Abstract
Here we report the successful three-dimensional crystallization of GlpT, the glycerol-3-phosphate transporter from Escherichia coli inner membrane. GlpT possesses 12 transmembrane α-helices and is a member of the major facilitator superfamily. It mediates the exchange of glycerol-3-phosphate for inorganic phosphate across the membrane. Approximately 20 phospholipid molecules per protein, identified as negatively charged phosphatidylethanolamine, phosphatidylglycerol, and cardiolipin, were required for the monodispersity of purified GlpT. Analytical size-exclusion chromatography proved to be efficient in identifying detergents for GlpT monodispersity. Nine such detergents were later used for GlpT crystallization. Screening for crystal nucleation was carried out with a variety of polyethylene glycols as the precipitant over a wide pH range. Subsequent identification of a rigid protein core by limited proteolysis and mass spectroscopy resulted in better-ordered crystals. These crystals exhibited order to 3.7 Å resolution in two dimensions. However, the stacking in the third dimension was partially disordered. This stacking problem was overcome by using a detergent mixture and manipulating the ionic interactions in the crystallization solution. The resulting GlpT crystals diffracted isotropically to 3.3 Å resolution and were suitable for structure determination by X-ray crystallography.
Keywords: Transporter, membrane protein, crystallization, detergent, lipid
GlpT, the sn-glycerol-3-phosphate (G3P) transporter from the inner membrane of Escherichia coli mediates the exchange of inorganic phosphate for G3P across the membrane (Silhavy et al. 1976; Larson et al. 1982; Ambudkar et al. 1986a). G3P functions as a precursor molecule for phospholipid biosynthesis (Rock and Cronan 1985), as well as a carbon and energy source (Elvin et al. 1985). The protein is a member of the major facilitator superfamily (MFS), the largest secondary active transporter family known to date (Henderson 1993; Reizer et al. 1993; Pao et al. 1998). Wild-type GlpT consists of 452 amino acids and has a molecular weight of 50.3 kD. A topology model has been proposed based on hydrophobicity analysis of GlpT sequence as well as phoA and lacZ fusion experiments (Gött and Boos 1988). According to this model, GlpT consist of 12 transmembrane α-helices, which accounts for >50% of its amino acids, and no large extramembrane domain exists in the protein. Although much is known about the transport kinetics of GlpT from whole-cell and reconstituted systems (Ambudkar et al. 1986a,b; Auer et al. 2001), little was understood about the mechanisms of substrate transport, largely due to the lack of structural information. With the recent structure determination of GlpT (Huang et al. 2003), the mechanism of substrate transport for this and other MSF proteins can be understood at an atomic level.
Membrane protein crystallization remains a challenge in structural biology, particularly for proteins that lack a large extramembrane domain. Packing in crystals with this type of protein is such that it typically produces anisotropic diffraction, making structure determination difficult or impossible (Michel 1983; Kühlbrandt 1987, 1988; Schertler et al. 1993). In addition, although the critical importance of detergent in membrane protein crystallization was realized over two decades ago (Garavito and Rosenbusch 1980; Michel and Oesterhelt 1980; Michel 1983), their selection remains mostly empirical. Recent advances in membrane protein crystallization include expression of protein paralogs and orthologs of a target protein (Chang et al. 1998; Locher et al. 2002), and identification and modification of a rigid protein core for crystallization (Doyle et al. 1998). However, detailed protocols for crystallization (Garavito and Rosenbusch 1980; Michel 1982; Schertler et al. 1993), in particular for optimization of crystallization, are sparse.
We previously overexpressed the GlpT protein in E. coli at a level of 1.8 mg/L of cell culture (Auer et al. 2001). A compact GlpT core (amino acids 2–448) was identified by limited proteolysis in combination with mass spectrometry and N-terminal peptide sequencing. This GlpT core was purified and extensively characterized. The protein remains monodisperse over a wide pH range and in a variety of detergents, and it is active upon reconstitution into proteoliposomes. In this article, we report the successful three-dimensional crystallization of the GlpT protein. The strategies used for crystal nucleation screening and optimization of crystal size and quality are described. Conditions for growing GlpT crystals that diffract X-rays to 3.3 Å resolution are reported, and the important factors for attaining high-quality crystals are discussed.
Results
GlpT expression and purification
Four GlpT constructs were expressed and purified: GlpT-myc-His, GlpT448, GlpT444, and GlpTΔ5-448, each with different N- or C-terminal truncations (Table 1). E. coli cell culture of 6 L typically produced 16 g of cells, resulting in 5 g of membrane after cell fractionation. From this, 10 to 12 mg of GlpT was eluted from a Ni2+-NTA affinity column, and 3 to 4 mg of stable and monomeric GlpT was recovered after preparative size-exclusion (SE) column on FPLC. GlpT fractions were collected at a protein concentration of 2 to 3 mg/mL, with a purity of 95% to 98%. Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectroscopy of the preparation yielded a sharp symmetrical peak with the expected molecular weight, indicating the absence of proteolysis. After thrombin digestion, GlpT proved to be resistant to further limited proteolytic treatment by trypsin, chymotrypsin, elastase, papain, subtilisin, or thermolysin (data not shown). The protein remained monodisperse in a number of non-ionic detergents for several weeks at 4°C and had a half-life of ~15 d at 20°C, as judged by analytical SE chromatography on HPLC. Only the GlpTΔ5-448 was less stable.
Table 1.
GlpT constructs used in crystallization trials
| Construct | Sequencea | Protein stability | Crystal and diffraction |
| GlpT-myc-His | L2-G452-myc-His | High | Yes, 25 Å |
| GlpT448 | L2-E448-LVPRGS-myc-Hisb | High | Yes, 3.2 Å |
| GlpT445 | L2-L445-LVPRGS-myc-Hisb | High | No |
| GlpTΔ5-448 | F5-E448-LVPRGS-myc-Hisb | Medium | No |
a The N-terminal methionine was posttranslationally removed in vivo (Auer et al. 2001).
b LVPRGS is the thrombin cleavage site that was cloned after E448 to remove the myc-His tags. The purified protein for crystallization therefore contained the LVPR in addition to the polypeptide sequence.
