Abstract
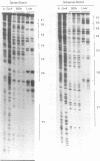
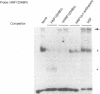
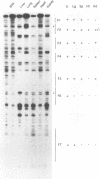
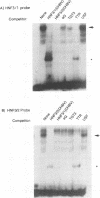
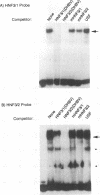
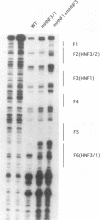
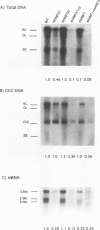
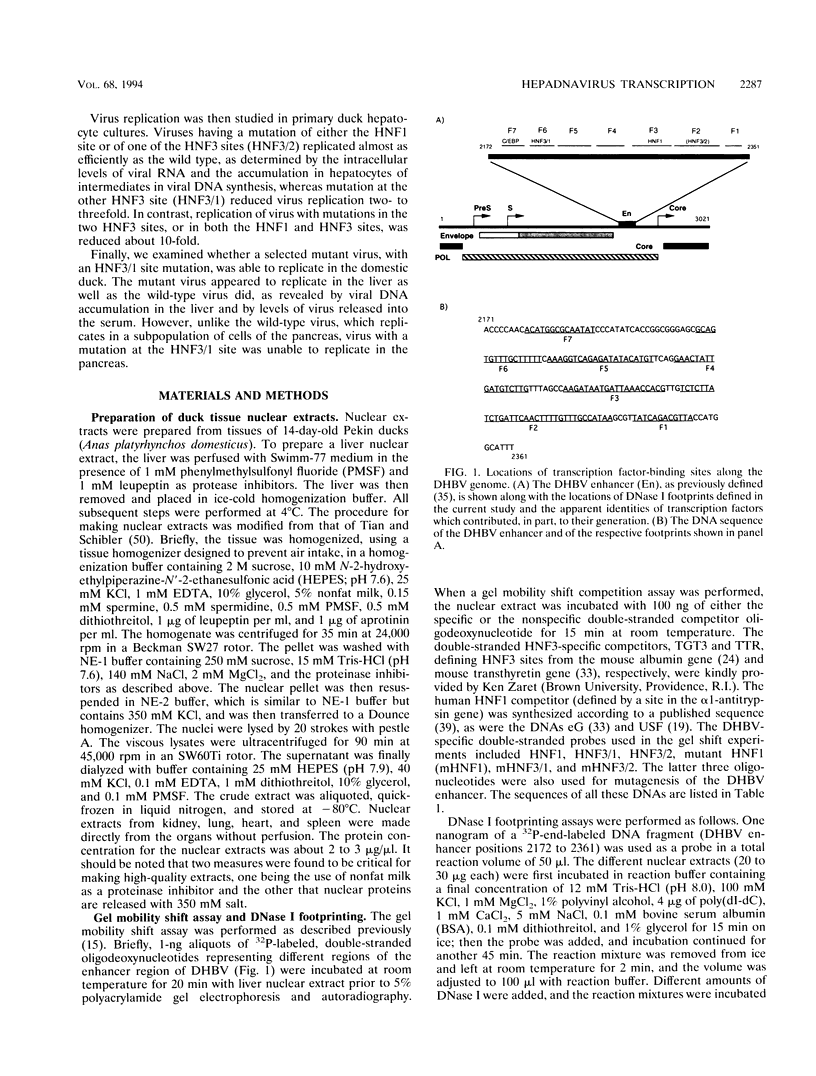
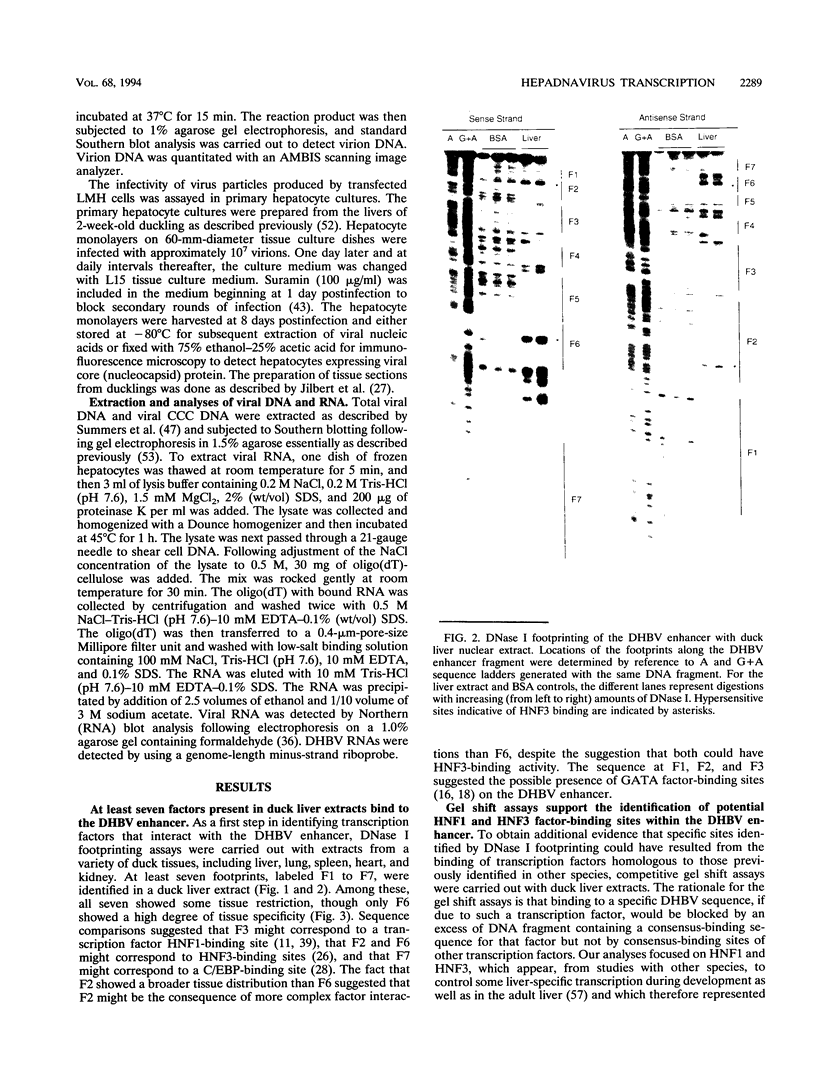
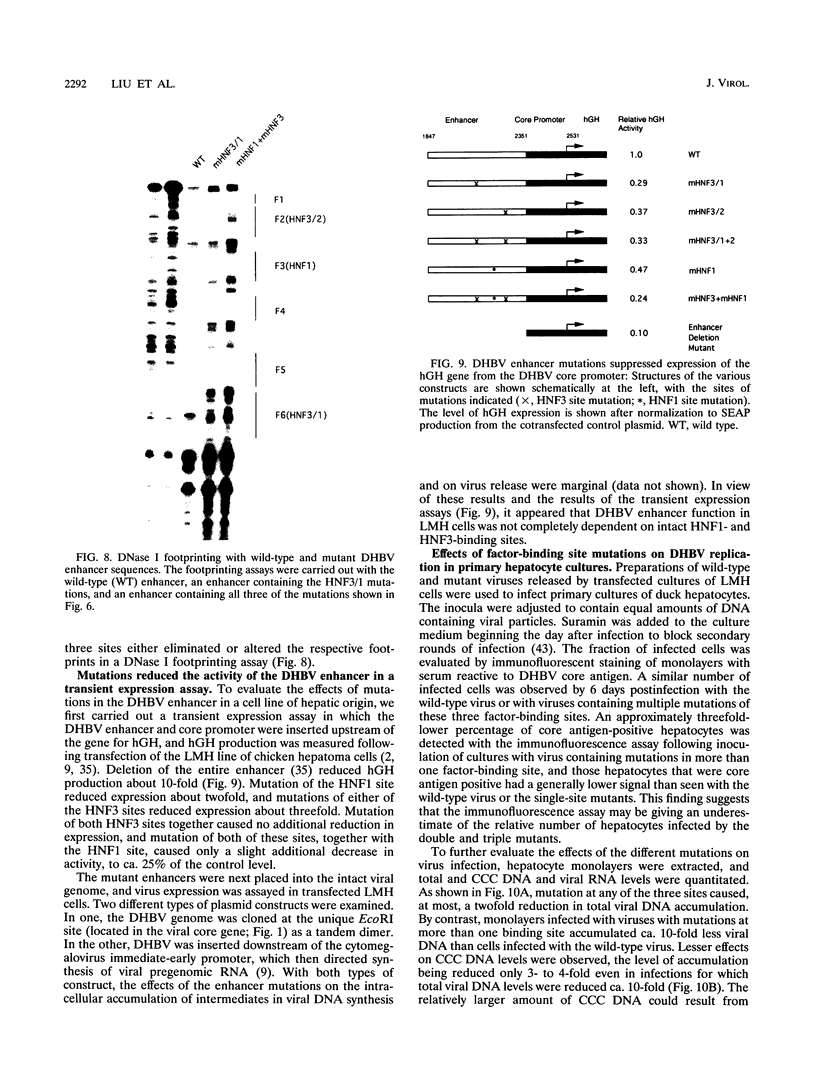
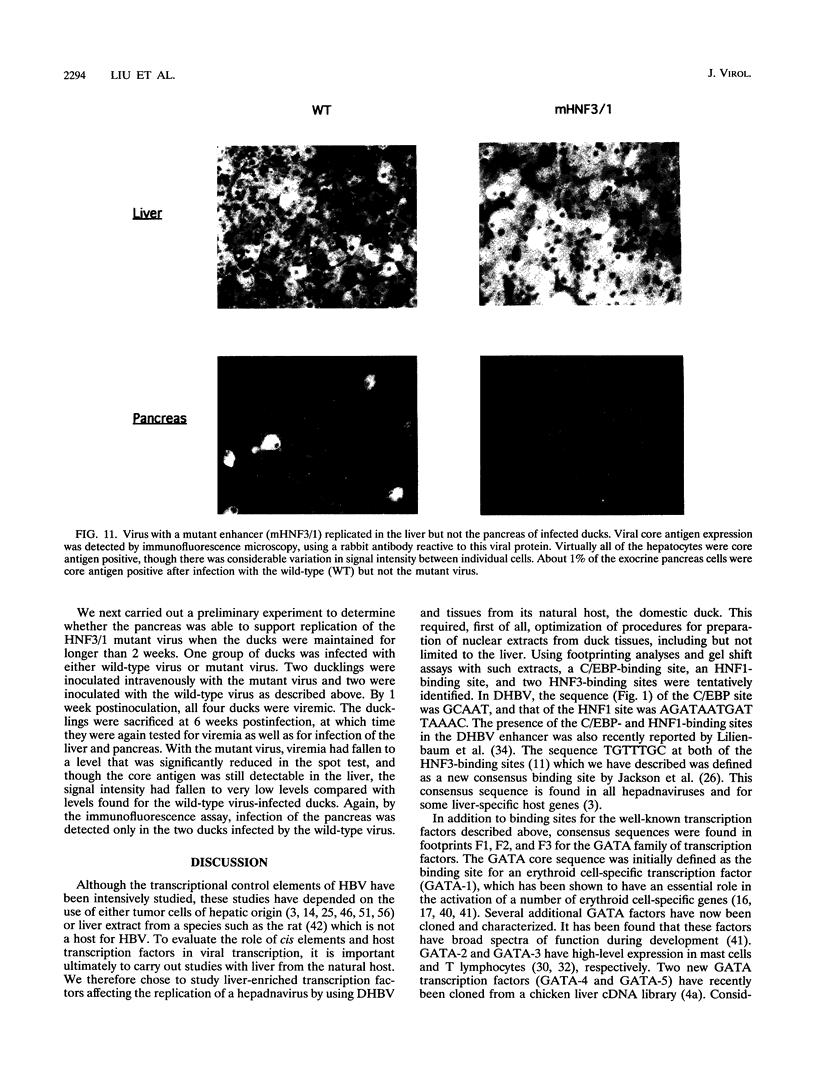
Hepatitis B viruses (hepadnaviruses) can cause chronic, productive infections of hepatocytes. Analyses of the enhancers and promoters of these viruses in cell lines have suggested a requirement of these elements for liver-enriched transcription factors. In this study, a minimum of seven factor-binding sites on the duck hepatitis B virus enhancer were detected by DNase I footprinting using duck liver nuclear extracts. Among the sites that were tentatively identified were one C/EBP-, one HNF1-, and two HNF3-binding sites. Mutations of the HNF1- and HNF3-like sites, which eliminated factor binding, as assessed by both DNase I footprinting and competitive gel shift assays, were evaluated for their effects on enhancer activity. Using a construct in which human growth hormone was expressed from the viral enhancer and core gene promoter, we found that all of the mutations, either alone or in combination, reduced expression two- to fourfold in LMH chicken hepatoma cells. The mutations in the HNF1 site and one of the HNF3 sites, when inserted into the intact viral genome, also suppressed virus RNA synthesis in primary hepatocyte cultures. Virus carrying the latter HNF3 mutation was also examined for its ability to infect and replicate in ducks. No significant inhibition of virus replication was observed in a short-term assay; however, virus with the HNF3 mutation was apparently unable to grow in the pancreas, a second site of duck hepatitis B virus replication in the duck.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arceci R. J., King A. A., Simon M. C., Orkin S. H., Wilson D. B. Mouse GATA-4: a retinoic acid-inducible GATA-binding transcription factor expressed in endodermally derived tissues and heart. Mol Cell Biol. 1993 Apr;13(4):2235–2246. doi: 10.1128/mcb.13.4.