Abstract
This study examined the efficacy of subthalamic nucleus (STN), deep brain stimulation (DBS), and medication for resting tremor during performance of secondary tasks. Hand tremor was recorded using accelerometry and electromyography (EMG) from 10 patients with Parkinson’s disease (PD) and ten matched control subjects. The PD subjects were examined off treatment, on STN DBS, on medication, and on STN DBS plus medication. In the first experiment, tremor was recorded in a quiet condition and during a cognitive task designed to enhance tremor. In the second experiment, tremor was recorded in a quiet condition and during isometric finger flexion (motor task) with the contralateral limb at 5% of the maximal voluntary contraction (MVC) that was designed to suppress tremor. Results showed that: (1) STN DBS and medication reduced tremor during a cognitive task that exacerbated tremor, (2) STN DBS normalized tremor frequency in both the quiet and cognitive task conditions, whereas tremor amplitude was only normalized in the quiet condition, (3) a secondary motor task reduced tremor in a similar manner to STN DBS. These findings demonstrate that STN DBS still suppresses tremor in the presence of a cognitive task. Furthermore, a secondary motor task of the opposite limb suppresses tremor to levels comparable to STN DBS.
Keywords: tremor, Parkinson’s disease, deep brain stimulation, subthalamic nucleus, secondary task
The cardinal signs of Parkinson’s disease (PD) are bradykinesia, rigidity, and tremor. It is well known that these signs worsen when patients perform two tasks simultaneously.1 Sturman et al.2 showed that STN DBS, unlike medication, reduced resting tremor amplitude in patients with PD to be comparable to healthy physiological tremor. However, the tremor testing condition was a quiet, controlled, laboratory setting free from secondary tasks. A characteristic of resting tremor is that its amplitude is enhanced by a cognitive secondary task, such as backward counting,3 and a cognitive task is often used during tremor evaluation. Since the STN has been hypothesized to play a role in tremor genesis4 and a cognitive task enhances tremor,3,5 STN DBS could exacerbate the cognitive circuitry responsible for enhancing parkinsonian tremor. In contrast, a motor task has been shown to improve tremor in patients with mild PD.6
Thus, the purpose of this study was twofold. The first experiment determined the effects of STN DBS and medication on tremor with and without a cognitive task and determined if STN DBS normalized tremor with and without a cognitive task. The second experiment compared the effects of STN DBS, medication, and a motor task, all known to reduce tremor, with the purpose of determining the comparative efficacy of each of the manipulations.
METHODS
Subjects
Ten consecutively chosen PD patients and ten age and gender matched control subjects participated in the study (Table 1). Patients were examined by a movement disorders neurologist and included in the study if they: (1) had PD as outlined by the Parkinson’s Disease Society Brain Bank diagnostic criteria,9,10 and (2) were deemed to have resting and/or postural tremor which is consistent with the Movement Disorders Consensus Statement on Tremor Type I or II classification.3 All subjects gave written informed consent consistent with the Declaration of Helsinki, which was approved by the local Institutional Review Board.
TABLE 1.
Patient characteristics
| Post-surgical UPDRS* Off Treatment
|
DBS Parameters
|
Anti-Parkinsonian Medications
|
|||||
|---|---|---|---|---|---|---|---|
| Age (years) | Gender | Item 20 | Item 21 | Stim Level (volts) | Electrode Contacts | Dose Prior to Testinga (mg) | Total Daily Doseb (mg/day) |
| 66 | F | 3 | 3 | 2.8 | 1−C+ | 320 | 960 |
| 64 | F | 3 | 3 | 3.0 | 1−C+ | 250 | 750 |
| 66 | F | 1 | 1 | 3.6 | 1−C+ | 370 | 1270 |
| 67 | F | 3 | 3 | 2.3 | 0−C+ | 280 | 780 |
| 72 | F | 4 | 2 | 4.0 | 0−C+ | 150 | 450 |
| 64 | F | 2 | 2 | 3.6 | 0−1−2−3+ | 250 | 850 |
| 50 | M | 3 | 3 | 2.5 | 1−C+ | 400 | 2400 |
| 64 | M | 1 | 1 | 1.7 | 1−C+ | 467 | 4200 |
| 57 | M | 1 | 0 | 3.0 | 2−C+ | 517 | 4667 |
| 70 | M | 3 | 0 | 3.5 | 1−C+ | 300 | 900 |
In 9 cases, the stimulation electrode was placed unilaterally contralateral to the most affected side, and 1 patient had electrodes implanted bilaterally. For this patient the most affected limb was studied (right limb), and the right DBS was deactivated 16 hr prior to testing and remained off throughout the entire 4 day study. Subsequently, only the left stimulator of this patient was manipulated during the 4 day study.
