Summary
Iron is an essential cofactor for several enzymes and metabolic pathways, in both microbes and in their eukaryotic hosts. To avoid toxicity, iron acquisition is tightly regulated. This represents a particular challenge for pathogens that reside within the endocytic pathway of mammalian cells, because endosomes and lysosomes are gradually depleted in iron by host transporters. An important player in this process is Nramp1 (Slc11a1), a proton efflux pump that translocates Fe2+ and Mn2+ ions from macrophage lysosomes/phagolysosomes into the cytosol. Mutations in Nramp1 cause susceptibility to infection with the bacteria Salmonella and Mycobacteria and the protozoan Leishmania, indicating that an available pool of intraphagosomal iron is critical for the intracellular survival and replication of these pathogens. Salmonella and Mycobacteria are known to express iron transporter systems that effectively compete with host transporters for iron. Until recently, however, very little was known about the molecular strategy used by Leishmania for survival in the iron-poor environment of macrophage phagolysosomes. It is now clear that intracellular residence induces Leishmania amazonensis to express LIT1, a ZIP family membrane Fe2+ transporter that is required for intracellular growth and virulence.
Introduction
Leishmania spp. are intracellular protozoan parasites that replicate within phagolysosomes of host macrophages. They are the causative agents of human leishmaniasis, which currently threatens around 350 million people throughout the world. Depending on the species involved, the human pathology resulting from Leishmania infections can vary from self-healing cutaneous lesions to very severe visceralizing disease. If left untreated, the visceral form of leishmaniasis can have a fatality rate as high as 100% within 2 years. One remarkable feature of Leishmania is its capacity to thrive within acidified, hydrolase-rich phagolysosomes, compartments that are usually involved in the destruction of invading pathogens (Antoine et al., 1998). Significant advances were made in our understanding of the role played by the host immune system in susceptibility to Leishmania infections (Sacks and Noben-Trauth, 2002; McMahon-Pratt and Alexander, 2004; Stanley and Engwerda, 2007). In contrast, little is known about the molecular strategies used by the parasites to survive and replicate within the hostile phagolysosomal environment. In particular, until recently it was not clear how Leishmania is equipped to deal with iron acquisition, which represents a key battleground for the survival of intracellular pathogens. Here we discuss the implications of a recent study identifying a ferrous iron transporter essential for Leishmania growth in macrophages.
Iron acquisition is a tightly regulated process
In humans and other vertebrates, iron assimilated by mucosal cells from dietary components passes into the blood stream, where it is chelated by transferrin. Transferrins are single chain glycoproteins with two binding sites for Fe3+, the ferric form of iron found in aerobic environments. At neutral pH transferrin binds Fe3+ atoms with high affinity, effectively ensuring that no free iron remains in circulation (Wessling-Resnick, 2000). After binding to specific cell surface receptors, the transferrin–Fe3+ complex (holotransferrin) is endocytosed. Endosomal acidification releases Fe3+ from transferrin, allowing its conversion into Fe2+ by endosomal reductases (Ohgami et al., 2005) (Fig. 1). Ferrous iron (Fe2+) is soluble in biological fluids, but can be very toxic because of the highly reactive hydroxyl radicals it generates in the presence of oxygen, by the Fenton reaction. For this reason, a number of mechanisms are in place for sensing the cellular iron concentration, and for responding appropriately by carefully modulating the uptake, storage and efflux of this metal (Wessling-Resnick, 2000).
Fig. 1.
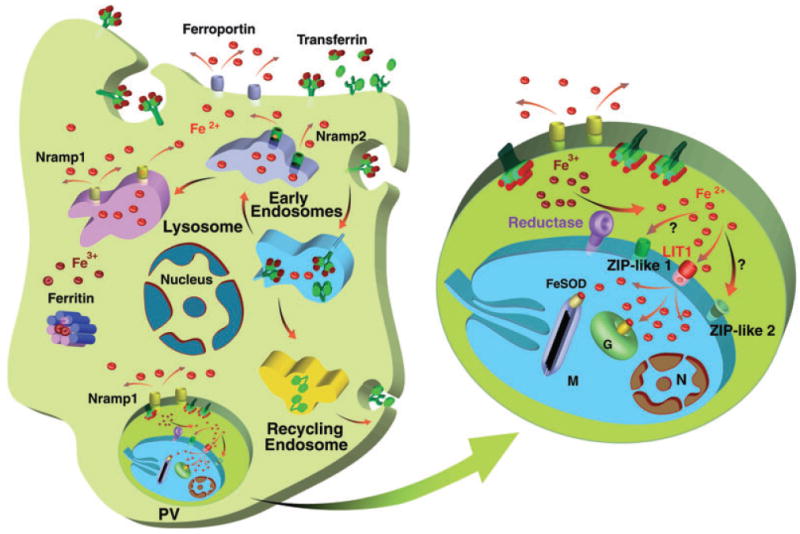
Diagram of iron import and export pathways in a Leishmania-infected macrophage. The enlarged parasitophorous vacuole (PV) shows an intracellular amastigote with the recently identified LIT1 transporter, the genome-encoded putative ZIP family transporters (ZIP-like 1 and 2), the putative reductase and the host divalent cation efflux pump Nramp1. G, glycosome; M, mitochondria, N, nucleus.
