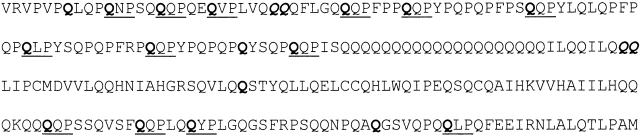Abstract
Celiac disease is a permanent immune-mediated food intolerance triggered by ingestion of wheat gliadins in genetically susceptible individuals. It has been reported that tissue transglutaminase plays an important role in the onset of celiac disease by converting specific glutamine residues within gliadin fragments into glutamic acid residues. This process increases binding affinity of gliadin peptides to HLA-DQ2/DQ8 molecules, thus enhancing the immune response. The aim of the present study was to achieve a detailed structural characterization of modifications induced by transglutaminase on gliadin peptides. Therefore, structural analyses were carried out on a recombinant α-gliadin and on a panel of 26 synthetic peptides, overlapping the complete protein sequence. Modified glutamine residues were identified by means of advanced mass-spectrometric methodologies on the basis of MALDI-TOF-MS and tandem mass spectrometry. Results led to the identification of 19 of 94 glutamine residues present in the recombinant α-gliadin, which were converted into glutamic acid residues by a transglutaminase-mediated reaction. This allowed us to achieve a global view of the modifications induced by the enzyme on this protein. Furthermore, results gathered could likely be utilized as relevant information for a better understanding of processes leading to T-cell recognition of gliadin peptides involved in celiac disease.
Keywords: Gliadin, transglutaminase, mass spectrometry, celiac disease, posttranslational modifications
Celiac disease (or gluten-sensitive enteropathy, CD) is a permanent intolerance to gluten, which is a complex mixture of storage proteins (glutenins and gliadins) found in wheat and in other grains like barley, rye, and oats. In many genetically susceptible individuals, dietary exposure to gluten induces an inflammatory response, causing destruction of the villous structure of the small intestine (Marsh 1992). Celiac disease is strongly associated with HLA-DQ2 (A1*0501, B1*0201) and HLA-DQ8 (A*0301, B*0302; Spurkland et al. 1997). In fact, gluten-specific HLA-DQ2 and/or HLA-DQ8-restricted CD4+ T lymphocytes were isolated from small intestinal biopsies of CD patients and gliadin-specific T-cell clones were prepared and tested (Lundin et al. 1993; van der Wal et al. 1998a). HLA class II molecules mediate the presentation of gliadin-derived peptides to T-cell leading to T-cell stimulation. CD is also characterized by secretion of specific antibodies against gliadin and tissue transglutaminase (tTGase), and in fact, the presence of antibodies specific for tTGase in serum from celiac patients is a strong indication for diagnosis (Dieterich et al. 1997, 1998; Sollid 2000).
Many reports have pointed to the involvement of tTGase in the generation of T-cell stimulatory gliadin peptides. This enzyme mainly catalyzes an acyl transfer reaction, in which the carboxamide group of a peptide-bound glutamine residue is the acyl donor and an appropriate primary amine, that is, the ɛ-amino group of a lysine residue, is the acyl acceptor, by which an isopeptide bond is formed (Folk 1983; Pucci et al. 1988; Leblanc et al. 2001). More recently, it has also been reported that tTGase converts glutamine residues of gliadin peptides in glutamic acid residues by means of a site-specific deamidation reaction (Molberg et al. 1998; Sjöstrom et al. 1998; van der Wal et al. 1998b). This reaction could introduce negatively charged residues in specific positions of the gliadin peptides, corresponding to anchor positions for binding to HLA-DQ2/DQ8 molecules (Johansen et al. 1996; Kwok et al. 1996; van der Wal et al. 1996; Wucherpfenning 2001). Therefore, modified peptides could exhibit an enhanced-binding affinity, leading to an increase of their immunogenicity (Quarsten et al. 1999; Anderson et al. 2000; Arentz-Hansen et al. 2000a).
However, identifying immunodominant gliadin epitopes has been difficult, essentially because of gliadin heterogeneity; that is to say, it is a complex mixture of proteins. According to the amino-terminal sequence and electrophoretic mobility, the components of gliadin can be divided into α-, γ-, and ω-gliadins, with each subgroup consisting of a mixture of varying proteins slightly differing in their primary structures. Another peculiarity of gliadin is found in its amino acid composition, as it is made up of a high percentage of glutamine (30%–40%) and proline (20%–25%) residues and a very low percentage of basic/acid aminoacid residues (2%–5%; Booth and Ewart 1970; Wieser et al. 1987). Hence, because of these structural features, the identification of all potential tTGase-specific deamidation sites becomes a complex task.
To overcome the difficulty, a recombinant α-gliadin representative of the whole group of α-gliadins has been cloned according to reported protocols (Arentz-Hansen et al. 2000b; S. Senger and M. Rossi, unpubl.). Our studies were aimed to identify all of the glutamine residues modified by tTGase in the recombinant protein. Gliadin peptide mixtures produced by enzymatic hydrolysis and treated with tTGase were submitted to MALDI-TOF-MS analyses and to tandem mass-spectrometric experiments performed on a hybrid quadrupole time-of-flight instrument equipped with a nanospray source (nanoESI-MS/MS). Structural data showed that tTGase recognized 19 of 94 glutamine residues and highlighted common structural features of glutamine residues involved in the enzymatic reaction. It is worth noting that this study stands as one of the first that reports a complete structural analysis of the modifications induced on a recombinant α-gliadin by tTGase.
