Abstract
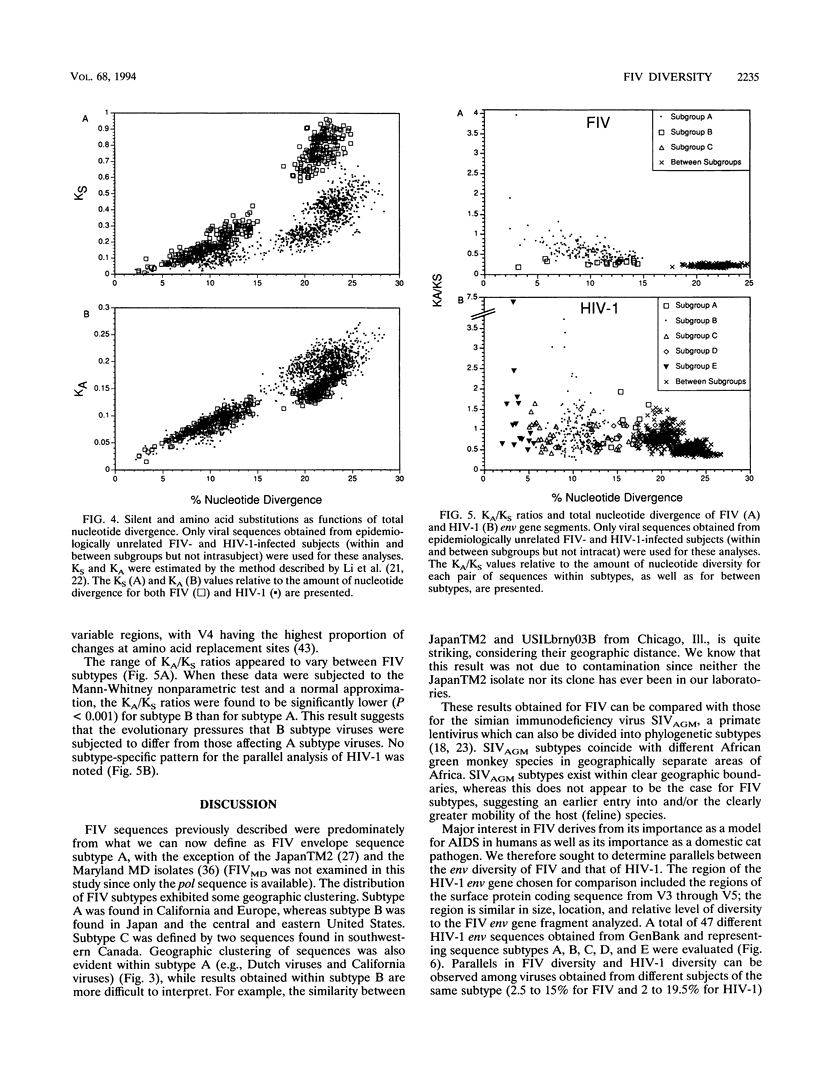
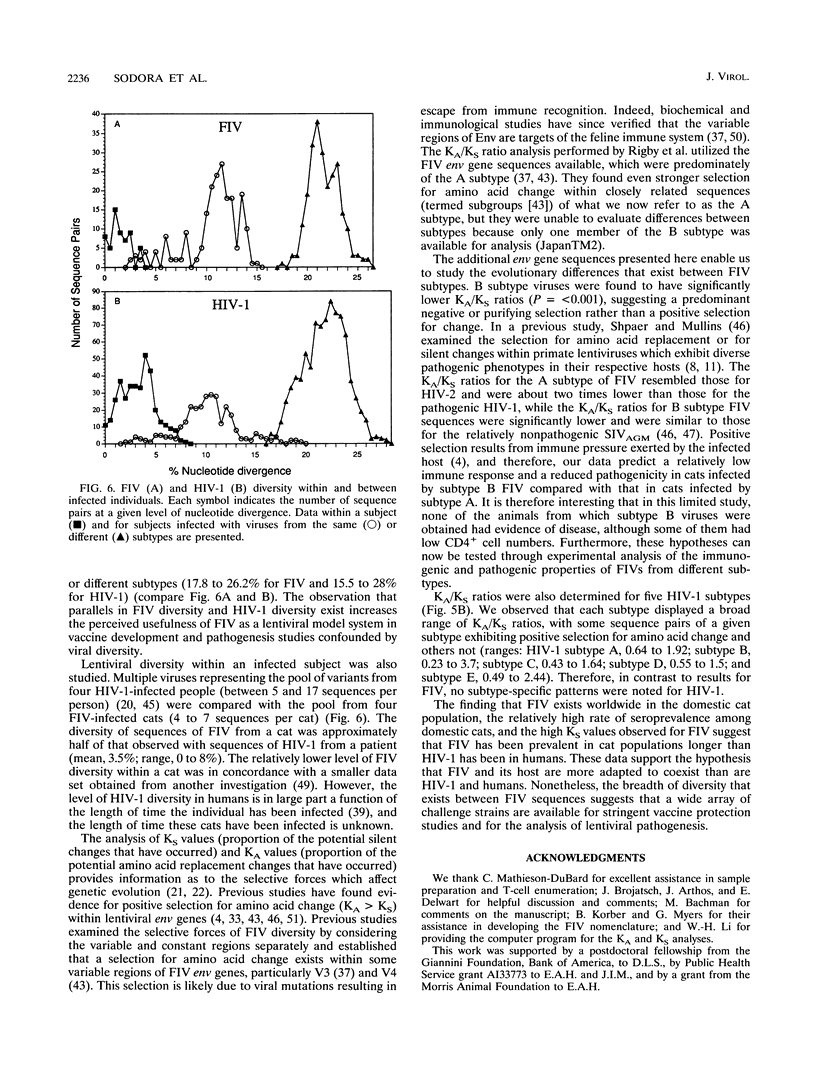
Feline immunodeficiency virus (FIV) is a lentivirus associated with AIDS-like illnesses in cats. As such, FIV appears to be a feline analog of human immunodeficiency virus (HIV). A hallmark of HIV infection is the large degree of viral genetic diversity that can develop within an infected individual and the even greater and continually increasing level of diversity among virus isolates from different individuals. Our goal in this study was to determine patterns of FIV genetic diversity by focusing on a 684-nucleotide region encompassing variable regions V3, V4, and V5 of the FIV env gene in order to establish parallels and distinctions between FIV and HIV type 1 (HIV-1). Our data demonstrate that, like HIV-1, FIV can be separated into distinct envelope sequence subtypes (three are described here). Similar to that found for HIV-1, the pairwise sequence divergence within an FIV subtype ranged from 2.5 to 15.0%, whereas that between subtypes ranged from 17.8 to 26.2%. However, the high number of synonymous nucleotide changes among FIV V3 to V5 env sequences may also include a significant number of back mutations and suggests that the evolutionary distances among FIV subtypes are underestimated. Although only a few subtype B viruses were available for examination, the pattern of diversity between the FIV A and B subtypes was found to be significantly distinct; subtype B sequences had proportionally fewer mutations that changed amino acids, compared with silent changes, suggesting a more advanced state of adaptation to the host. No similar distinction was evident for HIV-1 subtypes. The diversity of FIV genomes within individual infected cats was found to be as high as 3.7% yet twofold lower than that within HIV-1-infected people over a comparable region of the env gene. Despite these differences, significant parallels between patterns of FIV evolution and HIV-1 evolution exist, indicating that a wide array of potentially divergent virus challenges need to be considered in FIV vaccine and pathogenesis studies.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackley C. D., Yamamoto J. K., Levy N., Pedersen N. C., Cooper M. D. Immunologic abnormalities in pathogen-free cats experimentally infected with feline immunodeficiency virus. J Virol. 1990 Nov;64(11):5652–5655. doi: 10.1128/jvi.64.11.5652-5655.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlough J. E., Ackley C. D., George J. W., Levy N., Acevedo R., Moore P. F., Rideout B. A., Cooper M. D., Pedersen N. C. Acquired immune dysfunction in cats with experimentally induced feline immunodeficiency virus infection: comparison of short-term and long-term infections. J Acquir Immune Defic Syndr. 1991;4(3):219–227. [PubMed] [Google Scholar]
