Abstract
BACKGROUND
Due to dietary exposure of women to genistein, a soy-derived phytoestrogen, and the estrogen responsiveness of uterine leiomyomas ‘fibroids’, we evaluated the effects of genistein (0.001-50 μg/ml) on human uterine leiomyoma (UtLM) cells versus uterine smooth muscle cells (UtSMCs) in vitro.
METHODS
Light microscopy was used to determine the effects of genistein on cell morphology. Proliferation was assessed using a colorimetric assay and proliferating cell nuclear antigen (PCNA) immunocytochemistry. Flow cytometry was used to quantitate cells in the S-phase and those undergoing apoptosis. A fluorometric assay and confocal microscopy were used to detect caspase-3 activity and apoptotic bodies, respectively.
RESULTS
In UtLM cells, low concentrations (≤1 μg/ml) of genistein stimulated proliferation, increased PCNA labeling and the percentage of cells in the S-phase, but this did not occur in UtSMCs. Higher concentrations (≥10 μg/ml) of genistein adversely affected the morphology, significantly inhibited proliferation, decreased PCNA labeling, increased caspase-3 activity and induced apoptosis in both cell types.
CONCLUSIONS
Genistein’s effects are concentration-dependent in both cell lines. Lower concentrations elicit proliferative effects on UtLM cells only; whereas, higher concentrations alter morphology, inhibit proliferation, and increase caspase activity and apoptosis in both cell types, with the latter two effects being more extensive in UtSMCs.
Keywords: apoptosis, cell proliferation, genistein, uterine leiomyoma (UtLM) cells, uterine smooth muscle cells (UtSMCs)
Introduction
Uterine leiomyomas (fibroids; myomas) are the most common benign tumors occurring in premenopausal women. The incidence of these tumors has been reported to be as high as 77% based on pathological specimens (Cramer and Patel, 1990). The estimated cumulative incidence for fibroids is >80% for African-American women and approximately 70% for Caucasian women as evidenced by ultrasound screening (Day Baird et al., 2003). Although benign, they may cause health problems such as pelvic pain, menorrhagia and infertility (Buttram and Reiter, 1981; Haney, 2000; Stewart, 2001; Wegienka et al., 2003). The exact etiology of fibroids remains unknown, although studies have shown that these tumors are hormonally dependent (Andersen et al., 1995; Brandon et al., 1995; Englund et al., 1998), similar to breast and ovarian cancers (Chen and Anderson, 2001; O’Donnell et al., 2005).
Within the past decade, there has been an increased interest in phytoestrogens and their role in human health because of the possible protective role that some phytoestrogens may have in cancer. The flavonoids are the class of phytoestrogens most widely studied (King and Young, 1999). Isoflavones, a subclass of flavonoids, are thought to be the most well known of the plant estrogens (Ososki and Kennelly, 2003). Genistein, daidzein and their respective β-glucoside forms are the primary isoflavones in soybeans (Messina, 2002). The estimated intake of soy is 9-27 g/day for Asian populations (in China, Indonesia, Japan and Korea) (Soyatech Syndicated Report, 2006) compared with 1-3 g/day for Americans (Barnes et al., 1995).
At present, there are conflicting results in the literature regarding the protective versus adverse effects of soy in rodents and in vitro studies. Some rodent studies using cancer-inducing agents have shown that soy products are protective against cancers (Baggott et al., 1990; Murrill et al., 1996; Lamartiniere et al., 1998). However, another study has shown that when rodents are exposed during development, adverse effects may occur (Newbold et al., 2001). For example, mice treated neonatally with genistein developed uterine adenocarcinomas later in life (Newbold et al., 2001). In in vitro experiments, several investigators found that low concentrations (≤1 μM) of genistein (Wang et al., 1996; Le Bail et al., 1998; Maggiolini et al., 2001; Chen and Wong, 2004) stimulated growth of human breast cancer cells, whereas high concentrations (10 μM) of genistein (Le Bail et al., 1998; Maggiolini et al., 2001) inhibited growth of these cells.
Genistein is known to interact with estrogen receptors alpha and beta (ERα and β), but has a greater binding affinity for ERβ (Kuiper et al., 1998). Our laboratory has found that human uterine leiomyoma (UtLM) cells and uterine smooth muscle cells (UtSMCs; myometrial cells) express ERα and ERβ (Carney et al., 2002; data unpublished for ERβ). While the mechanism(s) by which genistein elicits its proliferative effects remains uncertain, several mechanisms such as interaction with the ER, activation of growth factor receptor pathways and acceleration of cells into the DNA synthesis phase (S-phase) have been proposed (Wang et al., 1996; Chen and Wong, 2004). Chen et al. (2004) investigated the effects of 1 μM genistein on cell proliferation induction in MCF-7 cells, and showed that increased cell proliferation can be measured by the percentage of cells in the S-phase of the cell cycle. Furthermore, Dees et al. (1997) showed that treatment of MCF-7 cells with 1 μM of genistein increased synthesis of cyclin D, induced activation of Cdk2 and stimulated hyperphosphorylation of pRb105, demonstrating genistein’s ability to stimulate MCF-7 cells to enter the cell cycle.
The inhibitory effects of genistein at high concentrations may involve non-estrogenic mechanisms, such as the inhibition of tyrosine-specific protein kinases (Akiyama et al., 1987; Akiyama and Ogawara, 1991) and topoisomerase II (Okura et al., 1988; Markovits et al., 1989). Genistein can also induce programmed cell death “apoptosis” (Constantinou et al., 1998; Fioravanti et al., 1998); it has been found that ≥50 μM genistein induces apoptosis in MCF-7 cells at 48 or 72 h (Pagliacci et al., 1994). Genistein has also been shown to inhibit the progression of MCF-7 cells through the cell cycle, ultimately, arresting the cells in the gap 2 to mitosis (G2/M) phase (Pagliacci et al., 1994; Constantinou et al., 1998).
Due to the dietary exposure of women to genistein and the estrogen responsiveness of uterine leiomyomas, we investigated the in vitro effects of genistein on UtLM cells and normal UtSMCs. In this study, we determined the effects of various concentrations of genistein on the morphology and proliferation of UtLM cells compared with UtSMCs and assessed mechanisms by which genistein’s proliferative or inhibitory effects occur.