Surgery was performed as part of standard clinical care.11,12 Following surgery, stimulation parameters were optimized over several visits by the same movement disorders neurologist to reduce pre-surgery scores on the motor section of the UPDRS (Table 1). Pre- and post-surgery anti-Parkinsonian medications were also optimized to reduce scores on the motor section of the UPDRS, specifically bradykinesia, tremor, and rigidity (Table 1). At the time of the experiments, the stimulation frequency was 185 Hz for all patients, and in all but four cases the pulse width was 60 μs. For subjects 3, 5, 6, and 9 the pulse width was 90 μs.
Procedures
For experiment 1 patients were tested on four consecutive days in the morning off treatment, on STN DBS, on medication (pre-surgery dose, Table 1), and on medication plus STN DBS. For experiment 2 patients were tested off treatment, on STN DBS, and on medication (pre-surgery dose, Table 1). Even though the dosages of anti-Parkinsonian medications were reduced for each of the patients post-surgery, we chose to study pre-surgery dosages of medication to ensure an adequate comparison between STN DBS and anti-Parkinsonian medications. The order of treatment conditions was randomized across subjects. The control subjects were tested on one morning.
Patients were tested after a 12 hr withdrawal from the specific treatment.13,14 Each patient was examined using a Medtronic Console Programmer (Model 7432) to ensure that the stimulator was off. STN DBS and medication were active for at least 12 hr prior to testing.
Apparatus
Subjects sat in a chair and the forearm, which was contralateral to the stimulator, was supported by the arm of the chair.15 A Coulbourn (V 94–41) uni-dimensional accelerometer was taped to the hand (2 cm proximal to the middle of the second metacarpophalangeal joint), and primarily measured the tremor that occurred in the vertical plane. A Coulbourn amplifier (V72–25A) amplified the acceleration signal in AC mode. The accelerometer’s resolution was 0.01 m/s2. A 12 bit A/D converter sampled the signal at 1,000 samples per second. Surface EMG of the extensor digitorum and the flexor digitorum superficialis was measured. The EMG signal was amplified (gain = 1,000) and bandpass filtered between 20 and 450 Hz (Delsys, Boston, MA).
Protocol
Subjects were instructed to relax their forearm and hand muscles, and allow the wrist to dangle unsupported over the edge of a supportive surface for 30 s.2,15,16 Subjects performed three trials for each treatment condition in experiments 1 and 2. Throughout each trial subjects were instructed not to suppress tremor.
In experiments 1 and 2, the quiet condition was conducted without mental or physical stressors. Subjects viewed a visual display on a computer monitor, which consisted of a stationary cursor and a target.
In experiment 1, in addition to the quiet condition, subjects counted backwards out loud from 100 by multiples of 3, 4, 6, or 7 (cognitive task). The order of counting conditions was randomized across trials. During the counting task, subjects viewed the same display computer as the quiet condition, except the cursor and target flashed every second to pace the counting of the subjects.
In experiment 2, in addition to the quiet condition, subjects performed sustained isometric index finger flexion with the hand ipsilateral to the stimulator (motor task). Isometric finger flexion was selected to examine the effect of a continuous descending motor command on tremor in the contralateral limb. Subjects were required to sustain a force, which was equal to 5% of their MVC (measured off treatment) against an Entran load cell (sensitivity = 190 mV/N) throughout the trial. Subjects received visual feedback from a display computer monitor identical to the quiet and cognitive conditions except that the cursor moved to the target position when the subject produced force. A high control-to-display visual feedback gain level was used.17 As such, small changes in the amount of force produced caused large cursor movement, which ensured that subjects attended to the display to maintain the cursor at the target.