Another reason for tightly regulating iron availability is related to the fact that this metal can also control important microbicidal responses, in a manner that has been often described as a double-edged sword (Weiss, 2002). Iron directly participates in the generation of toxic oxygen and nitrogen intermediates, by the Fenton reaction. However, production of nitric oxide by LPS/IFNγ-activated macrophages can downregulate the transferrin receptor and upregulate the synthesis of ferritin, the protein complex that chelates Fe3+ for storage in the cytosol. Both events are likely to restrict the access of pathogens to iron inside the endocytic compartment (Kim and Ponka, 2000). On the other hand, iron can also dampen the expression of inducible nitric oxide synthase by IFNγ-activated macrophages, favouring pathogen survival (Weiss et al., 1994; Oexle et al., 2003).
A major pathway for avoiding toxicity from intracellular iron accumulation is the translocation of Fe2+ into the cytosol, where it is safely stored in the oxidized Fe3+ form bound to ferritin. This function is in large part performed by the divalent cation efflux pump Nramp2 (Slc11a2/DMT1) (Gruenheid et al., 1999; Picard et al., 2000). Nramp2 deletion leads to postnatal lethality owing to a severe deficiency in intestinal iron absorption (Gunshin et al., 2005), highlighting the central role of this endosome transporter in providing iron for essential metabolic pathways in the cytosol. Macrophages express an additional metal transporter, Nramp1 (Slc11a1), in their late endosomes/lysosomes. Nramp1 was initially proposed to mediate iron influx into phagolysosomes (Goswami et al., 2001), but is now thought to function as a pH dependent divalent cation efflux pump, similar to Nramp2 (Jabado et al., 2000). Interestingly, mutations in Nramp1 do not cause iron absorption phenotypes but severely impair the ability of phagocytes to inhibit the growth of intracellular pathogens such as Salmonella, Mycobacteria and Leishmania (Blackwell et al., 2001; Forbes and Gros, 2001). The increased susceptibility to infection of Nramp−/− animals reinforces the view that transporters within the mammalian endocytic pathway can function in host defence, by restricting the access of pathogens to iron (Weinberg, 1992; Jabado et al., 2000; Schaible and Kaufmann, 2004). It is now clear that an efficient iron uptake mechanism capable of competing with mammalian transporters for the same substrates is critical for the survival and replication of several intravacuolar pathogens.
How pathogens compete with the host for iron
Many bacteria internalize iron sequestered by siderophores, secreted molecules with high affinity for iron that effectively compete with transferrin and other host proteins for Fe3+ (Crosa and Walsh, 2002). Macrophages counter attack by secreting lipocalin 2, an antimicrobial peptide that captures iron-loaded bacterial siderophores, delivering it to receptors on mammalian cells for internalization (Flo et al., 2004). In Salmonella enterica serovar Typhimurium, a Gram-negative facultative intracellular bacterium, secreted siderophores that bind Fe3+ with high affinity are internalized after binding to outer membrane receptors (Hantke et al., 2003; Rabsch et al., 2003). However, for full virulence Salmonella depends not only on siderophore-mediated Fe3+ internalization systems, but also on Fe2+ transmembrane transporters. Genes encoding several ATP-dependent or proton-coupled Fe2+ transporters have been identified in Salmonella, such as fepBCDG, sitA-D, feoABC, corAD and the Nramp homologue mntH (Marquis and Gros, 2007). Mutations in mntH and feoB or sitA-D inhibit Salmonella replication within macrophages, and virulence in Nramp1−/− 129 mice (Janakiraman and Slauch, 2000; Boyer et al., 2002). Thus, there is direct evidence that Salmonella has the molecular machinery required for competing with host cell transporters for Fe2+. A similar strategy appears to be used by the intracellular bacterium Mycobacteria, which depends on siderophores for growth in macrophages, and responds to changes in iron availability by mounting a complex transcriptional response that regulates iron uptake and adjusts the transcription of iron storage genes (De Voss et al., 2000; Rodriguez, 2006). Interestingly, M. tuberculosis also expresses a Nramp transporter homologue, named Mramp (Agranoff et al., 1999). Mutants in this gene have no growth phenotype in macrophages or mice (Boechat et al., 2002) and appear to accumulate similar amounts of iron in the phagosome (Agranoff et al., 1999). This observation suggests that the function of Mramp is redundant with that of other ferrous iron transporters, as observed with the Salmonella Nramp homologue dmntH.