Results
Structural analysis of the recombinant α-gliadin
Samples containing 10 μg of the reduced and alkylated recombinant α-gliadin were hydrolyzed with trypsin, elastase, and chymotrypsin, and the peptide mixtures obtained were analyzed by MALDI-TOF-MS. Although the analysis of the tryptic digest ensured that the primary structure of the expressed protein could be fully confirmed (data not shown), the tryptic peptides, because of their dimensions (most being longer than 30 residues), were not appropriate for the MS/MS experiments needed to identify the deamidation sites.
Furthermore, mass signals recorded in the MALDI spectrum obtained from the analysis of elastase peptide mixture could not be unambiguously assigned to the corresponding peptides along the protein sequence (Fig. 1 ▶). The signals, in fact, could be generated by more than one peptide. On the other hand, some peaks could not be assigned to any peptide on the basis of molecular mass and enzyme specificity. Therefore, the peptide mixture was analyzed by nanoESI-MS/MS. Doubly or triply charged ions originating from the gliadin peptides were sequentially selected and submitted to low-energy fragmentation experiments in order to acquire sequence information and to unambiguously identify all peptides generated out of enzymatic digestion. Similar experiments were carried out on the chymotryptic peptide mixture.
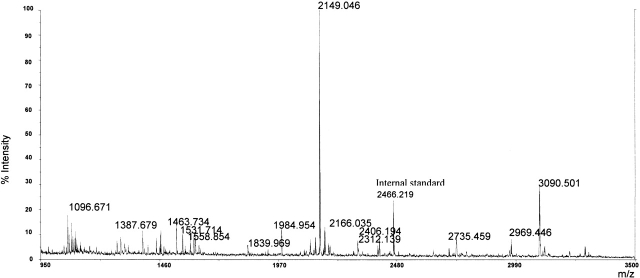
Figure 1.
MALDI-MS analysis of the peptide mixture obtained by elastase digestion of the recombinant α-gliadin.
Results of the tandem experiments on the elastase digest allowed us to detect peptides originated from all of the protein sequence, except from region 140–197. The peptides analyzed by MS/MS contain 76 of the 94 glutamine (Q) residues present in the whole recombinant α-gliadin, including a peptide containing a long polyglutamine stretch (peptide 95–120).
Analyses of the chymotryptic digest gave less informative results, which is why all of the following structural studies have been carried out on the peptide mixture generated by elastase hydrolysis.
Identification of tTGase deamidated peptides by MALDI-TOF-MS
The peptide mixture yielded by elastase digestion of α-gliadin was submitted to tTGase reaction and the deamidated mixture was analyzed by MALDI-TOF-MS.
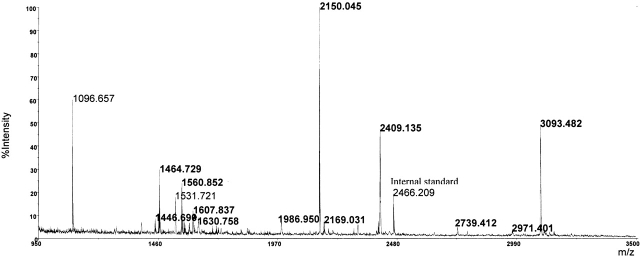
A direct comparison between the MALDI spectra acquired before and after tTGase reaction on the elastase peptide mixture led to the identification of peptides containing Q residues that were converted in glutamic acid (E) residues by tTGase-mediated deamidation; in fact, peaks originating from modified peptides showed a mass shift of 1 Da for each modified Q residue (Fig. 2 ▶). Results were confirmed by elaborating the MALDI spectrum using an instrument-specific software that transforms the isotopic cluster of each signal, formed by natural distribution of carbon isotopes in a single monoisotopic peak, whose molecular weight, in turn, corresponds to that of a theoretical peptide containing only the 12C isotope (Yergey 1983). It is worth noting that the peptides containing the polyglutamine stretch (i.e., 95–110 and 95–120) were not tTGase substrates.
Figure 2.
MALDI-MS analysis of the peptide mixture obtained by elastase digestion of the recombinant α-gliadin after tTGase treatment. The signals generated by some of the deamidated peptides are reported in bold.
Because several Q residues were present in each modified peptide, it was necessary to determine the entire peptide sequence by MS/MS experiments so that the deamidation sites could be identified.
Sequence analyses of the modified peptides by nanoESI-MS/MS
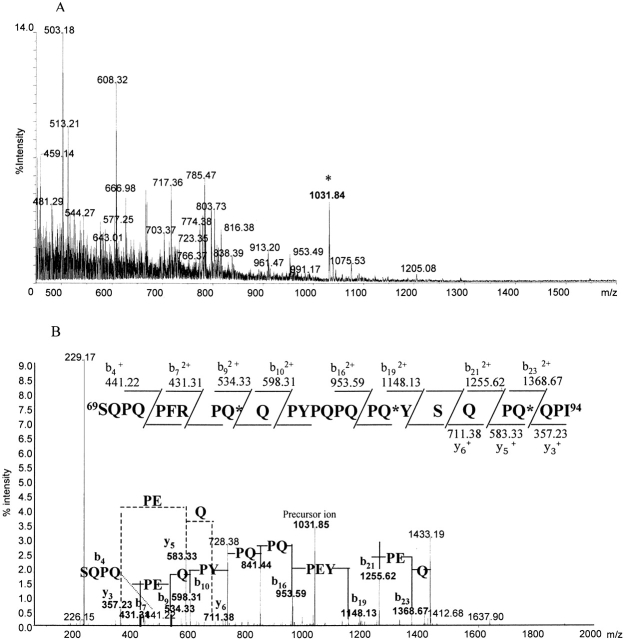
Elastase peptide mixture after tTGase reaction was submitted to tandem mass spectrometric experiments. Figure 3A ▶ reports MS spectrum containing all of the multiply charged ions originating from gliadin peptides. Doubly or triply charged ions of modified peptides, as identified previously by MALDI-TOF-MS analyses, were selected for CID experiments.