Data Analysis
The acceleration and EMG data were first conditioned by the following methods.2,13,18 The EMG signal was full-wave rectified and then both the acceleration and EMG data were downsampled to 200 Hz. The acceleration and EMG data were digitally filtered using a 4th order Butterworth filter with a lowpass cutoff frequency of 60 Hz for the EMG signals and 30 Hz for the acceleration signal. All data analyses were performed on the entire 30 s data segment—the same for each subject, condition, and trial.
Four derived measures of tremor were used. Full details of the calculation of each measure can be found in previous work.2 First, the amplitude of tremor was calculated and is reported in centimeter. Second, the regularity of tremor was quantified using approximate entropy (ApEn).19 This algorithm returns a value between 0 for highly regular signals (e.g. sine wave) and approximately 2 for irregular signals (e.g. white noise). Third, we calculated the coherence between the acceleration and EMG signals as an indirect estimate of the degree of motor unit entrainment affecting the acceleration signal.20 Finally, the dominant frequency in the EMG spectrum was examined. Each of these four derived measures of tremor were averaged across the three trials in each condition, and the average value for each of the four measures was subsequently analyzed in separate analyses of variance as described below.
Statistical Analysis
The clinical UPDRS hand tremor (item 20) scores were evaluated using a Wilcoxon matched pairs test. The dependent variables were evaluated using analyses of variance (ANOVA). For experiment 1 we used a within subject Medication (Off vs. On) by STN DBS (Off vs. On) by Task (Quiet vs. Cognitive) ANOVA and a between subject Group (PD on STN DBS vs. Control) by Task (Quiet vs. Cognitive) ANOVA. For experiment 2 we used a one factor within subject Treatment (Off vs. Motor vs. STN DBS vs. Medication) ANOVA. Significance was set at P < 0.05.
RESULTS
Clinical Effects
All 10 patients demonstrated positive clinical benefits from STN DBS (Table 2). Compared to off treatment, STN DBS [z = 2.8, P < 0.01], Meds [z = 2.52, P < 0.05], and STN DBS plus Meds [z = 2.8, P < 0.01] reduced unilateral, hand tremor UPDRS scores for item 20.
TABLE 2.
Post-surgical unilateral UPDRS scoresa
| Resting Tremor (item 20) | |
|---|---|
| Off | 2.4 (1.07) |
| STN DBS | 0.20 (0.42) |
| Meds | 1.50 (1.18) |
| STN DBS + Meds | 0 (0) |
Scores averaged across subjects ± standard deviation.
Experiment 1—Amplitude
Figure 1A and B and Table 3 depict increased tremor amplitude during the cognitive task compared with the quiet condition, and show that medication and STN DBS decreased tremor amplitude. Table 3 shows a medication by STN DBS interaction and further analysis of this interaction using Tukey’s post hoc test showed that medication (P < 0.05) reduced amplitude in comparison to when patients were off medication and off STN DBS. Medication (P = 0.99) did not reduce amplitude when patients were on STN DBS. STN DBS (P < 0.01) reduced amplitude in comparison to when patients were off medication and off STN DBS. STN DBS (P < 0.01) also reduced amplitude when patients were on medication.
FIG. 1.
