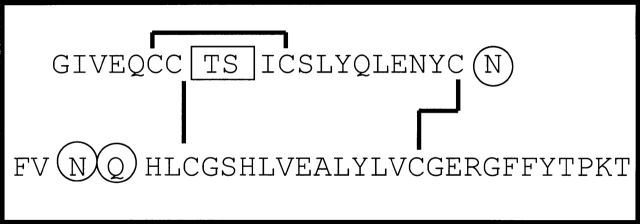Abstract
We have investigated the chemical modification of insulin under conditions that promote the conversion of the soluble protein into amyloid fibrils. The modifications that are incorporated into the fibrils include deamidation of Asn A21, Asn B3, and Gln B4. In order to prepare fibrils with minimal deamidation of these residues, the kinetics of aggregation were accelerated by seeding with aliquots of a solution containing preformed fibrils. The resulting fibrils were then reincubated to determine the extent to which chemical modification occurs in the fibril itself. The deamidation of Asn A21 in particular could be followed in detail. Deamidation of this residue in the fibrillar form of insulin was found to occur in only 52 ± 5% of molecules. This result indicates that there are at least two different packing environments of insulin molecules in the fibrils and suggests that the characterization of chemical modifications may be a useful probe of the environment of polypeptide chains within amyloid fibrils.
Keywords: Insulin, amyloid, deamidation, fibrils, symmetry
Amyloid fibrils are highly ordered aggregated states of peptides and proteins found in pathological deposits in a variety of diseases (Sipe 1994; Tan and Pepys 1994). Similar structures from a wide range of sequences can also be formed in vitro under appropriate conditions (Dobson 2001). Insulin fibrils, for example, have been found at the site of injection of a diabetic patient and in insulin infusion pumps, and can easily be produced in vitro by subjecting the protein to destabilizing conditions (Lougheed et al. 1980; Hutchison 1985; Dische et al. 1988; Sluzky et al. 1991; Bouchard et al. 2000). Insulin is also known to readily undergo minor chemical modifications that limit the shelf life of this therapeutically important hormone. Chemical modifications found in insulin preparations include covalent dimer formation, deamidation of the C-terminal asparagine in the A-chain (Asn A21), deamidation of Asn B3, and hydrolysis of the peptide bond between residues A8 and A9 (Fig. 1 ▶; Brange et al. 1992). The major chemical modification that occurs under conditions commonly used to induce fibril formation from insulin (pH 2.0, elevated temperature) is deamidation of Asn A21 to aspartic acid, but this is considered to be of minor significance because synthetic insulin Asp A21 has been found to have the same fibril-formation kinetics as unmodified insulin (Nielsen et al. 2001). Recent work, however, has demonstrated that even chemical modifications that occur in a minority of protein molecules can have a significant impact on amyloid fibril formation (Szendrei et al. 1994; Kubo et al. 2002; Nilsson et al. 2002). In the present study, amyloid fibrils of insulin were prepared, purified, and characterized in vitro to determine the chemical modifications present under these conditions. Furthermore, conditions were then established to make fibril preparations of greater homogeneity so that the chemical modifications that occur within the fibrils could be characterized.
Figure 1.
Insulin sequence and site of modifications. Primary structure of insulin with the A-chain shown above and the B-chain shown below. The A8–A9 split product (boxed) and asparagine deamidation (circled N) have been observed in therapeutic insulin preparations (Brange et al. 1992). In this work, the modified forms of insulin identified in insulin amyloid fibrils include both deamidated asparagines (positions A21 and B3) and deamidation of glutamine at position B4 (circled Q).
