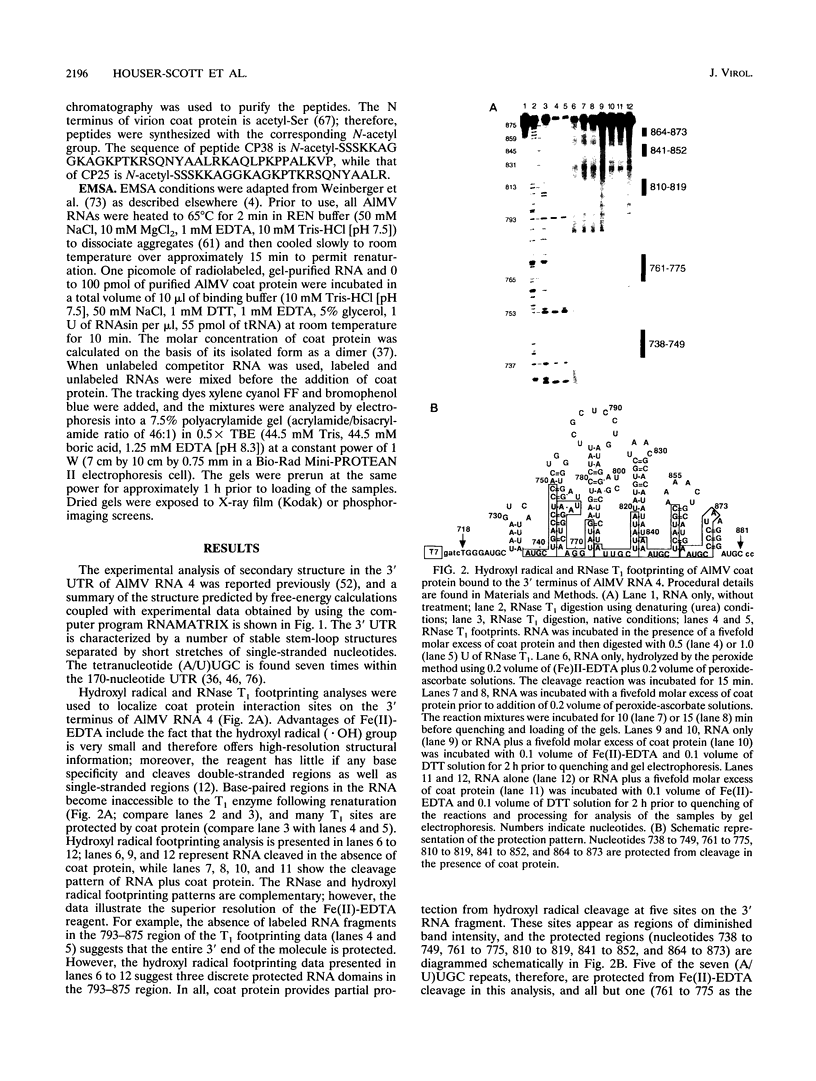
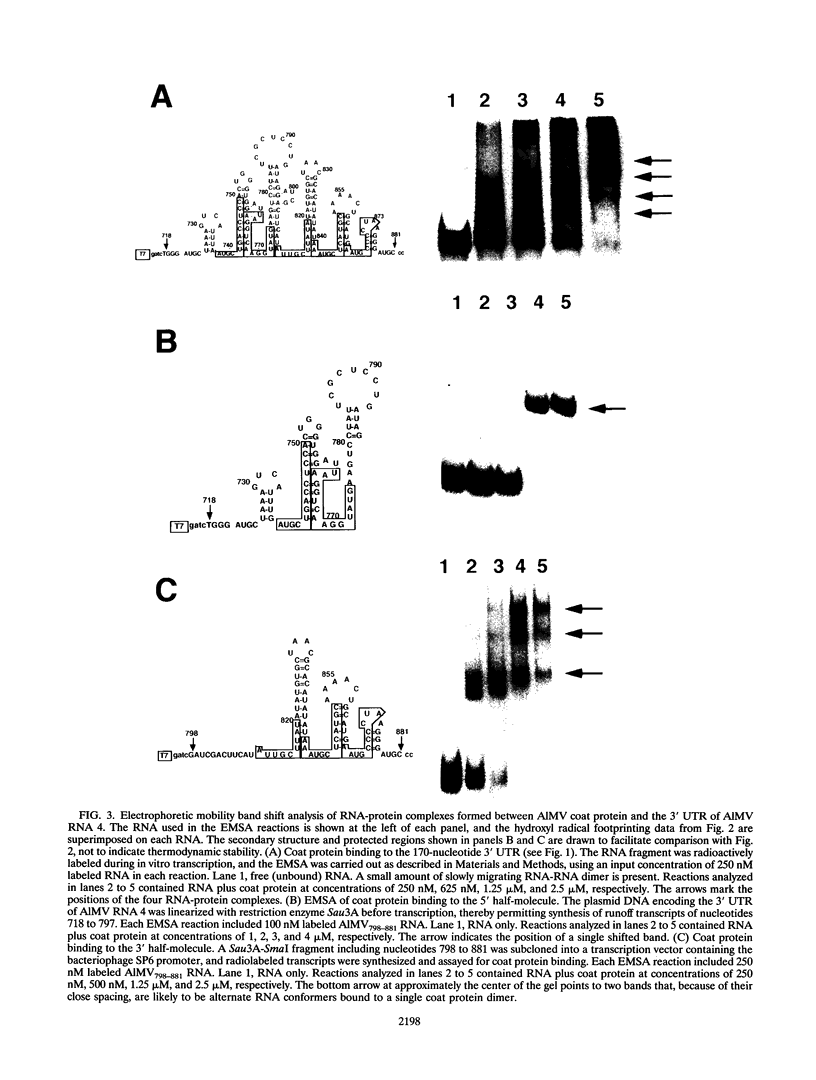
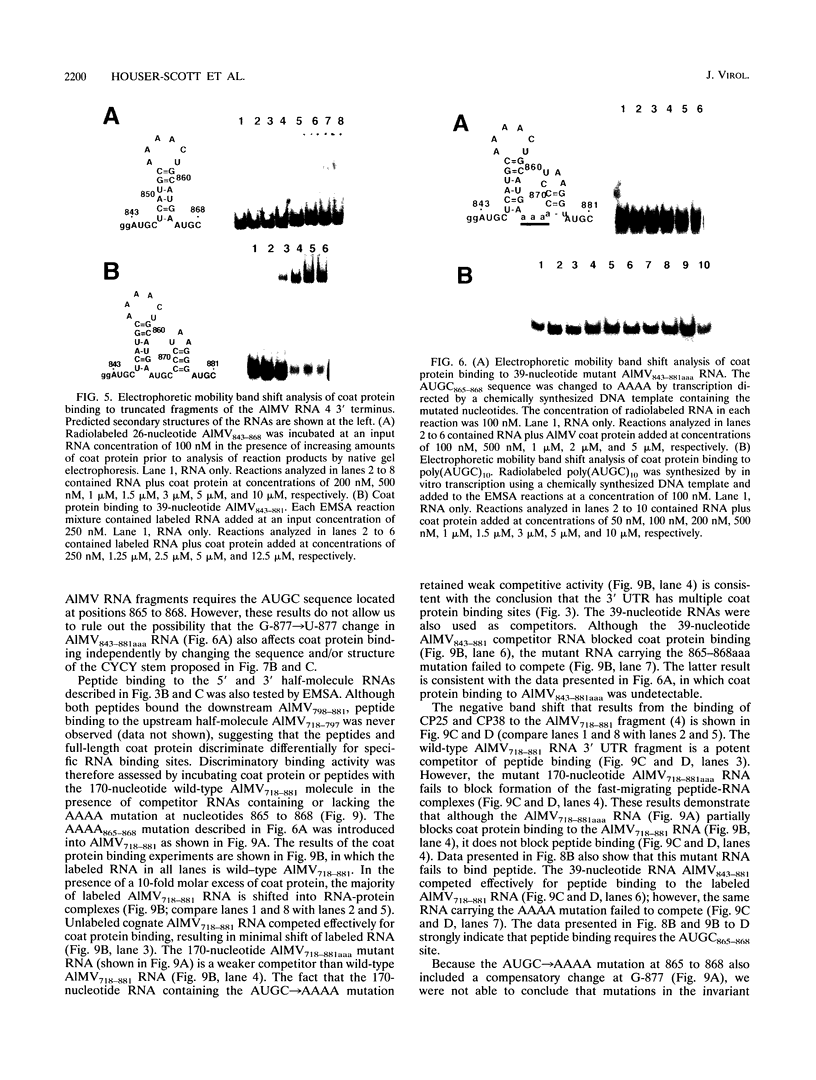
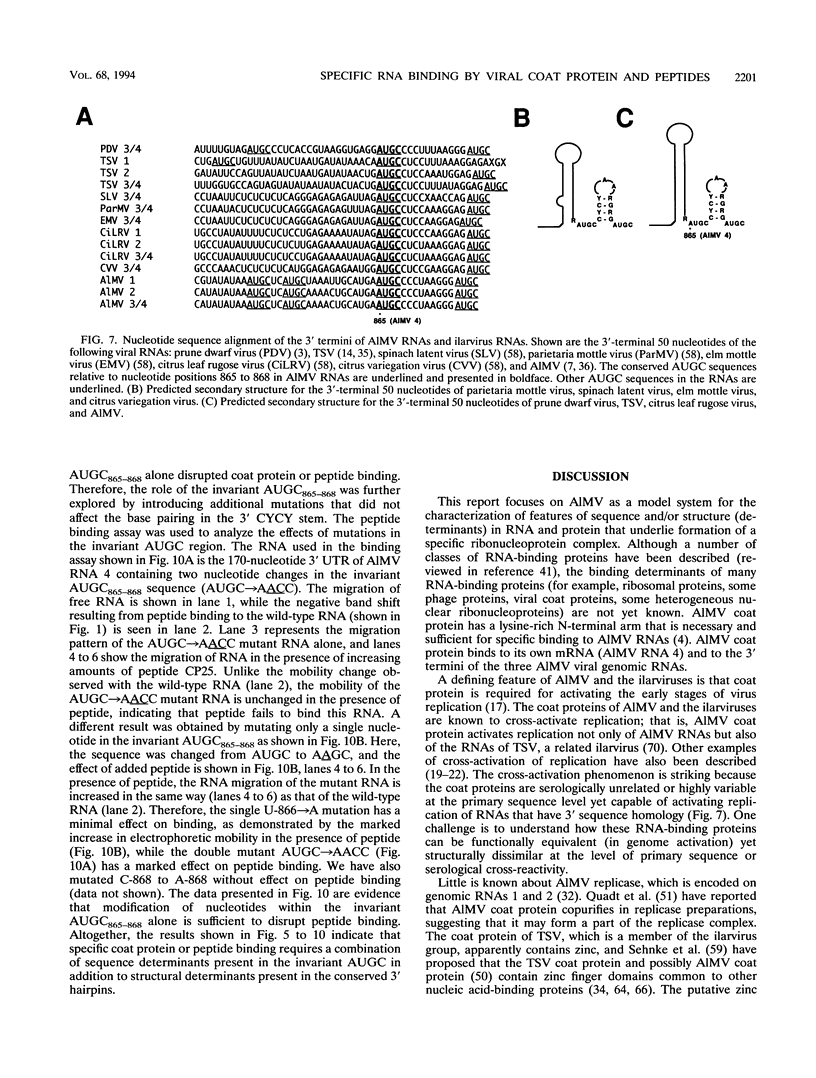
Abstract
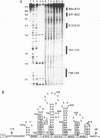
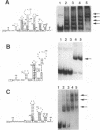
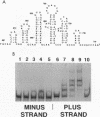
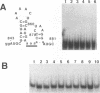
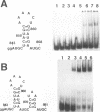
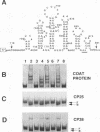
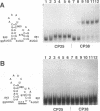
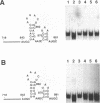
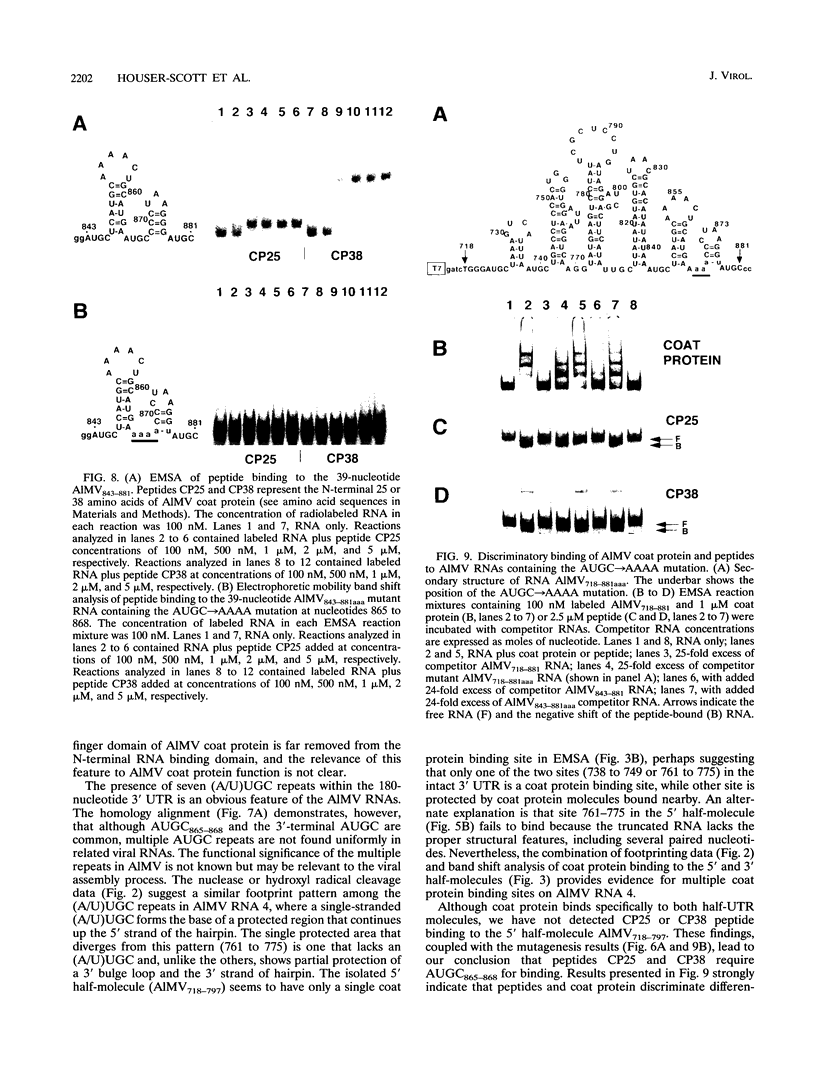
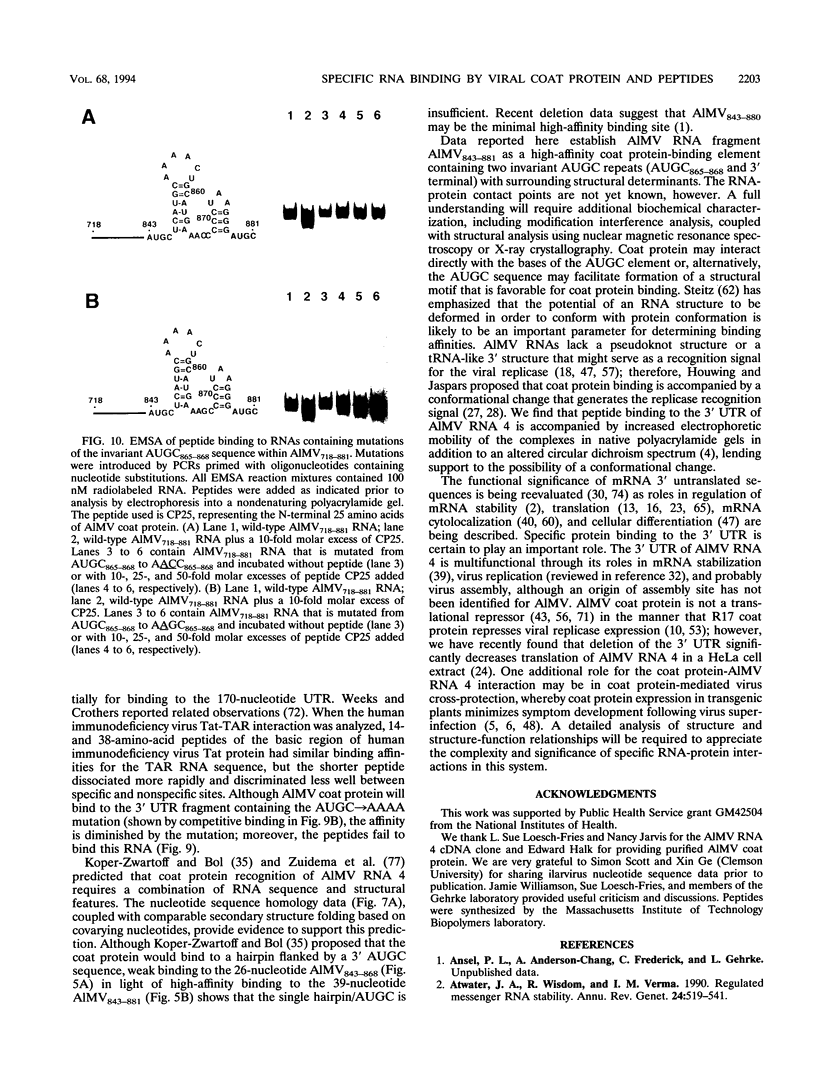
The specific binding of alfalfa mosaic virus coat protein to viral RNA requires determinants in the 3' untranslated region (UTR). Coat protein and peptide binding sites in the 3' UTR of alfalfa mosaic virus RNA 4 have been analyzed by hydroxyl radical footprinting, deletion mapping, and site-directed mutagenesis experiments. The 3' UTR has several stable hairpins that are flanked by single-stranded (A/U)UGC sequences. Hydroxyl radical footprinting data show that five sites in the 3' UTR of alfalfa mosaic virus RNA 4 are protected by coat protein, and four of the five protected regions contain AUGC or UUGC. Electrophoretic mobility band shift results suggest four coat protein binding sites in the 3' UTR. A 3'-terminal 39-nucleotide RNA fragment containing four AUGC repeats bound coat protein and coat protein peptides with high affinity; however, coat protein bound poorly to antisense 3' UTR transcripts and poly(AUGC)10. Site-directed mutagenesis of AUGC865-868 resulted in a loss of coat protein binding and peptide binding by the RNA fragment. Alignment of alfalfa mosaic RNA sequences with those from several closely related ilarviruses demonstrates that AUGC865-868 is perfectly conserved; moreover, the RNAs are predicted to form similar 3'-terminal secondary structures. The data strongly suggest that alfalfa mosaic virus coat protein and ilavirus coat proteins recognize invariant AUGC sequences in the context of conserved structural elements.