Figure 3.
Tandem mass spectrometric experiments carried out on the peptide mixture obtained by elastase digestion of the recombinant α-gliadin after tTGase treatment. (A) MS spectrum, the triply charged ion originating from peptide 69–94, is indicated by an asterisk. (B) MS/MS spectrum obtained from the fragmentation of the precursor ion at m/z 1031.51. The peptide sequence and interpretation is reported.
The results obtained by nanoESI-MS/MS experiments are summarized in Table 1. Through CID experiments carried out on the peptides present in the elastase digest treated with tTGase, 17 Q residues transformed in E residues by the enzymatic reaction have been identified. Unfortunately, it was not possible to detect peptides originating from residue 140 to residue 197 of the protein sequence containing the remaining 18 Q residues.
Table 1.
Identification of tTGase-modified peptides by MALDI-MS and definition of the deamidated glutamine residues present in the recombinant α-gliadin by nanoESI-MS/MS experiments
| MH+ | Precursor ion | Peptide | Deamidation | Sequence |
| 1560.88 | 780.96 MH22+ | 1–14 | 2 | VRVPVPQ*LQPQ*NPS |
| 2738.49 | 913.49 MH33+ | 1–24 | 4 | VRVPVPQ*LQPQ*NPSQQ*QPQEQ*VPL |
| 3497.85 | 950.00 MH44+ | 1–30 | 5 | VRVPVPQ*LQPQ*NPSQQ*QPQEQ*VPLVQQQQF |
| 1461.82 | 731.45 MH22+ | 2–14 | 2 | RVPVPQ*LQPQ*NPS |
| 2925.35 | 975.76 MH33+ | 31–55 | 3 | LGQQ*QPFPPQ*QPYPQPQPFPSQ*QPY |
| 2295.11 | 765.72 MH33+ | 32–51 | 2 | GQQ*QPFPPQ*QPYPQPQPFPS |
| 1569.84 | 785.47 MH22+ | 56–68 | 1 | LQLQPFPQPQ*LPY |
| 1404.75 | 702.87 MH2+2+ | 56–67 | 0 | LQLQPFPQPQLP |
| 1455.74 | 728.37 MH22+ | 57–68 | 1 | QLQPFPQPQ*LPY |
| 2149.09 | 717.03 MH33+ | 69–86 | 1 | SQPQPFRPQ*QPYPQPQPQ |
| 3092.55 | 1031.51 MH33+ | 69–94 | 3 | SQPQPFRPQ*QPYPQPQPQ*YSQPQ*QPI |
| 2312.13 | 771.33 MH33 | 70–88 | 1 | QPQPFRPQ*QPYPQPQPQYS |
| 1464.75 | 732.87 MH22+ | 75–86 | 1 | RPQ*QPYPQPQPQ |
| 2408.22 | 803.41 MH33+ | 75–94 | 3 | RPQ*QPYPQPQPQ*YSQPQ*QPI |
| 960.49 | 959.49 MH+ | 87–94 | 1 | YSQPQ*QPI |
| 1386.72 | 693.86 MH22+ | 95–105 | 0 | SQQQQQQQQQQ |
| 3262.47 | 816.37 MH44+ | 95–120 | 0 | SQQQQQQQQQQQQQQQQQQQQILQQI |
| 1311.72 | 656.87 MH22+ | 118–128 | 1 | QQILQQQLIPC |
| 943.63 | 942.63 MH+ | 121–128 | 1 | LQQQLIPC |
| 1267.67 | 634.34 MH22+ | 129–139 | 0 | MDVVLQQHNIA |
| 1592.83 | 796.83 MH22+ | 198–211 | 2 | SFQ*QPLQQ*YPLGQG |
| 1591.82 | 531.27 MH33+ | 199–212 | 1 | FQQPLQQ*YPLGQGS |
| 1444.73 | 722.86 MH22+ | 200–212 | 1 | QQPLQQ*YPLGQGS |
| 889.45 | 445.23 MH22+ | 204–211 | 0 | QQYPLGQG |
| 1444.71 | 722.85 MH22+ | 212–224 | 0 | SFRPSQQNPQAQG |
| 1531.74 | 766.87 MH22+ | 212–225 | 0 | SFRPSQQNPQAQGS |
| 1984.99 | 662.34 MH33+ | 212–229 | 1 | SFRPSQQNPQAQ*GSVQPQ |
| 2970.50 | 990.84 MH33+ | 212–237 | 2 | SFRPSQQNPQAQ*GSVQPQQ*LPQFEEI |
| 1456.76 | 728.88 MH22+ | 226–237 | 1 | VQPQQ*LPQFEEI |
| 1096.67 | 548.84 MH22+ | 238–247 | 0 | RNLALQTLPA |
| 2351.10 | 784.36 MH33+ | 248–268 | 0 | MCNVYIPPYCTITPFGIFGTN |
The deamidation sites are indicated with an asterisk. It was not possible to define which of the underlined Q residues is deamidated in peptide 1–30 and 118–128.
Representing an example of mass spectrometry applied to identify tTGase-modified Q residues, the analysis of the peptide 69–94 is reported in detail.
The triply charged ion at m/z 1031.51 (Fig. 3A ▶), generated by the deamidated peptide 69–94, is shifted by 1 Da/z compared with the m/z value of the unmodified peptide (m/z 1030.49). This confirms that this peptide contains three modification sites (as already determined by MALDI-TOF-MS, Figs. 1 and 2 ▶ ▶) of 10 Q residues present in the peptide sequence (Table 1).