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aufiero B., Schneider R. J. The hepatitis B virus X-gene product trans-activates both RNA polymerase II and III promoters. EMBO J. 1990 Feb;9(2):497–504. doi: 10.1002/j.1460-2075.1990.tb08136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Levy R., Faktor O., Berger I., Shaul Y. Cellular factors that interact with the hepatitis B virus enhancer. Mol Cell Biol. 1989 Apr;9(4):1804–1809. doi: 10.1128/mcb.9.4.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J., Hauber J., Hauber R., Geiger R., Cullen B. R. Secreted placental alkaline phosphatase: a powerful new quantitative indicator of gene expression in eukaryotic cells. Gene. 1988 Jun 15;66(1):1–10. doi: 10.1016/0378-1119(88)90219-3. [DOI] [PubMed] [Google Scholar]
- Büscher M., Reiser W., Will H., Schaller H. Transcripts and the putative RNA pregenome of duck hepatitis B virus: implications for reverse transcription. Cell. 1985 Mar;40(3):717–724. doi: 10.1016/0092-8674(85)90220-x. [DOI] [PubMed] [Google Scholar]
- Cascio S., Zaret K. S. Hepatocyte differentiation initiates during endodermal-mesenchymal interactions prior to liver formation. Development. 1991 Sep;113(1):217–225. doi: 10.1242/dev.113.1.217. [DOI] [PubMed] [Google Scholar]
- Clayton D. F., Darnell J. E., Jr Changes in liver-specific compared to common gene transcription during primary culture of mouse hepatocytes. Mol Cell Biol. 1983 Sep;3(9):1552–1561. doi: 10.1128/mcb.3.9.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. F., Harrelson A. L., Darnell J. E., Jr Dependence of liver-specific transcription on tissue organization. Mol Cell Biol. 1985 Oct;5(10):2623–2632. doi: 10.1128/mcb.5.10.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condreay L. D., Aldrich C. E., Coates L., Mason W. S., Wu T. T. Efficient duck hepatitis B virus production by an avian liver tumor cell line. J Virol. 1990 Jul;64(7):3249–3258. doi: 10.1128/jvi.64.7.3249-3258.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condreay L. D., Wu T. T., Aldrich C. E., Delaney M. A., Summers J., Seeger C., Mason W. S. Replication of DHBV genomes with mutations at the sites of initiation of minus- and plus-strand DNA synthesis. Virology. 1992 May;188(1):208–216. doi: 10.1016/0042-6822(92)90751-a. [DOI] [PubMed] [Google Scholar]
