Abstract
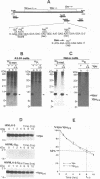
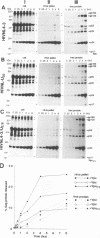
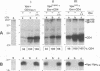
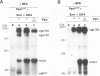
The human immunodeficiency virus type 1 (HIV-1)-specific Vpu is an 81-amino-acid amphipathic integral membrane protein with at least two different biological functions: (i) enhancement of virus particle release from the plasma membrane of HIV-1-infected cells and (ii) degradation of the virus receptor CD4 in the endoplasmic reticulum (ER). We have previously found that Vpu is phosphorylated in infected cells at two seryl residues in positions 52 and 56 by the ubiquitous casein kinase 2. To study the role of Vpu phosphorylation on its biological activity, a mutant of the vpu gene lacking both phosphoacceptor sites was introduced into the infectious molecular clone of HIV-1, pNL4-3, as well as subgenomic Vpu expression vectors. This mutation did not affect the expression level or the stability of Vpu but had a significant effect on its biological activity in infected T cells as well as transfected HeLa cells. Despite the presence of comparable amounts of wild-type and nonphosphorylated Vpu, decay of CD4 was observed only in the presence of phosphorylated wild-type Vpu. Nonphosphorylated Vpu was unable to induce degradation of CD4 even if the proteins were artificially retained in the ER. In contrast, Vpu-mediated enhancement of virus secretion was only partially dependent on Vpu phosphorylation. Enhancement of particle release by wild-type Vpu was efficiently blocked when Vpu was artificially retained in the ER, suggesting that the two biological functions of Vpu are independent, occur at different sites within a cell, and exhibit different sensitivity to phosphorylation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi A., Gendelman H. E., Koenig S., Folks T., Willey R., Rabson A., Martin M. A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986 Aug;59(2):284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barik S., Banerjee A. K. Phosphorylation by cellular casein kinase II is essential for transcriptional activity of vesicular stomatitis virus phosphoprotein P. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6570–6574. doi: 10.1073/pnas.89.14.6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beg A. A., Finco T. S., Nantermet P. V., Baldwin A. S., Jr Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of I kappa B alpha: a mechanism for NF-kappa B activation. Mol Cell Biol. 1993 Jun;13(6):3301–3310. doi: 10.1128/mcb.13.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. Y., Maldarelli F., Karczewski M. K., Willey R. L., Strebel K. Human immunodeficiency virus type 1 Vpu protein induces degradation of CD4 in vitro: the cytoplasmic domain of CD4 contributes to Vpu sensitivity. J Virol. 1993 Jul;67(7):3877–3884. doi: 10.1128/jvi.67.7.3877-3884.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane A., Kramer R., Ruben S., Levine J., Rosen C. A. The human immunodeficiency virus rev protein is a nuclear phosphoprotein. Virology. 1989 Jul;171(1):264–266. doi: 10.1016/0042-6822(89)90535-7. [DOI] [PubMed] [Google Scholar]
- Cohen E. A., Terwilliger E. F., Sodroski J. G., Haseltine W. A. Identification of a protein encoded by the vpu gene of HIV-1. Nature. 1988 Aug 11;334(6182):532–534. doi: 10.1038/334532a0. [DOI] [PubMed] [Google Scholar]
- Deen K. C., McDougal J. S., Inacker R., Folena-Wasserman G., Arthos J., Rosenberg J., Maddon P. J., Axel R., Sweet R. W. A soluble form of CD4 (T4) protein inhibits AIDS virus infection. Nature. 1988 Jan 7;331(6151):82–84. doi: 10.1038/331082a0. [DOI] [PubMed] [Google Scholar]
- Folks T., Benn S., Rabson A., Theodore T., Hoggan M. D., Martin M., Lightfoote M., Sell K. Characterization of a continuous T-cell line susceptible to the cytopathic effects of the acquired immunodeficiency syndrome (AIDS)-associated retrovirus. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4539–4543. doi: 10.1073/pnas.82.13.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier J., Matsukawa T., Nurse P., Maller J. Dephosphorylation and activation of Xenopus p34cdc2 protein kinase during the cell cycle. Nature. 1989 Jun 22;339(6226):626–629. doi: 10.1038/339626a0. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Phelps W., Feigenbaum L., Ostrove J. M., Adachi A., Howley P. M., Khoury G., Ginsberg H. S., Martin M. A. Trans-activation of the human immunodeficiency virus long terminal repeat sequence by DNA viruses. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9759–9763. doi: 10.1073/pnas.83.24.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Guy B., Kieny M. P., Riviere Y., Le Peuch C., Dott K., Girard M., Montagnier L., Lecocq J. P. HIV F/3' orf encodes a phosphorylated GTP-binding protein resembling an oncogene product. Nature. 1987 Nov 19;330(6145):266–269. doi: 10.1038/330266a0. [DOI] [PubMed] [Google Scholar]
