Abstract
The influence of latent virus on CD8+ T cell memory is poorly understood. HSV type 1 specifically establishes latency in trigeminal ganglia (TG) after corneal infection of mice. In latently infected TG, IL-15 deprivation reduced the following: 1) accumulation of HSV-specific CD8+ effector T cells (HSV-CD8eff), 2) accumulation of CD127+ putative HSV-CD8 memory precursors, and 3) the size and functionality of the memory (HSV-CD8mem) population. Although compromised in IL-15−/− mice, the HSV-CD8mem pool persisted in latently infected tissue, but not in noninfected tissue of the same mice. Anti-IL-2 treatment also dramatically reduced the size of the HSV-CD8eff population in the TG, but did not influence the concomitant generation of the CD127+ putative HSV-CD8mem precursor population or the size or functionality of the HSV-CD8mem pool. Thus, the size of the memory pool appears to be determined by the size of the CD127+ CD8mem precursor population and not by the size of the overall CD8eff pool. HSV-CD8mem showed a higher basal rate of proliferation in latently infected than noninfected tissue, which was associated with a reduced population of CD4+FoxP3+ regulatory T cells. Thus, the generation, maintenance, and function of memory CD8+ T cells is markedly influenced by latent virus.
One major advantage afforded by the adaptive immune system is its ability to develop an anamnestic memory response. The CD8mem response is stronger and more rapid than the primary response due to functional augmentation and increased frequency of Ag-specific cells within the T cell pool. CD8mem in both mice and humans is comprised of at least two subpopulations: 1) central CD8mem cells that express the lymph node homing receptors CD62L and CCR7 and, thus, reside primarily in secondary lymphoid tissues; and 2) effector CD8mem cells that lack lymph node homing receptors, favoring their presence in nonlymphoid tissues (1, 2). Both of these memory populations express IL-7Rα(CD127) and IL-15Rα and depend on IL-7 and IL-15 signals for survival and homeostatic growth (3–7). It appears that both central and effector CD8mem cells derive from precursors that are generated during the initial expansion of CD8eff cells. Although these CD8mem precursors express the functional and phenotypic characteristics of CD8eff, they are differentiated from true CD8eff by their expression of CD127, and typically represent <10% of the CD8eff population (8).
In most cases, pathogens are eliminated from the body following a brief acute infection, resulting in a prolonged period in which the CD8mem pool represents an armed and waiting population that is maintained by homeostatic proliferation in the absence of Ag. Homeostasis of this population appears to be dependent on the direct or indirect influence of at least three cytokines. IL-15 acts as a growth and survival factor, whereas IL-7 maintains viability by inhibiting the apoptosis of CD8mem (9). The third cytokine, IL-2, may indirectly inhibit homeostatic proliferation of CD8mem cells by maintaining the activity of CD4+CD25+ regulatory T cells (10, 11). The receptors for all three cytokines share a common γ-chain (CD132), but have unique α-chains. IL-15R and IL-2R are heterotrimers with two shared chains (CD122 and CD132). IL-15 can promote homeostatic proliferation of CD8mem that lack IL-15Rα through transpresentation to the low-affinity IL-2R comprised of CD122 and CD132 (7, 12–14).
In some cases, pathogens persist for prolonged periods within the host in the presence of nonsterilizing immunity. Such infections can be of two types: persistent or latent. In a persistent infection typified by the lymphocytic choriomeningitis virus (LCMV)3 clone 13 infection in mice, replicating pathogens remain in the host. Such infections produce a persistent, high-level antigenic load that profoundly influences the phenotypic and functional characteristics of the CD8mem population. The CD8mem pool generated during persistent infections by LCMV proliferate poorly in response to IL-7 and IL-15 due to down-regulation of their receptors, produce little IL-2, and express the inhibitory receptor PD-1 (15–17). Prolonged exposure to a high antigenic load during this chronic infection also leads to exhaustion of Ag-specific CD8mem (18).
The generation, maintenance, and function of the CD8mem population during latent viral infections remain largely unexplored. Following HSV-1 infection of the mouse cornea, the virus is harbored in a latent state in the sensory neurons of the trigeminal ganglion (TG). Virtually all of the HSV-1 specific CD8+ T cells in the infected TG recognize the immunodominant gB498–505 epitope (19, 20). For convenience, these gB498–505-specific CD8+ T cells will be referred to in this article as HSV-CD8. This population survives contraction and is maintained in the TG of latently infected mice for the life of the animal. Evidence from our laboratory and others suggests that low-level and perhaps intermittent expression of a limited array of viral genes persists in some latently infected neurons (19, 21–23).
The concept that these latently infected neurons are ignored by the host immune system is now giving way to recent findings that HSV-CD8mem cells surrounding these neurons express an activation phenotype (CD69+ and granzyme B+), and form an apparent immunologic synapse with neurons (19, 22). These cells also release lytic granules and produce IFN-γ when stimulated directly ex vivo (19, 24). These characteristics of CD8eff cells within a population that has undergone the expansion, contraction, and homeostasis phases are consistent with the conversion of CD8eff cells to an activated CD8mem population. Together these findings suggest that the low-level TCR stimulation that occurs within latently infected tissue results in the maintenance of an activated CD8mem population that lacks the functional impairment observed following long-term exposure to high antigenic loads during persistent infections. The fact that HSV-1 latency in this model is restricted to the sensory ganglia permits a comparison of the phenotypic and functional characteristics of the CD8mem pool in the latently infected TG to those in the noninfected lungs of the same mouse. We use this model to characterize within the same animal the involvement of IL-2 and IL-15 in regulating the generation and maintenance of the CD8eff and CD8mem pools in noninfected and latently infected tissue. Our data demonstrate that regulation of the CD8+ T cell response is markedly influenced by the presence of latent virus.
Materials and Methods
Mice and virus
HSV-1 strain RE was grown in Vero cells, and intact virions were isolated on Optiprep gradients according to the manufacturer’s instructions (Accurate Chemical & Scientific). Six- to 8-wk-old female wild-type (WT) C57BL/6 mice (The Jackson Laboratory) and IL-15−/− C57BL/6 mice (Taconic Farms) were anesthetized by i.p. injection of 2.0 mg of ketamine hydrochloride and 0.04 mg of xylazine (Phoenix Scientific) in 0.2 ml of HBSS (BioWhittaker). The abraded central corneas of anesthetized mice were infected by topical application of 3 µl of RPMI 1640 (BioWhittaker) containing 1 × 105 PFU of HSV-1. All animal experiments were conducted in accordance with guidelines established by the University of Pittsburgh Institutional Animal Care and Use Committee.
Tissue preparation
At the indicated days postinfection (dpi), anesthetized mice were injected with 0.3 ml of 1000 U/ml heparin and euthanized by exsanguination. Tissues were digested in 100 µl (TG) or 1 ml (lungs) of DMEM (BioWhittaker) containing 10% FCS and 400 U/ml collagenase type I (Sigma-Aldrich) for 1 h at 37°C. TGs and lungs were dispersed into single-cell suspensions and treated with RBC lysis buffer before staining with the designated Abs. Data were collected on a FACSAria cytometer and analyzed by FACSDiva software (BD Biosciences).
Reagents
The gB498–505 (SSIEFARL) peptide was purchased from Research Genetics (Invitrogen Life Technologies). PE-conjugated H-2Kb tetramers complexed with the gB498–505 peptide were provided by the National Institute of Allergy and Infectious Diseases Tetramer Core Facility (Emory University Vaccine Center, Atlanta, GA). PE-conjugated H-2Kb dimers were purchased from BD Pharmingen and were complexed with gB498–505 peptide at 37°C overnight before use. Rat antimouse allophycocyanin-Cy7-conjugated anti-CD8α (clone 53-6.7); FITC-conjugated anti-CD107a (1d45) and CD4 (RM4-5), allophycocyanin- conjugated anti-IFN-γ (XMG1.2), PE-conjugated anti-TNF-α (MP6-XT22), PerCP-conjugated anti-CD45 (30-F11), and the BrdU Flow Kit were purchased from BD Pharmingen. Allophycocyanin-conjugated anti-CD127 (A7R34) and the allophycocyanin anti-mouse/rat FoxP3 staining set were purchased from eBioscience. The appropriate isotype control Abs were purchased from BD Pharmingen or eBioscience.
Phenotypic analysis of T cells
For all phenotypic analyses, TG and lung cells were stained for CD45 to permit gating exclusively on infiltrating bone marrow-derived cells. For analysis of HSV-1-specific CD8+ T cells (HSV-CD8), cells were additionally stained with anti-CD8α and gB498–505 H-2Kb tetramers or gB498–505 H-2Kb dimers. The latter two reagents were used interchangeably in different experiments because, in our hands, they provided results that were indistinguishable. For analysis of regulatory T cells (Tregs), cells were stained with anti-CD45 and anti-CD4 followed by intracellular staining for FoxP3 using a FoxP3 staining kit according to the manufacturer’s directions (eBioscience).
