the 3,3′,5,5′ tetramethylbenzidine (TMB) peroxidase substrate (Pierce, Rockford, IL), and plates were analyzed spectrophotometrically at 450 nm. The standard curve for determination of anti-Aβ antibody concentrations in the sera was based on different known concentrations of monoclonal antibody 20.1 kindly provided by Dr. Van Nostrand (Stony Brook University, Stony Brook, NY). The concentrations of antibody were recorded in micrograms per milliliter. To determine the specific isotypes, pooled sera from mice were diluted 1:2500 and tested in duplicate. As we reported previously, mice of H2bxs immune haplotype (APP Tg 2576) do not express IgG2a, producing IgG2c anti-Aβ antibodies instead (Petrushina et al., 2003). Therefore, in our experiments we used anti-IgG2ab-specific antibodies (BD PharMingen) along with anti-IgG1-, IgG2b- and IgM- specific antibodies (Zymed, San Francisco, CA).
Detection of Aβ plaques in human brain tissues.
Sera from immunized mice were also screened for the ability to bind to Aβ plaques in the human brain as we described previously, using immunohistochemistry (Ghochikyan et al., 2003; Agadjanyan et al., 2005). A digital camera (Olympus, Tokyo, Japan) was used to capture images of the plaques at 20× magnification. The binding of antisera (dilution 1:1000) to the β-amyloid plaques was blocked by preabsorption of the sera with 5 μm Aβ1–15 peptide (1 h, 37°C).
Immunohistochemistry.
To analyze the effect of active immunization with the epitope vaccine on neuropathological changes in APP Tg 2576 mice (19.4 ± 0.9 months old), the brains were processed for immunohistochemistry and histochemistry by previously published methods (Ghochikyan et al., 2003; Agadjanyan et al., 2005). Animals were killed under deep Nembutal sodium solution (150 mg/kg, i.p.) anesthesia. To ensure proper fixation and immunostaining of brain tissues, mice were exsanguinated by transcardial perfusion with normal saline. Then brains were removed and bisected in midsagittal plane. The right hemisphere was snap frozen for biochemical analysis, whereas the left hemisphere was fixed in 4% paraformaldehyde for immunohistochemical analysis. Forty-micrometer-thick free-floating coronal sections of fixed hemibrains were collected using a vibratome. To assess the extent of neuropathology and neuroinflammation that occurs in the brains of mice, the following primary antibodies were used. Aβ deposits were detected with anti-Aβ42 (dilution, 1:2000; Invitrogen) and anti-Aβ40 (1:10000; Invitrogen). Activated microglia were detected with the anti-I-A/I-E [marker of major histocompatibility complex (MHC) II alloantigens; 1:200; BD PharMingen] and anti-CD45 (1:300; Serotec, Raleigh, NC) antibodies. Astrocytes were labeled with anti-glial fibrillary acidic protein (GFAP; 1:3000) antibodies (Eng et al., 2000). Infiltration of T cells and macrophages were analyzed using anti-CD3-ε (1:50; Santa Cruz Biotechnology, Santa Cruz, CA), anti-CD4, anti-CD8 (1:250; Novocastra Laboratories, Newcastle, UK), and anti-F4/80 (1:50; Serotec) antibodies, respectively. The tissues from all animals within a given experimental group were processed in parallel. Sections to be immunostained with anti-Aβ antibodies were pretreated in 90% formic acid for 4 min to enhance Aβ staining (Kitamoto et al., 1987). Sections for the staining with anti-CD45 and anti-I-A/I-E (anti-MHC II) were pretreated with proteinase K (0.03 mg/ml) for 5–7 min at room temperature. Hydrogen peroxide-quenched and blocked sections were incubated with primary antibody overnight at 4°C. Sections were then washed and incubated with appropriate biotinylated secondary antibodies (1 h at room temperature). After multiple washes, the tissues were incubated in ABC for 1 h, and color development was performed using DAB (3,3′-diaminobenzidine) substrate kit (Vector Laboratories, Burlingame, CA). Sections were mounted on Vectabond-coated slides (Vector Laboratories), dehydrated, and covered using DPX (BDH Laboratory Supplies, Poole, UK). Fibrillar Aβ deposits were visualized using Thioflavin S (ThS) as described by (Schmidt et al., 1995). Briefly, mouse brain sections were washed with Tris buffer and stained for 10 min with a solution of 0.5% ThS in 50% ethanol. Finally, sections were washed in 50% ethanol and Tris buffer, then dried and covered using Vectashield (Vector Laboratories).
Quantitative image analysis.
NIH imaging was used to analyze the area occupied by β-amyloid and glial reactivity as described previously (Head et al., 2001). Immunostaining was captured using a Sony (Tokyo, Japan) high-resolution CCD video camera (XC-77) and NIH image 1.59b5 software. For every animal, 12 images (525 × 410 μm each) of the frontal parietal region in the cortex at approximately the same plane (0.74 to −2.9 mm with respect to bregma) of two adjacent sections were captured with a 20× or 40× objective. The samples included six images from the superficial layer and the remaining six from the deep layer. NIH imaging was used to analyze the area occupied by β-amyloid (Aβ load) relative to the background, and expressed as the percentage of area occupied. The threshold for detection of immunoreactivity was established and then held constant throughout the image analysis. ThS-positive plaques were counted by visual inspection of cortical region of all stained sections although blind with respect to treatment condition; a mean semiquantitative score was independently determined for each slide by two observers.
Prussian blue staining for microhemorrhage.
Staining for hemosiderin deposits was performed on duplicate adjacent coronal sections of the mice brains containing similar regions located at approximately −1.5 bregma point, 50 μm thick, collected from immunized and naive, age-matched APP Tg 2576 mice. The sections were stained with Prussian blue working solution (equal parts of freshly made 5% potassium ferrocianide and 5% hydrochloric acid) for 30 min at room temperature, washed in deionized water, and counterstained with Nuclear fast red. Possible hemorrhage events in the form of the number of Prussian blue-positive profiles were counted in the brains of each mouse on all sections by two independent observers, and the average number of hemosiderin deposits was calculated per each brain hemisphere.
Biochemical analysis.
Biochemical analysis of the brain tissue was processed as described previously (Kawarabayashi et al., 2001), except that cortices of right hemispheres of brains were used. Briefly, frozen cortices were thawed, minced and then homogenized in 50 mm Tris-HCl buffer containing 2% SDS, pH 8.0, and a mixture of protease inhibitors (MP Biomedicals, Solon, OH). Homogenates were centrifuged (100,000 × g, 1 h, 4°C) and supernatants were stored at −70°C for additional analysis of soluble β-amyloid. Seventy percent formic acid was added to the pellets for extraction of SDS-insoluble β-amyloid. After sonication samples were centrifuged (100,000 × g, 1 h, 4°C) and supernatants were stored for analysis of insoluble β-amyloid. After neutralization of formic acid with 1.0 m Tris-base/0.5 m NaH2P04, concentrations of insoluble and soluble Aβ40 and Aβ42 were analyzed using β-amyloid ELISA kits (Invitrogen) according to the manufacturer's recommendations. Plates were analyzed spectrophotometrically at 450 nm via a microplate reader, and the concentrations of Aβ40 and Aβ42 were calculated using standard curves for Aβ40 and Aβ42 peptide by comparing the sample's absorbance with the absorbance of known concentrations of a standard. Using the wet weight of cortex region in the original homogenate, the final values of Aβ were expressed as micrograms per gram wet weight of cortex.
Dot blot assay and combination of IP and WB.
Soluble fractions from cortical homogenates of experimental and control mice used in ELISA were also used for dot blot assay and for both immunoprecipitation (IP) and Western blot (WB). Total protein concentration in the homogenates was determined using bicinchoninic acid assay (Pierce) and adjusted to 4 mg/ml with PBS.
Dot blot.