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abel P. P., Nelson R. S., De B., Hoffmann N., Rogers S. G., Fraley R. T., Beachy R. N. Delay of disease development in transgenic plants that express the tobacco mosaic virus coat protein gene. Science. 1986 May 9;232(4751):738–743. doi: 10.1126/science.3457472. [DOI] [PubMed] [Google Scholar]
- Atwater J. A., Wisdom R., Verma I. M. Regulated mRNA stability. Annu Rev Genet. 1990;24:519–541. doi: 10.1146/annurev.ge.24.120190.002511. [DOI] [PubMed] [Google Scholar]
- Brederode F. T., Koper-Zwarthoff E. C., Bol J. F. Complete nucleotide sequence of alfalfa mosaic virus RNA 4. Nucleic Acids Res. 1980 May 24;8(10):2213–2223. doi: 10.1093/nar/8.10.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhoff A. M., Tullius T. D. The unusual conformation adopted by the adenine tracts in kinetoplast DNA. Cell. 1987 Mar 27;48(6):935–943. doi: 10.1016/0092-8674(87)90702-1. [DOI] [PubMed] [Google Scholar]
- Calnan B. J., Biancalana S., Hudson D., Frankel A. D. Analysis of arginine-rich peptides from the HIV Tat protein reveals unusual features of RNA-protein recognition. Genes Dev. 1991 Feb;5(2):201–210. doi: 10.1101/gad.5.2.201. [DOI] [PubMed] [Google Scholar]
- Carey J., Cameron V., de Haseth P. L., Uhlenbeck O. C. Sequence-specific interaction of R17 coat protein with its ribonucleic acid binding site. Biochemistry. 1983 May 24;22(11):2601–2610. doi: 10.1021/bi00280a002. [DOI] [PubMed] [Google Scholar]
- Celander D. W., Cech T. R. Iron(II)-ethylenediaminetetraacetic acid catalyzed cleavage of RNA and DNA oligonucleotides: similar reactivity toward single- and double-stranded forms. Biochemistry. 1990 Feb 13;29(6):1355–1361. doi: 10.1021/bi00458a001. [DOI] [PubMed] [Google Scholar]