Intracellular cytokine staining and lytic granule exocytosis
TG cells were stimulated with 5 × 105 gB-transfected B6/T-350gB fibroblast cell line (25) pulsed with either 10−12 M (optimal) or 10−17 M (suboptimal) gB498–505 peptide in the presence of FITC-conjugated anti- CD107a mAb and GolgiPlug (BD Biosciences) for 6 h at 37°C/5% CO2. Following stimulation, cells were stained for surface expression of CD8α, followed by intracellular staining for IFN-γ and TNF-α after permeabilization and fixation via Cytofix/Cytoperm (BD Biosciences). CD107a capture on the cell surface during stimulation provides sensitive detection of lytic granule exocytosis as previously described (26, 27).
Administration of anti-IL-2 mAb and BrdU
In vivo, anti-IL-2 treatment was accomplished during the expansion of HSV-CD8eff by a single injection of 1 mg of anti-IL-2 mAb (clone S4B6-1) i.p. at 6 dpi followed by tissue excision at 8 dpi, or after establishment of HSV-CD8mem by injection of 1 mg of anti-IL-2 mAb i.p. every other day for 1 wk before excision of tissue. Initial experiments comparing mice treated with an isotype control mAb lacking specificity for mouse proteins to untreated mice revealed no difference in the HSV-specific CD8+ T cell response. Therefore, in repetitions of the experiments, anti-IL-2 mAbtreated mice were compared with untreated mice, and data from both groups were pooled. Mice were administered 1 mg of BrdU i.p. daily for 1 wk before tissue excision. Single-cell suspensions of indicated tissues were stained for expression of CD45, CD8, and gB498–505 H-2Kb tetramers or dimers. Intracellular BrdU staining was performed following the manufacturer’s protocol (BD Pharmingen).
Statistics
All statistical analyses were performed with GraphPad Prism 4 software using a two-tailed unpaired t test with 95% confidence intervals.
Results
The role of IL-15 and IL-2 in the generation of the HSV-CD8eff population in the infected TG
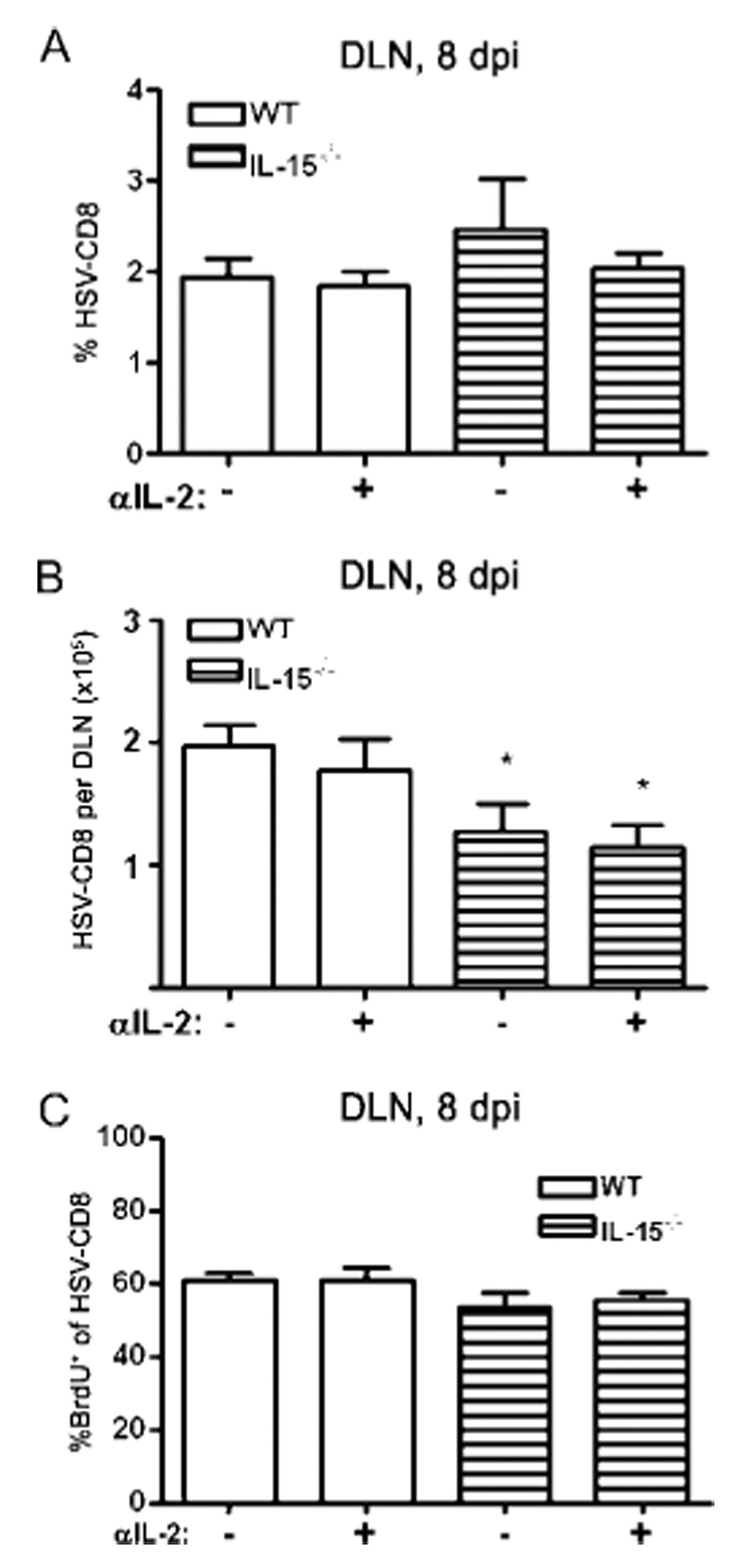
HSV-CD8eff cells accumulate to peak levels (representing about half of the CD8+ T cells present) in the TG 8 dpi, with most of the expansion occurring between 6 and 7 dpi (data not shown). A single treatment of WT mice with 1.0 mg of anti-IL-2 mAb on 6 dpi reduced the accumulation of HSV-CD8eff cells in the TG by 60% (Fig. 1A). Mice that received anti-IL-2 at 6 dpi showed comparable proliferation to control mice in both the draining lymph node (DLN) and TG at 8 dpi (Fig. 1B and Fig. 2C). The accumulation of HSV-CD8eff cells in the TG of IL-15−/− mice was reduced by 76% compared with that of untreated WT mice, but no further reduction was observed when IL-15−/− mice were treated with anti-IL-2 (Fig. 1A). The effect of anti-IL-2 treatment and of IL-15 deficiency was limited to accumulation of CD8+T cells in nonlymphoid organs because HSV-CD8eff expanded normally in the DLN of both IL-15−/− and anti-IL-2-treated mice (Fig. 2). Thus, IL-2 and IL-15 function in an overlapping or sequential manner to regulate the accumulation of HSV-CD8eff cells in the infected TG.
FIGURE 1.
Both IL-2 and IL-15 regulate the generation of the HSV-CD8eff response in the TG. WT and IL-15−/− mice received an i.p. injection of 1 mg of anti-IL-2 mAb at 6 dpi and in some cases 1 mg of BrdU i.p daily between 6 dpi and TG excision at 8 dpi. TG cells were simultaneously stained with anti-CD45, anti-CD8α, and gB498–505 H-2Kb tetramers or dimers to identify HSV-CD8, and in some cases with anti-BrdU. The entire TG sample was analyzed by flow cytometry, and pooled data are expressed as (A) the mean (±SEM) absolute number of HSV-CD8/TG or (B) the mean (±SEM) percentage of gB-specific CD8+ T cells that incorporate BrdU during a 48-h pulse. ***, p < 0.001 comparing WT anti-IL-2 treated to WT control, or IL-15−/− mice to comparably treated WT mice (n = 12–16 mice/group).
FIGURE 2.
Neither IL-2 nor IL-15 regulate the expansion of HSV-specific CD8+ T cells in the DLN. WT and IL-15−/− mice received an i.p. injection of 1 mg of anti-IL-2 mAb at 6 dpi and in some cases 1 mg of BrdU i.p daily between 6 dpi and DLN excision at 8 dpi. DLN cells were simultaneously stained with anti-CD8α, gB498–505 dimers, and in some cases with anti-BrdU. Pooled data are expressed as (A) the mean (±SEM) percent of CD8+ T cells that bind gB498–505 dimers (HSV-CD8); (B) the mean (±SEM) absolute number of HSV-CD8/DLN; or (C) the mean (±SEM) percentage of gB-specific CD8+ T cells that incorporate BrdU during a 48-h pulse. *, p < 0.05 comparing the IL-15−/− mice to comparably treated WT mice (n = 6 mice/group).