Two microliters of sequential dilutions of homogenates were applied to nitrocellulose membrane (GE Healthcare, Piscataway, NJ), air-dried, and blocked with 5% fat-free dry milk in TBST (10 mm Tris-HCl, pH 8.0, 150 mm NaCl, 0.05% Tween 20). Oligomers were detected by using HRP-conjugated A11 polyclonal antibody (kindly provided by Dr. C. Glabe, University of California, Irvine, Irvine, CA). Blots were developed with ECL detection system (Santa Cruz Biotechnology). Autoradiograms were scanned, and densitometry of Aβ oligomer spots was performed with NIH Image J software, version 1.36b. The relative optical density was calculated and presented as average value ±SD for each group.
IP and WB.
Aliquots of homogenates were pooled into four groups (based on low, moderate, high anti-Aβ antibody responders and control nonimmunized mice, 200 μg of total protein in each pooled aliquot), diluted to 500 μl with PBS and incubated (overnight at 4°C) with anti-Aβ 20.1 monoclonal antibody immobilized on protein G-sepharose. The beads were washed three times in PBS, and proteins were eluted in 20 μl of SDS-PAGE loading buffer by boiling. Samples were subjected to electrophoresis on 12.5% SDS-Tris polyacrylamide gel and proteins were electrotransferred to polyvinylidene difluoride membrane (GE Healthcare). The membrane was boiled for 2 min and blocked with 5% fat-free dry milk followed by detection of Aβ oligomers using anti-Aβ biotinylated 20.1 monoclonal antibody. Three experiments with the same homogenates were performed and Western blots were scanned and converted into digital files. Densitometry of Aβ oligomer bands were performed using NIH Image J software, version 1.36b and data (average ± SD) were presented from three experiments.
Statistical analysis.
All statistical parameters (mean, SD, significant difference, etc.) used in experiments were calculated using Prism 3.03 software (GraphPad Software, San Diego, CA). Statistically significant differences were examined using an ANOVA and post hoc comparisons were done using Tukey's test (p < 0.05 was considered as statistically different).
Results
The second generation epitope vaccine stimulates CD4+IFNγ+ T cells specific to PADRE without activation of autoreactive anti-Aβ T helper cells
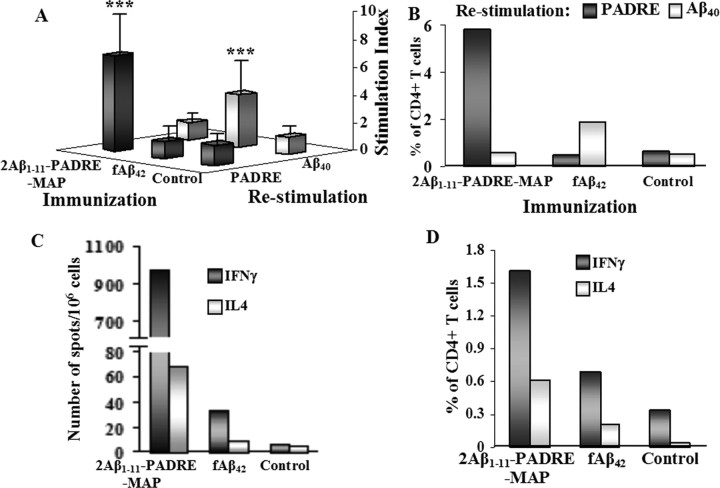
To circumvent the side effects of the AN-1792 vaccine against AD, we engineered a vaccine in which a foreign T helper cell epitope was incorporated with two copies of the B cell epitope of Aβ42. APP Tg 2576 mice vaccinated with 2Aβ1–11-PADRE-MAP induced T cell responses directed against the foreign antigenic determinant, PADRE, but not against self Aβ antigen. More specifically, splenocytes isolated from vaccinated animals proliferated after re-stimulation with PADRE, but not Aβ40 (Fig. 1A). In contrast, control APP Tg2576 mice vaccinated with fibrillar Aβ42 antigen (fAβ42) that has self B and T cell antigenic determinants induced only autoreactive T cells activated after restimulation with Aβ40 (Fig. 1A).
Figure 1.
The second generation peptide epitope vaccine induces T cell response specific to promiscuous and nonself epitope, PADRE. Splenocytes were isolated from individual mice (n = 5) after fourth immunization and restimulated in vitro with PADRE or Aβ40 peptides. A, Proliferation of splenocytes was detected by 3[H] thymidine incorporation. B, D, Proliferation of CD4+T cells (B) and production of cytokines by this T cell subset (D) were detected by flow cytometry in pooled splenocyte cultures. Production of IFNγ and IL4 cytokines by pooled immune splenocytes was detected by ELISPOT assay (C). The experiment was repeated after the 10th immunization with similar results. ***p < 0.001.
Direct measurement of activation of PADRE-specific CD4+ T helper cells in vaccinated APP Tg 2576 mice by a FACS assay confirmed these results. CD4+ T cells from mice vaccinated with the peptide epitope vaccine induced strong proliferation of this subset of T cells after restimulation with nonself PADRE peptide, but not Aβ40 (Fig. 1B). In contrast, vaccination with fAβ42 formulated in QuilA adjuvant induced proliferation of CD4+ T cells activated after re-stimulation with self-antigen Aβ40, but not with nonself T cell epitope PADRE. Of note, CD4+ T cells from mice injected with adjuvant did not proliferate after restimulation either with PADRE or Aβ40 peptides (Fig. 1B).
To further investigate T cell responses to vaccination, we analyzed Th1 (IFNγ) and Th2 (IL-4) cytokine production by splenocytes and CD4+ T cells from immunized and control APP Tg 2576 mice using ELISPOT and FACS assays, respectively (Fig. 1C,D). In these experiments, splenocytes from mice vaccinated with epitope vaccine were restimulated with PADRE, whereas splenocytes isolated from animals immunized with fAβ42 were restimulated with Aβ40. Groups of APP Tg 2576 mice injected with the epitope vaccine induced a strong IFNγ (Th1) response based on the number of splenocytes producing this lymphokine, whereas mice immunized with fAβ42 had low responses. Because we used a Th1 adjuvant in both the epitope and fAβ42 vaccinations, it was not surprising that both groups of mice generated less IL-4 (Th2) than IFNγ (Th1), whereas splenocytes from naive mice did not generate either IL4 or IFNγ cytokines (Fig. 1C). We confirmed these results by measuring the production of IL-4 or IFNγ cytokines in CD4+ T helper cells. We found that the number of PADRE-specific CD4+IFNγ+ T helper cells in mice immunized with the epitope vaccine was higher than the number of anti-Aβ CD4+IFNγ+ T cells in mice immunized with fAβ42 (Fig. 1D). The same was true for IL-4-producing anti-PADRE and anti-Aβ CD4+ T cells, although both groups had significantly lower number of CD4+T cells producing IL-4 than IFNγ (Fig. 1D). Thus, CD4+T cells and splenocytes isolated from both vaccinated groups predominantly produced a Th1-type cytokine, IFNγ, consistent with antigens being formulated in a Th1-type adjuvant, Quil A. Cellular immune responses analyzed after 4 (Fig. 1) and 10 (data not shown) immunizations showed the same specificity and profile. Collectively, the analyses of T cells demonstrated that the epitope vaccine did not activate autoreactive T cells, but stimulated nonself PADRE-specific CD4+IFNγ+T lymphocytes.
Anti-PADRE-specific CD4+IFNγ+ T lymphocytes help B cells to produce therapeutically potent anti-Aβ1–11 specific antibody in vaccinated APP Tg 2576
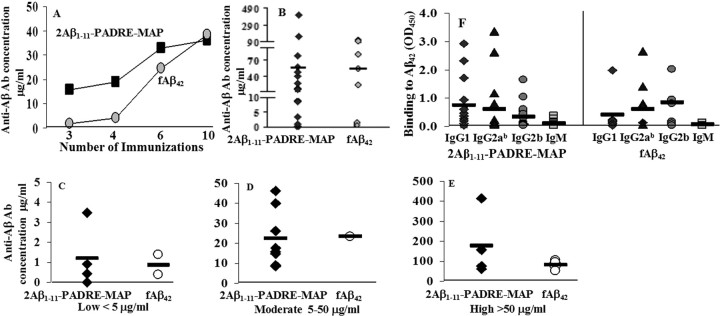
To determine the ability of activated PADRE-specific T cells to stimulate anti-Aβ B cells, we measured antibody concentrations in the sera pooled from each group of animals after three, four, six, and 10 immunizations. After three injections of the epitope vaccine the concentrations of anti-Aβ antibodies in the sera were higher than that in mice immunized with fAβ42. However, after the sixth immunization this difference in anti-Aβ antibody titers steadily diminished, becoming equal after the last two boosts in both groups (Fig. 2A). Of note, mice immunized with the Quil A adjuvant alone did not induce anti-Aβ antibodies (data not shown).