There is so far no evidence that protozoa can acquire Fe3+ through siderophores and siderophore receptors. A study using Leishmania chagasi showed that soluble molecules secreted by the parasites are not capable of removing iron from lactoferrin or transferrin (Wilson et al., 1994). In trypanosomatid protozoa, receptor-mediated uptake of Fe3+ chelated to transferrin has been clearly demonstrated only in the African trypanosome, Trypanosoma brucei (Steverding et al., 1995). This observation is consistent with the fact that African trypanosomes are exclusively extracellular in their mammalian host, and thus directly exposed to holotransferrin, the most abundant iron source in serum. Despite some suggestive evidence (Lima and Villalta, 1990; Voyiatzaki and Soteriadou, 1992; Borges et al., 1998; Britigan et al., 1998), it is still not clear whether a receptor-mediated transferrin uptake pathway is present in the two trypanosomatid parasites with an intracellular life style, Trypanosoma cruzi and Leishmania (Wilson and Britigan, 1998). After entering cells in lysosome-like vacuoles, T. cruzi escapes into the cytosol and replicates in direct contact with ferritin, the cytosolic Fe3+ carrier protein. In contrast, Leishmania spends its intracellular life cycle inside the endosomal pathway, where one of the major sources of iron is Fe3+ complexed to transferrin, or Fe2+ generated from Fe3+ by endosomal reductases (Fig. 1). As discussed below, such differences in environment may be reflected in specific mechanisms for iron acquisition in these parasites.
Iron uptake by Leishmania – how do they do it?
During phagocytosis macrophages generate an oxidative burst, which produces highly toxic reactive oxygen intermediates including superoxide anion (O2−) (Fang, 2004). The metalloenzyme superoxide dismutase (SOD) plays an important role in detoxifying O2−, by converting it into H2O2 and H2O (Fridovich, 1978). Two genes (SODA and SODB), which show complete conservation of a signature sequence for SODs utilizing iron as an essential cofactor, were initially identified in L. chagasi. These genes complemented Escherichia coli SOD-deficient mutants, and their overexpression in L. tropica protected the parasites against the free radical generating agents paraquat and nitroprusside (Paramchuk et al., 1997). Subsequent studies identified additional Leishmania SOD isoforms, and showed that LcFeSODB1 is expressed at high levels in L. chagasi stationary phase promastigote and amastigote stages, while LcFeSODB2 is more highly expressed in logarithmic growth promastigotes. Both LcFeSODB1 and LcFeSODB2 are targeted to glycosomes by the last three amino acids of their carboxyl termini, suggesting that these proteins may act to protect glycosomal enzymes from O2− toxicity (Plewes et al., 2003). Leishmania SODA, on the other hand, was reported to localize to the parasite's mitochondria (Getachew and Gedamu, 2007). Null mutants of LcFeSODB1 could not be obtained, but a single-allele knockout showed decreased growth in the presence of paraquat, and significantly reduced survival within macrophages (Plewes et al., 2003). Similar results were obtained with L. tropica (Ghosh et al., 2003). It is now clear that SOD genes are present in the genome of several Leishmania species, including L. donovani, L. tropica, L. aethiopica (Genetu et al., 2006) and L. major (Ivens et al., 2005). In several of these studies, resistance to oxidative stress correlated with the ability of Leishmania to proliferate within macrophages, consistent with the fact that generation of reactive oxygen species is a major microbicidal mechanism in these cells.
The uptake of iron required for the critical antioxidant function of SOD and for other essential metabolic reactions poses a particular problem for Leishmania. Although exogenously added holotransferrin is able to reach parasite intracellular compartments and promote the growth of Leishmania within macrophages (Borges et al., 1998), it is not clear whether there is sufficient Fe3+ complexed to transferrin available to the parasites under physiological conditions. Furthermore, as discussed above, the endosomal pathway of macrophages is actively depleted in Fe2+ by the divalent cation efflux pumps Nramp2 and Nramp1, so this metal is likely to be in very short supply inside Leishmania parasitophorous vacuoles. An important development in our understanding of how Leishmania acquires iron under these challenging conditions came from the observation that iron uptake in L. chagasi occurs preferentially in the reduced ferrous (Fe2+) form. This finding was followed by the demonstration that L. chagasi expresses a NADPH-dependent iron reductase, capable of converting oxidized, ferric Fe3+ into the more soluble Fe2+ (Wilson et al., 2002). There is extensive evidence that Fe3+ reduction is usually coupled to Fe2+ membrane transport in bacteria, yeast, plants and animal cells, so the discovery of a ferric reductase in Leishmania immediately suggested the potential existence of a ferrous iron transporter. As discussed below, our recent studies have identified such plasma membrane Leishmania transporter, LIT1, and shown that it is essential for intracellular replication and for virulence in animal models.
Identification and characterization of the Leishmania amazonensis ferrous iron transporter, LIT1
Leishmania major, L. infantum and L. braziliensis appear to encode in their genomes a single integral membrane ferric reductase, namely LmjF30.1610, LinJ30_V3.1630 and LbrM30_V2.1670 respectively (Ivens et al., 2005; Peacock et al., 2007). The predicted protein shows significant homology to the Fe3+ chelating reductase from Arabidopsis thaliana encoded by FRO2 (Robinson et al., 1999). The capability to reduce Fe3+ to Fe2+ is one of the main features of high affinity iron acquisition systems, so the presence of a putative ferric reductase across Leishmania species pointed to the probable existence of ferrous iron transporters. As discussed below, database searches followed by detailed analysis performed in our laboratory have identified LIT1, the first ferrous iron transporter to be detected in trypanosomatid protozoa.