- Brown A. L., Monaghan P. Evolution of the structural proteins of human immunodeficiency virus: selective constraints on nucleotide substitution. AIDS Res Hum Retroviruses. 1988 Dec;4(6):399–407. doi: 10.1089/aid.1988.4.399. [DOI] [PubMed] [Google Scholar]
- Clayton J., Kedes L. GEL, a DNA sequencing project management system. Nucleic Acids Res. 1982 Jan 11;10(1):305–321. doi: 10.1093/nar/10.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwart E. L., Shpaer E. G., Louwagie J., McCutchan F. E., Grez M., Rübsamen-Waigmann H., Mullins J. I. Genetic relationships determined by a DNA heteroduplex mobility assay: analysis of HIV-1 env genes. Science. 1993 Nov 19;262(5137):1257–1261. doi: 10.1126/science.8235655. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F. Immunodeficiency viruses: the simian-human connection. Nature. 1989 Jun 1;339(6223):338–339. doi: 10.1038/339338a0. [DOI] [PubMed] [Google Scholar]
- Fultz P. N., McClure H. M., Anderson D. C., Swenson R. B., Anand R., Srinivasan A. Isolation of a T-lymphotropic retrovirus from naturally infected sooty mangabey monkeys (Cercocebus atys). Proc Natl Acad Sci U S A. 1986 Jul;83(14):5286–5290. doi: 10.1073/pnas.83.14.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudsmit J., Kuiken C. L., Nara P. L. Linear versus conformational variation of V3 neutralization domains of HIV-1 during experimental and natural infection. AIDS. 1989;3 (Suppl 1):S119–S123. doi: 10.1097/00002030-198901001-00017. [DOI] [PubMed] [Google Scholar]
- Hoffmann-Fezer G., Thum J., Ackley C., Herbold M., Mysliwietz J., Thefeld S., Hartmann K., Kraft W. Decline in CD4+ cell numbers in cats with naturally acquired feline immunodeficiency virus infection. J Virol. 1992 Mar;66(3):1484–1488. doi: 10.1128/jvi.66.3.1484-1488.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosie M. J., Robertson C., Jarrett O. Prevalence of feline leukaemia virus and antibodies to feline immunodeficiency virus in cats in the United Kingdom. Vet Rec. 1989 Sep 9;125(11):293–297. doi: 10.1136/vr.125.11.293. [DOI] [PubMed] [Google Scholar]
- Ishida T., Taniguchi A., Kanai T., Kataoka Y., Aimi K., Kariya K., Washizu T., Tomoda I. Retrospective serosurvey for feline immunodeficiency virus infection in Japanese cats. Nihon Juigaku Zasshi. 1990 Apr;52(2):453–454. doi: 10.1292/jvms1939.52.453. [DOI] [PubMed] [Google Scholar]
- Johnson P. R., Fomsgaard A., Allan J., Gravell M., London W. T., Olmsted R. A., Hirsch V. M. Simian immunodeficiency viruses from African green monkeys display unusual genetic diversity. J Virol. 1990 Mar;64(3):1086–1092. doi: 10.1128/jvi.64.3.1086-1092.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumi K., Conway B., Cunningham S., Berson A., Evans C., Iversen A. K., Colvin D., Gallo M. V., Coutre S., Shpaer E. G. Human immunodeficiency virus type 1 envelope gene structure and diversity in vivo and after cocultivation in vitro. J Virol. 1992 Feb;66(2):875–885. doi: 10.1128/jvi.66.2.875-885.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W. H. Unbiased estimation of the rates of synonymous and nonsynonymous substitution. J Mol Evol. 1993 Jan;36(1):96–99. doi: 10.1007/BF02407308. [DOI] [PubMed] [Google Scholar]