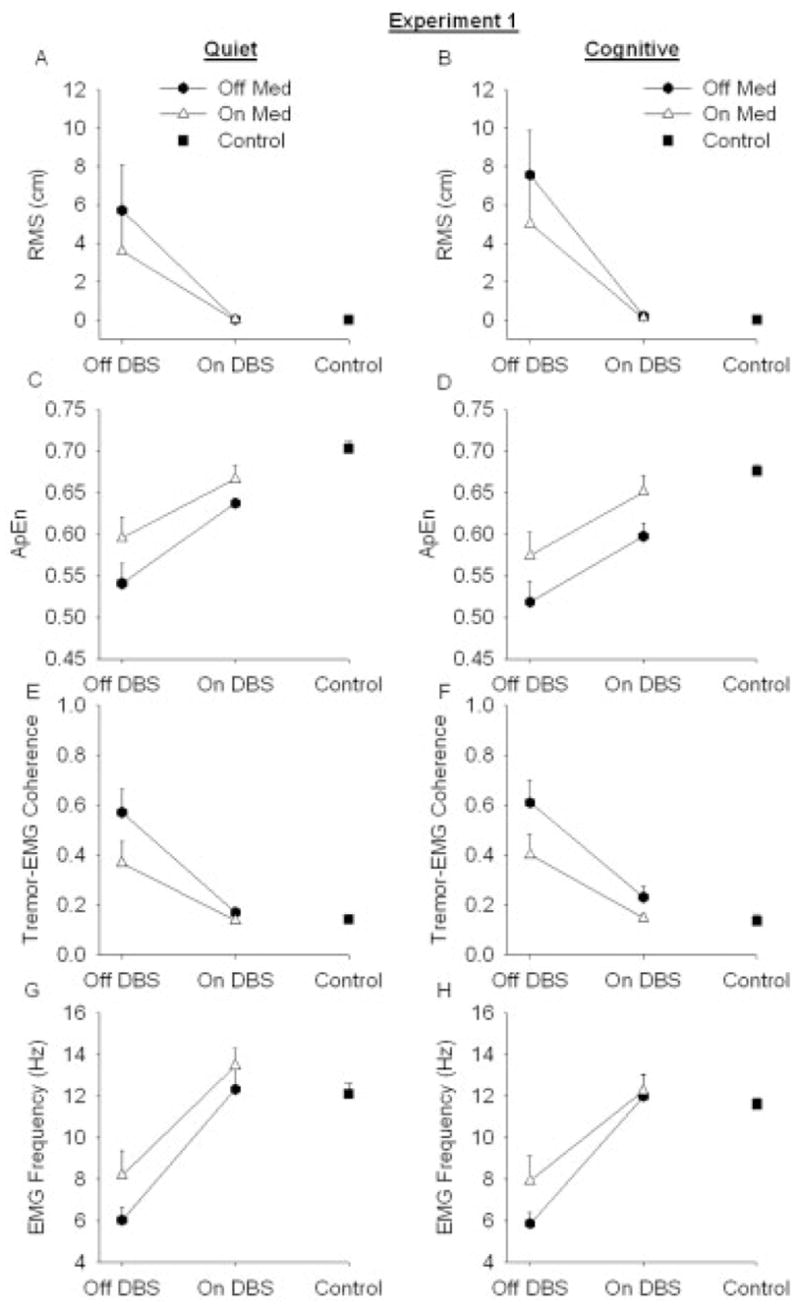
Resting tremor from PD patients (N = 10) off medication (closed hexagons), patients on medication (open triangles), and control subjects (closed squares) (N = 10). Each value was averaged across subjects (mean ± SE). (A) RMS amplitude during quiet task, (B) RMS amplitude during cognitive task, (C) Regularity (measured with ApEn) during quiet task, (D) Regularity (measured with ApEn) during cognitive task, (E) Tremor-EMG coherence during quiet task, (F) Tremor-EMG coherence during cognitive task, (G) Peak EMG frequency during quiet task, (H) Peak EMG frequency during cognitive task.
TABLE 3.
ANOVA results
| Amplitude | Regularity | Coherence | Frequency | |
|---|---|---|---|---|
| Experiment 1 | ||||
| ANOVA (i): Meds × DBS × Task | ||||
| Meds | P < 0.05 | P < 0.05 | P < 0.01 | P = 0.05 |
| DBS | P < 0.05 | P < 0.05 | P < 0.01 | P < 0.01 |
| Task | P < 0.05 | P < 0.05 | ns | ns |
| Meds × DBS Int | P < 0.05 | ns | P < 0.05 | ns |
| Meds × Task Int | ns | ns | ns | ns |
| DBS × Task Int | ns | ns | ns | ns |
| Meds × DBS × Task Int | ns | ns | ns | ns |
| ANOVA (ii): Group × Task | ||||
| Group | P < 0.01 | P < 0.01 | P < 0.05 | ns |
| Task | P < 0.05 | P < 0.05 | ns | ns |
| Group × Task Int | P < 0.05 | ns | ns | ns |
| Experiment 2 | ||||
| ANOVA: Treatment | P < 0.05 | P < 0.01 | P < 0.01 | P < 0.01 |
To determine if STN DBS reduced tremor amplitude to healthy levels, the STN DBS condition was compared with the control group under the quiet and cognitive task conditions. Table 3 shows an interaction between the group and task conditions. Further analysis of the interaction using Tukey’s post hoc test showed a significant difference between patients on STN DBS and healthy controls during the cognitive task (P < 0.01). Mean amplitude ± standard error from the control subjects during the cognitive condition was 0.02 cm ± 0.001 cm compared with 0.20 cm ± 0.06 cm for the patients. However, tremor amplitude during the quiet task was similar between the groups (P = 0.96). Mean amplitude ± standard error from the control subjects during the quiet condition was 0.01 cm ± 0.006 cm and amplitude was 0.03 cm ± 0.006 cm for the patients.