To identify the deamidation sites, the precursor ion at m/z 1031.51 was submitted to CID experiments, and Figure 3B ▶ reports the MS/MS spectrum obtained together with the sequence of the peptide and the fragment ions observed, which were diagnostic for determining the modification sites.
The m/z difference between the singly charged ions y5 (m/z 583.33) and y3 (m/z 357.23) was 226 Da/z, which corresponds to the sum of the molecular weights of P and E residues, thus demonstrating that the Q91 has been enzymatically converted in E. This was also confirmed by the m/z difference of 113 Da/z between the doubly charged ions b23 (m/z 1368.67) and b21 (m/z 1255.62). The m/z difference between doubly charged ions b19 (m/z 1148.13) and b16 (m/z 953.59) was 194.5 Da/z, and it corresponds to half of the sum of the molecular weight of P, E, and Y residues, thus showing that Q86 is deamidated. Finally, Q77 is also modified by the enzyme as indicated by the m/z difference between the doubly charged ions b9 (m/z 534.33) and b7 (m/z 421.31), which is 113 Da/z, corresponding to half of the sum of the molecular weights of P and E residues.
Determination of the complete sequence of the peptide confirmed that the other seven Q residues present in the peptide were not modified.
Structural analyses of gliadin syntetic peptides by mass spectrometry
A set of 26 synthetic peptides, formed by 20 amino acids, overlapping by 10 residues and spanning the entire protein sequence of the recombinant α-gliadin were analyzed before and after tTGase treatment by MALDI-TOF-MS and by nanoESI-MS/MS to determine the deamidation sites. The results obtained confirmed the modification sites identified previously on the recombinant protein and revealed two additional deamidation sites, Q148 and Q191, in the region 140–197 of α-gliadin (Table 2).
Table 2.
Identification of deamidation sites in the 26 synthetic peptides overlapping the entire sequence of the recombinant α-gliadin by nanoESI-MS/MS experiments
| Theoretical MW | Precursor ion (MH33+) | Peptide | Sequence | Deamidation | Modified Q |
| 2296.192 | 767.06 | 1–20 | VRVPVPQLQPQNPSQQQPQE | 2 | 7/11 |
| 2328.146 | 777.71 | 10–29 | PQNPSQQQPQEQVPLVQQQQ | 2 | 21/16 |
| 2335.196 | 780.40 | 20–39 | EQVPLVQQQQFLGQQQPFPP | 3 | 21/27 or 28/34 |
| 2368.164 | 791.09 | 30–49 | FLGQQQPFPPQQPYPQPQPF | 2 | 34/40 |
| 2411.191 | 805.06 | 40–59 | QQPYPQPQPFPSQQPYLQLQ | 1 | 52 |
| 2355.19 | 786.63 | 50–69 | PSQQPYLQLQPFPQPQLPYS | 2 | 52/65 |
| 2376.201 | 793.75 | 60–79 | PFPQPQLPYSQPQPFRPQQP | 1 | 65 |
| 2438.171 | 814.40 | 70–89 | QPQPFRPQQPYPQPQPQYSQ | 2 | 77/86 |
| 2397.135 | 800.70 | 80–99 | YPQPQPQYSQPQQPISQQQQ | 2 | 86/91 |
| 2461.169 | 90–109 | PQQPISQQQQQQQQQQQQQQ | 0 | ||
| 2549.233 | 100–119 | QQQQQQQQQQQQQQQQILQQ | * | ||
| 2436.260 | 110–129 | QQQQQQILQQILQQQLIPCM | * | ||
| 2303.210 | 769.45 | 120–139 | ILQQQLIPCMDVVLQQHNIA | 1 | 123 or 124 |
| 2256.172 | 130–149 | DVVLQQHNIAHGRSQVLQQS | 0 | ||
| 2324.125 | 766.04 | 141–160 | GRSQVLQQSTYQLLQELCCQ | 1 | 148 |
| 2459.161 | 150–169 | TYQLLQELCCQHLWQIPEQS | 0 | ||
| 2408.217 | 160–179 | QHLWQIPEQSQCQAIHKVVH | 0 | ||
| 2349.281 | 784.01 | 170–189 | QCQAIHKVVHAIILHQQQKQ | 0 | |
| 2322.208 | 775.40 | 180–199 | AIILHQQQKQQQQPSSQVSF | 1 | 191 |
| 2287.123 | 764.04 | 190–209 | QQQPSSQVSFQQPLQQYPLG | 2 | 200/205 |
| 2300.132 | 768.04 | 200–219 | QQPLQQYPLGQGSFRPSQQN | 1 | 205 |
| 2168.031 | 724.01 | 210–229 | QGSFRPSQQNPQAQGSVQPQ | 1 | 223 |
| 2293.140 | 766.05 | 220–239 | PQAQGSVQPQQLPQFEEIRN | 2 | 223/230 |
| 2314.181 | 230–249 | QLPQFEEIRNLALQTLPAMC | 0 | ||
| 2223.114 | 240–259 | LALQTLPAMCNVYIPPYCTI | 0 | ||
| 2116.034 | 250–268 | NVYIPPYCTITPFGIFGTN | 0 |
* Peptides not analyzed due to solubility problem are indicated with an asterisk. It was not possible to define which of the underlined Q residues is deamidated in peptides 20–39 and 120–139.
Discussion
Advanced mass-spectrometric methodologies have been applied extensively to achieve a detailed characterization of structural modifications induced by tTGase on peptides originating from a recombinant α-gliadin. The enzymatic reaction converts glutamine residues in glutamic acid, thereby increasing molecular mass of the transformed peptides of 1 Da for each modified residue. It was therefore necessary to use high-resolution mass-spectrometric techniques for the identification of deamidation sites. Our experiments were performed on a MALDI-TOF instrument equipped with reflectron and delay extraction and on a novel hybrid quadrupole/time-of-flight mass spectrometer equipped with a nanospray source (Mann and Talbo 1996; Morris et al. 1997).