Identification of phospholipids necessary for crystallization
After each purification step, the endogenous phospholipids copurifying with GlpT were quantified and correlated with its monodispersity and crystallization (Table 2). After Ni2+-NTA column purification in 0.1% dodecyl-maltoside (DDM), 41 ± 2 mole of phospholipid was found to associate with 1 mole GlpT. The amount of phospholipid after the SE column was reduced to 22 ± 1 and 24 ± 1 mole/mole of GlpT in 0.075% DDM and in a mixture of DDM/C12E9 (0.075% : 0.025%; 2.5 : 1, w/w; Table 2), respectively. GlpT was stable in these amounts of lipid and detergents after both the first and second columns. Interestingly, GlpT crystals formed only with samples after the second column. GlpT samples after a third chromatography column in DDM precipitated quickly out of solution. This indicates that the degree of delipidation, namely, the amount of phospholipids copurifying, was critical to the GlpT crystallization.
Table 2.
Endogenous phospholipid copurified with GlpT
| Detergent | Monodispersity | Lipid/GlpT | Crystal |
| Ni2+-NTA column DDMa | Yes | 41 ± 4 (n = 8) | No |
| Preparative size-exclusion column DDMa | Yes | 22 ± 1 (n = 8) | Yes 7.0 Å |
| DDM/C12E9b | Yes | 24 ± 1 (n = 4) | Yes 3.2 Å |
Lipid/protein ratio represents mol • Pi/mol GlpT determined by using a phosphorous assay, with “n” being the number of samples analyzed. Protein monodispersity was determined by analytical size-exclusion chromatography on HPLC.
a 0.075% DDM.
b 0.075% DDM and 0.025% C12E9.
We further identified the phospholipids necessary for GlpT crystallization by thin-layer chromatography. After the SE chromatography purification step, GlpT was found to copurify with approximately equal amounts of phosphatidylethanolamine (PE), phosphatidylglycerol (PG), and cardiolipin (CL).
Crystallization of various GlpT constructs
The full-length GlpT-myc-His, amino acids 2–552 plus the tags, yielded showers of microcrystals in polyethylene glycols (PEGs) 400 and 4000 and some of the conditions in the Hampton Crystal Screen I and II that use a PEG as the precipitant. Addition of glycerol and methyl-pentanediol (MPD) increased the size of the crystals, as did various drop dilutions such as 1 : 2 of protein to reservoir. Only GlpT purified after the SE column, but not the Ni2+-NTA column, produced crystals (Table 2). These crystals from the GlpT-myc-His construct, however, did not diffract.
After removal of the myc-His-tags, GlpT448 (aa 2–448) purified in DDM was subjected to a broader screen with various PEGs, including 400, 1000, 2000, and 2000MME (monomethyl ether), 3350, 4000, and 5000MME. This resulted in various crystal forms (Table 3), with the best obtained by using PEG 2000 and 2000MME. The first GlpT448 crystal to diffract X-rays was grown in 21% PEG 5000, 20% glycerol, 5% MPD, and 0.1 M succinate (pH 3.7 to 5.3). In contrast, GlpT445 (aa 2–445) did not produce any crystals, and GlpTΔ5-448 precipitated out in crystallization solution. We therefore focused our subsequent crystallization efforts on GlpT448.
Table 3.
Results of PEG/pH crystallization screen of GlpT in different detergents
| Detergent/PEG | DDM | UDM | DM | C12E9 | C12E8 | C12E6 | CYMAL-6 | CYMAL-5 | NG | HEGA-10 | FOS-MEA 10 |
| 400 | B, pH 5 | N, pH 6.5 | 0 | — | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1000 | G | 0 | 0 | — | 0 | 0 | G, pH 5 | 0 | — | 0 | 0 |
| 1500 | — | 0 | — | B, pH 5; B, pH 8-9 | 0 | 0 | — | B, pH 6 | 0 | 0 | 0 |
| 2000 | B/C pH 5 | — | — | — | N, pH 4 | C, pH 6; T, pH 8 | — | — | — | — | — |
| 2000MME | B, pH 5 | 0 | G, pH 4 + 9 | C/T, pH 5-6; H, pH 8-9 | B, pH 5; H, pH 5.5 | B, pH 5.5 | B, pH 4-6 | C, pH 6 | 0 | 0 | 0 |
| 3350 | B, pH 5; H, pH 8 | 0 | — | C, pH 4-7 | S, pH 7-8 | N, pH 6-7 | — | 0 | — | — | 0 |
| 4000 | B, pH 5; T, pH 9 | 0 | S, pH 9 | — | S, pH 6 | N, pH 8 | 0 | G, pH 6-7 | 0 | 0 | 0 |
| 5000MME | N, pH 5; N, pH 8 | 0 | — | C, pH 4-6 | S, pH 5-7 | H, pH 8-9; S, pH 7 | 0 | — | — | 0 | 0 |
| 8000 | — | S, pH 7 + 9 | 0 | C, pH 4-6 | S, pH 4-9 | S, pH 7-8; C, pH 4 | 0 | 0 | — | 0 | 0 |
| 10,000 | — | — | — | C, pH 4; N, pH 6 | — | S, pH 7 | — | — | — | — | 0 |
| 12,000 | — | — | — | — | — | S, pH 6 | — | — | — | — | 0 |
| 20,000 | — | — | — | — | — | S, pH 6 | — | — | — | — | — |
The above crystallization results were obtained with PEG/pH screening kits. Crystal morphology and corresponding pHs are indicated: 0, no crystals; —, not tested; N, needle; S, thin sheet; G, globular ball; B, bar; C, cube; T, triangle; and H, hexagonal.