- Ch'ng J. L., Shoemaker D. L., Schimmel P., Holmes E. W. Reversal of creatine kinase translational repression by 3' untranslated sequences. Science. 1990 May 25;248(4958):1003–1006. doi: 10.1126/science.2343304. [DOI] [PubMed] [Google Scholar]
- Cornelissen B. J., Janssen H., Zuidema D., Bol J. F. Complete nucleotide sequence of tobacco streak virus RNA 3. Nucleic Acids Res. 1984 Mar 12;12(5):2427–2437. doi: 10.1093/nar/12.5.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly T. J., Rusche J. R., Maione T. E., Frankel A. D. Circular dichroism studies of the HIV-1 Rev protein and its specific RNA binding site. Biochemistry. 1990 Oct 23;29(42):9791–9795. doi: 10.1021/bi00494a005. [DOI] [PubMed] [Google Scholar]
- Danthinne X., Seurinck J., Meulewaeter F., Van Montagu M., Cornelissen M. The 3' untranslated region of satellite tobacco necrosis virus RNA stimulates translation in vitro. Mol Cell Biol. 1993 Jun;13(6):3340–3349. doi: 10.1128/mcb.13.6.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Arenal F. Sequence and structure at the genome 3' end of the U2-strain of tobacco mosaic virus, a histidine-accepting tobamovirus. Virology. 1988 Nov;167(1):201–206. doi: 10.1016/0042-6822(88)90070-0. [DOI] [PubMed] [Google Scholar]
- Gonsalves D., Fulton R. W. Activation of Prunus necrotic ringspot virus and rose mosaic virus by RNA 4 components of some Ilarviruses. Virology. 1977 Sep;81(2):398–407. doi: 10.1016/0042-6822(77)90155-6. [DOI] [PubMed] [Google Scholar]
- Gonsalves D., Garnsey S. M. Functional equivalence of an RNA component and coat protein for infectivity of citrus leaf rugose virus. Virology. 1975 Mar;64(1):23–31. doi: 10.1016/0042-6822(75)90075-6. [DOI] [PubMed] [Google Scholar]
- Gonsalves D., Garnsey S. M. Infectivity of the multiple nucleoprotein and RNA components of citrus leaf rugose virus. Virology. 1974 Oct;61(2):343–353. doi: 10.1016/0042-6822(74)90272-4. [DOI] [PubMed] [Google Scholar]
- Gonsalves D., Garnsey S. M. Nucleic acid components of citrus variegation virus and their activation by coat protein. Virology. 1975 Oct;67(2):311–318. doi: 10.1016/0042-6822(75)90432-8. [DOI] [PubMed] [Google Scholar]