Results and Discussion
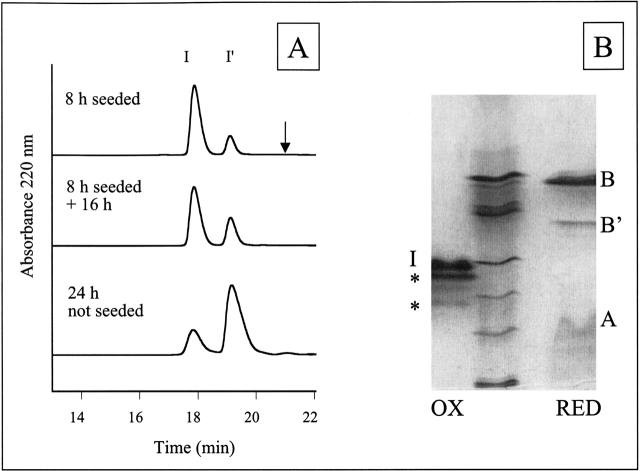
Insulin fibrils were prepared by incubation of a 2 mM solution of the protein at 65°C (pH 2.0) for 24 h. Aggregated protein was purified from soluble material by using a Centricon filter with a 50-kD molecular weight cut-off; this procedure will retain only aggregates containing ∼10 or more insulin molecules. The fibrils were solubilized in aqueous ammonia or Gdn HCl and analyzed using mass spectrometry, HPLC, electrophoresis, and N-terminal sequencing. Mass spectrometric analysis of the solubilized protein from the fibrils revealed a major peak corresponding to the molecular weight of monomeric insulin (measured 5808.8 Da, calculated 5807.7 Da). No peaks in the vicinity of 12 kD were observed from the recovered fibril material, confirming earlier reports that any covalent dimer formed in solution is not incorporated into the fibrils (Brange et al. 1997; Nettleton 1999). Under reducing conditions, the molecular weights correspond to the full-length A-chain and B-chain (measured A-chain 2383.2 Da, B-chain 3432.5 Da; calculated 2383.7 Da, 3430.0 Da). The hydrolysis product in which the A8–A9 bond is cleaved (observed in crystalline insulin preparations) was not present in the fibrils in detectable quantities because no A-chain fragments of molecular weight 852.0 Da and 1549.8 Da were observed after dissolution of the fibrils. This conclusion was confirmed by HPLC analysis; no peaks with the reported relative retention time of the split product were observed (Fig. 2A ▶; Brange et al. 1992).
Figure 2.
HPLC and IEF of solubilized insulin fibrils. (A) HPLC traces of solubilized insulin fibrils under nonreducing HPLC conditions reveal two peaks, I and I′. The peak labeled I corresponds to unmodified insulin and insulin with B-chain deamidation (determined by IEF). The I′ peak corresponds to insulin with A-chain deamidation and insulin with both A-chain deamidation and B-chain deamidation (IEF). The arrow indicates the expected relative retention time of the A8–A9 split product (Brange et al. 1992). The extent of Asn A21 deamidation can be approximated by the relative ratio of I′ to I. The Asn A21 deamidation in the 24 h unseeded fibrils is greater than the modification of the 8 h seeded + 16 h fibrils, indicating less chemical modification in the fibrils than in solution. (B) IEF gel electrophoresis of solubilized insulin amyloid fibrils formed by incubation for 24 h. IEF was performed under nonreducing (lane 1, OX) and reducing (lane 3, RED) conditions and compared with IEF marker proteins (lane 2) corresponding to pI 7.2, 6.4, 5.9, 5.1, 4.6, and 3.3 from top to bottom. Bands that occur in the same position as for an untreated insulin sample are labeled with I (insulin), A (A-chain), or B (B-chain) respectively. IEF under nonreducing conditions reveals insulin (calc. pI 5.4) and two additional bands. The dark band closest to insulin is assigned to insulin deamidated at position Asn A21 and the fainter bands to deamidation at Asn B3 (based on previous assignment by Brange et al. 1992) or insulin deamidated at more than one position (a single deamidation would reduce the pI by 0.4–0.5 and double deamidation by 0.7–0.8). Under reducing conditions, the B-chain (calc. pI 6.9) and a deamidated form of the B-chain (a single deamidation would reduce the pI by 0.9) are clearly distinguishable. These two bands were sequenced to reveal the presence of deamidated Asn B3 and Gln B4. The A-chain (both in control insulin solutions and in fibrils) smears on the gel, possibly due to reoxidation.