The initial GlpT448 crystals grew as rectangular rods with dimensions 50 to 100 μm in the longest dimension, and diffracted X-rays to 17 Å resolution. Importantly, the final detergent concentration prior to crystallization was found to be critical to crystallization: The best crystals were obtained with a range of 0.2% to 0.3% of DDM. When the level of DDM after concentrating the protein for crystallization was >0.5% to 0.6%, phase separation instead of crystallization occurred. Improvements in crystal order were made with several additives. The addition of 1 mM of DTT in the crystallization solution resulted in larger and more birefringent crystals. Crystals grown in the presence of detergent sulfobetaine 201 diffracted to 9 Å. Heavy-metal salts LuCl2, Cd(OCH3)2, and PbCl2 also improved crystal order to 11.6 Å, 8.6 Å, and 7.0 Å, respectively. These latter changes in detergent or salt, however, were less reproducible and were not included in the final crystallization experiments.
Use of crystallization screening kits
We prepared 12 PEG/pH screening kits (McPherson 1990) to search for nucleation of GlpT crystals in other detergents, based on the observations that GlpT crystals in DDM obtained with Crystal Screen Kits I and II and MemFrac grew only when PEGs were used as precipitants (Table 3). The precipitant concentrations in the kits ranged from 10% to 40% for low-molecular-weight PEGs, and 5% to 30% for those with high molecular weights. The pH range was between 3.5 and 9.0, at intervals of 0.5 units. Glycerol was included because it significantly improved GlpT monodispersity (Auer et al. 2001), and its concentration was varied between 0% to 20% to adjust the nucleation rate (McPherson 1999). MPD at 5% was included because reduced GlpT crystal nucleation rate and, hence, increased the crystal size. Overall, the constituents of each condition in the kits were kept at a minimum, allowing ample room for future refinement.
GlpT crystallization in other detergents
Exchange of DDM to other detergents resulted in new GlpT crystal forms (Fig. 1 ▶; Table 3). Detergents that retained GlpT monodispersity were chosen for detergent exchange (Auer et al. 2001): undecyl-maltoside (UDM), decyl-maltoside (DM), C12E9, C12E8, C12E6, Cymal-6, and Cymal-5. For comparison, nonyl-glucoside (NG), HEGA-10, and FOS-MEA 10, all from separate detergent families that were unable to preserve GlpT monodispersity, were also included in the current crystallization experiments. After solubilization and purification in DDM using Ni2+-NTA chromatography, DDM was exchanged to each of the above detergents by preparative SE chromatography. GlpT in the new detergent was then subjected to PEG/pH crystallization screens, and numerous crystal forms were obtained by using various PEGs and at different pH ranges (Table 3). Aside from DDM, protein crystals predominantly appeared with polyoxyethylene detergents C12E9, C12E8, and C12E6, and the crystals mostly crystallized at pH 5 to 6 and 8 to 9, as in the case of GlpT in DDM. Most interestingly, the hexagonal and triangular crystal form in C12E9 diffracted to 3.7 Å. These crystals were grown in 25% PEG 2000MME, 20% glycerol, 5% MPD, and 0.1 M Tris-HCl (pH 8.5). For comparison, crystals of GlpT grown in C12E8 and C12E6 diffracted to 8.4 and 7.0 Å, respectively.
Figure 1.
Gallery of various GlpT crystal forms after initial crystallization screen. Twenty percent glycerol and 5% MPD were present in all the drops including the following: (A) In DDM, 19% PEG 2000MME, and 100 mM Bis-Tris (pH 6.0); (B) In DDM, 19% PEG 2000MME, 100 mM Bis-Tris (pH 6.0), and 0.5 mM (NH4)2SO4; (C) In DDM, 25% PEG 3350 and 100 mM Tris (pH 8.0); (D) In C12E9, 25% PEG 2000MME, 100 mM Tris (pH 8.5). All pictures were recorded at the same magnification. Bar, 150 μm.
Reproduction of well-diffracting GlpT crystals in C12E9 was initially difficult due to variability in detergent exchange in the SE chromatography column. It was found that the GlpT peak retention time was dependent on the amount of protein loaded, indicating incomplete detergent exchange and variations in residual DDM amounts. We subsequently measured the DDM concentrations in GlpT samples eluted from the SE column and correlated them with the quality of the crystals produced. The sample that produced well-diffracting crystals had significant amounts of DDM present; smaller crystals were obtained from samples with less DDM; and no crystals were observed when DDM was absent. Therefore, detergent exchange on the SE column was often incomplete, and variations in the concentration of remaining DDM caused GlpT crystallization to be less reproducible, even though the presence of DDM was necessary for GlpT crystallization.
Subsequent crystallization experiments were carried out by adding C12E9 into GlpT samples purified with the SE column in DDM to ensure a well-defined detergent ratio and to improve reproducibility. Different detergent ratios of were examined, and the best crystals, obtained with DDM/C12E9 of 2.5 : 1 (w/w), diffracted X-rays at a synchrotron source beyond 3.2 Å. Importantly, a synchrotron source was necessary for GlpT crystal screening and optimization, because our crystals never diffracted >6 Å resolution with a rotating anode.