The first gene encoding a Fe2+ transporter to be cloned from plants or animals was the Arabidopsis thaliana IRT1, which encodes an integral membrane protein with eight predicted transmembrane domains and potential metal binding sites rich in histidine residues (Eide et al., 1996). Null mutants of IRT1 in Arabidopsis have a severe growth defect in normal soil, which is rescued by exogenous iron addition (Vert et al., 2002). Iron is the preferred metal substrate for IRT1, although cadmium, cobalt, manganese and zinc can also be transported (Eide et al., 1996; Rogers et al., 2000). This capacity for mediating the uptake of several transition metals is typical of ferrous iron transporters (Forbes and Gros, 2003), which are not as highly specific for iron as the ferric iron transport systems (Kaplan, 2002). IRT1 belongs to the ZIP family of iron transporters, present not only in plants but also in yeast, Drosophila, C. elegans and humans (Guerinot, 2000). Most members of the ZIP transporter family are predicted to have eight transmembrane domains and a similar membrane topology, in which the amino- and carboxy-terminal ends are located on the extracellular side of the plasma membrane (Fig. 2). The length of different family members ranges 309–476 amino acids, the difference being due to a cytoplasmic variable region between transmembrane domains III and IV. The most conserved portion of ZIP family proteins is in transmembrane domain IV, predicted to form an amphipathic helix with a fully conserved histidine residue and an adjacent semi-polar residue, and thought to be essential components of the heavy metal binding site (Eng et al., 1998; Guerinot, 2000).
Fig. 2.
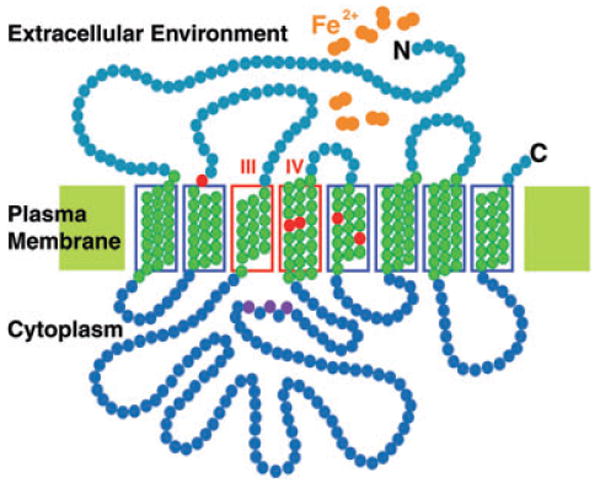
Topology of the LIT1 ferrous iron transporter on the plasma membrane of Leishmania. The amino acids shown in green form the transmembrane domains, the ones shaded in red correspond to conserved residues required for iron transport in Arabidopsis IRT1, and the ones in pink correspond to the conserved H×H×H motif present in the variable intracellular loop region. Transmembrane domains III and IV are indicated.
Database searches revealed that Leishmania also expresses metal transporters of the ZIP family. The L. major genome sequence contains two identical genes in tandem, LmjF31.3060 and Lmj31.3070 (LIT1-1 and LIT1-2) (Huynh et al., 2006), which share 30% identity and 53.8% similarity with Arabidopsis IRT1. Two additional genes containing predicted ZIP metal permease domains were also detected: LmjF28. 1930 on chromosome 28 and LmjF33.3200 on chromosome 33. Both share reasonable similarity with putative zinc transporters from Orysa sativa and Dictyostelium discoideum respectively. At least one of these additional putative ZIP family metal permeases can also be found in the T. cruzi and T. brucei genomes. However, interestingly, only LIT1-1 and LIT1-2 within the Leishmania genome have extensive similarity with the demonstrated Arabidopsis iron transporter, IRT1. This may reflect the specific need of Leishmania to compete with iron transporters present in the endocytic pathway of host cells. The Leishmania LIT1 protein is predicted to have a membrane topology similar to Arabidopsis IRT1, including the long intracellular variable loop region located between transmembrane domains III and IV (Huynh et al., 2006). This region shows little conservation among ZIP family members, but Leishmania LIT1 also contains a conserved H×H×H motif that has been proposed as a potential metal binding domain (Gitan and Eide, 2000; Gitan et al., 2003) (Fig. 2).