Experiment 1—Regularity
Figure 1C and D and Table 3 show that the cognitive task increased tremor regularity, and medication and STN DBS both decreased tremor regularity. To determine if STN DBS reduced tremor regularity to healthy levels, the STN DBS condition was compared with the control group under the quiet and cognitive task conditions. Patients on STN DBS exhibited more regular tremor compared with control subjects (Table 3), and the cognitive task increased regularity (Table 3). Mean ApEn ± standard error from the control subjects during the cognitive condition was 0.68 ± 0.008 compared with 0.59 ± 0.01 for the patients. Mean ApEn ± standard error from the control subjects during the quiet condition was 0.70 ± 0.008 compared with 0.63 ± 0.006 for the patients.
Experiment 1—Tremor-EMG Coherence
Figure 1E and F and Table 3 show that medication and STN DBS reduced tremor-EMG coherence. There was a medication by STN DBS interaction (Table 3). Further analysis of this interaction using Tukey’s post hoc test showed that medication (P < 0.01) reduced tremor-EMG coherence in comparison to when patients were off medication and off STN DBS. Medication (P = 0.42) did not reduce tremor-EMG coherence when patients were on STN DBS. STN DBS (P < 0.01) reduced tremor-EMG coherence when patients were off medication and off STN DBS. STN DBS (P < 0.01) also reduced tremor-EMG coherence when patients were on medication. To determine if STN DBS reduced tremor-EMG coherence to healthy levels, the STN DBS condition was compared with the healthy control group under the quiet and cognitive task conditions. Patients on STN DBS had higher tremor-EMG coherence compared with control subjects (Table 3). Mean tremor-EMG coherence ± standard error from the control subjects during the cognitive condition was 0.13 ± 0.02 compared with 0.23 ± 0.04 for the patients. Mean tremor-EMG coherence ± standard error from the control subjects during the quiet condition was 0.14 ± 0.009 compared with 0.17 ± 0.02 for the patients.
Experiment 1—Frequency
Figure 1G and H and Table 3 show that STN DBS and medication increased frequency. When frequency from PD patients on STN DBS was compared with healthy tremor, there was no difference between the groups in the quiet and cognitive task conditions (Table 3). Mean peak EMG frequency ± standard error from the control subjects during the cognitive condition was 11.60 ± 0.27 and it was 11.97 ± 1.07 for the patients. Mean peak EMG frequency ± standard error from the control subjects during the quiet condition was 12.11 ± 0.51 compared with 12.31 ± 0.94 for the patients.
Experiment 2
Figure 2 depicts amplitude, regularity, tremor-EMG coherence, and frequency from PD patients off treatment, on medication, off treatment performing the motor task, and on STN DBS. Treatment reduced amplitude, regularity, and tremor-EMG coherence, and increased frequency (Table 3). Further analysis of the treatment effect for amplitude, regularity, tremor-EMG coherence, and frequency using Tukey’s test showed a difference between the off treatment and STN DBS conditions (P’s < 0.03) and the off treatment and motor conditions (P’s < 0.05). In addition STN DBS (P < 0.01) increased frequency in comparison to the medication condition.
FIG. 2.

Resting tremor from PD patients (N = 10) off medication, on medication, performing the motor task, and on STN DBS. Each value was averaged across subjects (mean ± SE). (A) Amplitude, (B) Regularity (measured with ApEn), (C) Tremor-EMG coherence, and (D) EMG Frequency.