Figure 2.
The second generation epitope vaccine selectively initiates B cell responses toward an immunogenic epitope of Aβ1–11, whereas T cell help is provided by a genetically linked nonself T cell epitope, PADRE. A, B, Concentration of anti-Aβ antibody detected in the pooled sera after three, four, six, and 10 immunizations (A) or in individual animals after 10 immunizations (B). C–E, APP Tg 2576 mice immunized with epitope or fAβ42 vaccines generated low (C), moderate (D), or high (E) concentrations of anti-Aβ antibody. F, Detection of IgG1, IgG2ab, IgG2b, and IgM isotypes of anti-Aβ antibody.
As mentioned above, these data were generated with sera pooled from each of the group; however, in individual animals we found significant variability in anti-Aβ antibody responses (Fig. 2B). Concentrations of anti-Aβ1–11 antibodies after 10 immunizations were high in four mice immunized with the epitope vaccine (176.8 ± 163.56 μg/ml), whereas an additional eight and four vaccinated animals induced moderate (22.2 ± 14.16 μg/ml) and low levels (1.2 ± 1.5 μg/ml) of anti-Aβ1–11 antibodies, respectively (Fig. 2C–E). This individual variability in humoral immune responses was also detected in the group of mice immunized with fAβ42 antigen (Fig. 2C–E): four mice generated high (82.65 ± 22.68 μg/ml), one mouse had moderate (23.3 μg/ml), and two animals had low concentrations (0.9 ± 0.7 μg/ml) of anti-Aβ42 antibodies. Thus, the epitope vaccine was at least as effective as fAβ42 in induction of antibodies after 10 immunizations, but was significantly more effective at initiating the antibody immune responses (Fig. 2A).
To characterize the types of humoral immune responses from each vaccination group, we measured the production of IgG1, IgG2ab, IgG2b, and IgM anti-Aβ antibodies in the sera collected from individual animals after a total of 10 injections of the epitope vaccine or fAβ42. All mice vaccinated with the epitope vaccine generated comparable amounts of IgG1, IgG2ab, and as a result, the average of IgG1/IgG2ab ratio was close to 1 (Fig. 2F). This ratio implied that the epitope vaccine formulated in Th1 adjuvant induced a Th1 type humoral response. Similar results were observed in mice immunized with fAβ42; immunization in these animals induced anti-Aβ42 antibodies of all IgG isotypes tested, and IgG1/IgG2ab ratio was <1 (Fig. 2F). Antibodies generated by the epitope vaccine recognized human Aβ deposits when used in immunohistochemical experiments in an AD case, and this binding was blocked by preabsorption of sera with Aβ42, Aβ1–11, or 2Aβ1–11 peptides equally well (data not shown). Thus, as was expected, the epitope vaccine induced antibodies specific to the Aβ1–11 sequence of Aβ42.
The link between anti-Aβ1–11 antibody concentrations and clearance/reduction of AD-like neuropathology in the brains of APP Tg 2576 mouse model of AD
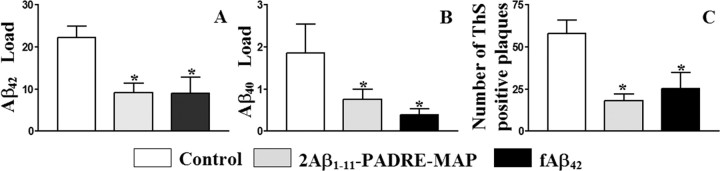
To demonstrate the therapeutic efficacy of anti-Aβ antibodies generated in response to the peptide epitope vaccine, we analyzed neuropathological changes in aged (19.4 ± 0.9 months old) experimental and control APP Tg 2576 mice (after 10 injections). Figure 3 shows a significant decrease in cortical plaque burden in APP Tg 2576 mice immunized with both the epitope vaccine and the fAβ42 antigen, compared with the control adjuvant-only injected group. More specifically, in brains of mice immunized with the epitope vaccine or fAβ42, we detected significantly less Aβ42 or Aβ40 load (diffuse and cored plaques) than in brains of control animals (Fig. 3A,B). Additionally, we demonstrated significant reduction of ThS-positive Aβ deposits (cored plaques) in the brains of experimental mice versus controls (Fig. 3C). Collectively, these results demonstrate that immunization with epitope vaccine is effective in reducing cerebral Aβ burden in APP Tg 2576 mice, which were ∼9 months old at the start of the study.
Figure 3.
A–C, The second generation epitope vaccine inhibits Aβ deposition in the brains of 19.4 ± 0.9-month-old APP Tg 2576 mice. Image analysis of Aβ42 (A), Aβ40 (B), and Thioflavin S (C) staining in cortex of mice vaccinated with epitope vaccine, fAβ42, or control mice. Vaccinated mice showed a significant reduction in Aβ42/40 load (cored and diffuse) and in the number of ThS-positive (cored) plaques (*p < 0.05) compared with the control animals. Error bars represent the average ± SE for n = 6 in the group of control mice, and n = 16 and n = 7 in the groups of mice vaccinated with epitope and fAβ42 vaccines, respectively.
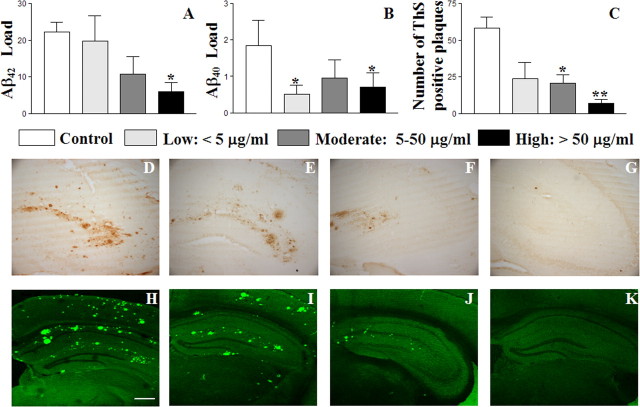
As described previously, data from the first human clinical trial indicated that AD patients responding to the AN-1792 vaccine and producing anti-Aβ antibody titers over 1:2200 showed some slowing of cognitive decline and localized reduction of plaques (Hock et al., 2003; Gilman et al., 2005; Masliah et al., 2005; Nicoll et al., 2006). To address this question in our preclinical trials, we grouped all mice vaccinated with the epitope vaccine based on the concentrations of generated anti-Aβ antibodies (Fig. 2C–E), and analyzed the levels of soluble and insoluble Aβ in the brains of experimental and control animals. The rationale for using Aβ as an outcome measure reflecting possible functional improvements was based on previous work showing that Aβ is correlated with behavior in APP Tg 2576 mice (Hsiao et al., 1996; Westerman et al., 2002) and that vaccination of APP Tg mice with fAβ42 leads to behavioral improvements and reduced Aβ (Chen et al., 2000; Janus et al., 2000; Morgan et al., 2000; Das et al., 2001, 2003). We observed a positive correlation between the concentration of anti-Aβ1–11 antibodies and reduction of cortical Aβ42 load in the brains of vaccinated APP Tg 2576 mice (coefficient of correlation is −0.83, p < 0.05). As shown in Figure 4A, there are no differences in Aβ42 load between the group of mice with low concentrations of anti-Aβ1–11 antibodies and control mice. Although Aβ42 load is noticeably reduced in the brains of mice with moderate concentration, this reduction was not significant, whereas in the mice with a high concentration of serum anti-Aβ1–11 antibodies Aβ42 load was reduced significantly (p = 0.018). Immunostaining of adjacent brain sections with anti-Aβ40-specific antibodies revealed a marked reduction of Aβ40 load in all groups of experimental mice versus the controls, independent of serum antibody concentrations (Fig. 4B). However, ThS staining also showed positive correlation between the concentrations of anti-Aβ1–11 antibodies and reduction of cortical cored plaques (Fig. 4C), which in APP Tg 2576 mice contain both Aβ40/42 peptides (Kawarabayashi et al., 2001). Similar effects were observed in the hippocampal regions of vaccinated APP Tg 2576 mice stained with anti-Aβ42. Representative images of hippocampal regions stained with anti Aβ42-specific monoclonal antibodies (D-G) or ThS (H-K) are shown in Figure 4D–K.