Complementation studies using an iron transport-deficient yeast strain, together with assays for direct uptake of 55Fe by Leishmania, directly demonstrated that LIT1 is a divalent metal transporter with preference for iron. LIT1 is localized on the Leishmania amazonensish plasma membrane, but it is detected only on intracellular amastigotes residing for several hours within parasitophorous vacuoles. This apparently exclusively intracellular expression suggests that LIT1 may be upregulated under conditions of low iron availability. Supporting this hypothesis, LIT1 expression is accelerated in amastigotes replicating within Nramp1+/+ macrophages (Huynh et al., 2006), which are predicted to have lower levels of intraphagosomal iron when compared to Nramp1−/− macrophages. Thus, similar to what was shown for Arabidopsis IRT1 (Connolly et al., 2002; Vert et al., 2002) and other eukaryotic divalent cation transporters, expression of LIT1 transporter may be upregulated by iron deprivation. Regulation of gene expression in Leishmania occurs largely at the post-transcriptional level, consistent with the constitutive transcription of gene clusters into large polycystronic units that is typical of trypanosomatid genomes (Clayton, 2002; Ivens et al., 2005). Studies of the mechanisms regulating amastigote-specific gene expression have implicated changes in mRNA stability (Charest et al., 1996; Wu et al., 2000) or translation efficiency (Zilka et al., 2001; Boucher et al., 2002). Future studies are needed to clarify the mechanism by which LIT1 expression is restricted to Leishmania intracellular amastigotes, and whether this process is triggered by the iron-poor environment of the parasitophorous vacuole. LIT1 is dispensable for L. amazonensis growth as promastigotes or amastigotes in axenic culture, suggesting the existence of alternative mechanisms for iron acquisition in the extracellular forms with access to a larger iron supply. This role may be fulfilled by the products of LmjF28.1330 and/or LmjF33.3200, the additional L. major genes with predicted ZIP metal permease domains. Alternatively, haeme may be acquired through the haemoglobin endocytic receptor identified in L. donovani promastigotes (Sengupta et al., 1999).
The in tandem localization of the two identical LIT1 genes within a 5425 bp region of chromosome 31 allowed generation of a L. amazonensis null mutant lacking both LIT1 copies. The LIT1−/− strain, although capable of normal growth and differentiation in axenic culture, lost the ability to replicate within macrophage parasitophorous vacuoles, and to induce cutaneous lesions in mice. Complementation of LIT1−/− with an episomal or integrated copy of LIT1 restored virulence and the capacity for intracellular growth (Huynh et al., 2006). Intriguingly, significant numbers of LIT1−/−L. amazonensis persisted for several months in mouse tissues, without causing pathology (Huynh et al., 2006). LIT1 null Leishmania strains may be useful for future studies of the mechanisms of in vivo persistence, which may involve infection of alternative cell types producing lower levels of reactive oxygen species.
Conclusion and perspectives
Major gaps still exist in our understanding of the biology of different Leishmania species, and how their properties correlate with the very different clinical forms of the disease. Before the overall process of Leishmania infection can be understood, and before the resulting pathology can be rationally treated, it will be necessary to understand how these parasites survive and replicate within acidified, hydrolase-rich phagolysosomes of macrophages. Our knowledge of the strategies used by Leishmania to survive within this harsh environment is still very limited. A significant insight into how Leishmania deals with the challenge of intracellular iron acquisition came from the identification of LIT1, a ferrous iron transporter that plays a critical role in intracellular growth and virulence of L. amazonensis. This transporter is expressed exactly when, and where it is needed to effectively compete with host iron transporters for this essential element. Identification of LIT1 has finally allowed Leishmania to be listed, along with Salmonella and Mycobacteria, as a pathogen known to possess molecular mechanisms for competing for iron inside the host endocytic pathway. Additional studies should now rapidly expand our knowledge of additional components in this critical pathway for Leishmania intracellular survival.
Acknowledgments
Work in our laboratory is supported by grants from the National Institutes of Health.
References
- Agranoff D, Monahan IM, Mangan JA, Butcher PD, Krishna S. Mycobacterium tuberculosis expresses a novel pH-dependent divalent cation transporter belonging to the Nramp family. J Exp Med. 1999;190:717–724. doi: 10.1084/jem.190.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoine JC, Prina E, Lang T, Courret N. The biogenesis and properties of the parasitophorous vacuoles that harbour Leishmania in murine macrophages. Trends Microbiol. 1998;6:392–401. doi: 10.1016/s0966-842x(98)01324-9. [DOI] [PubMed] [Google Scholar]