DISCUSSION
There were three novel findings from this study: (1) STN DBS and medication both reduced tremor during a cognitive task that exacerbated tremor, (2) STN DBS normalized frequency in the quiet and cognitive task conditions, whereas amplitude was only normalized in the quiet condition, (3) a secondary motor task reduced tremor in a similar manner to STN DBS.
STN and Cognitive Task
Our cognitive task was similar to neuropsychological tests that require suppression of habitual responses, such as counting backwards in increments of one, in order to intrinsically generate a response, which is known to involve the dorsolateral prefrontal cortex (DLPFC).21 The DLPFC receives thalamic projections from globus pallidus internal segment (GPi)/substantia nigra pars reticulata (SNr),22 and the dorsolateral prefrontal circuit also has connections to globus pallidus external segment (GPe).23,24 Since the STN has connections to both GPe and GPi/SNr it is able to regulate information processing at an intermediate level (GPe) and an output level (GPi/SNr).25–27 Therefore, it is possible that STN stimulation could modify DLPFC activation. This theory is supported by the finding that PD patients who received STN DBS had greater activation of the DLPFC than patients who received GPi stimulation.28 Our finding that STN DBS still successfully reduced tremor during the cognitive task lends support to the idea that STN DBS is not exacerbating the cognitive circuit(s) that enhance tremor. However, the fact that STN DBS was not able to approximate healthy levels for amplitude, regularity, and tremor-EMG coherence during the cognitive task suggests that the tremor circuitry was not normalized.
STN and Motor Task
The mechanism for tremor reduction in one hand during the performance of a motor task with the opposite hand may be due to transcallosal inhibition of the homologous motor cortex.6 For instance, tremor studies in PD using magnetoencephalography (MEG)29 and electroencephalography (EEG)30 have shown that the motor cortex and the corticospinal tract are integral parts of the subcortico-cortical tremor generator-loop. Therefore, the established inhibitory effects of a voluntary contraction on M1 ipsilateral to the movement should exert an inhibitory influence on the tremor generator-loop.6 The results from experiment 2 are consistent with the hypothesis that a motor task dampens tremor potentially at the level of M1. Since the motor task reduced tremor regularity and tremor-EMG coherence similar to STN DBS, the motor task may also alter the tremor oscillations in subcortical regions such as the basal ganglia.
STN DBS vs. Medication
One of the general findings in experiments 1 and 2 was that STN DBS was more effective in changing neurophysiological characteristics of tremor than medication. This is consistent with the findings of Deuschl and colleagues7 who showed that DBS was more effective than medical management alone. The ‘more or less’ model of high-frequency stimulation may help to explain the differential effect of STN DBS and medication for tremor.31 According to this model, STN DBS is hypothesized to silence low frequency neuronal activity (“less mechanism”) while driving high frequency gamma band activity (“more mechanism”). Levodopa is also known to promote gamma band oscillations in patients with PD, which increase before and during voluntary movement.32–34 While high frequency stimulation of the STN may mimic the effects of medication on movement tasks requiring high frequency basal ganglia activity, it may have a superior effect on the low frequency basal ganglia oscillations that lead to tremor suppression. Moreover, high frequency coherence between the basal ganglia and cortex during volitional movement may also provide a mechanism that enables the secondary motor task to suppress tremor so efficaciously.
Acknowledgments
This research was supported in part by grants from the National Institutes of Health (R01NS28127, R01NS40902, R01NS52318, T32MH067631). We thank Medtronic for donating the Medtronic Console Programmer (Model 7432) for use in this study. We also thank the staff at the Section for Movement Disorders in the Department of Neurological Sciences at Rush University Medical Center and the staff in the Department of Neurological Surgery at Rock-ford Health System. Finally, we would also like to acknowledge and thank all of the patients and their families who participated in this study.