- Li W. H., Wu C. I., Luo C. C. A new method for estimating synonymous and nonsynonymous rates of nucleotide substitution considering the relative likelihood of nucleotide and codon changes. Mol Biol Evol. 1985 Mar;2(2):150–174. doi: 10.1093/oxfordjournals.molbev.a040343. [DOI] [PubMed] [Google Scholar]
- Li Y., Naidu Y. M., Daniel M. D., Desrosiers R. C. Extensive genetic variability of simian immunodeficiency virus from African green monkeys. J Virol. 1989 Apr;63(4):1800–1802. doi: 10.1128/jvi.63.4.1800-1802.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louwagie J., McCutchan F. E., Peeters M., Brennan T. P., Sanders-Buell E., Eddy G. A., van der Groen G., Fransen K., Gershy-Damet G. M., Deleys R. Phylogenetic analysis of gag genes from 70 international HIV-1 isolates provides evidence for multiple genotypes. AIDS. 1993 Jun;7(6):769–780. doi: 10.1097/00002030-199306000-00003. [DOI] [PubMed] [Google Scholar]
- Maki N., Miyazawa T., Fukasawa M., Hasegawa A., Hayami M., Miki K., Mikami T. Molecular characterization and heterogeneity of feline immunodeficiency virus isolates. Arch Virol. 1992;123(1-2):29–45. doi: 10.1007/BF01317136. [DOI] [PubMed] [Google Scholar]
- Matsushita S., Robert-Guroff M., Rusche J., Koito A., Hattori T., Hoshino H., Javaherian K., Takatsuki K., Putney S. Characterization of a human immunodeficiency virus neutralizing monoclonal antibody and mapping of the neutralizing epitope. J Virol. 1988 Jun;62(6):2107–2114. doi: 10.1128/jvi.62.6.2107-2114.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa T., Fukasawa M., Hasegawa A., Maki N., Ikuta K., Takahashi E., Hayami M., Mikami T. Molecular cloning of a novel isolate of feline immunodeficiency virus biologically and genetically different from the original U.S. isolate. J Virol. 1991 Mar;65(3):1572–1577. doi: 10.1128/jvi.65.3.1572-1577.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraillon A., Barré-Sinoussi F., Parodi A., Moraillon R., Dauguet C. In vitro properties and experimental pathogenic effect of three strains of feline immunodeficiency viruses (FIV) isolated from cats with terminal disease. Vet Microbiol. 1992 Apr;31(1):41–54. doi: 10.1016/0378-1135(92)90140-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa S., Lutz H., Aubert A., Bishop D. H. Identification of conserved and variable regions in the envelope glycoprotein sequences of two feline immunodeficiency viruses isolated in Zurich, Switzerland. Virus Res. 1991 Sep;21(1):53–63. doi: 10.1016/0168-1702(91)90071-3. [DOI] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Myers G., MacInnes K., Korber B. The emergence of simian/human immunodeficiency viruses. AIDS Res Hum Retroviruses. 1992 Mar;8(3):373–386. doi: 10.1089/aid.1992.8.373. [DOI] [PubMed] [Google Scholar]