IL-15 is required for optimal generation of a CD127+ subpopulation of HSV-CD8eff in the TG
Within the HSV-CD8eff population present in the TG of WT mice at 8 dpi is a small subpopulation (~5%) that expresses CD127. In a LCMV infection model, this CD127+subpopulation was shown to represent the CD8mem precursor pool (8). Although a single treatment of WT mice with 1 mg of anti-IL-2 mAb at 6 dpi dramatically reduced the size of the HSV-CD8eff population in the TG (Fig. 1A), it did not influence the size of the CD127+CD8+ subpopulation (Fig. 3A). In contrast, this putative CD8mem precursor pool was significantly reduced in IL-15−/− mice (Fig. 3A).
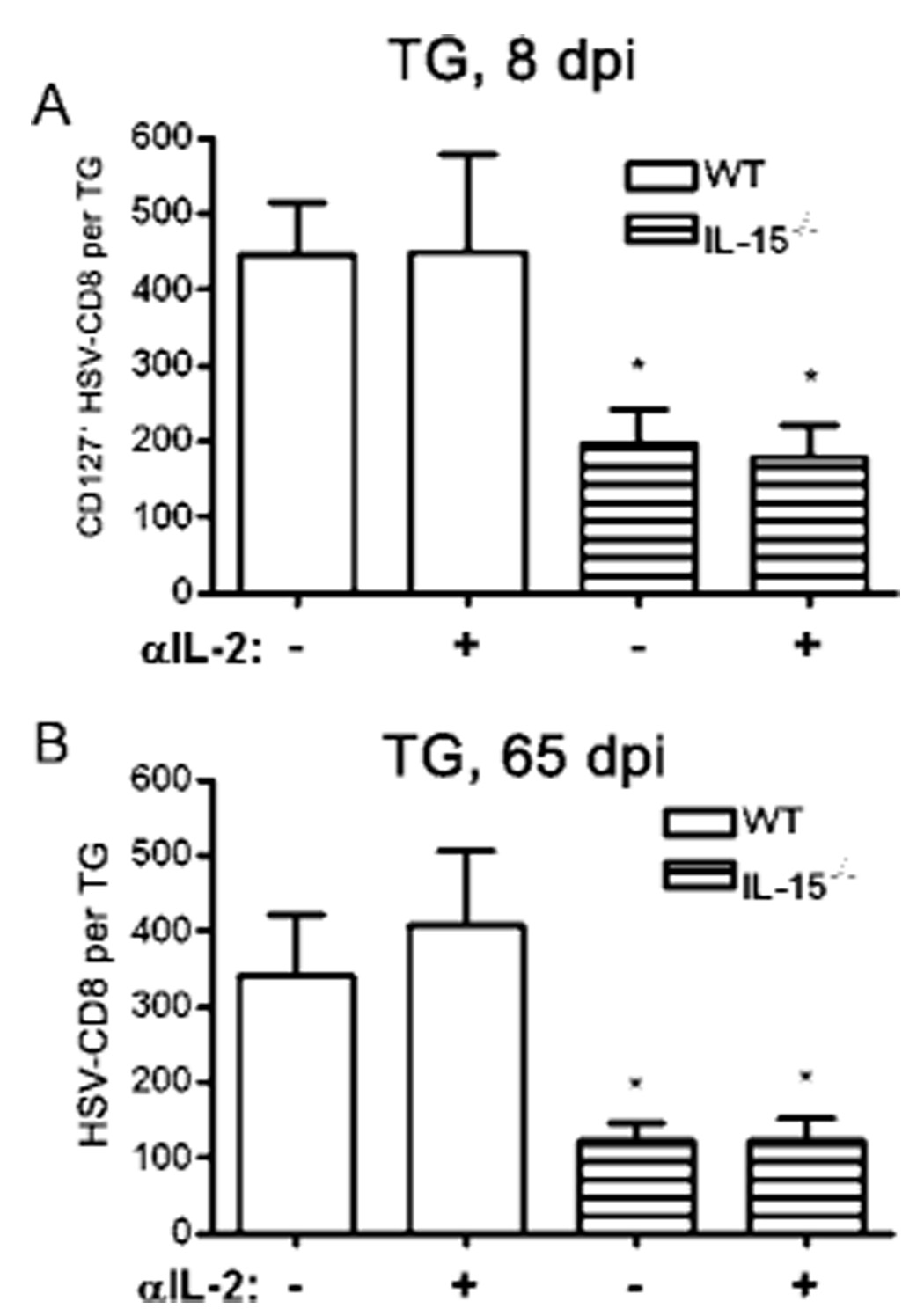
FIGURE 3.
The burst size of the putative HSV-CD8mem precursor pool correlates with the size of the HSV-CD8mem pool. A, WT and IL-15−/− mice received a single i.p. injection of 1 mg of anti-IL-2 mAb at 6 dpi and TG were excised at 8 dpi, simultaneously stained for CD45, CD8α, CD127, and gB498–505 H-2Kb tetramers or dimers (to identify HSV-CD8), and the entire TG sample was analyzed by flow cytometry. Pooled data are expressed as the mean (±SEM) absolute number of HSV-CD8+ T cells that expressed CD127 per TG. B, WT and IL-15−/− mice received a single i.p. injection of 1 mg of anti-IL-2 mAb at 6 dpi and TG were excised at 65 dpi and simultaneously stained with anti-CD45, anti-CD8α, and gB498–505 H-2Kb tetramers or dimers (to identify HSV-CD8). The entire TG sample was analyzed by flow cytometry. Pooled data are expressed as the mean (±SEM) absolute number of HSV-CD8+ T cells per TG. *, p < 0.05 comparing IL-15−/− mice to comparably treated WT mice (n = 3–8 mice per group).
The size of the HSV-CD8mem pool that is established in latently infected TG correlates with the size of the CD127+ putative HSV-CD8mem precursor pool
As noted above, the size of the CD127+ subpopulation of HSV-CD8eff (putative memory precursors) was reduced in IL-15−/− mice, but not in anti-IL-2-treated mice, even though the overall HSV-CD8eff population was dramatically reduced in both groups. Therefore, it was of interest to determine whether either the size of the overall HSV-CD8eff population or the size of the CD127+ subpopulation correlated with the size of the HSV-CD8mem pool that was retained in latently infected TG at 65 dpi. As noted in Fig. 3, in all four treatment groups (WT and IL-15−/−, untreated or treated with anti-IL-2) the size of the CD127+ HSV-CD8 subpopulation present in the TG at 8 dpi varied significantly, but in all cases correlated closely with the size of the HSV-CD8mem pool present at 65 dpi (Fig. 3). In contrast, the size of the overall CD8eff pool in the TG at 8 dpi, which also varied widely among the groups, did not correlate with the size of the HSV-CD8mem pool present at 65 dpi (Fig. 3). These findings are consistent with the notion that the HSV-CD8mem population retained in latently infected TG derives from a subpopulation of HSV-CD8eff cells that express CD127 and are dependent on IL-15.
Neither IL-15 nor IL-2 is required to maintain the HSV-CD8mem pool in latently infected TG
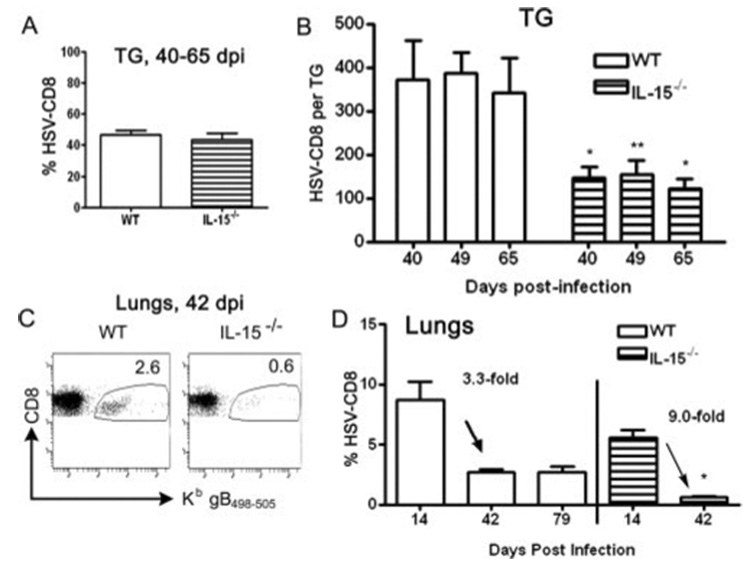
The restriction of latent HSV-1 to nervous tissue permits a comparison within the same animal of factors that regulate the size of the HSV-CD8mem pool in the presence or absence of latent virus and presumably low-level antigenic exposure. Following contraction of the CD8eff pool, a CD8+ T cell infiltrate consisting of 40–50% HSV-specific cells is established within latently infected TG of both WT and IL-15−/– mice (Fig. 4A). The reduced size of the CD8mem population in TG of IL-15−/− mice relative to WT mice resulted in a significantly reduced absolute number of HSV-CD8mem cells (Fig. 4B). However, once established the HSV-CD8mem pool was maintained at a constant level in latently infected TG of both WT and IL-15−/− mice, demonstrating that IL-15 is not required to maintain the HSV-CD8mem pool within latently infected tissue. The HSV-CD8mem pool was also reduced in the lungs of IL-15−/− mice relative to WT mice (Fig. 4, C and D). Furthermore, the HSV-CD8mem pool diminished to nearly undetectable levels in the lungs by 42 dpi, demonstrating that IL-15 is necessary for the homeostatic maintenance of HSV-CD8mem cells in the absence of latent virus.