Materials and Methods
Culture of human UtLM cells and UtSMCs
Human UtLM cells (GM10964; Coriell Institute for Medical Research, Camden, NJ, USA) and UtSMCs (Clonetics Corporation, San Diego, CA, USA) were kept in a standard tissue culture incubator at 37°C, with 95% humidity and 5% carbon dioxide. The UtLM cells were routinely cultured in Minimum Essential Medium (MEM) Eagle (Sigma Chemical Company, St Louis, MO, USA) supplemented with approximately 20% fetal bovine serum (FBS) (Sigma), 1X vitamins (Gibco, Grand Island, NY, USA), 1X essential amino acids (Gibco), 1X non-essential amino acids (Gibco) and 2X l-glutamine (Gibco). The UtSMCs were cultured in Smooth Muscle Cell Growth Media System (SmGM®-2 BulletKit; Clonetics). Twenty-four hours prior to genistein treatment, the media were changed to Dulbecco’s Modified Eagle’s Medium Nutrient Mixture F-12 Ham (DMEM/F-12) (Hyclone Laboratories, Logan, UT, USA) phenol red free with charcoal dextran treated (stripped) FBS (Hyclone) for both cell types.
Cell morphology
UtLM cells and UtSMCs were untreated [0 μg/ml; dimethylsulfoxide (DMSO); control] or treated with low (1 μg/ml) or high (50 μg/ml) concentrations of genistein for 168 h. Morphologic analysis was done using an Axiovert 25 inverted transmitted light microscope at 20X magnification, containing HAL 6 V 25 W illumination (Carl Zeiss, Inc, Thornwood, NY, USA). Axiovision 3.1 software was used to analyze the samples. Imaging was done using an AxioCam digital camera (Carl Zeiss, Inc).
Cell proliferation assay
UtLM cells and UtSMCs were seeded at 5 × 103 cells/well and 4 × 103 cells/well, respectively, in 96-well culturing plates (Corning, Corning, NY, USA). After 24 h, the media were changed to DMEM/F-12 (Hyclone) phenol red free with charcoal dextran treated (stripped) FBS (Hyclone) for both cell types. The cells were treated with genistein (4′,5,7-Trihydroxyisoflavone; 98% purity by HPLC; Sigma) every 2 days until day seven. Genistein was reconstituted using 0.1% DMSO (Sigma) before it was diluted in the media. The cells were exposed to 0 μg/ml (DMSO; control), 0.001 μg/ml (0.0037 μM), 0.01 μg/ml (0.037 μM), 0.1 μg/ml (0.37 μM), 1 μg/ml (3.7 μM), 10 μg/ml (37 μM), 25 μg/ml (93 μM) and 50 μg/ml (185 μM) genistein for either 24 or 168 h. The number of viable cells was determined using a colorimetric 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)2-(4-sulfophenyl)-2H-tetrazolium inner salt (MTS)-based CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega, Madison, WI, USA). The MTS combines with phenazine ethosulfate (PES), an electron coupling agent, and the MTS tetrazolium compound is bioreduced by viable cells into a colored formazan product, i.e. soluble in tissue culture medium. Dye intensity is proportional to the number of viable cells. The CellTiter 96® AQueous One Solution was added to the plate and allowed to incubate for 1 h and 30 min at 37°C, with 5% CO2, prior to reading the absorbance values at 490 nm using a plate reader (Molecular Devices Corporation, Sunnyvale, CA, USA). The samples were analyzed using SOFTmax Pro software (Molecular Devices Corporation).
Proliferating cell nuclear antigen (PCNA): immunocytochemistry and labeling
UtLM cells and UtSMCs were plated at densities of 15 × 104 cells/slides and 7.5 × 104 cells/slide, respectively, using chamber slides (Lab-tek; Nalge Nunc International, Naperville, IL, USA), and allowed to attach overnight. For each cell type, the culture medium was then changed to stripped serum DMEM/F12 (Hyclone) without phenol red followed by treatment of the cells with 0 μg/ml (DMSO; control), 1 μg/ml or 50 μg/ml genistein for 168 h. Prior to immunostaining, the cells were rinsed three times for 5 min in 1X automation buffer (Biomeda Corporation, Foster City, CA, USA). Both cell types were fixed consecutively with 70% methanol and 4% paraformaldehyde, for 10 min each. Anti-PCNA (Chemicon International, Temecula, CA, USA) (1:75 dilution) and goat antimouse IgM (Jackson Immunoresearch Laboratories, Incorporated) (1:400 dilution), were used as the primary and secondary antibodies, respectively. A Super Sensitive™ Link-Label IHC Detection System (BioGenex, San Ramon, CA, USA) was used according to the manufacturer’s recommendations. The kit contained 3,3′-diaminobenzidine (DAB) solution as the chromogen for visualization of staining. The cells were counterstained using Mayer’s hematoxylin (Poly Scientific, Bayshore, NY, USA). Percent PCNA labeling was determined by the number of cells having positively stained nuclei divided by 1000 total cells counted (i.e. labeled and unlabeled) and multiplied by 100.
Flow cytometric analysis
Flow cytometry was used as a second method of assessing cell proliferation, to determine the percentage of cells in various phases of the cell cycle. UtLM cells and UtSMCs were treated with 0 μg/ml (DMSO; control), 1 μg/ml or 50 μg/ml genistein (Sigma), then incubated in culture for 168 h. Both cell types were resuspended in 1 ml of Nuclear Isolation Media (NIM)-DAPI staining solution (NIM-DAPI, NPE Systems, Pembroke Pines, FL, USA) at a density of 500 000 cells/ml for 7 min at room temperature, and filtered through a 25 mm Filter Tip (NPE Systems). NIM-DAPI stained samples were run on a NPE Quanta™ flow analyzer fitted with a mercury arc lamp (HBO), a 365/546 nm dual exciter, a 565DCXR splitter (long pass) and a 450AF55 nm emission filter. A minimum of 10 000 nuclear signals was collected in list mode files. Modfit LT software (Verity Software House, Topsham, ME, USA) was used to analyze the samples.
Caspase fluorometric assay
A fluorometric CaspACE™ Assay System (Promega, Madison, WI, USA) was used to detect the activity of caspase-3 (CPP32) in UtLM cells and UtSMCs. In brief, the cells were treated with 0 μg/ml (DMSO; control) or 50 μg/ml genistein for 168 h, and total protein was isolated using RIPA buffer. From this, 50 μl of protein were added to the assay under conditions according to the manufacturer’s protocol, and 100 μl of the reaction mixture was added to the 96-well plate. The fluorescent activity of caspase-3 was determined after incubation of the samples with the CPP32 substrate (Ac-DEVD-AMC) using a microplate reader (Molecular Devices Corporation) at an excitation and emission wavelength of 360 and 460 nm, respectively, in conjunction with SOFTmax Pro software (Molecular Devices Corporation).