HPLC and IEF gel electrophoresis revealed, however, that despite the lack of chain cleavage, deamidated forms of the A-chain and B-chain were present in the fibrils (Fig. 2 ▶). HPLC analysis performed under nonreducing conditions revealed two major peaks, one with the same retention time as unmodified insulin (I) and a peak with a slightly longer retention time (I′; see Fig. 2 ▶). The second peak (I′) has been previously characterized as the species formed by deamidation of Asn A21 (Brange et al. 1992); this assignment is consistent with the analysis of insulin under reducing conditions, which demonstrated that the major new peak corresponds to a modified form of the A-chain. Modification of 8% of the B-chain molecules was evident from the HPLC analysis and was identified as deamidation by IEF (Fig. 2B ▶). The exact residue involved in the B-chain deamidation was characterized by N-terminal sequencing. Because only a small portion of the B-chain molecules was modified, the fibrils were analyzed using two different procedures. The first fibril sample was solubilized in aqueous ammonia, the B-chains separated by IEF (Fig. 2B ▶), transferred to a ProBlott membrane, and sequenced. The IEF band corresponding to deamidation of the B-chain revealed deamidation of Asn B3 and Gln B4. The second fibril sample was solubilized in Gdn HCl, reduced with DTT, and the HPLC peaks corresponding to the B-chain and modified B-chain were collected together and sequenced. The results revealed 3.8% deamidation of Asn B3 and 3.2% deamidation of Gln B4 in the sample.
The modifications of insulin described earlier could have occurred either in solution or in the fibril itself. If the modifications occurred in the fibril, then these chemical reactions may be useful as a structural probe of fibril symmetry. However, the development of this methodology requires several additional pieces of information, including (1) knowledge of the degree of insulin fibril solubility under the experimental conditions, (2) a method to prepare fibrils of greater homogeneity, and (3) careful characterization of the extent of chemical modification. Insulin fibrils are not particularly soluble under the conditions used in these experiments. The amount of insulin that remains in solution under the incubation conditions is very small (24 h no seed, 4.5 ± 0.5%; 2 h seeded, 2.5 ± 0.5%, 8 h seeded, 1.5 ± 0.9%; average ± standard deviation) and, even after 3 d at 65°C, the amount of insulin in solution is negligible (1.8 ± 0.3%). Therefore, the contribution to the analysis that results from any monomer/fibril equilibrium will be negligible. Insulin fibrils of greater homogeneity were formed by reducing the lag time in the aggregation kinetics by seeding with an aliquot of solution containing preformed fibrils. After 8 h at 65°C, insulin fibrils were recovered from the seeded solution and these fibrils contained significantly less deamidation (21 ± 3%) than the fibrils formed after 24 h without seeding (74 ± 3%). (The amount of Asn A21 deamidation of the insulin remaining in solution for the 24 h unseeded incubation is very similar to the amount in the fibrils [78 ± 1%], which indicates that in unseeded fibril formation, the equilibrium between insulin and Asn A21 deamidated insulin is reached during the >1 h lag phase prior to fibril formation and there is