- Blackwell JM, Goswami T, Evans CA, Sibthorpe D, Papo N, White JK, et al. SLC11A1 (formerly NRAMP1) and disease resistance. Cell Microbiol. 2001;3:773–784. doi: 10.1046/j.1462-5822.2001.00150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boechat N, Lagier-Roger B, Petit S, Bordat Y, Rauzier J, Hance AJ, et al. Disruption of the gene homologous to mammalian Nramp1 in Mycobacterium tuberculosis does not affect virulence in mice. Infect Immun. 2002;70:4124–4131. doi: 10.1128/IAI.70.8.4124-4131.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges VM, Vannier-Santos MA, de Souza W. Subverted transferrin trafficking in Leishmania-infected macrophages. Parasitol Res. 1998;84:811–822. doi: 10.1007/s004360050493. [DOI] [PubMed] [Google Scholar]
- Boucher N, Wu Y, Dumas C, Dube M, Sereno D, Breton M, Papadopoulou B. A common mechanism of stage-regulated gene expression in Leishmania mediated by a conserved 3′-untranslated region element. J Biol Chem. 2002;277:19511–19520. doi: 10.1074/jbc.M200500200. [DOI] [PubMed] [Google Scholar]
- Boyer E, Bergevin I, Malo D, Gros P, Cellier MF. Acquisition of Mn(II) in addition to Fe(II) is required for full virulence of Salmonella enterica serovar Typhimurium. Infect Immun. 2002;70:6032–6042. doi: 10.1128/IAI.70.11.6032-6042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britigan BE, Lewis TS, McCormick ML, Wilson ME. Evidence for the existence of a surface receptor for ferriclactoferrin and ferrictransferrin associated with the plasma membrane of the protozoan parasite Leishmania donovani. Adv Exp Med Biol. 1998;443:135–140. doi: 10.1007/978-1-4757-9068-9_16. [DOI] [PubMed] [Google Scholar]
- Charest H, Zhang WW, Matlashewski G. The developmental expression of Leishmania donovani A2 amastigote-specific genes is post-transcriptionally mediated and involves elements located in the 3′-untranslated region. J Biol Chem. 1996;271:17081–17090. doi: 10.1074/jbc.271.29.17081. [DOI] [PubMed] [Google Scholar]
- Clayton CE. Life without transcriptional control? From fly to man and back again. EMBO J. 2002;21:1881–1888. doi: 10.1093/emboj/21.8.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly EL, Fett JP, Guerinot ML. Expression of the IRT1 metal transporter is controlled by metals at the levels of transcript and protein accumulation. Plant Cell. 2002;14:1347–1357. doi: 10.1105/tpc.001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa JH, Walsh CT. Genetics and assembly line enzymology of siderophore biosynthesis in bacteria. Microbiol Mol Biol Rev. 2002;66:223–249. doi: 10.1128/MMBR.66.2.223-249.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Voss JJ, Rutter K, Schroeder BG, Su H, Zhu Y, Barry CE., 3rd The salicylate-derived mycobactin siderophores of Mycobacterium tuberculosis are essential for growth in macrophages. Proc Natl Acad Sci USA. 2000;97:1252–1257. doi: 10.1073/pnas.97.3.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide D, Broderius M, Fett J, Guerinot ML. A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc Natl Acad Sci USA. 1996;93:5624–5628. doi: 10.1073/pnas.93.11.5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng BH, Guerinot ML, Eide D, Saier MH., Jr Sequence analyses and phylogenetic characterization of the ZIP family of metal ion transport proteins. J Membr Biol. 1998;166:1–7. doi: 10.1007/s002329900442. [DOI] [PubMed] [Google Scholar]
- Fang FC. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol. 2004;2:820–832. doi: 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
- Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- Forbes JR, Gros P. Divalent-metal transport by NRAMP proteins at the interface of host–pathogen interactions. Trends Microbiol. 2001;9:397–403. doi: 10.1016/s0966-842x(01)02098-4. [DOI] [PubMed] [Google Scholar]
- Forbes JR, Gros P. Iron, manganese, and cobalt transport by Nramp1 (Slc11a1) and Nramp2 (Slc11a2) expressed at the plasma membrane. Blood. 2003;102:1884–1892. doi: 10.1182/blood-2003-02-0425. [DOI] [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978;201:875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Genetu A, Gadisa E, Aseffa A, Barr S, Lakew M, Jirata D, et al. Leishmania aethiopica: strain identification and characterization of superoxide dismutase-B genes. Exp Parasitol. 2006;113:221–226. doi: 10.1016/j.exppara.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Getachew F, Gedamu L. Leishmania donovani iron superoxide dismutase A is targeted to the mitochondria by its N-terminal positively charged amino acids. Mol Biochem Parasitol. 2007;154:62–69. doi: 10.1016/j.molbiopara.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Goswami S, Adhya S. Role of superoxide dismutase in survival of Leishmania within the macrophage. Biochem J. 2003;369:447–452. doi: 10.1042/BJ20021684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitan RS, Eide DJ. Zinc-regulated ubiquitin conjugation signals endocytosis of the yeast ZRT1 zinc transporter. Biochem J. 2000;346(Part 2):329–336. [PMC free article] [PubMed] [Google Scholar]