FIGURE 4.
IL-15 is not necessary to maintain the HSV-CD8mem pool at the site of the latent infection, but is required in noninfected tissues. The TG of WT and IL-15−/− mice were excised at 40–65 dpi (A) or at the designated time (B) and simultaneously stained with anti-CD8α and gB498–505 H-2Kb tetramers or dimers (to identify HSV-CD8), and the entire TG sample was analyzed by flow cytometry. Pooled data are expressed as (A) the mean (±SEM) percentage of cells in the CD8 gate that were HSV-CD8 or as (B) the mean (±SEM) absolute number of HSV-CD8+ T cells per TG. *, p < 0.05; **, p < 0.01 comparing the IL-15−/− mice to WT mice (n = 4–8 mice/group). Alternatively, lungs were excised at 42 dpi (C), or the designated time (D), simultaneously stained with anti-CD8α and gB498–505 H-2Kb tetramers or dimers (to identify HSV-CD8), and analyzed by flow cytometry. C, A representative dot plot with the percentage of HSV-CD8 indicated. D, Pooled data are expressed as the mean (±SEM) percentage of cells in the CD8 gate that were HSV-CD8+ T cells. *, p < 0.05 comparing the IL-15−/− mice to WT mice at each time (n = 4–6 mice/group).
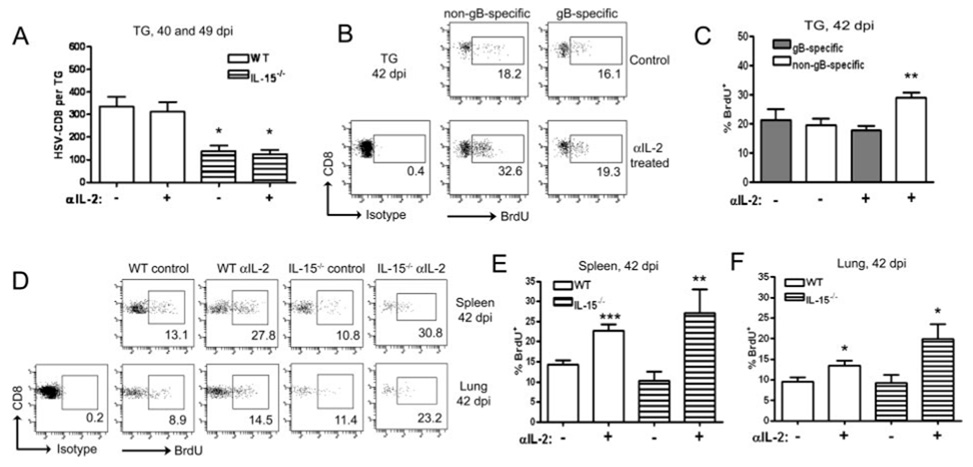
The presence of latent virus also appears to influence the regulatory effect of IL-2 on the proliferation of HSV-CD8mem cells. Treatment with anti-IL-2 after memory establishment had no effect on the size of the HSV-CD8mem pool in latently infected TG of WT or IL-15−/− mice (Fig. 5A). The basal rate of proliferation of the HSV-CD8mem population in latently infected TG was ~2-fold higher than that of their counterparts in the lungs, and 1.5-fold higher than those in the spleen (Fig. 5, B–E). Anti-IL-2 treatment after memory establishment had no effect on the proliferation of the HSV-CD8mem pool in latently infected TG, but significantly increased their proliferation in the lungs and spleen (Fig. 5, B–E). The increased proliferation of HSV-CD8mem cells in the lungs and spleen was particularly notable in the IL-15−/− mice, suggesting antagonistic roles for IL-2 and IL-15 in regulating homeostatic proliferation of the HSV-CD8mem pool. Although anti-IL-2 treatment after memory establishment had no effect on the proliferation of HSV-CD8mem cells in latently infected TG, it did significantly increase the proliferation in the TG of CD8mem cells that were not HSV-specific (Fig. 5, B and C). This latter observation demonstrates that anti-IL-2 treatment was effective in the TG, and further supports the notion that persistent Ag stimulation abrogates the regulatory effect of IL-2 on proliferation of the HSV-CD8mem cells within latently infected tissue.
FIGURE 5.
IL-2 regulates the HSV-CD8mem population in noninfected tissues and the non-gB498–505-specific CD8+ T cells within latently infected tissue. A, WT and IL-15−/− mice received injections of 1 mg of anti-IL-2 mAb every other day for 1 wk before TG excision at 40 or 49 dpi. TG cells were simultaneously stained with anti-CD8α and gB498–505 H-2Kb tetramers or dimers (to identify HSV-CD8) and analyzed by flow cytometry. Pooled data are expressed as the mean (±SEM) absolute number of HSV-CD8/TG. **, p < 0.01 comparing the IL-15−/− mice to comparably treated WT mice (n = 7–11 mice/group). WT mice (B and C) or WT and IL-15−/− mice (D–F) received injections of 1 mg of BrdU daily and 1 mg of anti-IL-2 mAb every other day for 1 wk before TG, spleen, and lung excision (40–49 dpi). TG cells were simultaneously stained with anti-CD45, anti-CD8α, gB498–505 H-2Kb tetramers or dimers, and anti-BrdU and analyzed by flow cytometry. B, Representative dot plots of TG cells from WT mice with the percentage of gB-specific and non-gB-specific CD8+ T cells that incorporated BrdU into cellular DNA indicated. C, Pooled data showing the mean (±SEM) percentage of gB-specific and non-gB-specific CD8+ T cells that incorporated BrdU into cellular DNA. **, p < 0.01 comparing anti-IL-2 treated non-gB-specific to control non-gB-specific (n = 5–6 mice/group). D, Representative dot plots of spleen and lung cells from WT and IL-15−/− mice with the percentage of gB-specific CD8+ T cells that incorporated BrdU into cellular DNA indicated. E and F, Pooled data showing the mean (±SEM) percentage of gB-specific CD8+ T cells that incorporated BrdU into cellular DNA in the spleen (E) and lungs (F). *, p < 0.05; **, p < 0.01; ***, p < 0.001 comparing anti-IL-2 treated to controls for each group (4–10 mice/group).
The elevated basal proliferation rate of HSV-CD8mem cells in latently infected TG is associated with a reduced pool of CD4+FoxP3+ Tregs
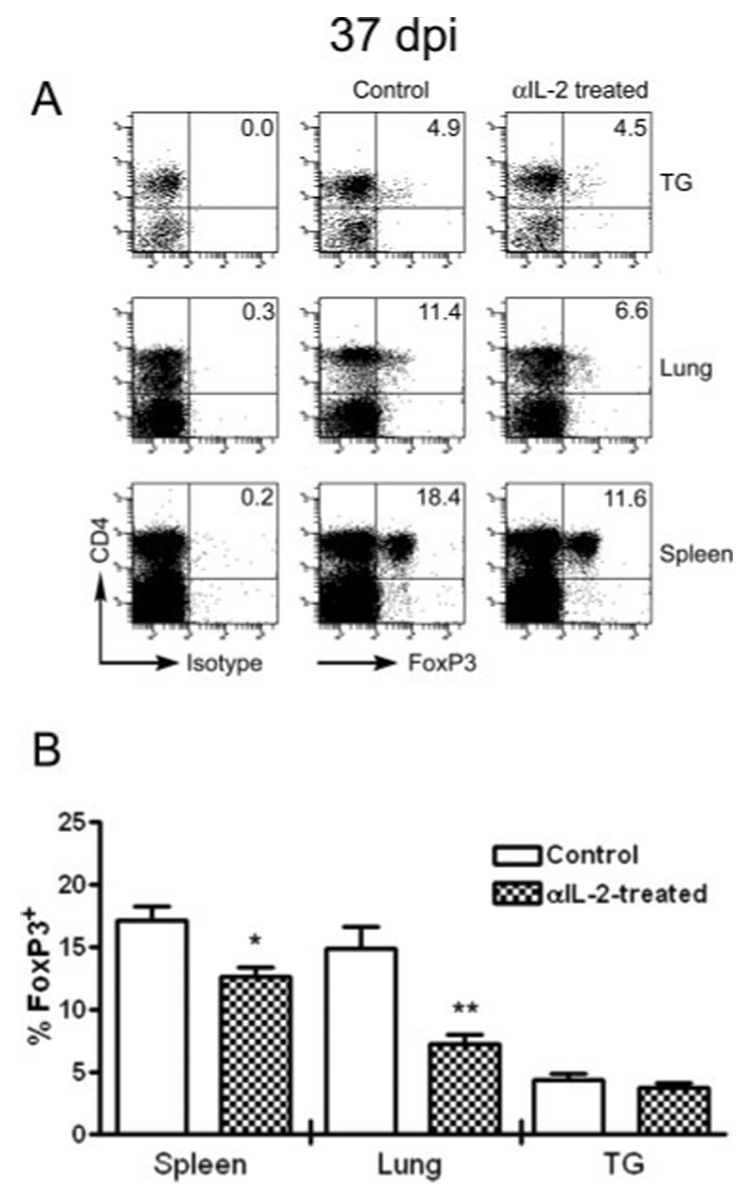
The expression of the transcription factor FoxP3 defines a population of CD4+ Treg cells, and these cells have been shown to regulate the homeostatic proliferation of the CD8mem pool (10, 11). Thirty days after HSV-1 corneal infection, FoxP3+ cells represented 17 and 15% of CD4+ T cells in the spleen and lungs, respectively, but only 4% of the CD4+ T cells in the TG (Fig. 6). Thus, the higher basal rate of proliferation of HSV-CD8mem cells in the TG is associated with a lower frequency of CD4+FoxP3+ Tregs. Injections of anti-IL-2 mAb every other day for 7 days before sacrifice significantly reduced the overall frequency of FoxP3+ cells in the spleen and lungs, while not influencing their frequency in the TG (Fig. 6). These findings are consistent with the hypothesis that IL-2 negatively regulates the homeostatic proliferation of HSV-CD8mem in noninfected tissue (spleen and lungs) by maintaining a resident population of FoxP3+ Tregs, although other possible explanations will be discussed.