Improvements in crystal packing
Triangular or hexagonal, plate-shaped crystals of GlpT in DDM/C12E9 were found to be not well-ordered along the threefold symmetry axis, and therefore, their diffraction patterns were not indexable (Fig. 2 ▶). When aligned perpendicular to the X-ray beam, the plate-shaped crystals diffracted to 3.8 to 4.0 Å, but lunes expected at borders between adjacent Laue zones from isotropically ordered crystals were missing. Streaky reflections were also observed when the crystal plate was parallel to the beam. These indicated the existence of disorder in the direction perpendicular to the plate. This stacking problem was solved by the use of DDM/C12E9 detergent mixture and addition of a divalent salt in the crystallization buffer. At a DDM/C12E9 ratio of 2.5 : 1 and in the presence of SrCl2 or MgCl2, thin GlpT crystals now grew as triangular bars, yielding isotropic diffraction.
Figure 2.
X-ray diffraction patterns of GlpT showing disorder in the third dimension. X-ray diffraction patterns collected from GlpT protein in C12E9 detergent. A and B were collected from a crystal, and the two patterns were recorded between a 90° rotation at the X25 beamline at the National Synchrotron Light Source in the Brookhaven National Laboratory. Diffraction spots are visible to 4.5 Å resolution.
GlpT crystals typically appeared on day 3 or 4 and continued growing for another 3 to 5 d, whereas crystal quality started to deteriorate 1 week later. The crystals were determined to have space group P3221, with unit cell dimensions a = b = 97.4 Å and c = 173.0 Å, and α = β = 90° and γ = 120°. The best crystals showed some weak reflections ~3.0 Å resolution (Fig. 3 ▶). Isotropic diffraction data sets complete to 3.3 Å had an Rmerge of 11%, with the outermost resolution shell at 50%. The final crystal conditions were GlpT at 6 mg/mL, 0.1% to 0.25% DDM, 0.04% to 0.1% C12E9, 25% to 27% PEG 2000MME, 0.1 M Tris (pH 8.5 to 8.9), 20% glycerol, 5% MPD, 5 to 100 mM NaCl, and 5 mM SrCl2 or MgCl2, grown at 15°C to 20°C.
Figure 3.
GlpT diffraction pattern and crystal. (A) 1° oscillation image depicting X-ray diffraction pattern from a GlpT crystal grown in DDM/C12E9. The space group of the crystals was P3221, with unit cell dimensions a = b = 97.4 Å and c = 173.0 Å, and α = β = 90° and γ = 120°. Isotropic diffraction data sets complete to 3.3 Å had an Rmerge of 11%. (B) Enlarged portion of A showing reflections at 3.2 Å resolution. (C) Photomicrograph of GlpT crystal from which the diffraction pattern was recorded. Bar, 50 μm.
Discussion
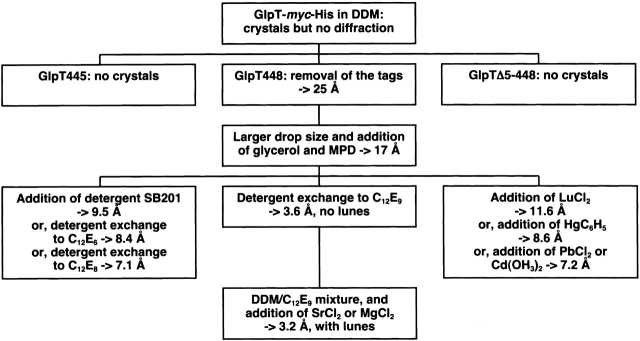
Inspired by the successful structure determination of the KscA K+-channel and the mechanosensitive channel (MscL; Chang 1998; Doyle et al. 1998), we took a similar approach to crystallize GlpT. Optimization of the GlpT crystallization is summarized in Figure 4 ▶. The key elements in our experiments were to (1) identify a rigid protein core by proteolysis and mass spectrometry and modify the protein construct accordingly, (2) prescreen detergents for their ability of maintaining protein monodispersity, (3) screen for crystal nucleation for every detergent identified in the previous step with PEG/pH crystallization kits to improve efficiency and reproducibility, and (4) improve protein contact in the crystals by using a detergent mixture. Hence, the emphasis was as much on the protein itself, as it was on detergent type or efficient screening and refinement of the crystallization conditions.
Figure 4.
Summary of steps required for gradually improving GlpT crystal quality.
Heterogeneity of protein, detergent, and lipid
The membrane protein molecule in solution exists as a protein-detergent-lipid complex. Specific lattice contacts in any protein crystal are made exclusively via protein–protein interactions, and too large a detergent micelle can be an obstacle for protein crystallization (Michel 1983). By reducing the heterogeneity of the protein surface and by optimizing the detergent micellar size and shape, we essentially increased the area available for the formation of lattice contacts, thereby improved the protein crystallizability (Kwong et al. 1999). Removal of the myc-His-tags markedly improved the GlpT crystal quality, possibly by reducing the conformational heterogeneity of the protein. Compared to soluble protein crystallization, the removal of these tags is more critical here because the total possible area accessible for potential crystal contacts is smaller for membrane proteins.
Another source of heterogeneity is the presence of unbound detergent in solution that, when in excess, interferes with proper packing of the protein-detergent micelles in the crystal lattice. Indeed, we have found that detergents with low critical-micellar concentration (DDM and C12E8) at a final concentration of 0.2% to 0.4% works best for protein concentrations of 5 to 10 mg/mL.
Lipid is another important factor for membrane protein crystallization. On the one hand, lipid can stabilize the membrane protein during purification, whereas on the other hand, complete delipidation led to aggregation of GlpT and other membrane transporters (Auer et al. 2001; Lemieux et al. 2002). Approximately 20 phospholipid molecules per GlpT molecule are required for its monodispersity and, hence, crystallization. In comparison, only five to 15 phospholipid molecules are required for the crystallization of the erythrocyte anion exchanger membrane domain (Lemieux et al. 2002). As to the types of phospholipids, comparable amounts of PE, PG, and CL were present during the crystallization of GlpT. Because the inner membrane composition of E. coli consists of 74% PE, 19% PG, and 3% CL (Devaux and Seigneuret 1985), purified GlpT was enriched in the negatively charged lipids PG and, especially, CL. Although it is clear that these lipids play role in the crystallization of the protein, no lipid was seen in the 3.3 Å crystal structure of GlpT (Huang et al. 2003), and it is unknown whether specific lipids are needed for a functional transporter. It is interesting to note that PG is critical also for the function and crystallization of the KscA K+-channel from E. coli (Valiyaveetil et al. 2002). Nonetheless, whether such negatively charged phospholipids are particularly suitable for protecting other purified membrane proteins remains to be seen.