- Han J., Huez G., Beutler B. Interactive effects of the tumor necrosis factor promoter and 3'-untranslated regions. J Immunol. 1991 Mar 15;146(6):1843–1848. [PubMed] [Google Scholar]
- Hershey J. W. Translational control in mammalian cells. Annu Rev Biochem. 1991;60:717–755. doi: 10.1146/annurev.bi.60.070191.003441. [DOI] [PubMed] [Google Scholar]
- Houwing C. J., Jaspars E. M. Coat protein binds to the 3'-terminal part of RNA 4 of alfalfa mosaic virus. Biochemistry. 1978 Jul 11;17(14):2927–2933. doi: 10.1021/bi00607a035. [DOI] [PubMed] [Google Scholar]
- Houwing C. J., Jaspars E. M. Complexes of alfalfa mosaic virus RNA 4 with one and three coat protein dimers. Biochemistry. 1980 Nov 11;19(23):5255–5260. doi: 10.1021/bi00564a016. [DOI] [PubMed] [Google Scholar]
- Houwing C. J., Jaspars E. M. Preferential binding of 3'-terminal fragments of alfalfa mosaic virus RNA 4 to virions. Biochemistry. 1980 Nov 11;19(23):5261–5264. doi: 10.1021/bi00564a017. [DOI] [PubMed] [Google Scholar]
- Houwing C. J., Jaspars E. M. Protein binding sites in nucleation complexes of alfalfa mosaic virus RNA 4. Biochemistry. 1982 Jul 6;21(14):3408–3414. doi: 10.1021/bi00257a025. [DOI] [PubMed] [Google Scholar]
- Jackson R. J. Cytoplasmic regulation of mRNA function: the importance of the 3' untranslated region. Cell. 1993 Jul 16;74(1):9–14. doi: 10.1016/0092-8674(93)90290-7. [DOI] [PubMed] [Google Scholar]
- Jaeger J. A., SantaLucia J., Jr, Tinoco I., Jr Determination of RNA structure and thermodynamics. Annu Rev Biochem. 1993;62:255–287. doi: 10.1146/annurev.bi.62.070193.001351. [DOI] [PubMed] [Google Scholar]