Hoechst stain for apoptosis
UtLM cells and UtSMCs were grown in glass bottom microwell dishes (MatTek, Ashaland, MA, USA) and treated with 0 μg/ml (DMSO; control) or 50 μg/ml genistein for 168 h. Prior to staining, the media was changed and the cells were incubated at 37°C, with 95% humidity and 5% carbon dioxide for 30 min using a 10 μg/ml solution of Hoechst 33342 dye (Invitrogen, Eugene, OR, USA). Nuclear changes and apoptotic bodies were observed using a LSM 510 UV laser scanning confocal microscope mounted on Axiovert 100 M microscope (Carl Zeiss, Inc.). The samples were analyzed using LSM Image Examiner version 3.2 software for Windows Network Technology (NT).
Statistical analysis
The statistical analyses were performed on data from three independent experiments for each assay. Descriptive statistics are expressed as mean ± SEM. For the cell proliferation MTS-based assay and PCNA labeling data, which were normally distributed, differences among concentrations were tested by parametric analysis of variance (ANOVA) (Neter et al., 1996). Significant overall differences were followed by Dunnett’s test, comparing each treated group with the control group. Percentages of cells within the S-phase, percentages of apoptotic cells and the caspase assay data, which were not normally distributed, were analyzed with the Kruskal-Wallis ANOVA (Conover, 1971). Significant overall concentration differences were examined by Mann-Whitney tests (Conover, 1971) to identify which treated groups differed from the control group. Differences between percentages of S-phase and apoptotic cells within the same sample were compared using paired t-tests (Snedecor and Cochran, 1976). All P-values are two-sided.
Results
Cell morphology in UtLM cells and UtSMCs at 168 h after treatment with genistein
Control UtLM cells appeared robust and were elongated with intact cigar shaped nuclei (Fig. 1A). The control UtSMCs were morphologically similar to the UtLM cells (Fig. 1B). UtLM cells treated with 1 μg/ml of genistein were morphologically similar to control UtLM cells, although there was cellular crowding, suggestive of proliferation (Fig. 1C). Compared to control UtSMCs, no morphological changes or cellular crowding was seen in UtSMCs treated with 1 μg/ml genistein (Fig. 1D). After treatment with 50 μg/ml of genistein, both UtLM cells and UtSMCs were shrunken and fewer in number (Fig. 1E and F). Furthermore, both cell types were no longer robust and elongated, and the nuclei were irregularly shaped.
Figure 1.
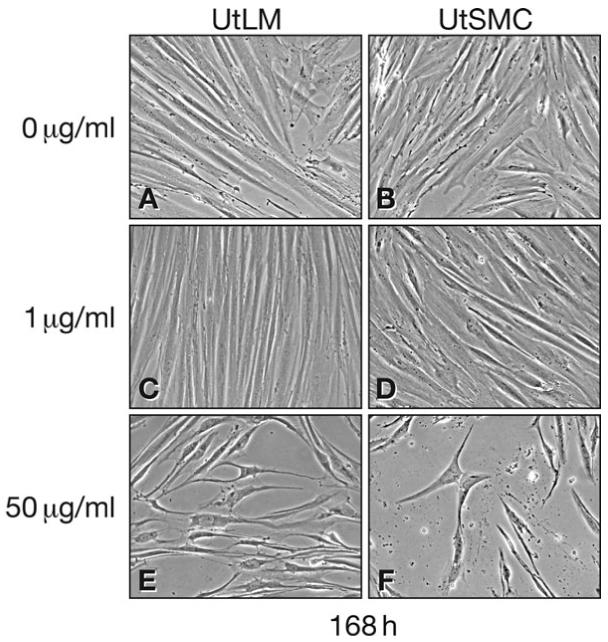
Morphology of UtLM cells and UtSMCs after treatment with 0 μg/ml (Control), 1 μg/ml and 50 μg/ml genistein at 168 h (A) UtLM cells and (B) UtSMCs at 0 μg/ml; (C) UtLM cells and (D) UtSMCs at 1 μg/ml; (E) UtLM cells and (F) UtSMCs at 50 μg/ml. Magnification ×20
Growth effects of genistein in UtLM cells and UtSMCs at 24 and 168 h
At 24 h, the proliferation of UtLM cells, as measured by the MTS-based assay, was significantly (P < 0.01) stimulated by treatment with 1 μg/ml of genistein, compared with controls (Fig. 2A). At 168 h, a significant (P < 0.001) stimulatory effect was observed with both 0.1 μg/ml and 1 μg/ml genistein compared with controls. However, higher concentrations of genistein (10, 25 and 50 μg/ml) significantly (P < 0.05 to P < 0.001) inhibited the proliferation of UtLM cells at 168 h compared with controls (Fig. 2A). UtSMCs were not stimulated by low concentrations of genistein at 24 or 168 h (Fig. 2B). Rather, treatment with 25 or 50 μg/ml of genistein caused significant (P < 0.001) decreases in UtSMC proliferation at 24 and 168 h, and by 168 h, 10 μg/ml of genistein also significantly (P < 0.05) inhibited the proliferation of UtSMCs (Fig. 2B).
Figure 2.
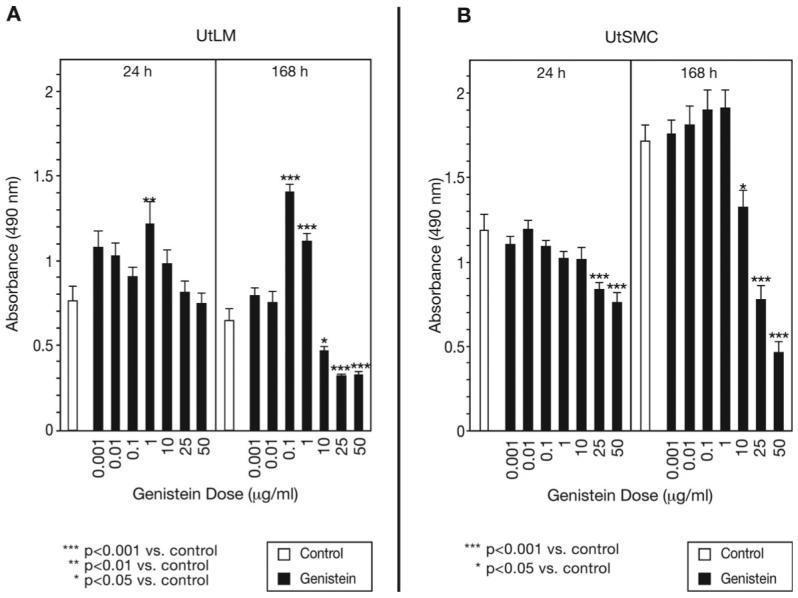
Effects of various concentrations of genistein on growth of UtLM cells and UtSMCs Evaluated by the MTS-based Cell Proliferation Assay at 24 h and 168 h
(A) UtLM Cells, (B) UtSMCs
The cells were treated with lower concentrations (0.001 μg/ml-1 μg/ml) and higher concentrations (10 μg/ml-50 μg/ml) of genistein and compared with controls. Results were obtained from three independent experiments and are expressed as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001 versus control
PCNA immunoexpression and labeling indices in UtLM cells and UtSMCs at 168 h
At 168 h, PCNA immunoexpression and labeling indices in UtLM cells treated with 1 μg/ml of genistein were increased compared with control cells (Fig. 3A1, A3 and B, P < 0.01), whereas no effect was observed in UtSMCs at this concentration (Figs. 3A2, A4 and B). Following treatment with 50 μg/ml of genistein, PCNA expression and labeling indices in both UtLM cells and UtSMCs were decreased compared with respective control cells (Fig. 3A5, A6 and B, P < 0.01).