Correlation of protein monodispersity and crystallizability
Crystallization requires the protein to be stable and monodisperse in solution. Previous work on membrane transporters has shown that the types of detergent and pH used are the most important parameters influencing the monodispersity of the protein (Boulter and Wang 2001; Li et al. 2001; Engel et al. 2002). A clear relationship was observed between the ability of a detergent to preserve the monodispersity of GlpT and the possibility of yielding protein crystals (Table 3). The nine detergents that retained GlpT monodispersity produced protein crystals of various qualities; those three detergents that were unable to preserve the monodispersity of the protein failed to yield any crystal. This clearly shows a strong correlation between the monodispersity and the crystallizability of a membrane protein in a particular detergent (Garavito et al. 1996; Rosenbusch et al. 2001). Furthermore, the monodispersity assay by analytical SE chromatography on HPLC (Wang et al. 2003a) proves to be a reliable technique for prescreening detergent for membrane protein crystallization.
Screening for crystal nucleation of GlpT
The availability of commercial protein crystallization kits has largely revolutionized the process of searching for nucleation of crystals for soluble proteins (McPherson 1999). These kits, however, are not suitable for membrane protein, presumably because the database available for membrane proteins is small and the kits are designed largely based experience with soluble proteins (Jancarik et al. 1991). Recently, efforts were made by various investigators to design general screening kits for crystallization of membrane proteins (Song and Gouaux 1997; Wiener and Snook 2001), based on the incomplete factorial experiments (Carter Jr. and Carter 1979) or detergent solution properties. In the current work, we took a different, more systematic approach.
A survey of published literature (http://www.mpibp-frankfurt.mpg.de/michel/public/memprotstruct.html) reveals that 77% of membrane protein crystals (51 out of 66) are grown with PEG, or its MME derivative, as the precipitant. Organic solvents tend to disturb the detergent micelles and, at high concentrations, denature membrane proteins. Salt, on the other hand, reduces the solubility of the detergent micelles (Zulauf 1991) and often precipitates the membrane protein embedded in the detergent micelle before crystallization occurs. PEGs are therefore a best choice for membrane protein crystallization. Another important factor is pH. More than 90% of soluble protein crystals are grown between pH 4 and 9 (McPherson 1999), and it is expected that this will be true for membrane proteins as well. Thus, assuming the ranges of GlpT crystallization conditions in different detergents would overlap, we used screening kits with 12 PEGs over the pH range of 3.5 to 9.0 (McPherson 1990) based on the initial crystallization results of the protein in DDM. Indeed, their application to GlpT purified in 11 detergents produced numerous hits, which were subsequently pursued for optimization. Furthermore, the utilization of these screening kits significantly improved the efficiency and reproducibility of crystallization experiments, which was particularly beneficial when a dozen of detergents were screened for crystallization. Finally, the 12 PEG/pH screening kits used to search for nucleation of GlpT crystals may serve as a starting point for other membrane proteins.
Improvement of crystal packing
The GlpT protein construct directly affected the crystal packing. One particular problem with membrane protein crystallization is anisotropy in resolution (Michel 1983), in which packing in two directions is well-ordered, but stacking of layers in the third dimension is less regular. Such crystal packing problems are more common for those proteins that lack a large extramembrane domain, as was observed with the plant light-harvesting complex II (Kühlbrandt 1987, 1988) and bacteriorhodopsin from Halobacterium (Schertler et al. 1993). Although both the lightharvesting complex and bacteriorhodopsin form type I crystals, which consist of stacked two-dimensional crystals (Michel 1983), the GlpT crystals in this work are true three-dimensional crystals (type II). Our work has shown that type II membrane crystals can also have a packing problem. The quality of the GlpT crystals was critically depended on the protein sequence at its C terminus, consistent with a later observation that the C terminus makes the only lattice contact in the C-direction in these crystals (Huang et al. 2003).
Another critical factor was the detergent. Changing the detergent greatly affected the crystallization of GlpT presumably by exposing different protein contacts. Most interesting was the requirement for a detergent mixture of DDM and C12E9 to give crystals that diffracted to 3.3 Å resolution. Although DDM alone resulted in crystalline order to 7 Å, complete detergent exchange to C12E9 gave no crystals. This is probably because DDM helped to fix GlpT into a particular conformation, as an ordered DDM molecule was found inside the substrate-translocation pore of GlpT (Huang et al. 2003). DDM alone and DDM/C12E9 mixture showed similar effects in delipidating GlpT (Table 2). Therefore, the improved crystal quality observed with DDM/C12E9 was probably due to more favorable packing and newly exposed protein surface areas for crystal contacts. GlpT crystal packing was also improved with the addition of divalent salts. The Sr2+ and Mg2+ ions probably interacted with hydrophilic protein domains that were involved in the packing between adjacent layers. It is noted that detergent mixtures were also used in the crystallization of fumarate reductase (Lancaster et al. 1999) and outer membrane protein TolC (Koronakis et al. 2000).
We and others have observed that obtaining three-dimensional crystals for a membrane protein that diffract to low resolution is not particularly difficult, but the process of improving the crystalline order from 15 Å to 3 Å is much harder. In this article we describe the successful crystallization of GlpT and the steps required for improving the crystalline order to a resolution suitable for structure determination by X-ray crystallography. Although the conditions for crystallizing GlpT may not be directly applicable to other membrane proteins, the strategy used here may however help other investigators in crystallizing their membrane proteins.