- Kjems J., Brown M., Chang D. D., Sharp P. A. Structural analysis of the interaction between the human immunodeficiency virus Rev protein and the Rev response element. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):683–687. doi: 10.1073/pnas.88.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koper-Zwarthoff E. C., Bol J. F. Nucleotide sequence of the putative recognition site for coat protein in the RNAs of alfalfa mosaic virus and tobacco streak virus. Nucleic Acids Res. 1980 Aug 11;8(15):3307–3318. doi: 10.1093/nar/8.15.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koper-Zwarthoff E. C., Brederode F. T., Walstra P., Bol J. F. Nucleotide sequence of the 3'-noncoding region of alfalfa mosaic virus RNA 4 and its homology with the genomic RNAs. Nucleic Acids Res. 1979 Dec 11;7(7):1887–1900. doi: 10.1093/nar/7.7.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruseman J., Kraal B., Jaspars E. M., Bol J. F., Brederode F. T., Veldstra H. Molecular weight of the coat protein of alfalfa mosaic virus. Biochemistry. 1971 Feb 2;10(3):447–455. doi: 10.1021/bi00779a015. [DOI] [PubMed] [Google Scholar]
- Latham J. A., Cech T. R. Defining the inside and outside of a catalytic RNA molecule. Science. 1989 Jul 21;245(4915):276–282. doi: 10.1126/science.2501870. [DOI] [PubMed] [Google Scholar]
- Macdonald P. M., Struhl G. cis-acting sequences responsible for anterior localization of bicoid mRNA in Drosophila embryos. Nature. 1988 Dec 8;336(6199):595–598. doi: 10.1038/336595a0. [DOI] [PubMed] [Google Scholar]
- Mattaj I. W. RNA recognition: a family matter? Cell. 1993 Jun 4;73(5):837–840. doi: 10.1016/0092-8674(93)90265-r. [DOI] [PubMed] [Google Scholar]
- Milligan J. F., Groebe D. R., Witherell G. W., Uhlenbeck O. C. Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res. 1987 Nov 11;15(21):8783–8798. doi: 10.1093/nar/15.21.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohier E., Hirth L., Meur M., Gerlinger P. Analysis of alfalfa mosaic virus 17 S RNA translational products. Virology. 1976 Jun;71(2):615–618. doi: 10.1016/0042-6822(76)90389-5. [DOI] [PubMed] [Google Scholar]