no significant kinetic partitioning of the Asn A21 deamidated insulin between the fibrils and solution.) The 8 h seeded fibrils were purified and reincubated at pH 2.0, 65°C for an additional 16 h to achieve a total incubation time of 24 h. A comparison of the 24 h unseeded, 8 h seeded, and 8 h seeded + 16 h additional incubation is shown in Figure 2A ▶. The extent of deamidation in the 24 h unseeded fibrils is significantly higher than in the 8 h seeded fibrils + 16 h incubation, despite the fact that both samples were incubated under the same solution conditions for a total of 24 h. These data clearly demonstrate that the deamidation in insulin amyloid fibrils is either generally slower or less residues are susceptible to this modification than in solution. A more extensive time course analysis over a period of 13 d clearly reveals an upper limit on the number of Asn A21 residues in the fibrils that are susceptible to modification (Fig. 3 ▶). The total percentage of Asn A21 residues in the fibrils that can be chemically modified is 52 ± 5% (Fig. 3 ▶). This number is very close to 50%, indicating the presence of two distinct packing arrangements of the A-chain in the fibril, one in which the C-terminal region of the polypeptide chain is buried and one in which it is solvent exposed. The fibrils used for the reincubation experiment (8 h seeded), however, contained ∼20% (21 ± 3%) deamidated insulin in the beginning of the experiment and, therefore, it is worthwhile to estimate the likely final percent deamidation, assuming a twofold symmetry. Assuming two packing orientations of insulin in the fibrils (surface exposed and buried), the initial ∼20% deamidated insulin could be in either position. If the deamidation occurred after the insulin was in the fibrils, then the ∼20% deamidated material would all be in surface-exposed positions; subsequent incubation of these fibrils would result in the deamidation of the remaining surface-exposed molecules to yield a final value of 50% deamidated insulin. If, however, all of the deamidation occurred before fibril formation and there are no other complicating factors, then 10% deamidated insulin would be in a buried position in the fibril and 10% in a surface-exposed position; reincubation of these fibrils would result in a total of 60% deamidated insulin (10% buried + 50% surface exposed). Therefore, because of the ∼20% deamidation in the initial fibrils, the final amount of deamidation for a twofold symmetry would be between 50% and 60%, which is consistent with the data in Figure 3 ▶.
Figure 3.
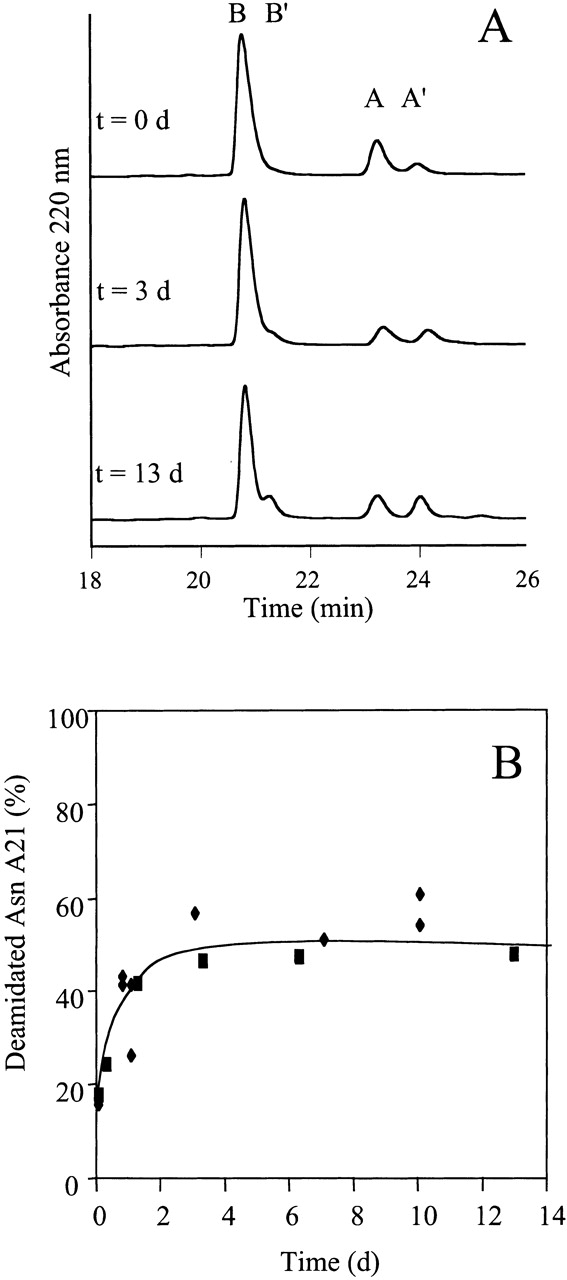
Time dependence of the deamidation of Asn A21 in insulin fibrils. (A) Insulin fibrils formed by seeding (top) and then reincubated at 65°C (pH 2.0) for 3 or 13 d (middle and bottom) were analyzed by HPLC under reducing conditions. The A-chains and B-chains are clearly resolvable (A,B) and the deamidation that occurs can be detected (A′, B′). After 13 d of incubation, the relative amount of A-chains that are deamidated at Asn A21 (A′) is very close to 50%. (B) Time course for deamidation of Asn A21 in the fibrils. The percent of deamidated Asn A21 was determined from HPLC analysis (e.g., Fig. 3A ▶); squares and diamonds represent two different batches of insulin. The maximum amount of deamidated Asn A21 in the fibrils is 52 ± 5%.