Figure 4.
A–C, The anti-Aβ antibody concentration positively correlates with the reduction of Aβ42 and Thioflavin S, but not Aβ40 load in the cortex areas of APP Tg 2576 mice immunized with epitope vaccine. Error bars represent the average ± SE for control mice (n = 6) and mice with low (n = 4), moderate (n = 8), and high (n = 4) anti-Aβ antibody concentrations. *p < 0.05; **p < 0.01). D–K, The same results were obtained after staining of hippocampal regions with anti-Aβ42-specific monoclonal antibody (D–G) or Thioflavin S (H–K). Representative images of hippocampal regions of control mice (D, H) and mice with low (E, I), moderate (F, J), and high (G, K) anti-Aβ antibody concentrations. Original magnifications, 4×. Scale bar, 500 μm.
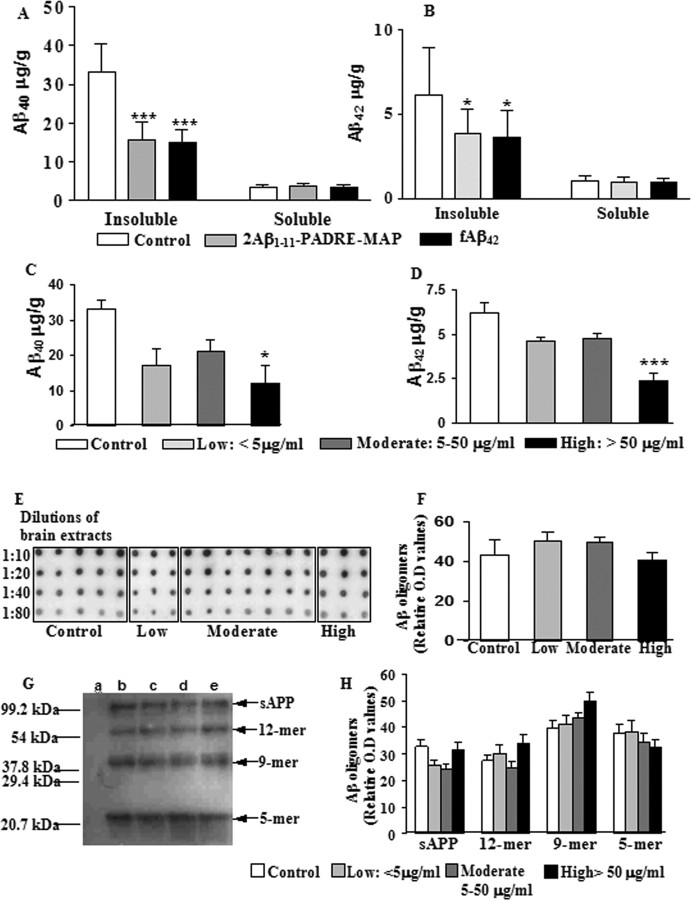
To further confirm an association between serum antibody concentrations and differential effects on reducing Aβ40 or Aβ42, we measured insoluble Aβ40 and Aβ42 in cortical homogenates of experimental and control mice by sandwich ELISA. Insoluble Aβ40 and Aβ42 concentrations were significantly lower in the cortex of mice vaccinated with the epitope vaccine or fAβ42, compared with control animals (Fig. 5A,B) (p < 0.001 and p < 0.05, respectively). However, when we compared Aβ levels in groups with low, moderate, and high titers of antibodies, we found a significant reduction of Aβ40 and Aβ42 only in mice with anti-Aβ1–11 antibody levels >50 μg/ml (Fig. 5C,D) (p < 0.05 and p < 0.001, respectively). Collectively, these results demonstrate that high concentrations of anti-Aβ antibody are critical for effective reduction of insoluble Aβ in APP Tg 2576 mice with pre-existing AD-like pathology.
Figure 5.
The reduction of insoluble, but not soluble Aβ40 and Aβ42 levels are positively correlated with the concentrations of anti-Aβ1–11 antibody. A, B, A significant decrease in the total (parenchymal and vascular) levels of insoluble Aβ40 and Aβ42 in cortical homogenates was observed after epitope vaccine and fAβ42 immunization. C, D, The significant reductions in insoluble Aβ40 and Aβ42 levels were observed only in groups with high titers of antibodies. *p < 0.05 and ***p < 0.001. However, the levels of soluble Aβ40 and Aβ42 in cortical homogenates did not differ between experimental and control groups (A, B). E–H, The level of oligomeric forms of Aβ detected in cortical homogenates using dot blot (E, F) or by combination of IP with WB (G, H) also was not changed after vaccination. In dot blot assay, homogenates from individual animals were diluted and analyzed using A11 oligomer-specific antibody (E), and relative optical density (F) was presented as the average ± SD. Pooled homogenates of each group of mice were analyzed by IP/WB (G), and no significant difference between the density and thickness of oligomeric bands (H) was detected (with the average ± SD calculated from three independent experiments). Lanes in WB: a, wild-type; b, control; c, low, <5 μg/ml; d, moderate, 5–50 μg/ml; e, high, > 50 μg/ml.
A potential problem of immunotherapy is that reduction of insoluble Aβ may lead to increased levels of soluble forms of this peptide (Patton et al., 2006), possibly oligomers, which are known to be more neurotoxic and can impair cognitive function (Klein et al., 2001; Gong et al., 2003; Cleary et al., 2005; Klyubin et al., 2005; Lesne et al., 2006). Accordingly, we measured soluble Aβ40 and Aβ42 levels in detergent-extracted samples of cortical homogenates of control mice and animals vaccinated with epitope vaccine or fAβ42 by capture ELISA (Fig. 5A,B). There were no statistically significant differences between experimental and control mice. In addition, we evaluated the levels of Aβ oligomers in the cortical homogenate by dot blot using A11 anti-oligomeric antibodies (Kayed et al., 2003). All available homogenates from low (n = 3), moderate (n = 7), and high (n = 3) responders as well as control (n = 5) mice were used in these experiments. No differences were found between all three groups of immunized mice as well as between vaccinated and control animals (Fig. 5E,F). Of note, no oligomers were detected in brain homogenates from wild-type mice (data not shown). To confirm these results and to measure the relative levels of high molecular weight (>5- to 6-mers) oligomers, the cortical homogenates were subjected to IP followed by WB. The levels of Aβ 5-mers, 9-mer, and 12-mer oligomers in detergent-extracted samples of cortical homogenates obtained from experimental and control mice did not significantly differ, and wild-type mice did not deposit Aβ-reactive oligomers (Fig. 5G,H). Therefore, the level of oligomeric, toxic forms of Aβ is not reduced in the brain of APP Tg 2576 mice at least when vaccination was initiated in mice with pre-existing AD-like pathology. These differences between reducing insoluble and soluble Aβ42 in vivo are not connected to the differences in binding affinity of anti-Aβ1–11 antibodies to these forms of β-amyloid as we previously demonstrated (Mamikonyan et al., 2007).