- Noller H. F., Woese C. R. Secondary structure of 16S ribosomal RNA. Science. 1981 Apr 24;212(4493):403–411. doi: 10.1126/science.6163215. [DOI] [PubMed] [Google Scholar]
- Pinck L., Pinck M. Sequence homology at the 3'-ends of alfalfa mosaic virus RNAs. FEBS Lett. 1979 Nov 1;107(1):61–65. doi: 10.1016/0014-5793(79)80463-9. [DOI] [PubMed] [Google Scholar]
- Pleij C. W., Rietveld K., Bosch L. A new principle of RNA folding based on pseudoknotting. Nucleic Acids Res. 1985 Mar 11;13(5):1717–1731. doi: 10.1093/nar/13.5.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglisi J. D., Tan R., Calnan B. J., Frankel A. D., Williamson J. R. Conformation of the TAR RNA-arginine complex by NMR spectroscopy. Science. 1992 Jul 3;257(5066):76–80. doi: 10.1126/science.1621097. [DOI] [PubMed] [Google Scholar]
- Quadt R., Jaspars E. M. Effect of removal of zinc on alfalfa mosaic virus RNA-dependent RNA polymerase. FEBS Lett. 1991 Jan 14;278(1):61–62. doi: 10.1016/0014-5793(91)80083-f. [DOI] [PubMed] [Google Scholar]
- Quadt R., Rosdorff H. J., Hunt T. W., Jaspars E. M. Analysis of the protein composition of alfalfa mosaic virus RNA-dependent RNA polymerase. Virology. 1991 May;182(1):309–315. doi: 10.1016/0042-6822(91)90674-z. [DOI] [PubMed] [Google Scholar]
- Quigley G. J., Gehrke L., Roth D. A., Auron P. E. Computer-aided nucleic acid secondary structure modeling incorporating enzymatic digestion data. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):347–366. doi: 10.1093/nar/12.1part1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romaniuk P. J., Lowary P., Wu H. N., Stormo G., Uhlenbeck O. C. RNA binding site of R17 coat protein. Biochemistry. 1987 Mar 24;26(6):1563–1568. doi: 10.1021/bi00380a011. [DOI] [PubMed] [Google Scholar]
- Rould M. A., Perona J. J., Söll D., Steitz T. A. Structure of E. coli glutaminyl-tRNA synthetase complexed with tRNA(Gln) and ATP at 2.8 A resolution. Science. 1989 Dec 1;246(4934):1135–1142. doi: 10.1126/science.2479982. [DOI] [PubMed] [Google Scholar]