FIGURE 6.
IL-2 differentially regulates the Treg population in latently infected and noninfected tissue. WT mice were left untreated or received injections of 1 mg of anti-IL-2 mAb every other day for 1 wk before TG, spleen, and lung excision. Single-cell suspensions of each tissue were stained for surface expression of CD45 and CD4, followed by intracellular staining for FoxP3. A, Representative dot plots of tissue excised at 37 dpi show that FoxP3 expression is limited almost entirely to CD4+ T cells, and the percentage of CD4+ T cells that express FoxP3 is indicated in the upper right quadrant. B, Pooled data from two experiments are expressed as the mean (±SEM) percentage of CD4+ T cells that express FoxP3. *, p < 0.05; **, p < 0.01 comparing the anti-IL-2-treated mice to untreated mice for each tissue (n = 3–8 mice/group).
Does anti-IL-2 treatment or the absence of IL-15 influence the functional program of HSV-CD8mem during latency?
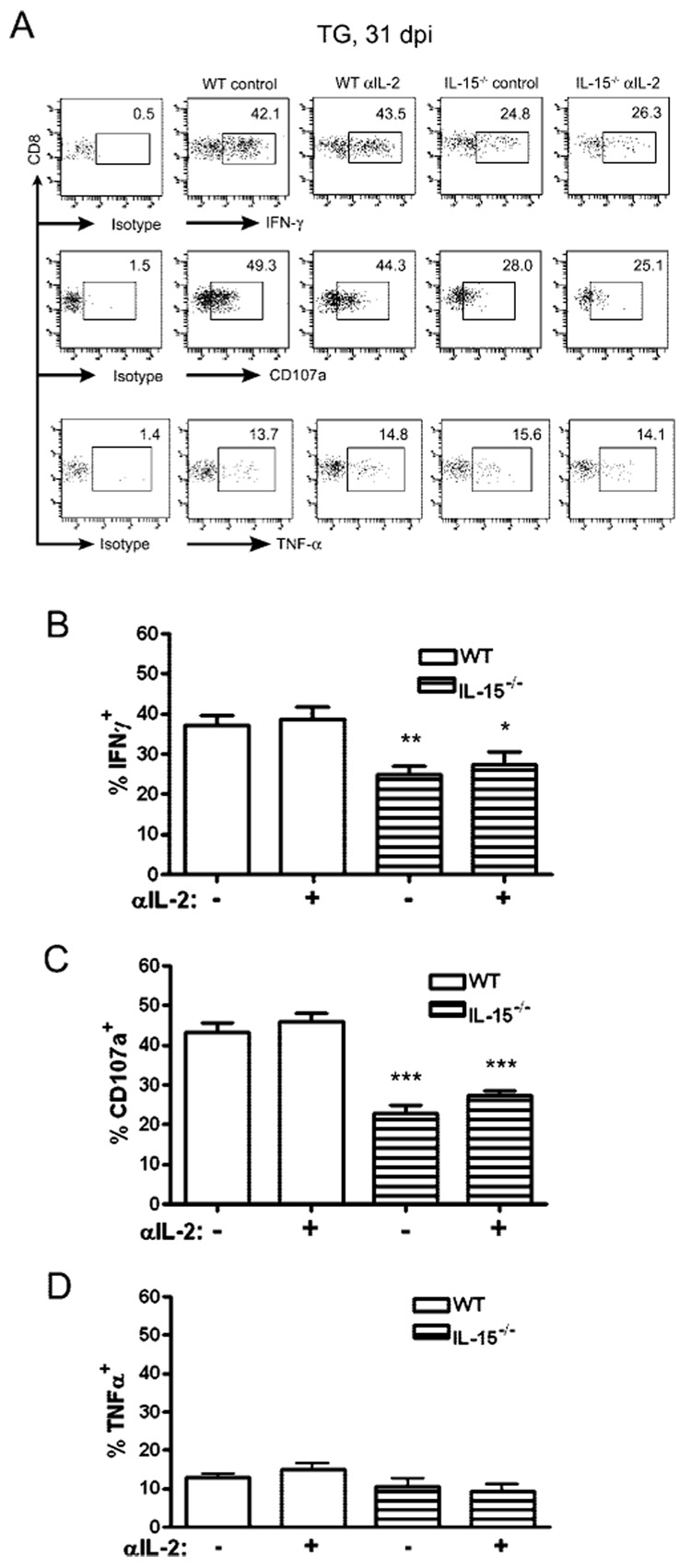
We tested whether anti-IL-2 treatment during latency or the persistent absence of IL-15 would impact the functional program of the CD8mem pool that is retained in latently infected TG. WT or IL-15−/− mice received injections of anti-IL-2 mAb every 48 h for 1 wk before harvest. CD8+ T cells were obtained from their TG at 31 dpi and tested for their capacity to produce the cytokines IFN-γ and TNF-α, or to release lytic granules (CD107a surface expression) in response to gB498–505 peptide-pulsed targets. As shown in Fig. 6, anti-IL-2 treatment did not alter the functional program of the HSV-CD8mem pool. In contrast, the HSV-CD8mem pool in the TG of IL-15−/− mice showed a significant functional compromise. In response to optimal stimulation (targets pulsed with 10−12 M gB498–505 peptide) the HSV-CD8mem pool in the TG of IL-15−/− mice exhibited a significantly diminished production of the cytokine IFN-γ and reduced lytic granule release, but normal production of TNF-α (Fig. 7).
FIGURE 7.
IL-15 deprivation impairs the function of HSV-CD8mem in the TG. WT and IL-15−/− mice received injections of 1 mg of anti-IL-2 mAb every other day for 1 wk before TG excision at 31 dpi. TG cells were optimally stimulated with gB-transfected B6/T-350gB fibroblasts pulsed with 10−12 M gB498–505 peptide in the presence of FITC-conjugated anti-CD107a mAb and GolgiPlug for 6 h. Following stimulation, the cells were stained for intracellular expression of IFN-γ and TNF-α. A, Representative dot plots gated on CD8 with the percentage of cells showing surface CD107a or intracellular expression of IFN-γ or TNF-α indicated. B–D, Pooled data from four similar analyses are expressed as the mean (±SEM) percentage of CD8+ T cells that express intracellular IFN-γ (B), surface CD107a (C), or intracellular TNF-α (D). *, p < 0.05; **, p < 0.01; ***, p < 0.001 comparing IL-15−/− mice to comparably treated WT mice (n = 4 mice/group).