Recent studies, most of which have been carried out on synthetic peptides, have reported on site specificity of tTGase-mediated deamidation (Novak et al. 2002; Piper et al. 2002; Vader et al. 2002). Our data are not only in good agreement with recently published results but also serve as an extension of information, as they allow to gather a global view of all structural modifications induced by the enzyme on the whole α-gliadin.
Moreover, aimed to obtain peptides appropriate for MS/MS analyses and covering almost the entire protein sequence, the recombinant α-gliadin has been digested by elastase, thus producing a complex peptide mixture. In fact, several peptides exhibiting different lengths were originated from regions of the proteins 1–94 and 198–237, due to the low enzyme specificity. The analyses of such overlapping peptides were relevant for gaining information on the structural requirement of tTGase action.
These studies led to the identification of 17 deamidation sites, whereas peptides originating from region 140–197 could not be disclosed in these experiments. The analyses of the synthetic peptides allowed us to confirm the previously defined deamidation sites and to add two more modified glutamine, Q148 and Q191.
A high percentage (20%) of all of the glutamine residues present in this protein is deamidated. Moreover, although the Q residues are almost evenly scattered throughout the entire primary structure of the protein, the deamidated Q residues are present mostly in the amino-terminal region (residues 1–94). Notably, in the long stretch of polyglutamine (95–115) present in the recombinant protein, there are no deamidation sites, as clearly demonstrated by tandem mass-spectrometric analyses carried out on peptides 95–105 and 95–120, generated by elastase digestion of recombinant gliadin.
Analyzing peptides covering the entire protein sequence, and of different lengths, it could be possible to evaluate the influence of the spacing of the target Q residues from amino and carboxyl termini. In fact, whereas peptide 69–94 includes three modification sites (Q77, Q86, Q91), one modified Q residue is present in the peptides 69–86 (Q77) and 70–88 (Q77), whereas Q91 is deamidated in the peptide 87–94. This seems to indicate that the target Q residue should be at least three residues away from the carboxyl terminus. On the other hand, Q residues that are neither present in amino-terminal nor in +2 position are modified, as highlighted by our results from the analysis of peptides originating from region 198–212. Peptide 198–211, in fact, has two deamidation sites (Q200 and Q205), whereas peptide 204–211 has no deamidation site, and only Q205 is deamidated in peptides 199–212 and 200–211.
Our results also evidence a strong influence of P in guiding deamidation, and as already reported (Vader et al 2002), identified Q residues in a QXP sequence as a preferential target of tTGase-mediated reaction. Actually, 13 Q residues of the 19 deamidated by tTGase-mediated reaction are in a QXP sequence, apart from the amino acid residue in position +3, for which there is a high variability. Without exception, Q residues followed in the peptide sequence by a proline residue or in a QXXP sequence are never deamidated (Fig. 4 ▶).
Figure 4.
Amino acid sequence of the recombinant α-gliadin. The QXP sequence deamidated by tTGase are underlined.
Moreover, the presence of a negatively charged residue (E residue) in a QXP sequence introduces in peptides structural features that agree with the peptide-binding motif of natural HLA-DQ2 ligands; that is, requiring specific residues in anchor positions, and in particular, preferentially a negative charge at p4, a proline residue at p6, and another negative charge or hydrophobic residue at p7 (Johansen et al. 1996; Kwok et al 1996; van der Wal et al. 1996). In our studies, there are examples of deamidated gliadin peptides containing the EXP sequence and a hydrophobic residue, such as F, Y, I, L at p7. Further, there are also examples of deamidated peptides exhibiting structural features in agreement with HLA-DQ8-binding motif (negatively charged residue in p1 and p9; Wucherpfenning 2001), such as the 20-mer peptide 1–20 (deamidated in Q7, Q16) and the 25-mer peptides 69–94 (deamidated in Q77, Q86). The binding motif is also present in the only HLA-DQ8-restricted gliadin epitope, reported previously as peptide 205–216 of an α-gliadin (van der Wal et al. 1998a).
The majority of celiac patients express HLA-DQ2 (<90% in most ethnic groups) and/or HLA-DQ8 molecules, and there is evidence that peptides that are not recognized in their native forms by T-cell clones isolated from intestinal biopsies of patients are able to trigger the immune response in their deamidated forms (Sollid 2000). These findings are strong indications that tTGase plays an essential role in the onset and development of CD by probably generating gliadin peptides that cause an increased immune response due to a stronger binding affinity to HLA-DQ2/DQ8 molecules.
Our results show that tTGase action on gliadin peptides is highly site specific and widely influenced by structural features present around the target glutamine residue, and confirm the key role of proline residue in guiding tTGase deamidation reaction. Our findings proved directly the presence of many deamidation sites in the recombinant α-gliadin and indicate their widespread distribution along the whole α-gliadin sequence. The presence of many potential deamidation sites has been already predicted for a γ-gliadin on the basis of tTGase specificity. Nevertheless, immunological analyses carried out on a set of 20-mer synthetic peptides covering nearly the complete protein sequence demonstrated that 8 peptides of 23 were recognized after tTGase treatment by T-cell lines generated from intestinal biopsies of CD patients (Fleckenstein et al. 2002). Moreover, recently, a 33-mer gliadin peptide was found to react with tTGase and was suggested as being the primary initiator of the inflammatory response to gluten (Shan et al. 2002). These findings and the centrality of this 33-mer, representing a small and well-located gliadin region in the pathogenesis of CD, lead to hypothize that more complex molecular mechanisms could be involved in T-cell recognition than tTGase-mediated modification of gliadin peptides.