- Ruff M., Krishnaswamy S., Boeglin M., Poterszman A., Mitschler A., Podjarny A., Rees B., Thierry J. C., Moras D. Class II aminoacyl transfer RNA synthetases: crystal structure of yeast aspartyl-tRNA synthetase complexed with tRNA(Asp). Science. 1991 Jun 21;252(5013):1682–1689. doi: 10.1126/science.2047877. [DOI] [PubMed] [Google Scholar]
- Schimmel P. RNA pseudoknots that interact with components of the translation apparatus. Cell. 1989 Jul 14;58(1):9–12. doi: 10.1016/0092-8674(89)90395-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehnke P. C., Mason A. M., Hood S. J., Lister R. M., Johnson J. E. A "zinc-finger"-type binding domain in tobacco streak virus coat protein. Virology. 1989 Jan;168(1):48–56. doi: 10.1016/0042-6822(89)90402-9. [DOI] [PubMed] [Google Scholar]
- Srinivasan S., Jaspars E. M. Influence of a few coat protein subunits on the base-paired structure of the RNA species of alfalfa mosaic virus. Biochim Biophys Acta. 1978 Aug 23;520(1):237–241. doi: 10.1016/0005-2787(78)90025-4. [DOI] [PubMed] [Google Scholar]
- Steitz T. A. Structural studies of protein-nucleic acid interaction: the sources of sequence-specific binding. Q Rev Biophys. 1990 Aug;23(3):205–280. doi: 10.1017/s0033583500005552. [DOI] [PubMed] [Google Scholar]
- Tan R., Frankel A. D. Circular dichroism studies suggest that TAR RNA changes conformation upon specific binding of arginine or guanidine. Biochemistry. 1992 Oct 27;31(42):10288–10294. doi: 10.1021/bi00157a016. [DOI] [PubMed] [Google Scholar]
- Theunissen O., Rudt F., Guddat U., Mentzel H., Pieler T. RNA and DNA binding zinc fingers in Xenopus TFIIIA. Cell. 1992 Nov 13;71(4):679–690. doi: 10.1016/0092-8674(92)90601-8. [DOI] [PubMed] [Google Scholar]
- Timmer R. T., Benkowski L. A., Schodin D., Lax S. R., Metz A. M., Ravel J. M., Browning K. S. The 5' and 3' untranslated regions of satellite tobacco necrosis virus RNA affect translational efficiency and dependence on a 5' cap structure. J Biol Chem. 1993 May 5;268(13):9504–9510. [PubMed] [Google Scholar]