Materials and methods
Materials
Restrictions enzymes were purchased from New England Biolabs, cloning kits from Qiagen, detergents from Anatrace, and PEG from Fluka. Crystallization supplies were from Hampton Research. All other chemicals were from Sigma and were of analytical grade or better.
GlpT overexpression and purification
GlpT-myc-His and GlpT448 (L2-E448) were overexpressed and purified according to previously published methods (Auer et al. 2001). In addition, two new protein constructs with different N- and/or C-terminal truncations (Table 1) were overexpressed and purified similarly as the wild type. Briefly, GlpT was cloned into pBAD-myc-His vector for overexpression in E. coli LMG194 strain (Invitrogen). A thrombin cleavage site was inserted upstream of the C-terminal myc-His-tags by using standard PCR methods. Colonies were selected for high expression by using a colony blot protocol (Qiagen 2001; Wang et al. 2003b). After cell breakage by three cycles of French Press and ultracentrifugation, the membrane fraction was solubilized for 30 min at 4°C with buffer containing 50 mM Tris (pH 8.0), 400 mM NaCl, 20% glycerol, and 1% DDM (Sol-grade), at 10 m/g membrane. GlpT was purified by using Ni2+-NTA affinity chromatography (Qiagen) in the presence of 50 mM Tris (pH 8.0), 100 mM NaCl, 20% glycerol, and 0.1% DDM. After thrombin digestion to remove the myc-His-tags, the protein was further purified using a SE column Superdex200 on FPLC (Amersham-Pharmacia), in 50 mM imidazole (pH 7.0), 100 mM NaCl, 0.5 mM EDTA, 20% glycerol, and one of the following detergents: 0.075% DDM (Ana-grade), 0.1% DM, 0.1% UDM, 0.1% C12E9, 0.1% C12E8, 0.15% C12E6, 0.25% Cymal-6, 0.5% Cymal-5, 0.25% NG, 0.22% HEGA-10, or 0.22% Fos-MEA-10. Protein purity and homogeneity were estimated by SDS-PAGE and MALDI-TOF mass spectrometry (Cadene and Chait 2000).
Detergent analysis by thin-layer chromatography
The completeness of detergent exchange was analyzed by one-dimensional thin-layer chromatography. Protein samples eluted from a SE column, along with DDM standards, were spotted directly onto silica glass plates (Fisher Scientific). The mobile solvent phase consisted of ethylacetate/methanol 4 : 1 (v/v). The plate was sprayed with 2N H2SO4 solution and then charred at 90°C for DDM detection (Reiss-Husson 1991).
Phospholipid quantification and identification
Total amount of phospholipid copurified with GlpT was measured by using a phosphorus assay after the Ni2+-NTA and SE chromatography columns (Chen et al. 1956). Briefly, samples of known protein concentration were treated with 50 μL perchloric acid at 200°C until they became transparent, followed by cooling to 4°C. A mixture of 100 μL of the digested protein and 900 μL of freshly prepared reaction solution (1.43% ascorbic acid, 0.32% ammonium molybdate, 3.39% H2SO4) was heated for 10 to 15 min at 75°C. Absorption was measured at 820 nm on an Agilent 8453 spectrophotometer at 20°C and quantified by using phosphate standard (Sigma).
Phospholipid copurified with GlpT was identified by thin-layer chromatography. Lipid was extracted from protein samples and separated by preparative TLC (Folch et al. 1957; Lemieux et al. 2002) using the following solvent system: chloroform-acetone-methanol-acetic acid-water, (6 : 8 : 2 : 2 : 1). Individual lipids along with the appropriate standards were then visualized by charring.
Crystallization and X-ray diffraction
GlpT purified in DDM was subjected to crystallization screens consisting of PEG400 and 4000 at intervals of 1.0 pH unit, as well as the three major Hampton screening kits: Crystal Screen I and II and MemFrac (Hampton Research). After detergent exchange on an SE chromatography column, crystallization conditions were more thoroughly screened with a series of screens that consisted of PEG or its MME derivative as the precipitant: 200, 400, 500, 2000, 2000MME, 5000MME, 4000, 8000, 10000, or 20000, and pH from 3.5 to 9.0 at 0.5 unit increments. Additives such as organics, detergents, and mono- and divalent salts were screened to improve crystal order and packing. Crystallization drops were assembled in a hanging drop fashion (McPherson 1999).
GlpT crystals were frozen in liquid ethane, liquid nitrogen, or a cold nitrogen stream. Crystals were examined by X-ray diffraction either with a rotating anode and an R-AXIS-II detector or, more often, with a synchrotron source at one of the following beamlines: X12B, X12C, and X25 at the National Synchrotron Light Source in Brookhaven National Laboratory; 19BM and 19ID at the Advanced Proton Source in Argonne National Laboratory; and 5.02 at the Advance Light Source in Lawrence Berkeley National Laboratory. The aperture size was 100 to 150 μm. Diffraction data were processed by using the HKL2000 program (Otwinowski and Miror 1997).
Acknowledgments
We are grateful to Dr. Michael Becker at the National Synchrotron Light Source in the Brookhaven National Laboratory, Drs. Frank Rotella and Norma Duke at the Advanced Proton Source in Argonne National Laboratory, and Dr. Gerry McDermott at the Advance Light Source in Lawrence Berkeley National Laboratory, for assistance in X-ray diffraction experiments. We would like to thank Ruthven Lewis and Dr. Ron McElhaney, University of Alberta, for assistance with analytical thin-layer chromatography; Yun Lu and Dr. T. Neubert for mass spectrometry; Drs. Regina Goetz, Lalji Kanbi, and Markus Safferling for synchrotron trips; and Heather Griffith for critical reading of the manuscript. M.A. thanks the Human Frontier Science Organization and the Agoroun Institute/Jane Coffin Childs Memorial Fund for postdoctoral fellowships. This work was financially supported by NIH (RO1-DK53973) and by the Diabetes Research Bridging Fund of the New York State Department of Health.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.03276603.