Conclusion
We have found that amyloid fibrils prepared from insulin under standard in vitro conditions can contain a number of chemical modifications, including deamidation of Asn A21, Asn B3, and Gln B4. The first two, but not the third, have been observed previously in therapeutic preparations of insulin (Brange et al. 1992). The extent of deamidation of these residues in the fibrils produced without seeding after incubation for 24 h was determined to be 74% Asn A21, 4% Asn B3, and 3% Gln B4. This result is consistent with the known trends of deamidation in which Asn is more labile than Gln, and a C-terminal Asn is more susceptible to deamidation than an Asn followed by another residue (for review, see Lindner and Helliger 2001). We have also observed, however, that deamidation can occur after fibril formation, a result consistent with a very early observation that ammonia (formed as a result of deamidation) was evolved from both insulin in solution and in aggregates (Du Vigneaud et al. 1933). However, only 52 ± 5% of the insulin was found to be deamidated at Asn A21 in the fibrils. This result indicates the existence of two distinct packing environments for this residue in the fibrils. The nature of the molecular packing of a protein into fibrils is important in determining the mechanism of fibril assembly and in identifying critical interactions that may be targeted for therapeutic intervention (Soto et al. 1998; Findeis et al. 1999; Poduslo et al. 1999; Reilly 2000; Rijkers et al. 2002). Although the structural information obtained with this technique is only with respect to isolated regions of the protein, it should be of value to test models of fibril structure (Jimenez et al. 2002). Furthermore, the methodology described in this paper should be of general value in the preparation of more homogenous fibril samples for analysis and in the differentiation between modifications that occur prior to fibril formation and after fibril formation.
Materials and methods
Materials
Fibril preparation
Human insulin was purchased from Sigma (>95% pure by analytical HPLC) and used within 3 mo of purchase. Insulin solutions (2 mM) were prepared in water and the pH adjusted to 2.0 using HCl. Unseeded fibrils were grown by incubation of 2 mM insulin (pH 2.0) at 65°C, for 24 h. Aggregates were separated from soluble material by Centricon (50 kD MW cutoff, Millipore), then washed and lyophilized to dryness. Seeded samples were prepared by incubation of insulin for 2 h or 8 h in a solution to which 5% w/w of a previously aggregated sample was added.
Fibril solubility
The amount of insulin remaining in solution after fibril formation and at equilibrium was quantified by measuring the absorbance at 280 nm of the solution (HP 8452A diode-array spectrophotometer) after removing the fibrils using an Anapore 0.02-μM microcentrifuge tube filter (Whatman). Data are reported as the average ± standard deviation, n = 3.
Fibril reincubation
Seeded fibrils were purified, resuspended in water at pH 2.0 to a concentration of 2 mM, and reheated for additional periods of time. After incubation, the fibril samples were solubilized and the integration of analytical HPLC traces was used to determine the relative proportions of modified and unmodified material. Data are reported as the average ± standard deviation, n = a minimum of 3.
Fibril dissolution
Insulin fibrils were solubilized by adding 7 M aqueous ammonia to a final pH of 10.5 followed by incubation for 30 min at room temperature. Insulin amyloid fibrils are soluble in basic solution (Waugh 1948) but these conditions can also promote deamidation. Control experiments, however, revealed that treatment of soluble insulin with aqueous ammonia (pH 10.5) for 1–2 h at room temperature does not lead to detectable deamidation of the A-chain by HPLC and only results in minor B-chain deamidation observable by IEF. In addition, experiments were performed in which fibrils were solubilized using 8 M Gdn HCl (pH 7.0), and produced comparable results to those in which the fibrils were solubilized with aqueous ammonia.