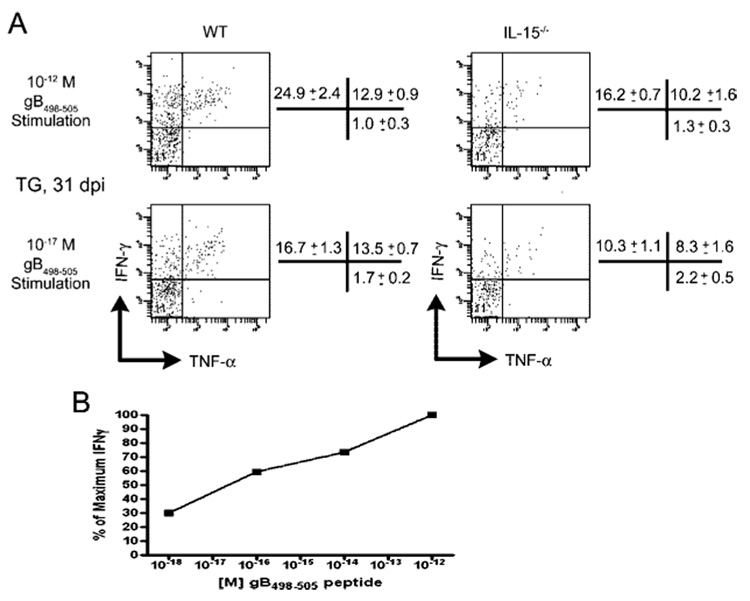
The pattern of cytokine production was of interest. Following optimal stimulation, ~37% of CD8+ T cells in the TG of WT mice produced IFN-γ (Fig. 8). Of these, approximately two-thirds produced IFN-γ only, whereas one-third produced both IFN-γ and TNF-α. Virtually all CD8+ T cells that produced TNF-α also produced IFN-γ. In contrast, following suboptimal stimulation (targets pulsed with 10−17 M peptide), overall production of IFN-γ was reduced to 30%, and the reduction was entirely within the population that produced IFN-γ but not TNF-α. Following optimal stimulation of CD8+ T cells from the TG of IL-15−/− mice, only 26% of CD8+ T cells produced IFN-γ (Fig. 8). The reduced IFN-γ production relative to comparably stimulated WT CD8+ T cells was largely accounted for by cells that produced IFN-γ but not TNF-α. The proportion of CD8+ T cells in the TG of IL-15−/− mice that produced only IFN-γ following optimal stimulation was comparable to that of WT mice following suboptimal stimulation. These findings are consistent with the notion that cells that produce both IFN-γ and TNF-α are more capable of responding to a reduced stimulus than those that produce IFN-γ alone. These data demonstrate that the reduced HSV-CD8mem population that develops in the TG of IL-15−/− mice is comprised of a higher proportion of the CD8 T cells capable of responding to low doses of Ag by producing both IFN-γ and TNF-α.
FIGURE 8.
Functional subpopulations of HSV-CD8mem in latently infected TG are differentially regulated by IL-15. TG were excised from WT and IL-15−/− mice at 31 dpi and TG cells were stimulated with 5 × 105 gB-transfected B6/T-350gB fibroblasts pulsed with either 10−12 M (optimal stimulation) or 10−17 M (suboptimal stimulation) gB498–505 peptide for 6 h in the presence of GolgiPlug. Following stimulation, the cells were stained for surface expression of CD45 and CD8 and for intracellular expression of IFN-γ and TNF-α. A, Representative dot plots are gated on CD45+CD8+ T cells. Pooled data from four such analyses are shown to the right of each dot plot and expressed as the mean (±SEM) percentage of gated cells that express IFN-γ only (upper left quadrant), IFN-γ and TNF-α (upper right quadrant), or TNF-α only (lower right quadrant), n = 4 mice/group. B, Pooled TG from five mice at 34 dpi were stimulated with gB498–505-pulsed B6/T-350 fibroblasts at indicated doses for 6 h before anti-IFN-γ intracellular stain. Data shown are a representative experiment of two such assays.
As illustrated in Table I, our findings contribute important additional details to the existing model of CD8+ T cell differentiation into effector and memory cells. The table illustrates how latent virus influences the requirements for maintaining CD8+ T cell memory.
Table I.
IL-2 and IL-15 regulation of the immune response to HSV-1
| Acute infection (1–8 dpi) |
Latent Infection (>30 dpi) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Optimal Effector Cell Expansion |
Memory Precursor |
Optimal Establishment of Memory |
Memory Maintenance |
|||||
| Treatment | LNa | TG | TG | Latently Infected Tissue | Noninfected Tissue | Latently Infected Tissue | Noninfected Tissue | Memory Function |
| Anti IL-2 | No effect | Inhibited | No effect | No effect | No effect | No effect | Enhanced | No effect |
| IL-15 knockout | No effect | Inhibited | Reduced | Inhibited | Inhibited | No effect | Reduced | Altered |
Lymph node.
Discussion
A hallmark of the herpesviruses is their capacity to induce recurrent disease, particularly in immunocompromised individuals. Recurrent disease results from the reactivation of these viruses from a latent state and subsequent viral proliferation resulting in tissue destruction. Accumulating evidence supports a dynamic view of herpesvirus latency in which CD8+ T cells play an important role in monitoring viral gene expression in latently infected cells and preventing virion formation (19, 23, 28). In mouse models of HSV-1 infection, latency is maintained in sensory neurons. CD8+ T cells have been shown to interact closely with latently infected neurons in vivo (19, 29), to form an apparent immunologic synapse with multiple neurons within each latently infected TG (19), and to prevent HSV-1 reactivation from latency in neurons in ex vivo TG cultures, at least in part through the production of IFN-γ (28, 30). In addition, recent findings in humans validate the murine HSV-1 model by demonstrating the persistence of a chronic immune infiltrate in the TG of infected individuals that is nearly identical to that seen in the TG of HSV-1-infected mice (31). Thus, the establishment and maintenance of an effective and persistently reactive HSV-specific CD8 T cell population within latently infected sensory ganglia might constitute an important protective mechanism to prevent viral reactivation and recurrent disease.
Distinct factors appear to contribute to the development and maintenance of the CD8 memory pool in different models of infectious disease. Within lymphoid organs, CD8+ T cells undergo an expansion, contraction, and homeostatic memory phase. These three phases are recapitulated within infected tissue. However, different cytokines appear to regulate the three phases of the CD8+ T cell response in lymphoid and nonlymphoid tissue (6, 32–34). In this study, we establish that IL-2 and IL-15 are of no or marginal importance in the initial expansion of HSV-CD8 T cells in the DLN following a localized HSV-1 corneal infection. However, treatment with anti-IL-2 mAb and/or deprivation of IL-15 had a dramatic inhibitory effect on the establishment of a HSV-CD8 population in the infected TG.
A recent study demonstrated that injection of certain anti-IL-2 mAb, including the one used in these studies, either alone or precomplexed with IL-2 directly augmented proliferation of memory phenotype CD8+ T cells in the spleens of treated mice (35). The IL-2/anti-IL-2 immune complexes apparently bound to Fc receptors and efficiently presented IL-2 to CD8+ memory phenotype T cells that express high levels of the IL-2R β-chain (CD122), but inefficiently to naive CD8+ T cells that are CD122low. Our observation that anti-IL-2 treatment did not influence the initial expansion of HSV-CD8 T cells in the lymph nodes of infected mice (Fig. 2) is consistent with that finding. However, the failure of anti-IL-2 treatment to augment the proliferation of HSV-CD8eff in the TG 8 dpi is not consistent with direct stimulation by IL-2 immune complexes, as CD8eff would be expected to express high levels of CD122 and thus be susceptible to direct stimulation by IL-2 immune complexes. Indeed, treatment with anti-IL-2 led to a dramatic reduction in the accumulation of HSV-CD8eff in the TG. Whether the accumulation of HSV-CD8eff in the TG is influenced by increased stimulation by IL-2 immune complexes or reduced IL-2 stimulation through neutralization remains to be determined.
Little is known of the factors that influence the generation of the CD8 memory precursor population during and shortly after the effector phase of the response to acute infection. However, identification of these factors might be aided by the recent observation that CD127 expression on activated CD8+ T cells marks a CD8 memory precursor subpopulation. Within lymphoid organs, the size of the memory precursor pool is proportional to the size of the effector pool (8). Based on this observation, one might predict that the factors that influence the initial expansion of CD8+ T cell effectors would also influence the generation of the memory precursors. Our findings establish that this is not necessarily true in nonlymphoid organs. In the TG at 8 dpi, IL-15 deprivation did proportionally reduce the size of the overall HSV-CD8eff pool and of the CD127+, putative HSV-CD8mem precursor pool. However, anti-IL-2 treatment also dramatically reduced the size of the HSV-CD8eff pool, but did not influence the size of the CD127+, putative HSV-CD8mem precursor pool. Thus, CD127− CD8+ effector T cells and CD127− putative CD8+ memory precursor T cells appear to be separate populations with distinct regulatory requirements.
Previous studies have suggested that the size of the CD8+ effector T cell population determines the size of the CD8 memory pool that survives contraction (36). In this study, we show that within the infected TG, mice developed a normal CD8 memory pool in the presence of a CD8+ effector T cell pool that was dramatically reduced by anti-IL-2 treatment. In contrast, mice whose HSV-CD8eff and putative HSV-CD8mem precursor pools were both reduced by IL-15 deprivation had significantly reduced numbers of HSV-CD8mem cells in their latently infected TG. Moreover, in four different treatment groups (WT and IL-15−/−, untreated or treated with anti-IL-2) the number of HSV-CD8mem cells retained in TG following contraction of the HSV-CD8eff cells varied widely, but in all cases closely approximated the number of putative HSV-CD8mem precursor cells present at the peak of HSV-CD8eff expansion. These data are consistent with the notion that in infected peripheral tissue, as in lymphoid organs, the size of the CD127+ putative memory precursor population and not the size of the overall effector population determines the size of the pool of Ag-specific memory CD8 T cells that survive contraction.