The effect of vaccination on microglial activation, lymphocyte infiltration, and microhemorrhages
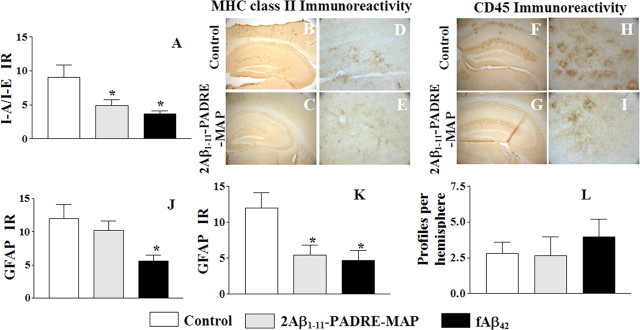
To detect inflammation-related pathology in the brains of animals immunized with the epitope vaccine, the same brain regions used for Aβ burden studies were evaluated for microglial activation (MHC class II, CD45), astrocyte hypertrophy (GFAP), and microhemorrhages in blood vessels (Prussian blue). Quantitative image analysis of MHC class II (I-Ab/I-Eb) positive cells demonstrated significantly decreased microglial activation in APP Tg 2576 mice vaccinated with the epitope vaccine, and this decrease was comparable with that seen in the cerebral cortex of mice immunized with fAβ42 (Fig. 6A). Representative MHC class II immunoreactivity is shown for mice vaccinated with the epitope vaccine for comparison with control animals (Fig. 6B–E). These data are similar to the results obtained after immunostaining of the brain tissues with anti-CD45 antibodies (Fig. 6F–I). Thus, immunization with the epitope vaccine decreased microglial activation in the brains of aged APP Tg 2576 mice.
Figure 6.
The second generation epitope vaccine reduces glial activation without increasing brain microhemorrhages. A, Image analysis of cortex areas from vaccinated or control mice was performed after staining with anti-I-A/I-E antibody. Vaccination either with the epitope vaccine, or fAβ42 decreased microglial activation in cortical region of immune mice (*p < 0.05) compared with that in control mice. B–I, Representative pictures of brain sections stained with anti-I-A/I-E (B–E) and anti-CD45 (F–I) antibodies showing a decreased immunoreactivity also in the hippocampal brain regions in mice immunized with the epitope vaccine (C, E, G, I) compared with control animals (B, D, F, H). Original magnifications: B, C, F, G, 5×; D, E, H, I, 40×. J, K, Image analysis of cortical regions from vaccinated or control mice was performed after staining with anti-GFAP antibody. Astrocytosis was significantly (*p < 0.05) decreased in mice immunized with fAβ42. A reduction in GFAP load was slightly reduced in all mice vaccinated with the epitope vaccine, but it was significant only in mice with high concentration anti-Aβ antibody. L, The number of Prussian blue-positive profiles in the brain hemispheres of experimental and control mice did not differ significantly.
Astrocytes in both experimental and control mice were detected using the astrocyte-specific marker (GFAP) and a quantitative image analysis indicated that vaccinated mice had a lesser degree of astrocytosis compared with the control group. However, immunization with epitope vaccine led to a 14% reduction, whereas immunization with fAβ42 resulted in ∼53% reduction in GFAP immunoreactivity (Fig. 6J). Importantly, immunization with the epitope vaccine reduced astrocytosis to ∼50% of the mice with high sera concentrations of antibodies and this decrease is statistically significant (Fig. 6K).
Previously, it was reported that very old APP Tg mice receiving passive transfer of anti-Aβmonoclonal antibodies developed cerebral microhemorrhages (Pfeifer et al., 2002; Wilcock et al., 2004). More recently, it was shown that cerebral amyloid angiopathy-associated microhemorrhages are increased in ∼20-month-old APP+presenilin 1 (PS1) transgenic mice immunized eight times with fAβ42 formulated in complete Freunds adjuvant (CFA)/incomplete Freunds adjuvant (IFA) (Wilcock et al., 2007). To assess this potential adverse side effect of epitope vaccine, we selected adjacent coronal sections of mouse brains located at −1.5 bregma and visualized microhemorrhages by detecting hemosiderin (byproduct of degradation of hemoglobin, occurring at the sites of previous microhemorrhages) deposits using Prussian blue staining. Characteristic blue hemosiderin-positive profiles were observed primarily in the neocortical, leptomeningeal, hippocampal and thalamic areas of the brain. These microhemorrhages were detected at a rate of 2.8 ± 0.8 per hemibrain in control nonimmunized mice and 2.6 ± 1.33 in animals immunized with the epitope vaccine. However, vaccination of a group of APP Tg 2576 mice with fAβ42 had resulted in 3.97 ± 1.24 hemosiderin-positive profiles. Although not statistically significant, there was nonetheless approximately a 41% increase compared with the control group, and these data resemble the results generated by (Wilcock et al., 2007) with APP+PS1 aged mice. Thus, immunization with the second generation epitope vaccine did not increase the incidence of cerebral microhemorrhages in ∼19-month-old APP Tg 2576 mice (Fig. 6L). Although there is only one unconfirmed report about lymphocyte infiltration detected in the brains of wild-type mice immunized with Aβ42 formulated in CFA/IFA followed by injection with pertussis toxin (Furlan et al., 2003), we also investigated whether immunization with the epitope vaccine formulated in Th1 adjuvant might also induce T cell infiltration into the brain. Our standard methodology for detecting T cells in brain vessels of mice (Ghochikyan et al., 2003) did not identify CD3-, CD4-, and CD8-positive T cells or F4/80-positive macrophages in the brains of mice immunized with our second generation epitope vaccine (data not shown). Thus, reduction of insoluble Aβ deposition in immunized APP Tg mice was not associated with deleterious side effects, including cerebral inflammation and microhemorrhages.
Discussion
Published results from the recently halted AN-1792 clinical trial in patients with AD suggested that aseptic meningoencephalitis was linked to an adverse T cell-mediated autoimmune response (Nicoll et al., 2003; Ferrer et al., 2004). Presence of anti-Aβ antibodies correlated with effective reduction of Aβ pathology in patients that came to autopsy (Nicoll et al., 2003; Ferrer et al., 2004; Masliah et al., 2005), suggesting a possible therapeutic benefit of vaccination (Bayer et al., 2005; Fox et al., 2005; Gilman et al., 2005). We analyzed the effect of different concentrations of anti-Aβ antibodies in serum on neuropathological changes in the brains of actively immunized APP Tg 2576 mice in the absence of an autoreactive anti-Aβ T cell response. We have obtained results somewhat similar to data described in the AN-1792 clinical trial: (1) the higher the concentrations of anti-Aβ antibody specific to the N terminus of Aβ42, the larger the therapeutic effect. The NTB composite z-scores were regressed on the geometric mean antibody titers, although this correlation was not significant (Gilman et al., 2005). (2) AN-1792 vaccine reduced the number of Aβ plaques, but not the level of soluble Aβ in the brains (Patton et al., 2006). To circumvent the side effects of the AN-1792 vaccine and to reduce the potential for cell-mediated autoimmune toxicity in AD patients, we engineered the first (Agadjanyan et al., 2005) generation of epitope vaccine and tested it in wild-type mice. Here, we report on design and testing of the second generation peptide epitope vaccine in APP Tg 2576 mouse model (Hsiao et al., 1996; Kawarabayashi et al., 2001; Westerman et al., 2002), and demonstrated that this prototype AD vaccine induced strong anti-PADRE cellular and anti-Aβ humoral responses (Figs. 1, 2). If this proves to be predictive for human trials and vaccinated individuals induce strong anti-Aβ antibody responses without generating potentially harmful autoreactive T helper cells, our epitope vaccine approach may represent a reasonable alternative to a passive vaccination strategy with humanized anti-Aβ antibody.