Analytical methods
Electrophoresis
Electrophoresis was performed using precast IEF gels (pH 3–10) under reducing or nonreducing conditions following the manufacturer’s instructions (Invitrogen). Staining was performed using either Coomassie brilliant blue or colloidal brilliant blue (Sigma).
HPLC
HPLC was performed on a Varian ProStar system with a dual wavelength (220 nm and 280 nm) detector. A C18 reversed phase column (Phenomenox) was used with a linear gradient (Buffer A = 5 mM HCl in water; Buffer B = 5 mM HCl in 80% acetonitrile). Peaks were integrated for analysis using the ProStar software.
Mass spectrometry
Insulin fibrils were dissolved in aqueous ammonia (pH 10.5) and one portion of the sample was reduced with DTT. The reduced and nonreduced samples were examined by MALDI mass spectrometry at the Protein and Nucleic Acid Chemistry Facility (PNAC) at the University of Cambridge.
N-terminal sequencing
The conditions used for N-terminal sequencing are known to be able to promote a small amount of deamidation. Therefore, the insulin B-chain from the fibril samples was compared with a control sample and the difference in the degree of deamidation was determined. All sequencing was performed at the PNAC Facility, University of Cambridge.
Acknowledgments
N-terminal sequencing was performed by Mike Weldon and mass spectrometry by Richard Turner at the PNAC Facility, University of Cambridge. This work was supported by a Programme Grant from the Wellcome Trust. We are also grateful to Carol Robinson and Mark Krebs for technical advice and helpful discussions.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Abbreviations
d, days
DTT, dithiothreitol
Gdn, guanidine
h, hours
IEF, isoelectric focusing
HPLC, high performance liquid chromatography
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.0360403.
References
- Bouchard, M., Zurdo, J., Nettleton, E.J., Dobson, C.M., and Robinson, C.V. 2000. Formation of insulin amyloid fibrils followed by FTIR simultaneously with CD and electron microscopy. Protein Sci. 9 1960–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brange, J., Lngkjaer, L., Havelund, S., and Volund, A. 1992. Chemical stability of insulin. 1. Hydrolytic degradation during storage of pharmaceutical preparations. Pharm. Res. 9 715–727. [DOI] [PubMed] [Google Scholar]
- Brange, J., Andersen, L., Laursen, E.D., Meyn, G., and Rasmussen, E. 1997. Toward understanding insulin fibrillation. J. Pharm. Sci. 86 517–525. [DOI] [PubMed] [Google Scholar]
- Dische, F.E., Wernstedt, C., Westermark, G.T., Westermark, P., Pepys, M.B., Rennie, J.A., Gilbey, S.G., and Watkins, P.J. 1988. Insulin as an amyloid-fibril protein at sites of repeated insulin injections in a diabetic patient. Diabetologia 31 158–161. [DOI] [PubMed] [Google Scholar]
- Dobson, C.M. 2001. The structural basis of protein folding and its links with human disease. Philos. Trans. R. Soc. Lond. B Biol. Sci. 356 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Vigneaud, V., Sifferd, R.H., and Sealock, R.R. 1933. The heat precipitation of insulin. J. Biol. Chem. 102 521–533. [Google Scholar]
- Findeis, M.A., Musso, G.M., Arico-Muendel, C.C., Benjamin, H.W., Hundal, A.M., Lee, J.J., Chin, J., Kelley, M., Wakefield, J., Hayward, N.J., et al. 1999. Modified-peptide inhibitors of amyloid β-peptide polymerization. Biochemistry 38 6791–6800. [DOI] [PubMed] [Google Scholar]
- Hutchison, K.G. 1985. Assessment of gelling in insulin solutions for infusion pumps. J. Pharm. Pharmacol. 37 528–531. [DOI] [PubMed] [Google Scholar]
- Jimenez, J.L., Nettleton, E.J., Bouchard, M., Robinson, C.V., Dobson, C.M., and Saibil, H.R. 2002. The protofilament structure of insulin amyloid fibrils. Proc. Natl. Acad. Sci. 99 9196–9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo, T., Nishimura, S., Kumagae, Y., and Kaneko, I. 2002. In vivo conversion of racemized β-amyloid ([D-Ser26]Aβ1–40) to truncated and toxic fragments ([D-Ser26]Aβ25–35/40) and fragment presence in the brains of Alzheimer’s patients. J. Neurosci. Res. 70 474–483. [DOI] [PubMed] [Google Scholar]
- Lindner, H. and Helliger, W. 2001. Age-dependent deamidation of asparagine residues in proteins. Exp. Gerontol. 36 1551–1563. [DOI] [PubMed] [Google Scholar]
- Lougheed, W.D., Woulfe-Flanagan, H., Clement, J.R., and Albisser, A.M. 1980. Insulin aggregation in artificial delivery systems. Diabetologia 19 1–9. [DOI] [PubMed] [Google Scholar]
- Nettleton, E.J. 1999. "Analysing protein conformations by mass spectrometry." D.Phil. thesis, Department of Chemistry, University of Oxford, Oxford.