References
- Ambudkar, S.V., Larson, T.J., and Maloney, P.C. 1986a. Reconstitution of sugar phosphate transport systems of Escherichia coli. J. Biol. Chem. 261 9083–9086. [PubMed] [Google Scholar]
- Ambudkar, S.V., Sonna, L.A., and Maloney, P.C. 1986b. Variable stoichiometry of phosphate-linked anion exchange in Streptococcus lactis: Implications for the mechanism of sugar phosphate transport by bacteria. Proc. Natl. Acad. Sci. 83 280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer, M., Kim, M.J., Lemieux, M.J., Villa, A., Song, J., Li, X.D., and Wang, D.N. 2001. High-yield expression and functional analysis of Escherichia coli glycerol-3-phosphate transporter. Biochemistry 40 6628–6635. [DOI] [PubMed] [Google Scholar]
- Boulter, J.M. and Wang, D.N. 2001. Purification and characterization of human erythrocyte glucose transporter in decylmaltoside detergent solution. Protein Expr. Purif. 22 337–348. [DOI] [PubMed] [Google Scholar]
- Cadene, M. and Chait, B. 2000. A robust, detergent friendly method for mass spectrometry analysis of integral membrane proteins. Anal. Chem. 72 5655–5658. [DOI] [PubMed] [Google Scholar]
- Carter Jr., C.W. and Carter, C.W. 1979. Protein crystallization using incomplete factorial experiments. J. Biol. Chem. 254 12219–12223. [PubMed] [Google Scholar]
- Chang, G., Spencer, R.H., Lee, A.T., Barclay, M.T., and Rees, D.C. 1998. Structure of the MscL homolog from Mycobacterium tubeculosis: A gated mechanosensitive ion channel. Science 282 2220–2226. [DOI] [PubMed] [Google Scholar]
- Chen, P.S., Toribara, T.Y., and Warner, H. 1956. Microdetermination of phosphorous. Anal. Chem. 28 1756–1758. [Google Scholar]
- Devaux, P.F. and Seigneuret, M. 1985. Specificity of lipid–protein interactions as determined by spectroscopic techniques. Biochim. Biophys. Acta 822 63–125. [DOI] [PubMed] [Google Scholar]
- Doyle, D.A., Morais Cabral, J., Pfuetzner, R.A., Kuo, A., Gulbis, J.M., Cohen, S.L., Chait, B.T., and MacKinnon, R. 1998. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science 280 69–77. [DOI] [PubMed] [Google Scholar]
- Elvin, C.M., Hardy, C.M., and Rosenberg, H. 1985. π exchange mediated by the GlpT-dependent sn-glycerol-3-phosphate transport system in Escherichia coli. J. Bacteriol. 161 1054–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel, C.K., Chen, L., and Prive, G.G. 2002. Stability of the lactose permease in detergent solutions. Biochim. Biophys. Acta 1564 47–56. [DOI] [PubMed] [Google Scholar]
- Folch, J., Lees, M., and Stanley, G.H.S. 1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226 497–509. [PubMed] [Google Scholar]
- Garavito, R.M. and Rosenbusch, J.P. 1980. Three-dimensional crystals of an integral membrane protein: An initial x-ray analysis. J. Cell. Biol. 86 327–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavito, R.M., Picot, D., and Loll, P.J. 1996. Strategies for crystallizing membrane proteins. J. Bioenerg. Biomemb. 28 13–27. [PubMed] [Google Scholar]
- Gött, P. and Boos, W. 1988. The transmembrane topology of the sn-glycerol-3-phosphate permease of Escherichia coli analysed by phoA and lacZ protein fusions. Mol. Microbiol. 2 655–663. [DOI] [PubMed] [Google Scholar]
- Henderson, P.J.F. 1993. The 12-transmembrane helix transporters. Curr. Opin. Cell Biol. 5 708–712. [DOI] [PubMed] [Google Scholar]
- Huang, Y., Lemieux, M.J., Song, J., Auer, M., and Wang, D.N. 2003. Structure and mechanism of the glycerol-3-phosphate transporter from Escherichia coli. Science 301 616–620. [DOI] [PubMed] [Google Scholar]
- Jancarik, J., Scott, W.G., Milligan, D.L., Koshland, D.E., and Kim, S.H. 1991. Crystallization and preliminary X-ray diffraction study of the ligand-binding domain of the bacterial chemotaxis-mediating aspartate receptor of Salmonella typhimurium. J. Mol. Biol. 221 31–34. [DOI] [PubMed] [Google Scholar]
- Koronakis, V., Sharff, A., Koronakis, E., Luisi, B., and Hughes, C. 2000. Crystal structure of the bacterial membrane protein TolC central to multidrug efflux and protein export. Nature 405 914–919. [DOI] [PubMed] [Google Scholar]
- Kühlbrandt, W. 1987. Three-dimensional crystals of the light-harvesting chlorophyll a/b protein complex from pea chloroplasts. J. Mol. Biol. 194 757–762. [DOI] [PubMed] [Google Scholar]
- ———. 1988. The structure of light-harvesting chlorophyll a/b protein complex from plant photosynthetic membranes at 7Å resolution in projection. J. Mol. Biol. 202 849–864. [DOI] [PubMed] [Google Scholar]
- Kwong, P.D., Wyatt, R., Desjardins, E., Robinson, J., Culp, J.S., Hellmig, B.D., Sweet, R.W., Sodroski, J., and Hendrickson, W.A. 1999. Probability analysis of variational crystallization and its application to gp120, the exterior envelope glycoprotein of type 1 human immunodeficiency virus (HIV-1). J. Biol. Chem. 274 4115–4123. [DOI] [PubMed] [Google Scholar]