Figure 3.
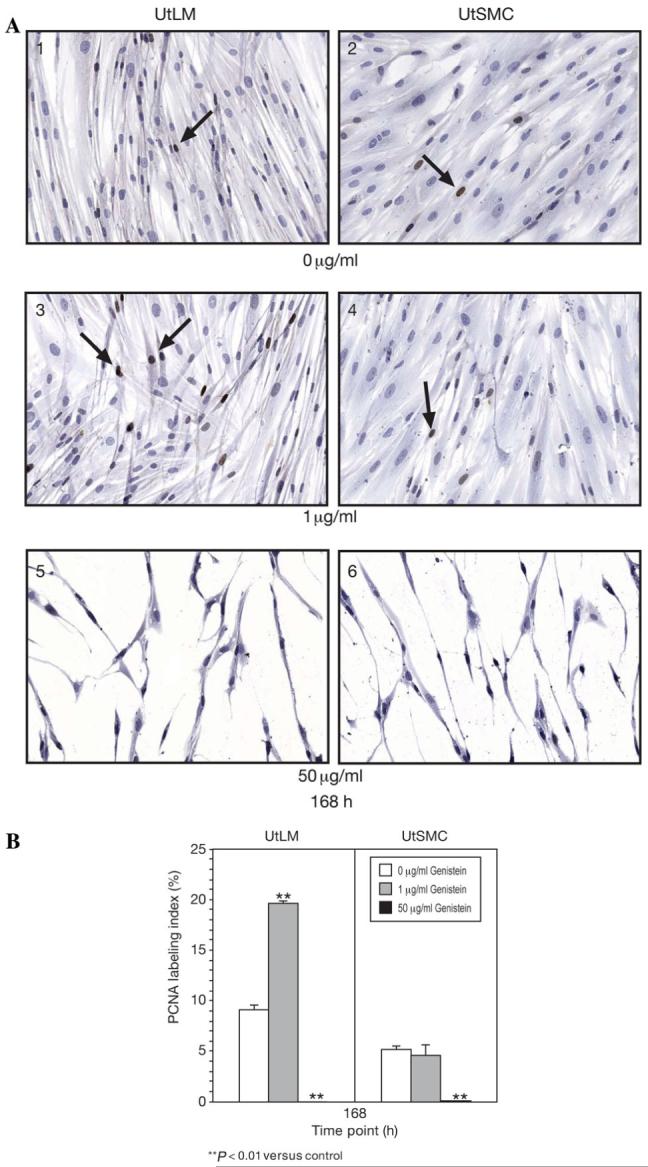
PCNA Immunoexpression and labeling at 168 h
(A) PCNA was immunoexpressed in the nuclei of both control UtLM cells (1) and (2) UtSMCs as indicated by dark brown staining (arrows). (3) UtLMs cells and (4) UtSMC treated with 1 μg/ml of genistein. (5) UtLM cells and (6) UtSMCs treated with 50 μg/ml of genistein. Original magnification 40. DAB with hematoxylin counterstain. (B) PCNA labeling in UtLM cells and UtSMCs after treatment with 0 μg/ml, 1 μg/ml and 50 μg/ml genistein. Results were obtained from three independent experiments and are expressed as mean ± SEM. **P < 0.01 versus control
Proliferation and apoptosis in UtLM cells and UtSMCs at 168 h
At 168 h, the percentage of UtLM cells in the S-phase of the cell cycle in the 1 μg/ml group was nearly double that in the control group, but this difference was not significant due to substantial variability in the control samples (Fig. 4A). UtLM cells treated with 50 μg/ml of genistein showed no increase in the percentage of S-phase cells compared with controls. The percentage of apoptotic UtLM cells was significantly higher (P < 0.05) in the 50 μg/ml group than in the control grouP < but apoptosis was similar in the control and 1 μg/ml groups (Fig. 4A). At 168 h, the percentages of UtSMCs in the S-phase were similar in the 0, 1 and 50 μg/ml of genistein groups (Fig. 4B). However, UtSMCs treated with 50 μg/ml of genistein showed a significant (P < 0.05) increase in apoptosis, which was far greater (16.3%) than that observed in the UtLM cells (2.0%) (Fig. 4B).
Figure 4.
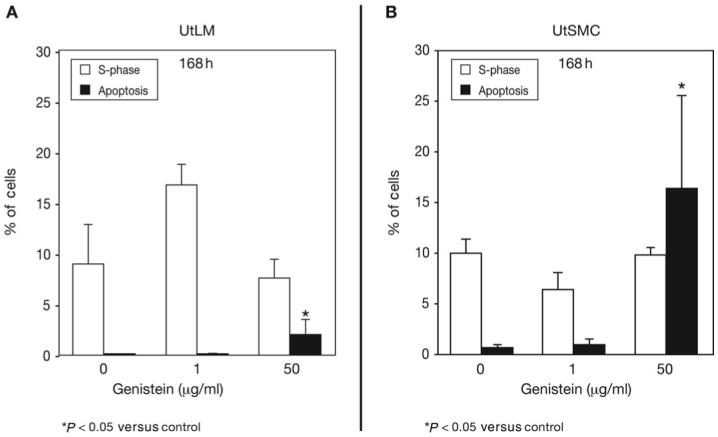
Proliferation and apoptosis in UtLM cells and UtSMCs at 168 h
(A) UtLM Cells and (B) UtSMCs. The percentage of cells in the S-phase and undergoing apoptosis after treatment with 1 μg/ml and 50 μg/ml of genistein compared with control cells. Results were obtained from three independent experiments and are expressed as mean ± SEM. *P < 0.05 versus control
A caspase assay and Hoechst stain for apoptosis in UtLM cells and UtSMCs at 168 h
At 168 h, caspase-3 activity in UtLM cells and UtSMCs treated with 50 μg/ml of genistein was significantly (P < 0.05) higher than in their respective controls (Fig. 5A). Additionally, caspase-3 activity in the UtSMCs was nearly double that in the UtLM cells after treatment with 50 μg/ml of genistein. A Hoechst stain was used to detect apoptotic nuclear changes in the UtLM cells and UtSMCs (Fig. 5B). The treated UtLM cells showed signs of nuclear swelling suggestive of necrosis, with few apoptotic cells (Fig. 5B1); whereas numerous apoptotic cells were observed in the treated UtSMCs (Fig. 5B2) compared with control UtSMCs and compared with treated UtLM cells (Fig. 5B1).