References
- 1.Benecke R, Rothwell JC, Dick JPR, Day BL, Marsden CD. Performance of simultaneous movements in patients with Parkinson’s disease. Brain. 1986;109:739–757. doi: 10.1093/brain/109.4.739. [DOI] [PubMed] [Google Scholar]
- 2.Sturman MM, Vaillancourt DE, Metman LV, Bakay RA, Corcos DM. Effects of subthalamic nucleus stimulation and medication on resting and postural tremor in Parkinson’s disease. Brain. 2004;127(Part 9):2131–2143. doi: 10.1093/brain/awh237. [DOI] [PubMed] [Google Scholar]
- 3.Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on tremor. Ad Hoc Scientific Committee Mov Disord. 1998;13(Suppl 3):2–23. doi: 10.1002/mds.870131303. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez MC, Guridi OJ, Alvarez L, et al. The subthalamic nucleus and tremor in Parkinson’s disease. Mov Disord. 1998;13(Suppl 3):111–118. doi: 10.1002/mds.870131320. [DOI] [PubMed] [Google Scholar]
- 5.Marsden CD, Owen DA. Mechanisms underlying emotional variation in parkinsonian tremor. Neurology. 1967;17:711–715. doi: 10.1212/wnl.17.7.711. [DOI] [PubMed] [Google Scholar]
- 6.Tamas G, Palvolgyi L, Takats A, Szirmai I, Kamondi A. Contralateral voluntary hand movement inhibits human parkinsonian tremor and variably influences essential tremor. Neurosci Lett. 2004;357:187–190. doi: 10.1016/j.neulet.2003.12.092. [DOI] [PubMed] [Google Scholar]
- 7.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deuschl G, Schade-Brittinger C, Krack P, et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2006;355:896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- 9.Olanow CW, Goetz CG, Kordower JH, et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann Neurol. 2003;54:403–414. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- 10.Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson’s disease: a clinicopathologic study. Neurology. 1992;42:1142–1146. doi: 10.1212/wnl.42.6.1142. [DOI] [PubMed] [Google Scholar]
- 11.Deep-Brain Stimulation for Parkinson’s Disease Study Group. Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson’s disease. N Engl J Med. 2001;345:956–963. doi: 10.1056/NEJMoa000827. [DOI] [PubMed] [Google Scholar]
- 12.Starr PA, Vitek JL, Bakay RA. Deep brain stimulation for movement disorders. Neurosurg Clin N Am. 1998;9:381–402. [PubMed] [Google Scholar]
- 13.Temperli P, Ghika J, Villemure JG, Burkhard PR, Bogousslavsky J, Vingerhoets FJ. How do parkinsonian signs return after discontinuation of subthalamic DBS? Neurology. 2003;60:78–81. doi: 10.1212/wnl.60.1.78. [DOI] [PubMed] [Google Scholar]
- 14.Langston JW, Widner H, Goetz CG, et al. Core assessment program for intracerebral transplantations (CAPIT) Mov Disord. 1992;7:2–13. doi: 10.1002/mds.870070103. [DOI] [PubMed] [Google Scholar]
- 15.Sturman MM, Vaillancourt DE, Corcos DM. Effects of aging on the regularity of physiological tremor. J Neurophysiol. 2005;93:3064–3074. doi: 10.1152/jn.01218.2004. [DOI] [PubMed] [Google Scholar]
- 16.Raethjen J, Pawlas F, Lindemann M, Wenzelburger R, Deuschl G. Determinants of physiologic tremor in a large normal population. Clin Neurophysiol. 2000;111:1825–1837. doi: 10.1016/s1388-2457(00)00384-9. [DOI] [PubMed] [Google Scholar]
- 17.Vaillancourt DE, Larsson L, Newell KM. Time-dependent structure in the discharge rate of human motor units. Clin Neurophysiol. 2002;113:1325–1338. doi: 10.1016/s1388-2457(02)00167-0. [DOI] [PubMed] [Google Scholar]
- 18.Vaillancourt DE, Sturman MM, Verhagen Metman L, Bakay RA, Corcos DM. Deep brain stimulation of the VIM thalamic nucleus modifies several features of essential tremor. Neurology. 2003;61:919–925. doi: 10.1212/01.wnl.0000086371.78447.d2. [DOI] [PubMed] [Google Scholar]