- Novotney C., English R. V., Housman J., Davidson M. G., Nasisse M. P., Jeng C. R., Davis W. C., Tompkins M. B. Lymphocyte population changes in cats naturally infected with feline immunodeficiency virus. AIDS. 1990 Dec;4(12):1213–1218. doi: 10.1097/00002030-199012000-00005. [DOI] [PubMed] [Google Scholar]
- Olmsted R. A., Barnes A. K., Yamamoto J. K., Hirsch V. M., Purcell R. H., Johnson P. R. Molecular cloning of feline immunodeficiency virus. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2448–2452. doi: 10.1073/pnas.86.7.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted R. A., Langley R., Roelke M. E., Goeken R. M., Adger-Johnson D., Goff J. P., Albert J. P., Packer C., Laurenson M. K., Caro T. M. Worldwide prevalence of lentivirus infection in wild feline species: epidemiologic and phylogenetic aspects. J Virol. 1992 Oct;66(10):6008–6018. doi: 10.1128/jvi.66.10.6008-6018.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancino G., Chappey C., Saurin W., Sonigo P. B epitopes and selection pressures in feline immunodeficiency virus envelope glycoproteins. J Virol. 1993 Feb;67(2):664–672. doi: 10.1128/jvi.67.2.664-672.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancino G., Fossati I., Chappey C., Castelot S., Hurtrel B., Moraillon A., Klatzmann D., Sonigo P. Structure and variations of feline immunodeficiency virus envelope glycoproteins. Virology. 1993 Feb;192(2):659–662. doi: 10.1006/viro.1993.1083. [DOI] [PubMed] [Google Scholar]
- Pang S., Shlesinger Y., Daar E. S., Moudgil T., Ho D. D., Chen I. S. Rapid generation of sequence variation during primary HIV-1 infection. AIDS. 1992 May;6(5):453–460. doi: 10.1097/00002030-199205000-00003. [DOI] [PubMed] [Google Scholar]
- Pedersen N. C., Ho E. W., Brown M. L., Yamamoto J. K. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science. 1987 Feb 13;235(4790):790–793. doi: 10.1126/science.3643650. [DOI] [PubMed] [Google Scholar]
- Phillips T. R., Lamont C., Konings D. A., Shacklett B. L., Hamson C. A., Luciw P. A., Elder J. H. Identification of the Rev transactivation and Rev-responsive elements of feline immunodeficiency virus. J Virol. 1992 Sep;66(9):5464–5471. doi: 10.1128/jvi.66.9.5464-5471.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips T. R., Talbott R. L., Lamont C., Muir S., Lovelace K., Elder J. H. Comparison of two host cell range variants of feline immunodeficiency virus. J Virol. 1990 Oct;64(10):4605–4613. doi: 10.1128/jvi.64.10.4605-4613.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby M. A., Holmes E. C., Pistello M., Mackay A., Brown A. J., Neil J. C. Evolution of structural proteins of feline immunodeficiency virus: molecular epidemiology and evidence of selection for change. J Gen Virol. 1993 Mar;74(Pt 3):425–436. doi: 10.1099/0022-1317-74-3-425. [DOI] [PubMed] [Google Scholar]