Figure 5.
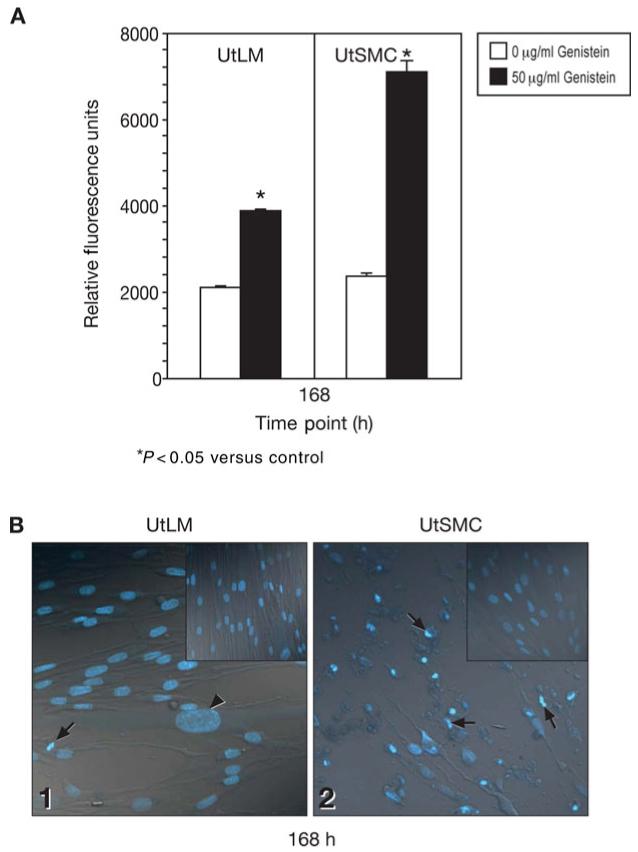
Effects of a high concentration of genistein on apoptosis at 168 h (A) Fluorometric caspase assay
Caspase-3 activity in the UtLM cells and UtSMCs after treatment with 0 μg/ml and 50 μg/ml genistein. Results were obtained from three independent experiments and are expressed as mean ± SEM. *P < 0.05 versus control. (B) Hoechst stain. UtSMCs (2) treated with 50 μg/ml genistein were apoptotic (arrows); whereas, few UtLM cells (1) were apoptotic (arrow), or had swollen nuclei (arrowhead). Insets are untreated UtLM cells (1 upper right) and UtSMCs (2 upper right). Original magnification × 40
Discussion
In the present study, we found that a low concentration of genistein (1 μg/ml) did not alter cell morphology in UtLM cells and UtSMCs; however, it did alter cell proliferation dynamics in UtLM cells, but not UtSMCs. Although in vitro and in vivo reports on the morphologic and proliferative effects of genistein in human uterine leiomyoma and myometrial cells are lacking, a few studies have assessed the effects of structurally similar compounds on these cell types. Kawaguchi et al. (1985) investigated the effects of estradiol (E2) (10-8 M) on smooth muscle cells from the explants of myometrium and leiomyomas, and found that a low concentration of E2 caused both cell types to proliferate, as indicated by a marked increase in cell numbers from day 7 to 14. Furthermore, both cell types treated with E2 showed similar ultrastructural features to controls, and E2 did not significantly affect the “differentiation” or change the shape or size of either cell type (Kawaguchi et al., 1985). Our findings are similar to the above study. However, the absence of a proliferative effect in the UtSMCs is in contrast with the E2-treated cells in the Kawaguchi et al. (1985) study and could be related to the fact that genistein is weakly estrogenic and a less potent inducer of cell growth in UtSMC. Our findings are also in agreement with an in vitro study of Eker rat leiomyoma cells in which low concentrations of genistein were proliferative and high concentrations were inhibitory (Hunter et al., 1999). Taken together, these data suggest that low concentrations of an estrogenic compound have no effect on the morphology of uterine leiomyoma or myometrial cells.
Newbold et al. (2002) found that CD-1 mice prenatally exposed to low (2.5 μg/kg and 10 μg/kg) concentrations of the synthetic, environmental estrogen, diethylstilbestrol (DES), had an increased incidence later in life of uterine leiomyomas that had histologic characteristics typical of uterine leiomyomas in women (Newbold et al., 2002). Interestingly, previous findings by McLachlan et al. (1980) revealed that low (2.5 μg/kg and 10 μg/kg) concentrations of DES in CD-1 mice treated at days 9 and 16 of gestation lead to the development of uterine leiomyomas in 12 to 18-month-old offspring; whereas a high (100 μg/kg) concentration of DES was often teratogenic and caused smooth muscle hypoplasia and underdevelopment, and thus no leiomyomas. The effects of prenatal DES exposure have also been evaluated in women. One study has shown that premenopausal women who reported prenatal exposure to DES show increased risk for fibroids compared with non-exposed women, and that the tumors tend to be larger in the exposed women (Baird and Newbold, 2005). In contrast, Wise et al. (2005) found no association between prenatal exposure and risk of fibroids in women, and instead determined that prenatal DES exposure was associated with an increased risk of paraovarian cysts. Collectively, however, the human data support the concept that estrogenic exposures during development can promote reproductive tract abnormalities later in life.