This conclusion is predicated on the assumption that the HSV-CD8mem pool that is retained in the TG is not greatly influenced by infiltration from the lymphoid organs. Two observations support this hypothesis. First, as noted above, the number of CD127+ putative HSV-CD8mem precursors in the TG at 8 dpi closely correlates with the number of HSV-CD8mem in the TG following contraction of the HSV-CD8eff pool. The second observation favoring the notion that the CD8mem pool in latently infected TG is maintained independent of infiltration is that in IL-15−/− mice HSV-CD8mem diminished to nearly undetectable levels in the lungs and spleen between 14 and 42 dpi, while remaining at constant levels in the TG.
The homeostatic proliferation of memory CD8+ T cells following removal of Ag is dependent on IL-15 (4, 6, 7, 33). However, CD8 T cells generated during a chronic infection are refractory to IL-15-induced homeostatic proliferation due to reduced expression of IL-15R (16). This difference in responsiveness to IL-15 following acute and chronic infection is presumed to be due to differences in TCR signaling. Other factors including differences intrinsic to the pathogens might also contribute. During HSV-1 latency, persistent TCR stimulation in the TG is strongly suggested by the activation phenotype of the HSV-CD8 cells surviving contraction, the apparent formation of an immunologic synapse between HSV-CD8 cells and neurons, and the persistent presence of inflammatory cytokines (19, 21, 29, 37–40). Our observation that IL-15 is required to maintain the HSV-CD8mem pool in the noninfected lungs, but not in latently infected TG within the same mouse, is consistent with the notion that periodic TCR stimulation can supplant the need for IL-15 in maintaining the HSV-CD8mem pool.
Although a small population of HSV-CD8mem cells was maintained in latently infected TG of IL-15−/− mice, these cells were functionally impaired. The HSV-1-specific CD8+ T cells in latently infected TG of WT mice fall into two functionally distinct populations: one that responds to a low concentration of antigenic peptide with production of both IFN-γ and TNF-α and a second that responds only to a high concentration of antigenic peptide with production of IFN-γ but not TNF-α. Thus, although the number of IFN-γ+ HSV-CD8mem is significantly reduced in the TG of IL-15−/− mice, those that do remain are sensitive to low concentrations of HSV-1 Ag and produce both IFN-γ and TNF-α. This functional population of HSV-CD8mem might be particularly critical in a latently infected tissue in which low levels of viral Ags are likely to be encountered.
Stimulation of dispersed TG cells with targets pulsed with an optimum concentration of gB498–505 peptide resulted in three functionally distinct populations of CD8+ T cells: 1) IFN-γ− and TNF-α−, 2) IFN-γ+ and TNF-α−, and 3) IFN-γ+ and TNF-α+. When optimally stimulated, fewer CD8+ T cells from the TG of IL-15−/− mice produced IFN-γ compared with those of WT mice. The reduction was almost exclusively in the IFN-γ+ TNF-α− population. This population appears to represent HSV-CD8 T cells capable of responding to a reduced stimulus as these cells isolated from WT mice failed to respond when stimulated with targets pulsed with a suboptimal concentration of gB peptide. Therefore, these data suggest that within latently infected tissue anti-viral CD8 memory cells capable of producing IFN-γ alone depend on IL-15 for optimal function. IL-15 appears to promote functional maturation of CD8+ T cells following acute infection by enhancing coreceptor expression and favoring the homeostatic proliferation of high-avidity cells by virtue of their elevated expression of IL-15Rα (41). These selective forces would not operate in an IL-15 deficient mouse. However, within latently infected tissue of IL-15−/− mice, persistent low-level expression of viral proteins might provide a selective force favoring maintenance of a functionally distinct IFN-γ+ TNF-α+ CD8+ T cell population.
We observed that anti-IL-2 treatment during the latent phase of the HSV-1 infection resulted in increased homeostatic proliferation of HSV-CD8 cells in the noninfected lungs and spleen, while not affecting the proliferation of those maintained within latently infected TG. Interestingly, anti-IL-2 treatment during latency did augment proliferation of the non-gB-specific CD8+ T cells within the TG. Our previous studies revealed that these non-gB-specific CD8+ T cells in latently infected TG lack detectable reactivity to HSV-1 proteins (19). These observations suggest that the rate of homeostatic proliferation of HSV-CD8mem cells is differentially regulated in latently infected and noninfected tissue. Because IL-2 differentially regulates proliferation of HSV-specific and non- HSV-specific CD8+ T cells within the same TG, a likely explanation would be that low-level TCR stimulation in the TG renders HSV-CD8mem cells refractory to regulation by IL-2.
A subpopulation of CD4+ T cells expressing the forkhead transcription factor FoxP3 has been identified as regulatory T cells capable of inhibiting proliferation and function of effector T cells (11). These CD4+ Treg cells have also been implicated in controlling the homeostatic proliferation of classical memory CD8 T cells (10). The survival and function of the CD4+ Treg cells is dependent on IL-2 (42–45). Thus, the negative regulation by IL-2 of the homeostatic proliferation of memory CD8+ T cells is thought to be mediated indirectly through CD4+ Treg cells. However, the above interpretation was based on the assumption that anti-IL-2 mAb neutralizes IL-2 function in vivo, an assumption that is now called into question by the recent observation that IL-2/anti-IL-2 immune complexes actually enhance IL-2 use by CD8 memory-phenotype cells, directly enhancing their proliferation. Arguing against direct stimulation of CD8mem in our model is the observation that treatment of IL-15−/− mice with anti-IL-2 also resulted in enhanced proliferation of HSV-CD8mem in the spleen and lung. A direct stimulatory effect of IL-2 immune complexes on IL-15−/− CD8 memory cells is unlikely because these cells have been shown to be CD122low(46).
During HSV-1 latency, we observed significantly higher levels of Treg cells in the noninfected lungs and spleen when compared with latently infected TG. After 1 wk of anti-IL-2 treatment in HSV-1 latently infected mice, the size of the Treg population was dramatically reduced in the lungs and spleen, and this correlated with a significant increase in the homeostatic proliferation of the HSV-CD8mem pool. In contrast, the Treg population in latently infected TG of the same mice was not diminished by anti-IL-2 treatment, and the homeostatic proliferation of HSV-CD8mem cells in the TG was also unaffected. These findings seem to suggest that the homeostatic proliferation of HSV-CD8mem in the lungs and spleen are a direct consequence of the reduction in numbers of Tregs following anti-IL-2 treatment. However, the alternative possibility that IL-2 immune complexes directly stimulated the proliferation of CD8mem in the lungs and spleen cannot be ruled out. In either case, it would appear that the homeostatic proliferation of HSV-specific, but not nonspecific CD8mem in latently infected TG is refractory to IL-2 regulation.
The available evidence in mouse models of HSV-1 infection suggest that quantitative and qualitative differences in the HSV-CD8mem pool maintained within latently infected sensory ganglia might influence susceptibility to HSV-1 reactivation from latency and recurrent disease. Our findings demonstrate unique regulatory requirements for generation and maintenance of this HSV-CD8 T cell pool in tissue that harbor latent virus. These findings have important implications for the design of immunology-based intervention in recurrent herpetic disease.
Acknowledgments
We thank Theresa Lee and Dawn McKissic for technical assistance, Nancy Zurowski for flow cytometry acquisition, and the National Institute of Allergy and Infectious Diseases Tetramer Core Facility (Emory University Vaccine Center, Atlanta, GA) for supplying the tetramers.
Footnotes
Support for this work was provided by National Institutes of Health Grants EY05945, P30 EY08098, and T32 AI060525, and an unrestricted grant from Research to Prevent Blindness (New York, NY) and the Eye and Ear Foundation of Pittsburgh.
Abbreviations used in this paper: LCMV, lymphocytic choriomeningitis virus; TG, trigeminal ganglion; WT, wild type; dpi, days postinfection; HSV-CD8, HSV-1-specific CD8+ T cell; Treg, regulatory T cell; DLN, draining lymph node.
Disclosures The authors have no financial conflict of interest.