- Costa R. H., Grayson D. R., Darnell J. E., Jr Multiple hepatocyte-enriched nuclear factors function in the regulation of transthyretin and alpha 1-antitrypsin genes. Mol Cell Biol. 1989 Apr;9(4):1415–1425. doi: 10.1128/mcb.9.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtois G., Morgan J. G., Campbell L. A., Fourel G., Crabtree G. R. Interaction of a liver-specific nuclear factor with the fibrinogen and alpha 1-antitrypsin promoters. Science. 1987 Oct 30;238(4827):688–692. doi: 10.1126/science.3499668. [DOI] [PubMed] [Google Scholar]
- Crescenzo-Chaigne B., Pillot J., Lilienbaum A., Levrero M., Elfassi E. Identification of a strong enhancer element upstream from the pregenomic RNA start site of the duck hepatitis B virus genome. J Virol. 1991 Jul;65(7):3882–3886. doi: 10.1128/jvi.65.7.3882-3886.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPersio C. M., Jackson D. A., Zaret K. S. The extracellular matrix coordinately modulates liver transcription factors and hepatocyte morphology. Mol Cell Biol. 1991 Sep;11(9):4405–4414. doi: 10.1128/mcb.11.9.4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T., Felsenfeld G. The erythroid-specific transcription factor Eryf1: a new finger protein. Cell. 1989 Sep 8;58(5):877–885. doi: 10.1016/0092-8674(89)90940-9. [DOI] [PubMed] [Google Scholar]
- Evans T., Felsenfeld G. trans-Activation of a globin promoter in nonerythroid cells. Mol Cell Biol. 1991 Feb;11(2):843–853. doi: 10.1128/mcb.11.2.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T., Reitman M., Felsenfeld G. An erythrocyte-specific DNA-binding factor recognizes a regulatory sequence common to all chicken globin genes. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5976–5980. doi: 10.1073/pnas.85.16.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor P. D., Sawadogo M., Roeder R. G. The adenovirus major late transcription factor USF is a member of the helix-loop-helix group of regulatory proteins and binds to DNA as a dimer. Genes Dev. 1990 Oct;4(10):1730–1740. doi: 10.1101/gad.4.10.1730. [DOI] [PubMed] [Google Scholar]