The AN-1792 clinical trial data demonstrated significant individual variability in anti-Aβ antibody responses in vaccinated AD patients (Bayer et al., 2005; Gilman et al., 2005). As mentioned by Patton et al. (2006), only 59 individuals in the immunized cohort induced desirable antibody titers to immunization with fAβ42. Importantly, some of these AD patients showed a trend toward slowing of cognitive decline associated with the disease (Hock et al., 2003), improvement in the memory domain of the NTB and the decreased CSF tau levels (Gilman et al., 2005). Collectively, these data suggested that higher titers of antibodies might be beneficial for AD patients. Because the concentrations of anti-Aβ antibodies in individual APP Tg 2576 mice vaccinated with our epitope vaccine were not uniform either (Fig. 2B) we were able to analyze the association between the neuropathological changes in vaccinated APP Tg 2576 mice with the concentration of anti-Aβ antibodies in the sera of these animals. We observed that the concentration of anti-Aβ antibodies was associated with a therapeutic effect in APP Tg 2576 mice (Fig. 4). Our data also indicated that high concentrations of anti-Aβ antibodies are critical, selectively for the reduction of Aβ42 deposits, whereas Aβ40 deposits and cored plaques are more responsive to Aβimmunotherapy overall and can be cleared even with low levels of anti-Aβantibodies (Fig. 4). Previous data with APP Tg mice have demonstrated that although Aβ plaques were reduced, the total level of soluble and insoluble Aβ did not differ between immunized and control mice (Janus et al., 2000). Clinical studies demonstrated that immunizations with AN-1792 vaccine mobilize parenchymal plaques and reduce the level of insoluble Aβ, but increase the level of vascular and soluble β-amyloid (Masliah et al., 2005; Nicoll et al., 2006; Patton et al., 2006). These data suggest that the increased level of soluble Aβ may produce conditions that favor formation of toxic oligomeric species of Aβ. To address this issue, we analyzed the levels of soluble and insoluble Aβ40 and Aβ42 in cortex homogenates obtained from immunized and control APP Tg 2576 mice and demonstrated that vaccination significantly decreased the levels of insoluble Aβ40 and Aβ42. Importantly, vaccination did not increase the levels of the more toxic soluble Aβ in mouse model of AD (Fig. 5A,B) in contrast to data reported with the AN-1792 vaccine (Patton et al., 2006). Additionally, we demonstrated that only the high concentrations of anti-Aβ antibodies significantly decrease both insoluble Aβ40 and Aβ42 in cortical tissues of experimental mice (Fig. 5C,D). Thus, our vaccine did not affect the levels of soluble Aβ40 and Aβ42 in the brains of mice with pre-existing AD-like pathology. Previously it was demonstrated that Aβ neurotoxicity requires insoluble fibril formation (Loo et al., 1993; Lorenzo and Yankner, 1994). However, emphasis has shifted to soluble oligomers as pathological species of Aβ42 (Gong et al., 2003; Cleary et al., 2005; Klyubin et al., 2005; Lesne et al., 2006), although aggregation-related toxicity was reported almost a decade earlier (Pike et al., 1991). In APP Tg 2576 mice, memory deficits are first detected in 6-month-old mice accumulating 12-mer oligomers (Lesne et al., 2006) and before overt plaque deposition. Accordingly, we analyzed the levels of oligomers in the brains of APP Tg 2576 mice using an anti-oligomeric antibody (A11), and have demonstrated that immunization with the epitope vaccine did not induce a reduction in immunized compared with control animals (Fig. 5E,F). We further confirmed these by dot blot experiments using Aβ fractions immunoprecipitated from cortical homogenates and then detecting oligomers by Western blotting. Of note, in these experiments we used 12.5% SDS-Tris polyacrylamide gel that allowed the detection of oligomers ≥20 kDA. The levels of Aβ oligomers in the brains of experimental and control mice were not significantly different and no oligomers were detected in wild-type mice (Fig. 5G, lane a). These results suggest that either higher concentration of anti-Aβ antibodies are needed to significantly reduce oligomeric Aβ in brains of immunized mice, or more likely that immunization should be initiated at an earlier age, in mice either without pre-existing Aβ pathology or with early-stage AD-like pathology. Whereas our ongoing prevention immunization studies with the DNA epitope vaccine may allow us to address these hypotheses, we need to mention that multiple transcutaneous immunizations of young PSAPP mice without pre-existing AD-like pathology with fAβ42 and cholera toxin generated high titers of anti-Aβ antibodies (>250 μg/ml) that reduced the levels of both insoluble and soluble cerebral Aβ detected in aged mice (Nikolic et al., 2007). Interestingly, analysis from two cases from the AN-1792 clinical trial report also suggest that “anti-amyloid immunization may be most effective not as therapeutic or mitigating measures, but as a prophylactic measure when Aβ deposition is still minimal” (Patton et al., 2006).
It is difficult to predict the effects of frequent passive delivery of high concentrations of humanized anti-Aβ antibodies in ongoing passive immunotherapy trials with AD patients because this treatment approach may still induce undesirable side effects, for example microhemorrhages. Although these clinical studies are important for the future of Aβ immunotherapy, an effective active immunization approach is still feasible providing that the AD vaccine is safe, induces an adequate antibody response to important B cell epitope of Aβ, and is free of harmful autoreactive T cell responses. The second generation epitope vaccine described in this study induced peripheral anti-PADRE-specific Th1-type proinflammatory responses without infiltration of T cells or macrophages in the brains of vaccinated APP Tg2576 mice. It is known that AN-1792 vaccine caused an increase in cerebral vasculature deposition of Aβ (Masliah et al., 2005; Nicoll et al., 2006; Patton et al., 2006). Previously it was also shown that active Aβ42 vaccination of double transgenic (APP+PS1) mice resulted in significantly increased cerebral amyloid angiopathy and associated microhemorrhages (Wilcock et al., 2007). Although we did not directly investigate the effect of epitope vaccine on Aβ deposition in cerebral vasculature, we demonstrated that anti-Aβ1–11 antibodies did not increase the incidence of cerebral microhemorrhages in the brains of immunized mice (Fig. 6L). Thus, we suggest that an epitope vaccine could be used as a safe and effective measure for the treatment of people with early preclinical stage AD especially if they can be diagnosed by measuring Tau/Aβ and pTau/Aβ ratio in CSF (de Jong et al., 2006; Fagan et al., 2007a,b) and/or detecting of accumulation of Aβ in the brains using Pittsburgh Compound-B positron emission tomography scan (Klunk et al., 2004).
Footnotes
This work was supported by National Institutes of Health Grants AG20241 and NS50895 (D.H.C.) and Alzheimer's Association Grant IIRG-03-6279 (M.G.A.). Additional support for mice and the production of peptides was provided by University of California, Irvive Alzheimer's Disease Research Center Grant P50 AG16573. Dr. Nina Movsesyan was supported by National Institute on Aging training Grant AG00096. We thank Adrine Karapetyan for technical help and valuable comments.