References
- 1.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 2.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 3.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 4.Goldrath AW, Sivakumar PV, Glaccum M, Kennedy MK, Bevan MJ, Benoist C, Mathis D, Butz EA. Cytokine requirements for acute and basal homeostatic proliferation of naive and memory CD8+T cells. J. Exp. Med. 2002;195:1515–1522. doi: 10.1084/jem.20020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kieper WC, Tan JT, Bondi-Boyd B, Gapin L, Sprent J, Ceredig R, Surh CD. Overexpression of interleukin (IL)-7 leads to IL-15-independent generation of memory phenotype CD8+ T cells. J. Exp. Med. 2002;195:1533–1539. doi: 10.1084/jem.20020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schluns KS, Williams K, Ma A, Zheng XX, Lefrancois L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J. Immunol. 2002;168:4827–4831. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- 7.Burkett PR, Koka R, Chien M, Chai S, Chan F, Ma A, Boone DL. IL-15Rα expression on CD8+ T cells is dispensable for T cell memory. Proc. Natl. Acad. Sci. USA. 2003;100:4724–4729. doi: 10.1073/pnas.0737048100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 9.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu. Rev. Immunol. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 10.Murakami M, Sakamoto A, Bender J, Kappler J, Marrack P. CD25+ CD4+ T cells contribute to the control of memory CD8+ T cells. Proc. Natl. Acad. Sci. USA. 2002;99:8832–8837. doi: 10.1073/pnas.132254399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suvas S, Kumaraguru U, Pack CD, Lee S, Rouse BT. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J. Exp. Med. 2003;198:889–901. doi: 10.1084/jem.20030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Rα recycles and presents IL-15 in trans to neighboring cells. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 13.Schluns KS, Klonowski KD, Lefrancois L. Transregulation of memory CD8 T-cell proliferation by IL-15Rα+ bone marrow-derived cells. Blood. 2004;103:988–994. doi: 10.1182/blood-2003-08-2814. [DOI] [PubMed] [Google Scholar]
- 14.Burkett PR, Koka R, Chien M, Chai S, Boone DL, Ma A. Coordinate expression and trans presentation of interleukin (IL)-15Rα and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J. Exp. Med. 2004;200:825–834. doi: 10.1084/jem.20041389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wherry EJ, Barber DL, Kaech SM, Blattman JN, Ahmed R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc. Natl. Acad. Sci. USA. 2004;101:16004–16009. doi: 10.1073/pnas.0407192101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 18.Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 19.Khanna KM, Bonneau RH, Kinchington PR, Hendricks RL. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity. 2003;18:593–603. doi: 10.1016/s1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallace ME, Keating R, Heath WR, Carbone FR. The cytotoxic T-cell response to herpes simplex virus type 1 infection of C57BL/6 mice is almost entirely directed against a single immunodominant determinant. J. Virol. 1999;73:7619–7626. doi: 10.1128/jvi.73.9.7619-7626.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen SH, Garber DA, Schaffer PA, Knipe DM, Coen DM. Persistent elevated expression of cytokine transcripts in ganglia latently infected with herpes simplex virus in the absence of ganglionic replication or reactivation. Virology. 2000;278:207–216. doi: 10.1006/viro.2000.0643. [DOI] [PubMed] [Google Scholar]
- 22.van Lint AL, Kleinert L, Clarke SR, Stock A, Heath WR, Carbone FR. Latent infection with herpes simplex virus is associated with ongoing CD8+ T-cell stimulation by parenchymal cells within sensory ganglia. J. Virol. 2005;79:14843–14851. doi: 10.1128/JVI.79.23.14843-14851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu T, Khanna KM, Chen X, Fink DJ, Hendricks RL. CD8+ T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensrons. J. Exp. Med. 2000;191:1459–1466. doi: 10.1084/jem.191.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suvas S, Azkur AK, Rouse BT. Qa-1b and CD94-NKG2a interaction regulate cytolytic activity of herpes simplex virus-specific memory CD8+ T cells in the latently infected trigeminal ganglia. J. Immunol. 2006;176:1703–1711. doi: 10.4049/jimmunol.176.3.1703. [DOI] [PubMed] [Google Scholar]
- 25.Bonneau RH, Salvucci LA, Johnson DC, Tevethia SS. Epitope specificity of H-2Kb-restricted, HSV-1-, and HSV-2-cross-reactive cytotoxic T lymphocyte clones. Virology. 1993;195:62–70. doi: 10.1006/viro.1993.1346. [DOI] [PubMed] [Google Scholar]
- 26.Wolint P, Betts MR, Koup RA, Oxenius A. Immediate cytotoxicity but not degranulation distinguishes effector and memory subsets of CD8+ T cells. J. Exp. Med. 2004;199:925–936. doi: 10.1084/jem.20031799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Betts MR, Price DA, Brenchley JM, Lore K, Guenaga FJ, Smed-Sorensen A, Ambrozak DR, Migueles SA, Connors M, Roederer M, et al. The functional profile of primary human antiviral CD8+ T cell effector activity is dictated by cognate peptide concentration. J. Immunol. 2004;172:6407–6417. doi: 10.4049/jimmunol.172.10.6407. [DOI] [PubMed] [Google Scholar]
- 28.Decman V, Kinchington PR, Harvey SA, Hendricks RL. Gamma interferon can block herpes simplex virus type 1 reactivation from latency, even in the presence of late gene expression. J. Virol. 2005;79:10339–10347. doi: 10.1128/JVI.79.16.10339-10347.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu T, Tang Q, Hendricks RL. Inflammatory infiltration of the trigeminal ganglion after herpes simplex virus type 1 corneal infection. J. Virol. 1996;70:264–271. doi: 10.1128/jvi.70.1.264-271.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu T, Khanna KM, Carriere BN, Hendricks RL. Gamma interferon can prevent herpes simplex virus type 1 reactivation from latency in sensory neurons. J. Virol. 2001;75:11178–11184. doi: 10.1128/JVI.75.22.11178-11184.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Theil D, Derfuss T, Paripovic I, Herberger S, Meinl E, Schueler O, Strupp M, Arbusow V, Brandt T. Latent herpesvirus infection in human trigeminal ganglia causes chronic immune response. Am. J. Pathol. 2003;163:2179–2184. doi: 10.1016/S0002-9440(10)63575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D’Souza WN, Schluns KS, Masopust D, Lefrancois L. Essential role for IL-2 in the regulation of antiviral extralymphoid CD8 T cell responses. J. Immunol. 2002;168:5566–5572. doi: 10.4049/jimmunol.168.11.5566. [DOI] [PubMed] [Google Scholar]
- 33.Becker TC, Wherry EJ, Boone D, Murali-Krishna K, Antia R, Ma A, Ahmed R. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J. Exp. Med. 2002;195:1541–1548. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D’Souza WN, Lefrancois L. IL-2 is not required for the initiation of CD8 T cell cycling but sustains expansion. J. Immunol. 2003;171:5727–5735. doi: 10.4049/jimmunol.171.11.5727. [DOI] [PubMed] [Google Scholar]
- 35.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 36.Hou S, Hyland L, Ryan KW, Portner A, Doherty PC. Virus-specific CD8+ T-cell memory determined by clonal burst size. Nature. 1994;369:652–654. doi: 10.1038/369652a0. [DOI] [PubMed] [Google Scholar]
- 37.Cantin EM, Hinton DR, Chen J, Openshaw H. Gamma interferon expression during acute and latent nervous system infection by herpes simplex virus type 1. J. Virol. 1995;69:4898–4905. doi: 10.1128/jvi.69.8.4898-4905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halford WP, Gebhardt BM, Carr DJJ. Persistent cytokine expression in trigeminal ganglion latently infected with herpes simplex virus type 1. J. Immunol. 1996;157:3542–3549. [PubMed] [Google Scholar]
- 39.Halford WP, Gebhardt BM, Carr DJ. Acyclovir blocks cytokine gene expression in trigeminal ganglia latently infected with herpes simplex virus type 1. Virology. 1997;238:53–63. doi: 10.1006/viro.1997.8806. [DOI] [PubMed] [Google Scholar]
- 40.Theil D, Derfuss T, Paripovic I, Herberger S, Meinl E, Schueler O, Strupp M, Arbusow V, Brandt T. Latent herpesvirus infection in human trigeminal ganglia causes chronic immune response. Am. J. Pathol. 2003;163:2179–2184. doi: 10.1016/S0002-9440(10)63575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oh S, Perera LP, Burke DS, Waldmann TA, Berzofsky JA. IL-15/IL-15Rα-mediated avidity maturation of memory CD8+ T cells. Proc. Natl. Acad. Sci. USA. 2004;101:15154–15159. doi: 10.1073/pnas.0406649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Furtado GC, de Lafaille MAC, Kutchukhidze N, Lafaille JJ. Interleukin 2 signaling is required for CD4+ regulatory T cell function. J. Exp. Med. 2002;196:851–857. doi: 10.1084/jem.20020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 44.D’Cruz LM, Klein L. Development and function of agonist-induced CD25+Foxp3+ regulatory T cells in the absence of interleukin 2 signaling. Nat. Immunol. 2005;6:1152–1159. doi: 10.1038/ni1264. [DOI] [PubMed] [Google Scholar]
- 45.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3+CD25+CD4+ regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J. Exp. Med. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Judge AD, Zhang X, Fujii H, Surh CD, Sprent J. Interleukin 15 controls both proliferation and survival of a subset of memory-phenotype CD8+T cells. J. Exp. Med. 2002;196:935–946. doi: 10.1084/jem.20020772. [DOI] [PMC free article] [PubMed] [Google Scholar]