- Halpern M. S., Egan J., Mason W. S., England J. M. Viral antigen in endocrine cells of the pancreatic islets and adrenal cortex of Pekin ducks infected with duck hepatitis B virus. Virus Res. 1984;1(3):213–223. doi: 10.1016/0168-1702(84)90040-6. [DOI] [PubMed] [Google Scholar]
- Halpern M. S., Egan J., McMahon S. B., Ewert D. L. Duck hepatitis B virus is tropic for exocrine cells of the pancreas. Virology. 1985 Oct 15;146(1):157–161. doi: 10.1016/0042-6822(85)90064-9. [DOI] [PubMed] [Google Scholar]
- Halpern M. S., England J. M., Deery D. T., Petcu D. J., Mason W. S., Molnar-Kimber K. L. Viral nucleic acid synthesis and antigen accumulation in pancreas and kidney of Pekin ducks infected with duck hepatitis B virus. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4865–4869. doi: 10.1073/pnas.80.15.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern M. S., McMahon S. B., Mason W. S., O'Connell A. P. Viral antigen expression in the pancreas of DHBV-infected embryos and young ducks. Virology. 1986 Apr 15;150(1):276–282. doi: 10.1016/0042-6822(86)90288-6. [DOI] [PubMed] [Google Scholar]
- Hu K. Q., Siddiqui A. Regulation of the hepatitis B virus gene expression by the enhancer element I. Virology. 1991 Apr;181(2):721–726. doi: 10.1016/0042-6822(91)90906-r. [DOI] [PubMed] [Google Scholar]
- Jackson D. A., Rowader K. E., Stevens K., Jiang C., Milos P., Zaret K. S. Modulation of liver-specific transcription by interactions between hepatocyte nuclear factor 3 and nuclear factor 1 binding DNA in close apposition. Mol Cell Biol. 1993 Apr;13(4):2401–2410. doi: 10.1128/mcb.13.4.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilbert A. R., Wu T. T., England J. M., Hall P. M., Carp N. Z., O'Connell A. P., Mason W. S. Rapid resolution of duck hepatitis B virus infections occurs after massive hepatocellular involvement. J Virol. 1992 Mar;66(3):1377–1388. doi: 10.1128/jvi.66.3.1377-1388.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. F., Landschulz W. H., Graves B. J., McKnight S. L. Identification of a rat liver nuclear protein that binds to the enhancer core element of three animal viruses. Genes Dev. 1987 Apr;1(2):133–146. doi: 10.1101/gad.1.2.133. [DOI] [PubMed] [Google Scholar]