References
- Agadjanyan MG, Ghochikyan A, Petrushina I, Vasilevko V, Movsesyan N, Mkrtichyan M, Saing T, Cribbs DH. Prototype Alzheimer's disease vaccine using the immunodominant B cell epitope from beta-amyloid and promiscuous T cell epitope pan HLA DR-binding peptide. J Immunol. 2005;174:1580–1586. doi: 10.4049/jimmunol.174.3.1580. [DOI] [PubMed] [Google Scholar]
- Alexander J, Sidney J, Southwood S, Ruppert J, Oseroff C, Maewal A, Snoke K, Serra HM, Kubo RT, Sette A, Grey HM. Development of high potency universal DR-restricted helper epitopes by modification of high affinity DR-blocking peptides. Immunity. 1994;1:751–761. doi: 10.1016/s1074-7613(94)80017-0. [DOI] [PubMed] [Google Scholar]
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- Bard F, Barbour R, Cannon C, Carretto R, Fox M, Games D, Guido T, Hoenow K, Hu K, Johnson-Wood K, Khan K, Kholodenko D, Lee C, Lee M, Motter R, Nguyen M, Reed A, Schenk D, Tang P, Vasquez N, et al. Epitope and isotype specificities of antibodies to beta-amyloid peptide for protection against Alzheimer's disease-like neuropathology. Proc Natl Acad Sci USA. 2003;100:2023–2028. doi: 10.1073/pnas.0436286100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer AJ, Bullock R, Jones RW, Wilkinson D, Paterson KR, Jenkins L, Millais SB, Donoghue S. Evaluation of the safety and immunogenicity of synthetic Abeta42 (AN1792) in patients with AD. Neurology. 2005;64:94–101. doi: 10.1212/01.WNL.0000148604.77591.67. [DOI] [PubMed] [Google Scholar]
- Boche D, DeBeer S, Cox A, Wilkinson D, Holmes C, Neal J, Love S, Esiri M, Bridges L, Weller R, Nicoll JA. Eighth International Conference Alzheimer's and Parkinson's Disease: Progress and New Perspectives. Salzburg, Austria: Kenes; 2007. Evidence of a transient increase in cerebral amyloid angiopathy after Abeta42 immunization in human Alzheimer's disease. [Google Scholar]
- Chai SK, Clavijo P, Tam JP, Zavala F. Immunogenic properties of multiple antigen peptide systems containing defined T and B epitopes. J Immunol. 1992;149:2385–2390. [PubMed] [Google Scholar]
- Chen G, Chen KS, Knox J, Inglis J, Bernard A, Martin SJ, Justice A, McConlogue L, Games D, Freedman SB, Morris RGM. A learning deficit related to age and beta-amyloid plaques in a mouse model of Alzheimer's disease. Nature. 2000;408:975–979. doi: 10.1038/35050103. [DOI] [PubMed] [Google Scholar]
- Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- Cribbs DH, Ghochikyan A, Tran M, Vasilevko V, Petrushina I, Sadzikava N, Kesslak P, Kieber-Emmons T, Cotman CW, Agadjanyan MG. Adjuvant-dependent modulation of Th1 and Th2 responses to immunization with beta-amyloid. Int Immunol. 2003;15:505–514. doi: 10.1093/intimm/dxg049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P, Murphy MP, Younkin LH, Younkin SG, Golde TE. Reduced effectiveness of Abeta1–42 immunization in APP transgenic mice with significant amyloid deposition. Neurobiol Aging. 2001;22:721–727. doi: 10.1016/s0197-4580(01)00245-7. [DOI] [PubMed] [Google Scholar]
- Das P, Howard V, Loosbrock N, Dickson D, Murphy MP, Golde TE. Amyloid-β immunization effectively reduces amyloid deposition in FcRγ−/− knock-out mice. J Neurosci. 2003;23:8532–8538. doi: 10.1523/JNEUROSCI.23-24-08532.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong D, Jansen RW, Kremer BP, Verbeek MM. Cerebrospinal fluid amyloid beta42/phosphorylated tau ratio discriminates between Alzheimer's disease and vascular dementia. J Gerontol A Biol Sci Med Sci. 2006;61:755–758. doi: 10.1093/gerona/61.7.755. [DOI] [PubMed] [Google Scholar]
- DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer's disease. Proc Natl Acad Sci USA. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodart JC, Bales KR, Gannon KS, Greene SJ, DeMattos RB, Mathis C, DeLong CA, Wu S, Wu X, Holtzman DM, Paul SM. Immunization reverses memory deficits without reducing brain Aβ burden in Alzheimer's disease model. Nat Neurosci. 2002;5:452–457. doi: 10.1038/nn842. [DOI] [PubMed] [Google Scholar]
- Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000) Neurochem Res. 2000;25:1439–1451. doi: 10.1023/a:1007677003387. [DOI] [PubMed] [Google Scholar]
- Esler WP, Wolfe MS. A portrait of Alzheimer secretases-new features and familiar faces. Science. 2001;293:1449–1454. doi: 10.1126/science.1064638. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Eighth International Conference Alzheimer's and Parkinson's Disease: Progress and New Perspectives. Salzburg, Austria: Kenes; 2007a. Cerebrospinal fluid biomarkers of early stage Alzheimer disease. [Google Scholar]
- Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007b;64:343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Rovira MB, Guerra ML, Rey MJ, Costa-Jussa F. Neuropathology and pathogenesis of encephalitis following amyloid-beta immunization in Alzheimer's disease. Brain Pathol. 2004;14:11–20. doi: 10.1111/j.1750-3639.2004.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NC, Black RS, Gilman S, Rossor MN, Griffith SG, Jenkins L, Koller M. Effects of Abeta immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology. 2005;64:1563–1572. doi: 10.1212/01.WNL.0000159743.08996.99. [DOI] [PubMed] [Google Scholar]
- Furlan R, Brambilla E, Sanvito F, Roccatagliata L, Olivieri S, Bergami A, Pluchino S, Uccelli A, Comi G, Martino G. Vaccination with amyloid-beta peptide induces autoimmune encephalomyelitis in C57/BL6 mice. Brain. 2003;126:285–291. doi: 10.1093/brain/awg031. [DOI] [PubMed] [Google Scholar]
- Ghochikyan A, Vasilevko V, Petrushina I, Tran M, Sadzikava N, Babikyan D, Movsesyan N, Tian W, Ross TM, Cribbs DH, Agadjanyan MG. Generation and chracterization of the humoral immune response to DNA immunization with a chimeric β-amyloid-interleukin-4 minigene. Eur J Immunol. 2003;33:3232–3241. doi: 10.1002/eji.200324000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, Eisner L, Kirby L, Rovira MB, Forette F, Orgogozo JM. Clinical effects of Aβ immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- Gong Y, Chang L, Viola KL, Lacor PN, Lambert MP, Finch CE, Krafft GA, Klein WL. Alzheimer's disease-affected brain: presence of oligomeric A beta ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proc Natl Acad Sci USA. 2003;100:10417–10422. doi: 10.1073/pnas.1834302100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Head E, Garzon-Rodriguez W, Johnson JK, Lott IT, Cotman CW, Glabe C. Oxidation of Aβ and plaque biogenesis in Alzheimer's disease and Down syndrome. Neurobiol Dis. 2001;8:792–806. doi: 10.1006/nbdi.2001.0431. [DOI] [PubMed] [Google Scholar]
- Hock C, Konietzko U, Streffer JR, Tracy J, Signorell A, Muller-Tillmanns B, Lemke U, Henke K, Moritz E, Garcia E, Wollmer MA, Umbricht D, de Quervain DJ, Hofmann M, Maddalena A, Papassotiropoulos A, Nitsch RM. Antibodies against beta-amyloid slow cognitive decline in Alzheimer's disease. Neuron. 2003;38:547–554. doi: 10.1016/s0896-6273(03)00294-0. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, Schmidt SD, Chishti MA, Horne P, Heslin D, French J, Mount HT, Nixon RA, Mercken M, Bergeron C, Fraser PE, St George-Hyslop P, Westaway D. A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer's disease. Nature. 2000;408:979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. Age-dependent changes in brain, CSF, and plasma amyloid β protein in the Tg2576 transgenic mouse model of Alzheimer's disease. J Neurosci. 2001;21:372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- Kitamoto T, Ogomori K, Tateishi J, Prusiner SB. Formic acid pretreatment enhances immunostaining of cerebral and systemic amyloids. Lab Invest. 1987;57:230–236. [PubMed] [Google Scholar]