Notwithstanding the several studies aimed to identify immunodominant gliadin peptides, few γ and α gliadin epi-topes have been determined to date (Arentz-Hansen et al. 2002). Therefore, immunological studies are in progress to identify epitopes originating from the recombinant α-gliadin and to better define the crucial role of the enzymatic deamidation in enhancing the immunogenicity of modified gliadin peptides. The identification of immunodominant epitope will represent the basis of alternative therapies aimed to restore in celiac patients a tolerance to gliadin by means of immunomodulatory protocols.
Materials and methods
Materials
The recombinant α-gliadin (EMBL accession no. AJ130948) was cloned and expressed in Escherichia coli (Arentz-Hansen et al. 2000b; S. Senger and M. Rossi, unpubl.). Guinea-pig liver tissue transglutaminase, trypsin, chymotrypsin and elastase, dithiothreitol (DTT), iodoacetamide, α-cyano-4-hydroxycinnamic acid, angiotensin, and ACTH were purchased from Sigma. The RP-HPLC columns were from Vydac, whereas the C18 ZipTip pipette tips were from Millipore. Nanotips used for nanoESI-MS/MS experiments were from Protana. All of the other reagents and solvents were of the highest purity available from Carlo Erba.
Reduction and alkylation of recombinant α-gliadin
Protein samples were reduced in 0.1 M Tris-HCl (pH 8.5), containing 100 mM EDTA and 6 M guanidinium chloride with a 10:1 molar excess of DTT over the SH groups for 2 h at 37°C in nitrogen atmosphere. Alkylation of cysteine residues was carried out in the same buffer with a 5:1 molar excess of iodoacetamide over the total SH groups for 30 min at room temperature in the dark. Alkylated protein samples were purified by RP-HPLC on a Vydac C18 column (25 × 0.46 cm, 5 μ) using a Waters HPLC instrument and an elution system consisting of 15% isopropyl alcohol, 0.1% trifluoroacetic acid (TFA, solvent A), and 0.07% TFA in 95% acetonitrile (ACN, solvent B). The protein was eluted by means of a linear gradient from 30% to 60% solvent B over 30 min. Fraction containing α-gliadin was collected and analyzed by electrospray mass spectrometry on a single quadrupole instrument (Platform, Micromass) to verify the purity and identity of the sample. Protein samples were dried in a Speed-Vac centrifuge (Savant), lyophilized twice and stored at −20°C.
Production and analysis of synthetic peptides
A set of 26 synthetic peptides, 20 amino acids long, encompassing the entire protein sequence of the recombinant protein and overlapping by 10 amino acids were produced by solid-phase Fmoc synthesis by means of a Pioneer Peptide Synthesis System 9050 instrument (PE-Biosystems). Their purity was assessed by means of RP-HPLC analyses performed on a Vydac C18 (25 × 0.46 cm, 5 μ) column using 0.1% TFA (solvent A) and 0.07% TFA in 95% ACN (solvent B) and a linear gradient from 20% to 60% solvent B over 40 min, and their identity was assessed by means of MALDI-TOF analyses, performed as described below.
Enzymatic reactions
Elastase, chymotrypsin, and trypsin digestions were performed in 50 mM ammonium bicarbonate (pH 8.5), at 37°C for 2 h using an enzyme to substrate ratio of 1:100 w/w.
Deamidation reaction was carried out in 0.125 M Tris-HCl (pH 8.5), containing 2.5 mM calcium chloride and 10 mM DTT, using a concentration of 0.2 μg/μL for tTGase and of 2 μg/μL for the different substrates (synthetic peptides or gliadin peptide mixtures) for 4 h.
MALDI-TOF analyses
Gliadin peptides generated by enzymatic hydrolysis before and after tTGase treatment were analyzed by MALDI-TOF mass spectrometry. A total of 100 fmole of peptide mixtures mixed with α-cyano-4-hydroxycynnamic acid (10 mg/mL in 50% ACN, 50% H2O, containing 125 fmole/μL ACTH and 25 fmole/μL angiotensin as internal standards) were deposited onto MALDI sample probe and dried under ambient conditions. All mass spectra were generated on a MALDI-TOF mass spectrometer Voyager DE PRO (Applied Biosystems), operating in positive-ion reflectron mode. The laser intensity (N2, 337 nsec) was set just above the ion generation threshold and pulsed every 10 nsec. Mass spectra were acquired from each sample by accumulating 100 laser shots, and were calibrated using as internal standards the monoisotopic peak of angiotensin (m/z 931.5154) and that of ACTH (m/z 2465.1989), so that the error on the experimental mass measurements is <20 ppm, and therefore, in perfect agreement with the theoretical molecular weight of the analyzed peptides. All mass values are reported as monoisotopic masses.
Deisotoping spectra were obtained by elaborating the raw data by means of a specific program provided by the manufacturers.
Synthetic peptides were analyzed by use of the same experimental conditions.
Tandem mass-spectrometric experiments
Deamidation sites were defined by MS/MS experiments performed on hybrid quadrupole/orthogonal time-of-flight instrument (QStar Pulsar, Applied Biosystems) equipped with nanospray source. Samples containing 100 fmole of peptide mixtures obtained from enzymatic digestion of the recombinant protein or synthetic peptides, before and after tTGase treatment, were desalted by solid-phase extraction using C18 ZipTip pipette tips eluted in 1–2 μL of ACN/H20 (1:1 v/v) and introduced directly into nanospray tips. The Analyst QS software was used for data acquisition and processing. MS spectra were acquired in the m/z range 400–2000 and the mass selection window for precursor ion was set to 1–2 Da and centered on the 12C isotope of the pertinent ion. Helium gas served as the collision gas for the collision-induced dissociation (CID) experiments and MS/MS spectra, acquired in the m/z range 100–2000, were obtained setting collision energies variable in the range 20–40 V, just above the ion generation. All spectra were acquired with resolution between 8000–10,000 FWHM.