- Gitan RS, Shababi M, Kramer M, Eide DJ. A cytosolic domain of the yeast Zrt1 zinc transporter is required for its post-translational inactivation in response to zinc and cadmium. J Biol Chem. 2003;278:39558–39564. doi: 10.1074/jbc.M302760200. [DOI] [PubMed] [Google Scholar]
- Goswami T, Bhattacharjee A, Babal P, Searle S, Moore E, Li M, Blackwell JM. Natural-resistance-associated macrophage protein 1 is an H+/bivalent cation antiporter. Biochem J. 2001;354:511–519. doi: 10.1042/0264-6021:3540511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenheid S, Canonne-Hergaux F, Gauthier S, Hackam DJ, Grinstein S, Gros P. The iron transport protein NRAMP2 is an integral membrane glycoprotein that colocalizes with transferrin in recycling endosomes. J Exp Med. 1999;189:831–841. doi: 10.1084/jem.189.5.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerinot ML. The ZIP family of metal transporters. Biochim Biophys Acta. 2000;1465:190–198. doi: 10.1016/s0005-2736(00)00138-3. [DOI] [PubMed] [Google Scholar]
- Gunshin H, Fujiwara Y, Custodio AO, Direnzo C, Robine S, Andrews NC. Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. J Clin Invest. 2005;115:1258–1266. doi: 10.1172/JCI24356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K, Nicholson G, Rabsch W, Winkelmann G. Salmochelins, siderophores of Salmonella enterica and uropathogenic Escherichia coli strains, are recognized by the outer membrane receptor IroN. Proc Natl Acad Sci USA. 2003;100:3677–3682. doi: 10.1073/pnas.0737682100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh C, Sacks DL, Andrews NW. A Leishmania amazonensis ZIP family iron transporter is essential for parasite replication within macrophage phagolysosomes. J Exp Med. 2006;203:2363–2375. doi: 10.1084/jem.20060559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivens AC, Peacock CS, Worthey EA, Murphy L, Aggarwal G, Berriman M, et al. The genome of the kinetoplastid parasite, Leishmania major. Science. 2005;309:436–442. doi: 10.1126/science.1112680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabado N, Jankowski A, Dougaparsad S, Picard V, Grinstein S, Gros P. Natural resistance to intracellular infections: natural resistance-associated macrophage protein 1 (Nramp1) functions as a pH-dependent manganese transporter at the phagosomal membrane. J Exp Med. 2000;192:1237–1248. doi: 10.1084/jem.192.9.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janakiraman A, Slauch JM. The putative iron transport system SitABCD encoded on SPI1 is required for full virulence of Salmonella typhimurium. Mol Microbiol. 2000;35:1146–1155. doi: 10.1046/j.1365-2958.2000.01783.x. [DOI] [PubMed] [Google Scholar]
- Kaplan J. Mechanisms of cellular iron acquisition: another iron in the fire. Cell. 2002;111:603–606. doi: 10.1016/s0092-8674(02)01164-9. [DOI] [PubMed] [Google Scholar]
- Kim S, Ponka P. Effects of interferon-gamma and lipopolysaccharide on macrophage iron metabolism are mediated by nitric oxide-induced degradation of iron regulatory protein 2. J Biol Chem. 2000;275:6220–6226. doi: 10.1074/jbc.275.9.6220. [DOI] [PubMed] [Google Scholar]
- Lima MF, Villalta F. Trypanosoma cruzi receptors for human transferrin and their role. Mol Biochem Parasitol. 1990;38:245–252. doi: 10.1016/0166-6851(90)90027-j. [DOI] [PubMed] [Google Scholar]
- McMahon-Pratt D, Alexander J. Does the Leishmania major paradigm of pathogenesis and protection hold for New World cutaneous leishmaniases or the visceral disease? Immunol Rev. 2004;201:206–224. doi: 10.1111/j.0105-2896.2004.00190.x. [DOI] [PubMed] [Google Scholar]
- Marquis JF, Gros P. Intracellular Leishmania: your iron or mine? Trends Microbiol. 2007;15:93–95. doi: 10.1016/j.tim.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Oexle H, Kaser A, Most J, Bellmann-Weiler R, Werner ER, Werner-Felmayer G, Weiss G. Pathways for the regulation of interferon-gamma-inducible genes by iron in human monocytic cells. J Leukoc Biol. 2003;74:287–294. doi: 10.1189/jlb.0802420. [DOI] [PubMed] [Google Scholar]
- Ohgami RS, Campagna DR, Greer EL, Antiochos B, McDonald A, Chen J, et al. Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells. Nat Genet. 2005;37:1264–1269. doi: 10.1038/ng1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paramchuk WJ, Ismail SO, Bhatia A, Gedamu L. Cloning, characterization and overexpression of two iron superoxide dismutase cDNAs from Leishmania chagasi: role in pathogenesis. Mol Biochem Parasitol. 1997;90:203–221. doi: 10.1016/s0166-6851(97)00141-2. [DOI] [PubMed] [Google Scholar]