- Klein WL, Krafft GA, Finch CE. Targeting small Abeta oligomers: the solution to an Alzheimer's disease conundrum? Trends Neurosci. 2001;24:219–224. doi: 10.1016/s0166-2236(00)01749-5. [DOI] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang GF, Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Langstrom B. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Klyubin I, Walsh DM, Lemere CA, Cullen WK, Shankar GM, Betts V, Spooner ET, Jiang L, Anwyl R, Selkoe DJ, Rowan MJ. Amyloid beta protein immunotherapy neutralizes Abeta oligomers that disrupt synaptic plasticity in vivo. Nat Med. 2005;11:556–561. doi: 10.1038/nm1234. [DOI] [PubMed] [Google Scholar]
- Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- Loo DT, Copani A, Pike CJ, Whittemore ER, Walencewicz AJ, Cotman CW. Apoptosis is induced by beta-amyloid in cultured central nervous system neurons. Proc Natl Acad Sci USA. 1993;90:7951–7955. doi: 10.1073/pnas.90.17.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo A, Yankner BA. Beta-amyloid neurotoxicity requires fibril formation and is inhibited by congo red. Proc Natl Acad Sci USA. 1994;91:12243–12247. doi: 10.1073/pnas.91.25.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamikonyan G, Necula M, Mkrtichyan M, Ghochikyan A, Petrushina I, Movsesyan N, Mina E, Kiyatkin A, Glabe C, Cribbs DH, Agadjanyan MG. Anti-Abeta 1–11 antibody binds to different beta-amyloid species, inhibits fibril formation, and disaggregates preformed fibrils, but not the most toxic oligomers. J Biol Chem. 2007;282:22376–22386. doi: 10.1074/jbc.M700088200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Hansen L, Adame A, Crews L, Bard F, Lee C, Seubert P, Games D, Kirby L, Schenk D. Abeta vaccination effects on plaque pathology in the absence of encephalitis in Alzheimer disease. Neurology. 2005;64:129–131. doi: 10.1212/01.WNL.0000148590.39911.DF. [DOI] [PubMed] [Google Scholar]
- McLaurin J, Cecal R, Kierstead ME, Tian X, Phinney AL, Manea M, French JE, Lambermon MH, Darabie AA, Brown ME, Janus C, Chishti MA, Horne P, Westaway D, Fraser PE, Mount HT, Przybylski M, St George-Hyslop P. Therapeutically effective antibodies against amyloid-beta peptide target amyloid-beta residues 4–10 and inhibit cytotoxicity and fibrillogenesis. Nat Med. 2002;8:1263–1269. doi: 10.1038/nm790. [DOI] [PubMed] [Google Scholar]
- Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, Duff K, Jantzen P, DiCarlo G, Wilcock D, Connor K, Hatcher J, Hope C, Gordon M, Arendash GW. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer's disease. Nature. 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- Nikolic WV, Bai Y, Obregon D, Hou H, Mori T, Zeng J, Ehrhart J, Shytle RD, Giunta B, Morgan D, Town T, Tan T. Transcutaneous β-amyloid peptide immunization of transgenic Alzheimer's mice results in reduced cerebral b-amyloid deposits in the absence of T cell infiltration and microhemorrhage. Proc Natl Acad Sci USA. 2007;104:2507–2512. doi: 10.1073/pnas.0609377104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO, et al. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat Med. 2003;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- Nicoll JA, Barton E, Boche D, Neal JW, Ferrer I, Thompson P, Vlachouli C, Wilkinson D, Bayer A, Games D, Seubert P, Schenk D, Holmes C. Abeta species removal after abeta42 immunization. J Neuropathol Exp Neurol. 2006;65:1040–1048. doi: 10.1097/01.jnen.0000240466.10758.ce. [DOI] [PubMed] [Google Scholar]
- Nitsch RM, Hock C. Eighth International Conference Alzheimer's and Parkinson's Disease: Progress and New Perspectives. Salzburg, Austria: Kenes; 2007. Immunotherapy against beta-amyloid in Alzheimer's disease. [Google Scholar]
- Orgogozo JM, Gilman S, Dartigues JM, Laurent B, Puel M, Kirby LC, Jouanny P, Dubois B, Eisner L, Flitman S, Michel BF, Boada M, Frank A, Hock C. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003;61:46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- Pala P, Hussell T, Openshaw PJ. Flow cytometric measurement of intracellular cytokines. J Immunol Methods. 2000;243:107–124. doi: 10.1016/s0022-1759(00)00230-1. [DOI] [PubMed] [Google Scholar]
- Patton RL, Kalback WM, Esh CL, Kokjohn TA, Van Vickle GD, Luehrs DC, Kuo YM, Lopez J, Brune D, Ferrer I, Masliah E, Newel AJ, Beach TG, Castano EM, Roher AE. Amyloid-beta peptide remnants in AN-1792-immunized Alzheimer's disease patients: a biochemical analysis. Am J Pathol. 2006;169:1048–1063. doi: 10.2353/ajpath.2006.060269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrushina I, Tran M, Sadzikava N, Ghochikyan A, Vasilevko V, Agadjanyan MG, Cribbs DH. Importance of IgG2c isotype in the immune response to b-amyloid in APP Tg mice. Neurosci Lett. 2003;338:5–8. doi: 10.1016/s0304-3940(02)01357-5. [DOI] [PubMed] [Google Scholar]
- Pfeifer M, Boncristiano S, Bondolfi L, Stalder A, Deller T, Staufenbiel M, Mathews PM, Jucker M. Cerebral hemorrhage after passive anti-Abeta immunotherapy. Science. 2002;298:1379. doi: 10.1126/science.1078259. [DOI] [PubMed] [Google Scholar]
- Pike CJ, Walencewicz AJ, Glabe CG, Cotman CW. Aggregation-related toxicity of synthetic β-amyloid protein in hippocampal cultures. Euro J Pharm. 1991;207:367–368. doi: 10.1016/0922-4106(91)90014-9. [DOI] [PubMed] [Google Scholar]
- Price DL, Sisodia SS. Cellular and molecular biology of Alzheimer's disease and animal models. Annu Rev Med. 1994;45:435–446. doi: 10.1146/annurev.med.45.1.435. [DOI] [PubMed] [Google Scholar]
- Racke MM, Boone LI, Hepburn DL, Parsadainian M, Bryan MT, Ness DK, Piroozi KS, Jordan WH, Brown DD, Hoffman WP, Holtzman DM, Bales KR, Gitter BD, May PC, Paul SM, DeMattos RB. Exacerbation of cerebral amyloid angiopathy-associated microhemorrhage in amyloid precursor protein transgenic mice by immunotherapy is dependent on antibody recognition of deposited forms of amyloid β. J Neurosci. 2005;25:629–636. doi: 10.1523/JNEUROSCI.4337-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk D. Opinion: amyloid-beta immunotherapy for Alzheimer's disease: the end of the beginning. Nat Rev Neurosci. 2002;3:824–828. doi: 10.1038/nrn938. [DOI] [PubMed] [Google Scholar]
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse [see comments] Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- Schmidt ML, Robinson KA, Lee VM, Trojanowski JQ. Chemical and immunological heterogeneity of fibrillar amyloid in plaques of Alzheimer's disease and Down's syndrome brains revealed by confocal microscopy. Am J Pathol. 1995;147:503–515. [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. The molecular pathology of Alzheimer's disease. Neuron. 1991;6:487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease: a central role for amyloid. J Neuropathol Exp Neurol. 1994;53:438–447. doi: 10.1097/00005072-199409000-00003. [DOI] [PubMed] [Google Scholar]
- Tam JP. Synthetic peptide vaccine design: synthesis and properties of a high-density multiple antigenic peptide system. Proc Natl Acad Sci USA. 1988;85:5409–5413. doi: 10.1073/pnas.85.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner HL, Selkoe DJ. Inflammation and therapeutic vaccination in CNS diseases. Nature. 2002;420:879–884. doi: 10.1038/nature01325. [DOI] [PubMed] [Google Scholar]
- Westerman MA, Cooper-Blacketer D, Mariash A, Kotilinek L, Kawarabayashi T, Younkin LH, Carlson GA, Younkin SG, Ashe KH. The relationship between Aβ and memory in the Tg2576 mouse model of Alzheimer's disease. J Neurosci. 2002;22:1858–1867. doi: 10.1523/JNEUROSCI.22-05-01858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock DM, Rojiani A, Rosenthal A, Subbarao S, Freeman MJ, Gordon MN, Morgan D. Passive immunotherapy against Abeta in aged APP-transgenic mice reverses cognitive deficits and depletes parenchymal amyloid deposits in spite of increased vascular amyloid and microhemorrhage. J Neuroinflammation. 2004;1:24. doi: 10.1186/1742-2094-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock DM, Jantzen PT, Li Q, Morgan D, Gordon MN. Amyloid-beta vaccination, but not nitro-NSAID treatment, increases vascular amyloid and microhemorrhage while both reduce parenchymal amyloid. Neuroscience. 2007;144:950–960. doi: 10.1016/j.neuroscience.2006.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]