Genistein is believed to elicit a concentration dependent dual threshold effect with regard to growth stimulation or inhibition. Studies using MCF-7 cells have shown that concentrations ≤1 μM genistein stimulate cell growth (Wang et al., 1996; Chen and Wong, 2004), whereas concentrations ≥10 μM inhibit growth (Le Bail et al., 1998; Maggiolini et al., 2001). The lower concentrations of 0.001 (0.0037 μM)-1 μg/ml (3.7 μM) of genistein, selected for our study, are within the physiological range (reported to be ≤1 μM) (Bouker and Hilakivi-Clarke, 2000), and the higher concentrations of 10 (37 μM)-50 μg/ml (185 μM) are within pharmacological (>10 μM) concentrations (Bouker and Hilakivi-Clarke, 2000). It appears to be unclear whether high in vitro concentrations can be achieved in vivo (de Lemos, 2001). Most of the in vitro experiments have used concentrations that have exceeded 10 μM; however, based on pharmacokinetic calculations involving daily intake of isoflavones, following absorption from the gut, distribution to peripheral tissues and excretion, it is unlikely that blood isoflavone concentrations even in high soy consumers would be greater than in the 1-5 μM range (Barnes et al., 1996). Although a study has shown that healthy Japanese men and women consuming a single, high dose of genistein have plasma concentrations of >20 μM at 4 h after initial consumption, but by 24 h the plasma levels decrease (< 5 μM) (Izumi et al., 2000) and are within the physiological range.
In order to ascertain a mechanism by which genistein produced stimulatory and inhibitory effects in the UtLM cells and UtSMC, we assessed cell proliferation using a MTS-based assay and PCNA labeling. Flow cytometry was used to evaluate percentage of cells in the S-phase and apoptosis. We found that UtLM cells, but not UtSMC, treated with a low concentration of genistein showed significant increases in cell proliferation and PCNA labeling; whereas a high concentration of genistein resulted in significant decreases in cell proliferation and PCNA labeling and significant increases in apoptosis for both cell types, with the percentage of apoptosis in UtSMC being triple that of UtLM cells. Our findings are in agreement with two studies using MCF-7 cells in that treatment of MCF-7 cells with a low concentration (1 μM) of genistein stimulated cell growth (Le Bail et al., 1998) and increased the percentage of S-phase cells, while decreasing the percentage of quiescent to gap 1 (G0/G1; resting) phase cells (Chen and Wong, 2004). Chen and Wong (2004) attributed the increased proliferation to the mitogenic ability of genistein to stimulate gap 1 to synthesis (G1/S) transition in the cells. However, another study has shown that treatment of MCF-7 cells with 1 μM of genistein caused increased synthesis of cyclin D1, activation of Cdk2 and induced hyperphosphorylation of pRb105, indicating genistein stimulated the entrance of MCF-7 cells into the cell cycle (Dees et al., 1997). Our results suggest that genistein induces the progression of UtLM cells from the G0/G1 phase to the S-phase, ultimately leading to cell proliferation. Contrary to the effects of a low dose of genistein, in our study, higher concentrations (10-50 μg/ml) of genistein reduced cell growth in both the UtLM cells and UtSMCs, which is similar to what has been reported for MCF-7 cells (Le Bail et al., 1998). Po et al. (2002) also showed that treatment of MCF-7 cells with 10150 μM genistein resulted in a significant increase in apoptosis compared with control cells.
Indicators of apoptosis such as caspase-3 activity and nuclear morphology were assessed in both cell types in our study. Caspase-3 is an important biological mediator in the apoptotic pathway (Thornberry et al., 1997). This executioner protein is involved in the extrinsic and intrinsic apoptotic pathways (Boatright and Salvesen, 2003). We found that a high concentration of genistein significantly increased caspase-3 activity and apoptosis in both cell types. Furthermore, at the high concentration, UtSMCs caspase-3 activity was nearly double that observed in UtLM cells and supports the flow cytometry data in which we found nearly eight times the percentage of apoptotic UtSMCs compared with UtLM cells. Confocal microscopy and the Hoechst stain confirmed the presence of apoptotic bodies, primarily in the UtSMCs, whereas the UtLM cells did show some characteristics of apoptotic cells, although this was not a predominant feature of dying cells.
In summary, genistein’s concentration determines its effects on the morphology and cell proliferation of uterine leiomyoma and normal myometrial cells. Low (0.1 μg/ml and 1.0 μg/ml) concentrations of genistein significantly stimulated UtLM cell growth, but not UtSMC growth; high concentrations (10-50 μg/ml) of genistein inhibited the growth of both UtLM cells and UtSMCs. Our data show that UtLM cells are more responsive than UtSMCs to the proliferative effects of a low concentration of genistein, as indicated by an increase absorbance of cells in the MTS-based assay, enhanced PCNA labeling and the increased percentage of cells in the S-phase of the cell cycle. The increased responsiveness observed in UtLM cells could be due, in part, to enhanced transactivation of the ER, and/or activation or up-regulation of transcription factors, growth factor peptides, receptor tyrosine kinases and their downstream effector kinases (MAPK/ERK 1/2), which have all been shown to be up-regulated or activated in response to treatment with 17β E2 in UtLM cells, but not in UtSMCs (Barbarisi et al., 2001; Swartz et al., 2005). Conversely, a high dose of genistein induced more severe apoptotic effects in the UtSMCs possibly due to early (data not shown) and sustained up-regulation of caspase-3 activity leading to activation of apoptotic pathways which was not observed in the UtLM cells. Although cell death occurred in the UtLM cells at the higher concentration, it appears that the mechanism of death was mainly by a non-apoptotic route. UtLM cells are neoplastic cells and may be programmed towards cell survival and proliferation; therefore, they might be less susceptible to the apoptotic inducing effects of genistein. Our in vitro model provides some insight on the differential effects of low and high concentrations of a phytoestrogen on uterine leiomyoma and uterine smooth muscle cell proliferation and death.