- Johnson P. F. Transcriptional activators in hepatocytes. Cell Growth Differ. 1990 Jan;1(1):47–52. [PubMed] [Google Scholar]
- Joulin V., Bories D., Eléouet J. F., Labastie M. C., Chrétien S., Mattéi M. G., Roméo P. H. A T-cell specific TCR delta DNA binding protein is a member of the human GATA family. EMBO J. 1991 Jul;10(7):1809–1816. doi: 10.1002/j.1460-2075.1991.tb07706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley C., Blumberg H., Zon L. I., Evans T. GATA-4 is a novel transcription factor expressed in endocardium of the developing heart. Development. 1993 Jul;118(3):817–827. doi: 10.1242/dev.118.3.817. [DOI] [PubMed] [Google Scholar]
- Ko L. J., Yamamoto M., Leonard M. W., George K. M., Ting P., Engel J. D. Murine and human T-lymphocyte GATA-3 factors mediate transcription through a cis-regulatory element within the human T-cell receptor delta gene enhancer. Mol Cell Biol. 1991 May;11(5):2778–2784. doi: 10.1128/mcb.11.5.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai E., Prezioso V. R., Smith E., Litvin O., Costa R. H., Darnell J. E., Jr HNF-3A, a hepatocyte-enriched transcription factor of novel structure is regulated transcriptionally. Genes Dev. 1990 Aug;4(8):1427–1436. doi: 10.1101/gad.4.8.1427. [DOI] [PubMed] [Google Scholar]
- Lilienbaum A., Crescenzo-Chaigne B., Sall A. A., Pillot J., Elfassi E. Binding of nuclear factors to functional domains of the duck hepatitis B virus enhancer. J Virol. 1993 Oct;67(10):6192–6200. doi: 10.1128/jvi.67.10.6192-6200.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Condreay L. D., Burch J. B., Mason W. Characterization of the core promoter and enhancer of duck hepatitis B virus. Virology. 1991 Sep;184(1):242–252. doi: 10.1016/0042-6822(91)90841-x. [DOI] [PubMed] [Google Scholar]