- Göttlinger H. G., Dorfman T., Cohen E. A., Haseltine W. A. Vpu protein of human immunodeficiency virus type 1 enhances the release of capsids produced by gag gene constructs of widely divergent retroviruses. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7381–7385. doi: 10.1073/pnas.90.15.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauber J., Bouvier M., Malim M. H., Cullen B. R. Phosphorylation of the rev gene product of human immunodeficiency virus type 1. J Virol. 1988 Dec;62(12):4801–4804. doi: 10.1128/jvi.62.12.4801-4804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henklein P., Schubert U., Kunert O., Klabunde S., Wray V., Klöppel K. D., Kiess M., Portsmann T., Schomburg D. Synthesis and characterization of the hydrophilic C-terminal domain of the human immunodeficiency virus type 1-encoded virus protein U (Vpu). Pept Res. 1993 Mar-Apr;6(2):79–87. [PubMed] [Google Scholar]
- Huet T., Cheynier R., Meyerhans A., Roelants G., Wain-Hobson S. Genetic organization of a chimpanzee lentivirus related to HIV-1. Nature. 1990 May 24;345(6273):356–359. doi: 10.1038/345356a0. [DOI] [PubMed] [Google Scholar]
- Klimkait T., Strebel K., Hoggan M. D., Martin M. A., Orenstein J. M. The human immunodeficiency virus type 1-specific protein vpu is required for efficient virus maturation and release. J Virol. 1990 Feb;64(2):621–629. doi: 10.1128/jvi.64.2.621-629.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maldarelli F., Chen M. Y., Willey R. L., Strebel K. Human immunodeficiency virus type 1 Vpu protein is an oligomeric type I integral membrane protein. J Virol. 1993 Aug;67(8):5056–5061. doi: 10.1128/jvi.67.8.5056-5061.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda Z., Chou M. J., Matsuda M., Huang J. H., Chen Y. M., Redfield R., Mayer K., Essex M., Lee T. H. Human immunodeficiency virus type 1 has an additional coding sequence in the central region of the genome. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6968–6972. doi: 10.1073/pnas.85.18.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mervis R. J., Ahmad N., Lillehoj E. P., Raum M. G., Salazar F. H., Chan H. W., Venkatesan S. The gag gene products of human immunodeficiency virus type 1: alignment within the gag open reading frame, identification of posttranslational modifications, and evidence for alternative gag precursors. J Virol. 1988 Nov;62(11):3993–4002. doi: 10.1128/jvi.62.11.3993-4002.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles K., Huganir R. L. Regulation of nicotinic acetylcholine receptors by protein phosphorylation. Mol Neurobiol. 1988 Summer;2(2):91–124. doi: 10.1007/BF02935341. [DOI] [PubMed] [Google Scholar]
- Münstermann U., Fritz G., Seitz G., Lu Y. P., Schneider H. R., Issinger O. G. Casein kinase II is elevated in solid human tumours and rapidly proliferating non-neoplastic tissue. Eur J Biochem. 1990 Apr 30;189(2):251–257. doi: 10.1111/j.1432-1033.1990.tb15484.x. [DOI] [PubMed] [Google Scholar]
- Niefind K., Schomburg D. Amino acid similarity coefficients for protein modeling and sequence alignment derived from main-chain folding angles. J Mol Biol. 1991 Jun 5;219(3):481–497. doi: 10.1016/0022-2836(91)90188-c. [DOI] [PubMed] [Google Scholar]
- Padmanabha R., Chen-Wu J. L., Hanna D. E., Glover C. V. Isolation, sequencing, and disruption of the yeast CKA2 gene: casein kinase II is essential for viability in Saccharomyces cerevisiae. Mol Cell Biol. 1990 Aug;10(8):4089–4099. doi: 10.1128/mcb.10.8.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R., Mumbauer S., Hoke G. M., Takatsuki A., Sarngadharan M. G. Brefeldin A inhibits the processing and secretion of envelope glycoproteins of human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 1991 Aug;7(8):707–712. doi: 10.1089/aid.1991.7.707. [DOI] [PubMed] [Google Scholar]
- Pinna L. A. Casein kinase 2: an 'eminence grise' in cellular regulation? Biochim Biophys Acta. 1990 Sep 24;1054(3):267–284. doi: 10.1016/0167-4889(90)90098-x. [DOI] [PubMed] [Google Scholar]
- Pinto L. H., Holsinger L. J., Lamb R. A. Influenza virus M2 protein has ion channel activity. Cell. 1992 May 1;69(3):517–528. doi: 10.1016/0092-8674(92)90452-i. [DOI] [PubMed] [Google Scholar]