Acknowledgements
The authors would like to thank Drs. Ronald Herbert and Wendy Jefferson for their critical review of this manuscript. This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
References
- Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein. A specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- Akiyama T, Ogawara H. Use and specificity of genistein as inhibitor of protein-tyrosine kinases. Methods Enzymol. 1991;201:362–370. doi: 10.1016/0076-6879(91)01032-w. [DOI] [PubMed] [Google Scholar]
- Andersen J, DyReyes VM, Barbieri RL, Coachman DM, Miksicek RJ. Leiomyoma primary cultures have elevated transcriptional response to estrogen compared with autologous myometrial cultures. J Soc Gynecol Investig. 1995;2:542–551. doi: 10.1016/1071-5576(94)00053-4. [DOI] [PubMed] [Google Scholar]
- Baggott JE, Ha T, Vaughn WH, Juliana MM, Hardin JM, Grubbs CJ. Effect of miso (Japanese soybean paste) and NaCl on DMBA-induced rat mammary tumors. Nutr Cancer. 1990;14:103–109. doi: 10.1080/01635589009514083. [DOI] [PubMed] [Google Scholar]
- Baird DD, Newbold R. Prenatal diethylstilbestrol (DES) exposure is associated with uterine leiomyoma development. Reprod Toxicol. 2005;20:81–84. doi: 10.1016/j.reprotox.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Barbarisi A, Petillo O, Di Lieto A, Melone MA, Margarucci S, Cannas M, Peluso G. 17-beta estradiol elicits an autocrine leiomyoma cell proliferation: evidence for a stimulation of protein kinase-dependent pathway. J Cell Physiol. 2001;186:414–424. doi: 10.1002/1097-4652(2000)9999:999<000::AID-JCP1040>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Barnes S, Peterson TG, Coward L. Rationale for the use of genistein-containing soy matrices in chemoprevention trials for breast and prostate cancer. J Cell Biochem Suppl. 1995;22:181–187. doi: 10.1002/jcb.240590823. [DOI] [PubMed] [Google Scholar]
- Barnes S, Sfakianos J, Coward L, Kirk K. Soy isoflavonoids and cancer prevention. Underlying biochemical and pharmacological issues. Adv Exp Med Biol. 1996;401:87–100. doi: 10.1007/978-1-4613-0399-2_7. [DOI] [PubMed] [Google Scholar]
- Boatright KM, Salvesen GS. Mechanisms of caspase activation. Curr Opin Cell Biol. 2003;15:725–731. doi: 10.1016/j.ceb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Bouker KB, Hilakivi-Clarke L. Genistein: does it prevent or promote breast cancer? Environ Health Perspect. 2000;108:701–708. doi: 10.1289/ehp.00108701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon DD, Erickson TE, Keenan EJ, Strawn EY, Novy MJ, Burry KA, Warner C, Clinton GM. Estrogen receptor gene expression in human uterine leiomyomata. J Clin Endocrinol Metab. 1995;80:1876–1881. doi: 10.1210/jcem.80.6.7775635. [DOI] [PubMed] [Google Scholar]
- Buttram VC, Jr, Reiter RC. Uterine leiomyomata: etiology, symptomatology, and management. Fertil Steril. 1981;36:433–445. doi: 10.1016/s0015-0282(16)45789-4. [DOI] [PubMed] [Google Scholar]
- Carney SA, Tahara H, Swartz CD, Risinger JI, He H, Moore AB, Haseman JK, Barrett JC, Dixon D. Immortalization of human uterine leiomyoma and myometrial cell lines after induction of telomerase activity: molecular and phenotypic characteristics. Lab Invest. 2002;82:719–728. doi: 10.1097/01.lab.0000017499.51216.3e. [DOI] [PubMed] [Google Scholar]
- Chen X, Anderson JJ. Isoflavones inhibit proliferation of ovarian cancer cells in vitro via an estrogen receptor-dependent pathway. Nutr Cancer. 2001;41:165–171. doi: 10.1080/01635581.2001.9680628. [DOI] [PubMed] [Google Scholar]
- Chen WF, Wong MS. Genistein enhances insulin-like growth factor signaling pathway in human breast cancer (MCF-7) cells. J Clin Endocrinol Metab. 2004;89:2351–2359. doi: 10.1210/jc.2003-032065. [DOI] [PubMed] [Google Scholar]
- Conover W. Practical Nonparametric Statistics. John Wiley & Sons, Inc.; New York, NY: 1971. [Google Scholar]
- Constantinou AI, Kamath N, Murley JS. Genistein inactivates bcl-2, delays the G2/M phase of the cell cycle, and induces apoptosis of human breast adenocarcinoma MCF-7 cells. Eur J Cancer. 1998;34:1927–1934. doi: 10.1016/s0959-8049(98)00198-1. [DOI] [PubMed] [Google Scholar]
- Cramer SF, Patel A. The frequency of uterine leiomyomas. Am J Clin Pathol. 1990;94:435–438. doi: 10.1093/ajcp/94.4.435. [DOI] [PubMed] [Google Scholar]
- Day Baird D, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: Ultrasound evidence. Am J Obstet Gynecol. 2003;188:100–107. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- Dees C, Foster JS, Ahamed S, Wimalasena J. Dietary estrogens stimulate human breast cells to enter the cell cycle. Environ Health Perspect. 1997;105(Suppl 3):633–636. doi: 10.1289/ehp.97105s3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deLemos ML. Effects of soy phytoestrogens genistein and daidzein on breast cancer growth. Ann Pharmacother. 2001;35:1118–1121. doi: 10.1345/aph.10257. [DOI] [PubMed] [Google Scholar]
- Englund K, Blanck A, Gustavsson I, Lundkvist U, Sjoblom P, Norgren A, Lindblom B. Sex steroid receptors in human myometrium and fibroids: changes during the menstrual cycle and gonadotropin-releasing hormone treatment. J Clin Endocrinol Metab. 1998;83:4092–4096. doi: 10.1210/jcem.83.11.5287. [DOI] [PubMed] [Google Scholar]
- Fioravanti L, Cappelletti V, Miodini P, Ronchi E, Brivio M, Di Fronzo G. Genistein in the control of breast cancer cell growth: insights into the mechanism of action in vitro. Cancer Lett. 1998;130:143–152. doi: 10.1016/s0304-3835(98)00130-x. [DOI] [PubMed] [Google Scholar]
- Haney AF. Clinical decision making regarding leiomyomata: what we need in the next millenium. Environ Health Perspect. 2000;108(Suppl 5):835–839. doi: 10.1289/ehp.00108s5835. [DOI] [PubMed] [Google Scholar]
- Hunter DS, Hodges LC, Vonier PM, Fuchs-Young R, Gottardis MM, Walker CL. Estrogen receptor activation via activation function 2 predicts agonism of xenoestrogens in normal and neoplastic cells of the uterine myometrium. Cancer Res. 1999;59:3090–3099. [PubMed] [Google Scholar]