- 19.Pincus SM. Approximate entropy as a measure of system complexity. Proc Natl Acad Sci USA. 1991;88:2297–2301. doi: 10.1073/pnas.88.6.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elble RJ, Randall JE. Motor-unit activity responsible for 8- to 12-Hz component of human physiological finger tremor. J Neurophysiol. 1976;39:370–383. doi: 10.1152/jn.1976.39.2.370. [DOI] [PubMed] [Google Scholar]
- 21.Jahanshahi M, Ardouin CM, Brown RG, et al. The impact of deep brain stimulation on executive function in Parkinson’s disease. Brain. 2000;123(Part 6):1142–1154. doi: 10.1093/brain/123.6.1142. [DOI] [PubMed] [Google Scholar]
- 22.Hedreen JC, DeLong MR. Organization of striatopallidal, striatonigral, and nigrostriatal projections in the macaque. J Comp Neurol. 1991;304:569–595. doi: 10.1002/cne.903040406. [DOI] [PubMed] [Google Scholar]
- 23.Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- 24.Parent A, Hazrati L. Functional anatomy of the basal ganglia. I The cortical-basal ganglia-thalamo-cortical loop. Brain Res Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- 25.Benazzouz A, Piallat B, Pollack P, Benabid A. Responses of substantia nigra pars reticulata and globus pallidus complex to high frequency stimulation of the subthalamic nucleus in rats: electro-physiological data. Neurosci Lett. 1995;189:77–80. doi: 10.1016/0304-3940(95)11455-6. [DOI] [PubMed] [Google Scholar]
- 26.Ni Z, Bouali-Benazzouz R, Gao D, Benabid AL, Benazzouz A. Changes in the firing pattern of globus pallidus neurons after the degeneration of nigrostriatal pathway are mediated by the subthalamic nucleus in the rat. Eur J Neurosci. 2000;12:4338–4344. [PubMed] [Google Scholar]
- 27.Benazzouz A, Gao DM, Ni Z, Piallat B, Bouali-Benazzouz R, Benabid AL. Effect of high frequency stimulation of the subthalamic nucleus on the neuronal activities of the substantia nigra pars reticulata and ventrolateral nucleus of the thalamus in the rat. Neuroscience. 2000;99:289–295. doi: 10.1016/s0306-4522(00)00199-8. [DOI] [PubMed] [Google Scholar]
- 28.Limousin P, Greene J, Pollak P, Rothwell J, Benabid AL, Frackowiak R. Changes in cerebral activity pattern due to subthalamic nucleus or internal pallidum stimulation in Parkinson’s disease. Ann Neurol. 1997;42:283–291. doi: 10.1002/ana.410420303. [DOI] [PubMed] [Google Scholar]
- 29.Timmermann L, Gross J, Dirks M, Volkmann J, Freund HJ, Schnitzler A. The cerebral oscillatory network of parkinsonian resting tremor. Brain. 2003;126(Part 1):199–212. doi: 10.1093/brain/awg022. [DOI] [PubMed] [Google Scholar]
- 30.Hellwig B, Haussler S, Lauk M, et al. Tremor-correlated cortical activity detected by electroencephalography. Clin Neurophysiol. 2000;111:806–809. doi: 10.1016/s1388-2457(00)00248-0. [DOI] [PubMed] [Google Scholar]
- 31.Garcia L, D’Alessandro G, Bioulac B, Hammond C. High-frequency stimulation in Parkinson’s disease: more or less? Trends Neurosci. 2005;28:209–216. doi: 10.1016/j.tins.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Brown P, Oliviero A, Mazzone P, Insola A, Tonali P, Di Lazzaro V. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson’s disease. J Neurosci. 2001;21:1033–1038. doi: 10.1523/JNEUROSCI.21-03-01033.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cassidy M, Mazzone P, Oliviero A, et al. Movement-related changes in synchronization in the human basal ganglia. Brain. 2002;125(Part 6):1235–1246. doi: 10.1093/brain/awf135. [DOI] [PubMed] [Google Scholar]
- 34.Williams D, Tijssen M, Van Bruggen G, et al. Dopamine-dependent changes in the functional connectivity between basal ganglia and cerebral cortex in humans. Brain. 2002;125(Part 7):1558–1569. doi: 10.1093/brain/awf156. [DOI] [PubMed] [Google Scholar]