- Lancaster, C.R., Kroger, A., Auer, M., and Michel, H. 1999. Structure of fumarate reductase from Wolinella succinogenes at 2.2 Å resolution. Nature 402 377–385. [DOI] [PubMed] [Google Scholar]
- Larson, T.J., Schumacher, G., and Boos, W. 1982. Identification of the glpT-encoded sn-glycerol-3-phosphate permease of Escherichia coli, an oligomeric integral membrane protein. J. Bacteriol. 152 1008–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux, M.J., Reithmeier, R.A., and Wang, D.N. 2002. Importance of detergent and phospholipid in the crystallization of the human erythrocyte anion-exchanger membrane domain. J. Struct. Biol. 137 322–332. [DOI] [PubMed] [Google Scholar]
- Li, X.D., Villa, A., Gownley, C., Kim, M.J., Song, J.M., Auer, M., and Wang, D.N. 2001. Monomeric state and ligand binding of recombinant GABA transporter from Escherichia coli. FEBS Lett. 494 165–169. [DOI] [PubMed] [Google Scholar]
- Locher, K.P., Lee, A.T., and Rees, D.C. 2002. The E. coli BtuCD structure: A framework for ABC transporter architecture and mechanism. Science 296 1091–1098. [DOI] [PubMed] [Google Scholar]
- McPherson, A. 1990. Current approaches to macromolecular crystallization. Eur. J. Biochem. 189 1–23. [DOI] [PubMed] [Google Scholar]
- ———. 1999. Crystallization of biological macromolecules. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Michel, H. 1982. Three-dimensional crystals of a membrane protein complex. J. Mol. Biol. 158 567–572. [DOI] [PubMed] [Google Scholar]
- ———. 1983. Crystallization of membrane proteins. Trends Biochem. Sci. 8 56–59. [Google Scholar]
- Michel, H. and Oesterhelt, D. 1980. Three-dimensional crystals of membrane proteins: Bacteriorhodopsin. Proc. Natl. Acad. Sci. 77 1283–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otwinowski, Z. and Miror, W. 1997. Processing of X-ray diffraction data collected in oscillation mode. Meth. Enzymol. 276 307–326. [DOI] [PubMed] [Google Scholar]
- Pao, S.S., Paulsen, I.T., and Saier Jr., M.H. 1998. Major facilitator superfamily. Microbiol. Mol. Biol. Rev. 62 1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiagen. 2001. QIAgen detection and assay handbook, pp. 35–37. Qiagen, Hilden, Germany.
- Reiss-Husson, F. 1991. Crystallization of membrane proteins. In Crystallization of nucleic acids and proteins (eds. A. Ducruix and R. Geige), pp. 175–193. IRL Press, Oxford.
- Reizer, J., Finley, K., Kakuda, D., MacLeod, C.L., Reizer, A., and Saier Jr., M.H. 1993. Mammalian integral membrane receptors are homologous to facilitators and antiporters of yeast, fungi, and eubacteria. Protein Sci. 2 20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock, C.O. and Cronan, J.E. 1985. Lipid metabolism in prokaryotes. In Biochemistry of lipids and membranes (eds. D.E. Vance and J.E. Vance), pp. 73–115. Benjamin/Cummings, Menlo Park, CA.
- Rosenbusch, J.P., Lustig, A., Grabo, M., Zulauf, M., and Regenass, M. 2001. Approaches to determining membrane protein structures to high resolution: Do selections of subpopulations occur? Micron 32 75–90. [DOI] [PubMed] [Google Scholar]
- Schertler, G.F.X., Bartunik, H.D., Michel, H., and Oesterhelt, D. 1993. Orthorhombic crystal form of bacteriorhodopsin nucleated on benzamidine diffracting to 3.6 Å resolution. J. Mol. Biol. 234 156–164. [DOI] [PubMed] [Google Scholar]
- Silhavy, T.J., Hartig-Beecken, I., and Boos, W. 1976. Periplasmic protein related to the sn-glycerol-3-phosphate transport system of Escherichia coli. J. Bacteriol. 126 951–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, L. and Gouaux, J.E. 1997. Membrane protein crystallization: Application of sparse matrices to the a-hemolysin heptamer. In Methods in enzymology (eds. C.W. Carter Jr. and R.M. Sweet), pp. 60–74. Academic Press, San Diego, CA. [DOI] [PubMed]
- Valiyaveetil, F.I., Zhou, Y., and MacKinnon, R. 2002. Lipids in the structure, folding, and function of the KcsA K+ channel. Biochemistry 41 10771–10777. [DOI] [PubMed] [Google Scholar]
- Wang, D.N., Lemieux, M.J., and Boulter, J.M. 2003a. Purification and characterization of transporter proteins from human erythrocyte membrane. In Membrane protein protocols: Expression, purification and characterization (ed. B. Selinsky), pp. 239–256. Humana Press, Totowa, NJ. [DOI] [PubMed]
- Wang, D.N., Safferling, M., Lemieux, M.J., Griffith, H., Chen, Y., and Li, X.D. 2003b. Practical aspects of overexpressing bacterial secondary membrane transporters for structural studies. Biochim. Biophys. Acta 1610 23–36. [DOI] [PubMed] [Google Scholar]
- Wiener, M.C. and Snook, C.F. 2001. The development of membrane protein crystallization screens based on detergent solution properties. J. Cryst. Growth 232 426–431. [Google Scholar]
- Zulauf, M. 1991. Detergent phenomena in membrane protein crystallization. In Crystallization of membrane proteins (ed. H. Michel), pp. 53–72. CRC Press, Boca Raton, FL.