- Ratka M., Lackmann M., Ueckermann C., Karlins U., Koch G. Poliovirus-associated protein kinase: destabilization of the virus capsid and stimulation of the phosphorylation reaction by Zn2+. J Virol. 1989 Sep;63(9):3954–3960. doi: 10.1128/jvi.63.9.3954-3960.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarre T. F. The phosphorylation of eukaryotic initiation factor 2: a principle of translational control in mammalian cells. Biosystems. 1989;22(4):311–325. doi: 10.1016/0303-2647(89)90053-1. [DOI] [PubMed] [Google Scholar]
- Schneider T., Hildebrandt P., Rokos K., Schubert U., Rönspeck W., Grund C., Beck A., Blesken R., Kulins G., Oldenburg H. Expression of nef, vpu, CA and CD4 during the infection of lymphoid and monocytic cell lines with HIV-1. Arch Virol. 1992;125(1-4):161–176. doi: 10.1007/BF01309635. [DOI] [PubMed] [Google Scholar]
- Schneider T., Hildebrandt P., Rönspeck W., Weigelt W., Pauli G. The antibody response to the HIV-1 specific "out" (vpu) protein: identification of an immunodominant epitope and correlation of antibody detectability to clinical stages. AIDS Res Hum Retroviruses. 1990 Jul;6(7):943–950. doi: 10.1089/aid.1990.6.943. [DOI] [PubMed] [Google Scholar]
- Schubert U., Henklein P., Boldyreff B., Wingender E., Strebel K., Porstmann T. The human immunodeficiency virus type 1 encoded Vpu protein is phosphorylated by casein kinase-2 (CK-2) at positions Ser52 and Ser56 within a predicted alpha-helix-turn-alpha-helix-motif. J Mol Biol. 1994 Feb 11;236(1):16–25. doi: 10.1006/jmbi.1994.1114. [DOI] [PubMed] [Google Scholar]
- Schubert U., Schneider T., Henklein P., Hoffmann K., Berthold E., Hauser H., Pauli G., Porstmann T. Human-immunodeficiency-virus-type-1-encoded Vpu protein is phosphorylated by casein kinase II. Eur J Biochem. 1992 Mar 1;204(2):875–883. doi: 10.1111/j.1432-1033.1992.tb16707.x. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Shin J., Doyle C., Yang Z., Kappes D., Strominger J. L. Structural features of the cytoplasmic region of CD4 required for internalization. EMBO J. 1990 Feb;9(2):425–434. doi: 10.1002/j.1460-2075.1990.tb08127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strebel K., Klimkait T., Maldarelli F., Martin M. A. Molecular and biochemical analyses of human immunodeficiency virus type 1 vpu protein. J Virol. 1989 Sep;63(9):3784–3791. doi: 10.1128/jvi.63.9.3784-3791.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strebel K., Klimkait T., Martin M. A. A novel gene of HIV-1, vpu, and its 16-kilodalton product. Science. 1988 Sep 2;241(4870):1221–1223. doi: 10.1126/science.3261888. [DOI] [PubMed] [Google Scholar]
- Terwilliger E. F., Cohen E. A., Lu Y. C., Sodroski J. G., Haseltine W. A. Functional role of human immunodeficiency virus type 1 vpu. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5163–5167. doi: 10.1073/pnas.86.13.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey R. L., Bonifacino J. S., Potts B. J., Martin M. A., Klausner R. D. Biosynthesis, cleavage, and degradation of the human immunodeficiency virus 1 envelope glycoprotein gp160. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9580–9584. doi: 10.1073/pnas.85.24.9580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey R. L., Buckler-White A., Strebel K. Sequences present in the cytoplasmic domain of CD4 are necessary and sufficient to confer sensitivity to the human immunodeficiency virus type 1 Vpu protein. J Virol. 1994 Feb;68(2):1207–1212. doi: 10.1128/jvi.68.2.1207-1212.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey R. L., Maldarelli F., Martin M. A., Strebel K. Human immunodeficiency virus type 1 Vpu protein induces rapid degradation of CD4. J Virol. 1992 Dec;66(12):7193–7200. doi: 10.1128/jvi.66.12.7193-7200.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey R. L., Maldarelli F., Martin M. A., Strebel K. Human immunodeficiency virus type 1 Vpu protein regulates the formation of intracellular gp160-CD4 complexes. J Virol. 1992 Jan;66(1):226–234. doi: 10.1128/jvi.66.1.226-234.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X. J., Göttlinger H., Haseltine W. A., Cohen E. A. Envelope glycoprotein and CD4 independence of vpu-facilitated human immunodeficiency virus type 1 capsid export. J Virol. 1992 Aug;66(8):5119–5126. doi: 10.1128/jvi.66.8.5119-5126.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandomeni R., Zandomeni M. C., Shugar D., Weinmann R. Casein kinase type II is involved in the inhibition by 5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole of specific RNA polymerase II transcription. J Biol Chem. 1986 Mar 5;261(7):3414–3419. [PubMed] [Google Scholar]