Acknowledgments
The present was supported by European Laboratory for the Investigation of Food-Induced Disease (ELFID). We thank Dr. L. Longorbardo and Dr. O. Fierro (Laboratorio di Sintesi Organica, Istituto di Scienze dell’Alimentazione, CNR, Avellino) for peptides synthesis, and M. De Vito for manuscript revision.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Abbreviations
CD, celiac disease
CID, collision induced dissociation
MALDI-TOF-MS, matrix-assisted laser desorption ionization–time of flight–mass spectrometry
MS/MS, tandem mass spectrometry
nanoESI-MS/MS, tandem mass spectrometric experiments performed on a hybrid quadrupole time-of-flight instrument equipped with nano-electrospray source
RP-HPLC, reverse phase high performance liquid chromatography
tTGase, tissue transglutaminase
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.03185903.
References
- Anderson, R.P., Degano, P., Godkin, A.J., Jewell, D.P., and Hill, A.V.S. 2000. In vivo antigen challenge in celiac disease identifies a single transglutaminase-modified peptide as the dominant A-gliadin T-cell epitope. Nat. Med. 6 337–342. [DOI] [PubMed] [Google Scholar]
- Arentz-Hansen, E.H., Körne, R., Molberg, Ø., Quarsten, H., Vader, W., Kooy, Y.M., Lundin, K.E., Koning, F., Roepstorff, P., Sollid, L.M., et al. 2000a. The intestinal T cell response to α-gliadin in adult celiac disease is focused on a single deamidated glutamine targeted by tissue transglutaminase. J. Exp. Med. 191 603–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arentz-Hansen, E.H., McAdam, S.N., Molberg, O., Kristiansen, C., and Sollid, L.M. 2000b. Production of a panel of recombinant gliadins for the characterization of T cell reactivity in coeliac disease. Gut 46 46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arentz-Hansen, E.H., McAdam, S.N.R., Molberg, Ø., Fleckenstein, B., Lundin, K.E., Jorgensen, T.J.D., Jung, G., Roepstorff, P., and Sollid, L.M. 2002. Celiac lesion T-cells recognize epitopes that cluster in regions of gliadins rich in proline residues. Gastroentoerology 123 803–809. [DOI] [PubMed] [Google Scholar]
- Booth, M.R. and Ewart, J.A. 1970. Modification of wheat gliadin by γ-rays. J. Sci. Food Agric. 21 145–147. [DOI] [PubMed] [Google Scholar]
- Dieterich, W., Enhis, T., Bauer, M., Donner, P., Volta, U., Riecken, E.O., and Schuppan, D. 1997. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat. Med. 3 797–801. [DOI] [PubMed] [Google Scholar]
- Dieterich, W., Laag, E., Schopper, H., Volta, U., Ferguson, A., Gillett, H., Riecken, E.O., and Schuppan, D. 1998. Autoantibodies to tissue transglutaminase as predictors of celiac disease. Gastroenterology 115 1317–1321. [DOI] [PubMed] [Google Scholar]
- Fleckenstein, B., Molberg, Ø., Qiao, S.W., Schmid, D.G., von der Mulbe, F., Elgstoen, K., Jung, G., and Sollid, L.M. 2002. Gliadin T cell epitope selection by tissue transglutaminase in celiac disease. Role of enzyme specificity and pH influence on the transamidation versus deamidation process. J. Biol. Chem. 277 34109–34116. [DOI] [PubMed] [Google Scholar]
- Folk, J.E. 1983. Mechanism and basis for specificity of transglutaminase-catalyzed ɛ-(γ-glutamyl) lysine bond formation. Adv. Enzymol. Relat. Areas Mol. Biol. 54 1–56. [DOI] [PubMed] [Google Scholar]
- Johansen, B.H., Jensen, T., Thorpe, C.J., Vartdal, F., Thorsby, E., and Sollid, L.M. 1996. Both α and β chain polymorphisms determine the specificity of the disease-associated HLA-DQ2 molecules, with β chain residues being most influential. Immunogenetics 45 142–150. [DOI] [PubMed] [Google Scholar]
- Kwok, W.W., Domeier, M.L., Raymond, F.C., Byers, P., and Nepom, G.T. 1996. Allele-specific motifs characterize HLA-DQ interactions with a diabetes-associated peptide derived from glutamic acid decarboxylase. J. Immunol. 156 2171–2177. [PubMed] [Google Scholar]
- Leblanc, A., Gravel, C., Labelle, J., and Keillor, J.W. 2001. Kinetic studies of guinea pig liver transglutaminase reveal a general-base-catalyzed deacylation mechanism. Biochemistry 40 8335–8342. [DOI] [PubMed] [Google Scholar]
- Lundin, K.E., Scott, H., Hansen, T., Paulsen, G., Halstensen, T.S., Fausa, O., Thorsby, E., and Sollid, L.M. 1993. Gliadin-specific, HLA-DQ(α 1*0501, β 1*0201) restricted T cells isolated from the small intestinal mucosa of celiac disease patients. J. Exp. Med. 178 187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann, M. and Talbo, G. 1996. Developments in matrix-assisted laser desorption/ionization peptide mass spectrometry. Curr. Opin. Biotechnol. 7 11–19. [DOI] [PubMed] [Google Scholar]