- Nielsen, L., Frokjaer, S., Carpenter, J.F., and Brange, J. 2001. Studies of the structure of insulin fibrils by Fourier transform infrared (FTIR) spectroscopy and electron microscopy. J. Pharm. Sci. 90 29–37. [DOI] [PubMed] [Google Scholar]
- Nilsson, M.R., Driscoll, M., and Raleigh, D.P. 2002. Low levels of asparagine deamidation can have a dramatic effect on aggregation of amyloidogenic peptides: Implications for the study of amyloid formation. Protein Sci. 11 342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poduslo, J.F., Curran, G.L., Kumar, A., Frangione, B., and Soto, C. 1999. β-Sheet breaker peptide inhibitor of Alzheimer’s amyloidogenesis with increased blood-brain barrier permeability and resistance to proteolytic degradation in plasma. J. Neurobiol. 39 371–382. [PubMed] [Google Scholar]
- Reilly, C.E. 2000. β-Sheet breaker peptides reverse conformation of pathogenic prion proteins. J. Neurol. 247 319–320. [DOI] [PubMed] [Google Scholar]
- Rijkers, D.T., Hoppener, J.W., Posthuma, G., Lips, C.J., and Liskamp, R.M. 2002. Inhibition of amyloid fibril formation of human amylin by N-alkylated amino acid and α-hydroxy acid residue containing peptides. Chemistry 8 4285–4291. [DOI] [PubMed] [Google Scholar]
- Sipe, J.D. 1994. Amyloidosis. Crit. Rev. Clin. Lab. Sci. 31 325–354. [DOI] [PubMed] [Google Scholar]
- Sluzky, V., Tamada, J.A., Klibanov, A.M., and Langer, R. 1991. Kinetics of insulin aggregation in aqueous solutions upon agitation in the presence of hydrophobic surfaces. Proc. Natl. Acad. Sci. 88 9377–9381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto, C., Sigurdsson, E.M., Morelli, L., Kumar, R.A., Castano, E.M., and Frangione, B. 1998. β-Sheet breaker peptides inhibit fibrillogenesis in a rat brain model of amyloidosis: Implications for Alzheimer’s therapy. Nat. Med. 4 822–826. [DOI] [PubMed] [Google Scholar]
- Szendrei, G.I., Fabian, H., Mantsch, H.H., Lovas, S., Nyeki, O., Schon, I., and Otvos Jr., L. 1994. Aspartate-bond isomerization affects the major conformations of synthetic peptides. Eur. J. Biochem. 226 917–924. [DOI] [PubMed] [Google Scholar]
- Tan, S.Y. and Pepys, M.B. 1994. Amyloidosis. Histopathology 25 403–414. [DOI] [PubMed] [Google Scholar]
- Waugh, D.F. 1948. Regeneration of insulin from insulin fibrils by the action of alkali. J. Am. Chem. Soc. 70 1850–1857. [DOI] [PubMed] [Google Scholar]