- Mason W. S., Halpern M. S., England J. M., Seal G., Egan J., Coates L., Aldrich C., Summers J. Experimental transmission of duck hepatitis B virus. Virology. 1983 Dec;131(2):375–384. doi: 10.1016/0042-6822(83)90505-6. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Monaci P., Nicosia A., Cortese R. Two different liver-specific factors stimulate in vitro transcription from the human alpha 1-antitrypsin promoter. EMBO J. 1988 Jul;7(7):2075–2087. doi: 10.1002/j.1460-2075.1988.tb03047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S. H. GATA-binding transcription factors in hematopoietic cells. Blood. 1992 Aug 1;80(3):575–581. [PubMed] [Google Scholar]
- Orkin S. H. Globin gene regulation and switching: circa 1990. Cell. 1990 Nov 16;63(4):665–672. doi: 10.1016/0092-8674(90)90133-y. [DOI] [PubMed] [Google Scholar]
- Patel N. U., Jameel S., Isom H., Siddiqui A. Interactions between nuclear factors and the hepatitis B virus enhancer. J Virol. 1989 Dec;63(12):5293–5301. doi: 10.1128/jvi.63.12.5293-5301.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petcu D. J., Aldrich C. E., Coates L., Taylor J. M., Mason W. S. Suramin inhibits in vitro infection by duck hepatitis B virus, Rous sarcoma virus, and hepatitis delta virus. Virology. 1988 Dec;167(2):385–392. [PubMed] [Google Scholar]
- Pognonec P., Roeder R. G. Recombinant 43-kDa USF binds to DNA and activates transcription in a manner indistinguishable from that of natural 43/44-kDa USF. Mol Cell Biol. 1991 Oct;11(10):5125–5136. doi: 10.1128/mcb.11.10.5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R., Will H. Regulatory sequences of duck hepatitis B virus C gene transcription. J Virol. 1991 Nov;65(11):5693–5701. doi: 10.1128/jvi.65.11.5693-5701.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegrist C. A., Durand B., Emery P., David E., Hearing P., Mach B., Reith W. RFX1 is identical to enhancer factor C and functions as a transactivator of the hepatitis B virus enhancer. Mol Cell Biol. 1993 Oct;13(10):6375–6384. doi: 10.1128/mcb.13.10.6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers J., Smith P. M., Horwich A. L. Hepadnavirus envelope proteins regulate covalently closed circular DNA amplification. J Virol. 