- Marsh, M.N. 1992. Gluten, major histocompatibility complex, and small intestine. A molecular and immunobiological approach to the spectrum of gluten sensitivity (‘celiac sprue’). Gastroenterology 102 330–354. [PubMed] [Google Scholar]
- Molberg, Ø., McAdam, S.N., Körner, R., Quarsten, H., Kristiansen, C., Madsen, L., Fugger, L., Scott, H., Noren, O., Roepstorff, P., et al. 1998. Tissue transglutaminase selectively modifies gliadin peptides, that are recognized by gut-derived T cells in celiac disease. Nat. Med. 4 713–717. [DOI] [PubMed] [Google Scholar]
- Morris, H.R., Paxton, T., Panico, M., McDowell, R., and Dell, A. 1997. A novel geometry mass spectrometer, the quadrupole ortogonal accelleration time of flight instrument, for low femtomole/attomole range biopolymer sequencing. J. Protein Chem. 16 469–479. [DOI] [PubMed] [Google Scholar]
- Novak, P., Man, P., Tuckova, L., Tlaskalova-Hogenova, H., Bezouska, K., and Havlicek, V. 2002. Monitoring of in vitro deamidation of gliadin peptic fragment by mass spectrometry may reflect one of the molecular mechanisms taking place in celiac disease development. J. Mass Spectrom. 37 507–511. [DOI] [PubMed] [Google Scholar]
- Piper, J.L., Gray, G.M., and Khosla, C. 2002. High selectivity of human tissue transglutaminase for immunoactive gliadin peptides: Implications for celiac sprue. Biochemistry 41 386–393. [DOI] [PubMed] [Google Scholar]
- Pucci, P., Malorni, A., Marino, G., Metafora, S., Esposito, C., and Porta, R. 1988. β-endorphin modification by transglutaminase in vitro: Identification by FAB/MS of glutamine-11 and lysine-29 as acyl donor and acceptor sites. Biochem. Byophys. Res. Commun. 154 737–740. [DOI] [PubMed] [Google Scholar]
- Quarsten, H., Molberg, Ø., Fugger, L., McAdam, S.N., and Sollid, L.M. 1999. HLA binding and T cell recognition of a tissue transglutaminase-modified gliadin epitope. Eur. J. Immunol. 29 2506–2514. [DOI] [PubMed] [Google Scholar]
- Shan, L., Molberg, O., Parrot, I., Hausch, F., Filiz, F., Gray, G.M., Sollid, L.M., and Khosla, C. 2002. Structural basis for gluten intolerance in celiac sprue. Science 297 2275–2279. [DOI] [PubMed] [Google Scholar]
- Sjöstrom, H., Lundin, K.E., Molberg, Ø., Körner, R., McAdam, S.N., Anthonsen, D., Quarsten, H., Noren, O., Roepstorff, P., Thorsby E., et al. 1998. Identification of a gliadin T-cell epitope in coelic disease: General importance of gliadin deamidation for intestinal T-cell recognition. Scand. J. Immunol. 48 111–115. [DOI] [PubMed] [Google Scholar]
- Sollid, M.L. 2000. Molecular basis of celiac disease. Annu. Rev. Immunol. 18 53–81. [DOI] [PubMed] [Google Scholar]
- Spurkland, A., Ingvarsson, G., Falk, E.S., Knutsen, I., Sollid, L.M., and Thorsby, E. 1997. Dermatitis herpetiformis and celiac disease are both primarily associated with the HLA-DQ (α 1*0501, β 1*02) or the HLA-DQ (α 1*03, β 1*0302) heterodimers. Tissue Antigens 49 29–34. [DOI] [PubMed] [Google Scholar]
- Vader, L.W., de Rue, A., van der Wal, Y., Kooy, Y.M.C., Benckhuijsen, W.E., Mearin, M.L., Drijfhout, J.W., van Veelen, P., and Koning, F. 2002. Specificity of tissue transglutaminase explains cereal toxicity in celiac disease. J. Exp. Med. 195 643–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wal, Y., Kooy, Y.M., Drijfhout, J.W., Amons, R., and Koning, F. 1996. Peptide binding characteristics of the celiac disease-associated DQ(α1*0501, β1*0201) molecule. Immunogenetics 44 246–253. [DOI] [PubMed] [Google Scholar]
- van der Wal, Y., Kooy, Y., van Veelen, P., Peňa, S.A., Mearin, L.M., Molberg, Ø., Lundin, K.E., Sollid, L.M., Mutis, T., Benckhuijsen, et al. 1998a. Small intestinal T cells of celiac disease patients recognize a natural pepsin fragment of gliadin. Proc. Natl. Acad. Sci. 95 10050–10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wal, Y., Kooy, Y., van Veelen, P., Pena, S., Mearin, L., Papadopoulos, G., and Koning, F. 1998b. Selective deamidation by tissue transglutaminase strongly enhances gliadin-specific T cell reactivity. J. Immunol. 161 1585–1588. [PubMed] [Google Scholar]
- Wieser, H., Mödl, A., Seilmeier, W., and Belitz, H.D. 1987. High-perfomance liquid chromatography of gliadins from different wheat varieties: Amino acid composition and N-terminal amino acid sequence of components. Z. Lebens. Unters. Forsch. 185 371–378. [DOI] [PubMed] [Google Scholar]
- Wucherpfenning, K.W. 2001. Insights into autoimmunity gained from structural analysis of MCH-peptide complexes. Curr. Opinion Immunol. 13 650–656. [DOI] [PubMed] [Google Scholar]
- Yergey, J.A. 1983. A general approach to calculating isotopic distributions for mass spectrometry. Int. J. Mass Spectrom. Ion Phys. 52 337–349. [DOI] [PubMed] [Google Scholar]