- Shpaer E. G., Mullins J. I. Rates of amino acid change in the envelope protein correlate with pathogenicity of primate lentiviruses. J Mol Evol. 1993 Jul;37(1):57–65. doi: 10.1007/BF00170462. [DOI] [PubMed] [Google Scholar]
- Siebelink K. H., Chu I. H., Rimmelzwaan G. F., Weijer K., Osterhaus A. D., Bosch M. L. Isolation and partial characterization of infectious molecular clones of feline immunodeficiency virus obtained directly from bone marrow DNA of a naturally infected cat. J Virol. 1992 Feb;66(2):1091–1097. doi: 10.1128/jvi.66.2.1091-1097.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebelink K. H., Chu I. H., Rimmelzwaan G. F., Weijer K., van Herwijnen R., Knell P., Egberink H. F., Bosch M. L., Osterhaus A. D. Feline immunodeficiency virus (FIV) infection in the cat as a model for HIV infection in man: FIV-induced impairment of immune function. AIDS Res Hum Retroviruses. 1990 Dec;6(12):1373–1378. doi: 10.1089/aid.1990.6.1373. [DOI] [PubMed] [Google Scholar]
- Siebelink K. H., Rimmelzwaan G. F., Bosch M. L., Meloen R. H., Osterhaus A. D. A single amino acid substitution in hypervariable region 5 of the envelope protein of feline immunodeficiency virus allows escape from virus neutralization. J Virol. 1993 Apr;67(4):2202–2208. doi: 10.1128/jvi.67.4.2202-2208.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds P., Balfe P., Ludlam C. A., Bishop J. O., Brown A. J. Analysis of sequence diversity in hypervariable regions of the external glycoprotein of human immunodeficiency virus type 1. J Virol. 1990 Dec;64(12):5840–5850. doi: 10.1128/jvi.64.12.5840-5850.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott R. L., Sparger E. E., Lovelace K. M., Fitch W. M., Pedersen N. C., Luciw P. A., Elder J. H. Nucleotide sequence and genomic organization of feline immunodeficiency virus. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5743–5747. doi: 10.1073/pnas.86.15.5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torten M., Franchini M., Barlough J. E., George J. W., Mozes E., Lutz H., Pedersen N. C. Progressive immune dysfunction in cats experimentally infected with feline immunodeficiency virus. J Virol. 1991 May;65(5):2225–2230. doi: 10.1128/jvi.65.5.2225-2230.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verschoor E. J., Hulskotte E. G., Ederveen J., Koolen M. J., Horzinek M. C., Rottier P. J. Post-translational processing of the feline immunodeficiency virus envelope precursor protein. Virology. 1993 Mar;193(1):433–438. doi: 10.1006/viro.1993.1140. [DOI] [PubMed] [Google Scholar]
- Yamamoto J. K., Hohdatsu T., Olmsted R. A., Pu R., Louie H., Zochlinski H. A., Acevedo V., Johnson H. M., Soulds G. A., Gardner M. B. Experimental vaccine protection against homologous and heterologous strains of feline immunodeficiency virus. J Virol. 1993 Jan;67(1):601–605. doi: 10.1128/jvi.67.1.601-605.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]