1990 Jun;64(6):2819–2824. doi: 10.1128/jvi.64.6.2819-2824.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers J., Smith P. M., Huang M. J., Yu M. S. Morphogenetic and regulatory effects of mutations in the envelope proteins of an avian hepadnavirus. J Virol. 1991 Mar;65(3):1310–1317. doi: 10.1128/jvi.65.3.1310-1317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagawa M., Omata M., Marion P. L. Open reading frames on plus strand genome of duck hepatitis B virus. Gastroenterol Jpn. 1990 Sep;25 (Suppl 2):20–22. doi: 10.1007/BF02779923. [DOI] [PubMed] [Google Scholar]
- Tian J. M., Schibler U. Tissue-specific expression of the gene encoding hepatocyte nuclear factor 1 may involve hepatocyte nuclear factor 4. Genes Dev. 1991 Dec;5(12A):2225–2234. doi: 10.1101/gad.5.12a.2225. [DOI] [PubMed] [Google Scholar]
- Trujillo M. A., Letovsky J., Maguire H. F., Lopez-Cabrera M., Siddiqui A. Functional analysis of a liver-specific enhancer of the hepatitis B virus. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3797–3801. doi: 10.1073/pnas.88.9.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttleman J. S., Pugh J. C., Summers J. W. In vitro experimental infection of primary duck hepatocyte cultures with duck hepatitis B virus. J Virol. 1986 Apr;58(1):17–25. doi: 10.1128/jvi.58.1.17-25.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. Y., Zhou Z. Y., Judd A., Cartwright C. A., Robinson W. S. The hepatitis B virus-encoded transcriptional trans-activator hbx appears to be a novel protein serine/threonine kinase. Cell. 1990 Nov 16;63(4):687–695. doi: 10.1016/0092-8674(90)90135-2. [DOI] [PubMed] [Google Scholar]
- Wu T. T., Condreay L. D., Coates L., Aldrich C., Mason W. Evidence that less-than-full-length pol gene products are functional in hepadnavirus DNA synthesis. J Virol. 1991 May;65(5):2155–2163. doi: 10.1128/jvi.65.5.2155-2163.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee J. K. A liver-specific enhancer in the core promoter region of human hepatitis B virus. Science. 1989 Nov 3;246(4930):658–661. doi: 10.1126/science.2554495. [DOI] [PubMed] [Google Scholar]