- Izumi T, Piskula MK, Osawa S, Obata A, Tobe K, Saito M, Kataoka S, Kubota Y, Kikuchi M. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J Nutr. 2000;30:1695–1699. doi: 10.1093/jn/130.7.1695. [DOI] [PubMed] [Google Scholar]
- Kawaguchi K, Fujii S, Konishi I, Okamura H, Mori T. Ultrastructural study of cultured smooth muscle cells from uterine leiomyoma and myometrium under the influence of sex steroids. Gynecol Oncol. 1985;21:32–41. doi: 10.1016/0090-8258(85)90229-x. [DOI] [PubMed] [Google Scholar]
- King A, Young G. Characteristics and occurrence of phenolic phytochemicals. J Am Diet Assoc. 1999;99:213–218. doi: 10.1016/S0002-8223(99)00051-6. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Lamartiniere CA, Zhang JX, Cotroneo MS. Genistein studies in rats: potential for breast cancer prevention and reproductive and developmental toxicity. Am J Clin Nutr. 1998;68:1400S–1405S. doi: 10.1093/ajcn/68.6.1400S. [DOI] [PubMed] [Google Scholar]
- Le Bail JC, Varnat F, Nicolas JC, Habrioux G. Estrogenic and antiproliferative activities on MCF-7 human breast cancer cells by flavonoids. Cancer Lett. 1998;130:209–216. doi: 10.1016/s0304-3835(98)00141-4. [DOI] [PubMed] [Google Scholar]
- Maggiolini M, Bonofiglio D, Marsico S, Panno ML, Cenni B, Picard D, Ando S. Estrogen receptor alpha mediates the proliferative but not the cytotoxic dose-dependent effects of two major phytoestrogens on human breast cancer cells. Mol Pharmacol. 2001;60:595–602. [PubMed] [Google Scholar]
- Markovits J, Linassier C, Fosse P, Couprie J, Pierre J, Jacquemin-Sablon A, Saucier JM, Le Pecq JB, Larsen AK. Inhibitory effects of the tyrosine kinase inhibitor genistein on mammalian DNA topoisomerase II. Cancer Res. 1989;49:5111–5117. [PubMed] [Google Scholar]
- McLachlan JA, Newbold RR, Bullock BC. Long-term effects on the female mouse genital tract associated with prenatal exposure to diethylstilbestrol. Cancer Res. 1980;40:3988–3999. [PubMed] [Google Scholar]
- Messina M. Brief historical overview of isoflavone research. In: Gilani GS, Anderson JJBJ, editors. Phytoestrogens and Health. AOCS Press; Champaign, Illinois: 2002. pp. 1–31. [Google Scholar]
- Murrill WB, Brown NM, Zhang JX, Manzolillo PA, Barnes S, Lamartiniere CA. Prepubertal genistein exposure suppresses mammary cancer and enhances gland differentiation in rats. Carcinogenesis. 1996;17:1451–1457. doi: 10.1093/carcin/17.7.1451. [DOI] [PubMed] [Google Scholar]
- Neter J, Kutner M, Nachtsheim C, Wasserman W. Applied Linear Statistical Models. 4th edn. WCB McGraw-Hill; Boston: 1996. [Google Scholar]
- Newbold RR, Banks EP, Bullock B, Jefferson WN. Uterine adenocarcinoma in mice treated neonatally with genistein. Cancer Res. 2001;61:4325–4328. [PubMed] [Google Scholar]
- Newbold RR, Moore AB, Dixon D. Characterization of uterine leiomyomas in CD-1 mice following developmental exposure to diethylstilbestrol (DES) Toxicol Pathol. 2002;30:611–616. doi: 10.1080/01926230290105839. [DOI] [PubMed] [Google Scholar]
- O’Donnell AJM, Macleod KG, Burns DJ, Smyth JF, Langdon SP. Estrogen receptor-a mediates gene expression changes and growth response in ovarian cancer cells exposed to estrogen. Endocr Relat Cancer. 2005;12:851–866. doi: 10.1677/erc.1.01039. [DOI] [PubMed] [Google Scholar]
- Okura A, Arakawa H, Oka H, Yoshinari T, Monden Y. Effect of genistein on topoisomerase activity and on the growth of [Val 12]Ha-ras-transformed NIH 3T3 cells. Biochem Biophys Res Commun. 1988;157:183–189. doi: 10.1016/s0006-291x(88)80030-5. [DOI] [PubMed] [Google Scholar]
- Ososki AL, Kennelly EJ. Phytoestrogens: a review of the present state of research. Phytother Res. 2003;17:845–869. doi: 10.1002/ptr.1364. [DOI] [PubMed] [Google Scholar]
- Pagliacci MC, Smacchia M, Migliorati G, Grignani F, Riccardi C, Nicoletti I. Growth-inhibitory effects of the natural phyto-oestrogen genistein in MCF-7 human breast cancer cells. Eur J Cancer. 1994;30A:1675–1682. doi: 10.1016/0959-8049(94)00262-4. [DOI] [PubMed] [Google Scholar]
- Po LS, Wang TT, Chen ZY, Leung LK. Genistein-induced apoptosis in MCF-7 cells involves changes in Bak and Bcl-x without evidence of anti-oestrogenic effects. Br J Nutr. 2002;88:463–469. doi: 10.1079/BJN2002693. [DOI] [PubMed] [Google Scholar]
- Snedecor GW, Cochran WG. Statistical Methods. The Iowa State University Press; 1976. [Google Scholar]
- Soyatech Syndicated Report. Lafayette, CA: 2006. P.O. Box 84, Bar Harbor, ME, and Soyfoods Center Survey, P.O. Box 234. [Google Scholar]
- Swartz CD, Afshari CA, Yi L, Hall KE, Dixon D. Estrogen-induced changes in IGF-I, Myb family and MAP kinase pathway genes in human uterine leiomyoma and normal uterine smooth muscle cell lines. Mol Hum Reprod. 2005;11:4441–4450. doi: 10.1093/molehr/gah174. [DOI] [PubMed] [Google Scholar]
- Stewart EA. Uterine fibroids. Lancet. 2001;357:293–298. doi: 10.1016/S0140-6736(00)03622-9. [DOI] [PubMed] [Google Scholar]
- Thornberry NA, Rano TA, Peterson EP, Rasper DM, Timkey T, Garcia-Calvo M, Houtzager VM, Nordstrom PA, Roy S, Vaillancourt JP, et al. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J Biol Chem. 1997;272:17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- Wang TT, Sathyamoorthy N, Phang JM. Molecular effects of genistein on estrogen receptor mediated pathways. Carcinogenesis. 1996;17:271–275. doi: 10.1093/carcin/17.2.271. [DOI] [PubMed] [Google Scholar]
- Wegienka G, Baird DD, Hertz-Picciotto I, Harlow SD, Steege JF, Hill MC, Schectman JM, Hartmann KE. Self-reported heavy bleeding associated with uterine leiomyomata. Obstet Gynecol. 2003;101:431–437. doi: 10.1016/s0029-7844(02)03121-6. [DOI] [PubMed] [Google Scholar]
- Wise LA, Palmer JR, Rowlings K, Kaufman RH, Herbst AL, Noller KL, Titus-Ernstoff, Troisi R, Hatch EE, Robboy SJ. Risk of benign gynecologic tumors in relation to prenatal diethylstilbestrol exposure. Obstet Gynecol. 2005;105:167–173. doi: 10.1097/01.AOG.0000147839.74848.7c. [DOI] [PubMed] [Google Scholar]