- Vallee B. L., Coleman J. E., Auld D. S. Zinc fingers, zinc clusters, and zinc twists in DNA-binding protein domains. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):999–1003. doi: 10.1073/pnas.88.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kuyl A. C., Neeleman L., Bol J. F. Role of alfalfa mosaic virus coat protein in regulation of the balance between viral plus and minus strand RNA synthesis. Virology. 1991 Nov;185(1):496–499. doi: 10.1016/0042-6822(91)90807-n. [DOI] [PubMed] [Google Scholar]
- Weeks K. M., Crothers D. M. RNA binding assays for Tat-derived peptides: implications for specificity. Biochemistry. 1992 Oct 27;31(42):10281–10287. doi: 10.1021/bi00157a015. [DOI] [PubMed] [Google Scholar]
- Weinberger J., Baltimore D., Sharp P. A. Distinct factors bind to apparently homologous sequences in the immunoglobulin heavy-chain enhancer. 1986 Aug 28-Sep 3Nature. 322(6082):846–848. doi: 10.1038/322846a0. [DOI] [PubMed] [Google Scholar]
- Wickens M. Messenger RNA. Springtime in the desert. Nature. 1993 May 27;363(6427):305–306. doi: 10.1038/363305a0. [DOI] [PubMed] [Google Scholar]
- Zuidema D., Bierhuizen M. F., Cornelissen B. J., Bol J. F., Jaspars E. M. Coat protein binding sites on RNA 1 of alfalfa mosaic virus. Virology. 1983 Mar;125(2):361–369. doi: 10.1016/0042-6822(83)90208-8. [DOI] [PubMed] [Google Scholar]
- van Beynum G. M., de Graaf J. M., Castel A., Kraal B., Bosch L. Structural studies on the coat protein of alfalfa mosaic virus. The complete primary structure. Eur J Biochem. 1977 Jan 3;72(1):63–78. doi: 10.1111/j.1432-1033.1977.tb11225.x. [DOI] [PubMed] [Google Scholar]
- van Vloten-Doting L. Coat protein is required for infectivity of tobacco streak virus: biological equivalence of the coat proteins of tobacco streak and alfalfa mosaic viruses. Virology. 1975 May;65(1):215–225. doi: 10.1016/0042-6822(75)90022-7. [DOI] [PubMed] [Google Scholar]