- Peacock CS, Seeger K, Harris D, Murphy L, Ruiz JC, Quail MA, et al. Comparative genomic analysis of three Leishmania species that cause diverse human disease. Nat Genet. 2007;39:839–847. doi: 10.1038/ng2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard V, Govoni G, Jabado N, Gros P. Nramp 2 (DCT1/DMT1) expressed at the plasma membrane transports iron and other divalent cations into a calcein-accessible cytoplasmic pool. J Biol Chem. 2000;275:35738–35745. doi: 10.1074/jbc.M005387200. [DOI] [PubMed] [Google Scholar]
- Plewes KA, Barr SD, Gedamu L. Iron superoxide dismutases targeted to the glycosomes of Leishmania chagasi are important for survival. Infect Immun. 2003;71:5910–5920. doi: 10.1128/IAI.71.10.5910-5920.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabsch W, Methner U, Voigt W, Tschape H, Reissbrodt R, Williams PH. Role of receptor proteins for enterobactin and 2,3-dihydroxybenzoylserine in virulence of Salmonella enterica. Infect Immun. 2003;71:6953–6961. doi: 10.1128/IAI.71.12.6953-6961.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson NJ, Procter CM, Connolly EL, Guerinot ML. A ferric-chelate reductase for iron uptake from soils. Nature. 1999;397:694–697. doi: 10.1038/17800. [DOI] [PubMed] [Google Scholar]
- Rodriguez GM. Control of iron metabolism in Mycobacterium tuberculosis. Trends Microbiol. 2006;14:320–327. doi: 10.1016/j.tim.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Rogers EE, Eide DJ, Guerinot ML. Altered selectivity in an Arabidopsis metal transporter. Proc Natl Acad Sci USA. 2000;97:12356–12360. doi: 10.1073/pnas.210214197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol. 2002;2:845–858. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- Schaible UE, Kaufmann SH. Iron and microbial infection. Nat Rev Microbiol. 2004;2:946–953. doi: 10.1038/nrmicro1046. [DOI] [PubMed] [Google Scholar]
- Sengupta S, Tripathi J, Tandon R, Raje M, Roy RP, Basu SK, Mukhopadhyay A. Hemoglobin endocytosis in Leishmania is mediated through a 46-kDa protein located in the flagellar pocket. J Biol Chem. 1999;274:2758–2765. doi: 10.1074/jbc.274.5.2758. [DOI] [PubMed] [Google Scholar]
- Stanley AC, Engwerda CR. Balancing immunity and pathology in visceral leishmaniasis. Immunol Cell Biol. 2007;85:138–147. doi: 10.1038/sj.icb7100011. [DOI] [PubMed] [Google Scholar]
- Steverding D, Stierhof YD, Fuchs H, Tauber R, Overath P. Transferrin-binding protein complex is the receptor for transferrin uptake in Trypanosoma brucei. J Cell Biol. 1995;131:1173–1182. doi: 10.1083/jcb.131.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vert G, Grotz N, Dedaldechamp F, Gaymard F, Guerinot ML, Briat JF, Curie C. IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell. 2002;14:1223–1233. doi: 10.1105/tpc.001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyiatzaki CS, Soteriadou KP. Identification and isolation of the Leishmania transferrin receptor. J Biol Chem. 1992;267:9112–9117. [PubMed] [Google Scholar]
- Weinberg ED. Iron depletion: a defense against intracellular infection and neoplasia. Life Sci. 1992;50:1289–1297. doi: 10.1016/0024-3205(92)90279-x. [DOI] [PubMed] [Google Scholar]
- Weiss G. Iron and immunity: a double-edged sword. Eur J Clin Invest. 2002;32 1:70–78. doi: 10.1046/j.1365-2362.2002.0320s1070.x. [DOI] [PubMed] [Google Scholar]
- Weiss G, Werner-Felmayer G, Werner ER, Grunewald K, Wachter H, Hentze MW. Iron regulates nitric oxide synthase activity by controlling nuclear transcription. J Exp Med. 1994;180:969–976. doi: 10.1084/jem.180.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessling-Resnick M. Iron transport. Annu Rev Nutr. 2000;20:129–151. doi: 10.1146/annurev.nutr.20.1.129. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Britigan BE. Iron acquisition by parasitic protozoa. Parasitol Today. 1998;14:348–353. doi: 10.1016/s0169-4758(98)01294-0. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Vorhies RW, Andersen KA, Britigan BE. Acquisition of iron from transferrin and lactoferrin by the protozoan Leishmania chagasi. Infect Immun. 1994;62:3262–3269. doi: 10.1128/iai.62.8.3262-3269.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ME, Lewis TS, Miller MA, McCormick ML, Britigan BE. Leishmania chagasi: uptake of iron bound to lactoferrin or transferrin requires an iron reductase. Exp Parasitol. 2002;100:196–207. doi: 10.1016/s0014-4894(02)00018-8. [DOI] [PubMed] [Google Scholar]
- Wu Y, El Fakhry Y, Sereno D, Tamar S, Papadopoulou B. A new developmentally regulated gene family in Leishmania amastigotes encoding a homolog of amastin surface proteins. Mol Biochem Parasitol. 2000;110:345–357. doi: 10.1016/s0166-6851(00)00290-5. [DOI] [PubMed] [Google Scholar]
- Zilka A, Garlapati S, Dahan E, Yaolsky V, Shapira M. Developmental regulation of heat shock protein 83 in Leishmania. 3′ processing and mRNA stability control transcript abundance, and translation id directed by a determinant in the 3′-untranslated region. J Biol Chem. 2001;276:47922–47929. doi: 10.1074/jbc.M108271200. [DOI] [PubMed] [Google Scholar]


