Abstract
Humans and monkeys mislocalize targets flashed around the time of a saccade. Here, we present data from three monkeys on a double-step task with a 100 ms target duration. All three subjects mislocalized targets that were flashed around the time of the first saccade, in spite of long intersaccadic intervals. The error was consistently in the direction opposite that of the saccade, and occurred in some cases when the target presentation was entirely presaccadic. This is inconsistent with a theory invoking a damped representation of eye position, but it is consistent with the hypothesis that it is due to an error in peri-saccadic remapping.
Keywords: Saccade, Oculomotor, Monkey, Psychophysics, Localization
1. Introduction
The primate visual system maintains an accurate representation of space despite a constantly moving eye. This accuracy has commonly been attributed to the visual system’s compensation for retinal shifts evoked by eye movements, an idea that was first proposed by von Helmholtz (1963) in 1866, who called the adjusting factor the “sense of effort.” More recent accounts of this compensation were later given by Sperry (1950), who called it “corollary discharge,” and by von Holst and Mittelstaedt (1950), who called it “efference copy.” In both of these accounts, the central idea is that the oculomotor system sends a signal to the visual system when it initiates a movement, and that the change of the retinal position of the visual world is canceled by the change in eye position caused by the motor signal, resulting in a stable visual image. Recently, however, it has become apparent that spatial accuracy is not maintained perfectly in the period immediately surrounding a saccade. The observed patterns of errors can generally be divided into two types. The first is a compression of visual space around the saccade target (Awater, Burr, Lappe, Morrone, & Goldberg, 2005; Awater, Krekelberg, & Lappe, 2000; Burr, Morrone, & Ross, 2001; Honda, 1993; Lappe, Awater, & Krekelberg, 2000; Morrone, Ross, & Burr, 1997; Ross, Morrone, & Burr, 1997) which is predominately parallel to the saccade vector but which has recently been shown to have an orthogonal component, as well (Kaiser & Lappe, 2004). Compression appears to be dependent on the presence of visual references, especially during the post-saccadic interval (Lappe et al., 2000), although it has been observed in total darkness when mislocalization of the saccade target was considered (Awater et al., 2000). The second pattern, which occurs in the absence of visual references, consists of a biphasic shift of the entire visual field, so that targets flashed before or at the beginning of a saccade are mislocalized in the direction of the saccade (pro-directional mislocalization), whereas those flashed later than this are mislocalized in the opposite direction (anti-directional mislocalization) (Dassonville, Schlag, & Schlag-Rey, 1992, 1995; Honda, 1989, 1991; Schlag & Schlag-Rey, 1995; Sogo & Osaka, 2001; Watanabe, Noritake, Maeda, Tachi, & Nishida, 2005). This shift mislocalization is generally seen over a 200 ms interval starting roughly 100 ms before saccade onset (Ross, Morrone, Goldberg, & Burr, 2001), and is manifested across a wide range of tasks, including perceptual localization tasks (Honda, 1989, 1991; Sogo & Osaka, 2001) and eye movement tasks (Boucher, Groh, & Hughes, 2001; Dassonville et al., 1992, Dassonville, Schlag, & Schlag-Rey, 1995; Schlag & Schlag-Rey, 1995). It has also been demonstrated for hand-pointing under certain conditions (Bockisch & Miller, 1999; Sogo & Osaka, 2002; Watanabe et al., 2005). However, Burr et al. (2001) found no errors in pointing once visual references were removed.
The most widely accepted current model of corollary discharge is one in which a continuously varying eye position signal adjusts the retinal signal (Matin, 1976). Because a veridical eye position signal would result in accurate localization, it is assumed that the eye position signal is both anticipatory and damped, so that it begins to move before saccade onset and does not reach its new steady state until after saccade termination. Such an eye position signal, when combined with a veridical retinal signal, would produce the biphasic pattern of errors described above. This damped eye position model has been widely cited (Bockisch & Miller, 1999; Dassonville et al., 1992, 1995; Honda, 1989, 1991; Schlag & Schlag-Rey, 1995; Sogo & Osaka, 2001) to explain the phenomenon of peri-saccadic mislocalization.
A major shortcoming of this model is that it relies on the existence of a continuously varying eye position signal. While there is physiological (Duhamel, Colby, & Goldberg, 1992) and lesion (Sommer & Wurtz, 2002) evidence to support the idea of corollary discharge, and psychophysical evidence suggesting importance of such a signal in peri-saccadic mislocalization (Honda, 1995; Morrone et al., 1997), there is none that specifically supports a continuously-varying eye position signal. The damped eye position model is therefore based on a purely hypothetical neuronal signal. Another hypothesis is that the retinal signal is adjusted by a displacement signal. It is well established that the receptive fields of multiple retinotopically organized areas involved in visual attention and the planning of both eye movements and arm movements, including LIP (Duhamel et al., 1992), FEF (Umeno & Goldberg, 1997), and SC (Walker, Fitzgibbon, & Goldberg, 1995), and the parietal reach region (Snyder, Batista, & Andersen, 2000) begin to shift before the onset of a saccade. This means that neurons with nothing in their receptive fields may begin to respond prior to the saccade, if the saccade will bring a stimulus into the receptive field. These anticipatory responses avoid long visual processing delays and may help to mediate visual stability across saccades. It is therefore reasonable to think that perisaccadic errors of localization may represent a breakdown or even a byproduct of this mechanism. For example, it has been found that during the period when activity is remapped to the post-saccadic receptive field, the neuron is still responsive to stimuli flashed for 100 ms in its current, or pre-saccadic, receptive field (Kusunoki & Goldberg, 2003). This means that a visual target flashed for 100 ms in the immediate presaccadic period can stimulate populations of neurons with non-overlapping receptive fields: those with the stimulus in their pre-saccadic receptive fields, and those with the stimulus in their post-saccadic receptive fields (Kusunoki & Goldberg, 2003). This period of neuronal ambiguity corresponds roughly to the period during which mislocalization is observed. If the average of the retinotopic vectors of these populations is taken as the true target location, it could result in errors of localization similar to those seen experimentally.
Previous mislocalization experiments have typically used target durations of not more than 2 ms (Bockisch & Miller, 1999; Dassonville et al., 1992, 1995; Honda, 1989, 1991; Schlag & Schlag-Rey, 1995; Sogo & Osaka, 2001; Watanabe et al., 2005). Conversely, ambiguous remapping of activity in LIP has been demonstrated using visual stimuli with durations of 100 ms (Kusunoki & Goldberg, 2003). To determine whether peri-saccadic mislocalization would occur under conditions that are known to produce neuronal ambiguity, we carried out a variation of the double-step task on three rhesus monkeys using the 100 ms stimulus duration which was used in the physiological experiments (Kusunoki & Goldberg, 2003). All three subjects consistently mislocalized targets that were flashed around the time of the initial saccade. Surprisingly, the mislocalization we observed was exclusively in the direction opposite that of the first saccade. Furthermore, this anti-directional mislocalization occurred in some cases when the target presentation was entirely pre-saccadic, which directly contradicts the damped eye-position theory. We also found that the mislocalization persists in spite of long intersaccadic intervals, suggesting that the error occurs before the level of motor execution.
2. Methods
2.1. Animal methods
The subjects for this study were two adult male and one adult female rhesus monkeys (Macaca mulatta), weighing between 5 kg and 12 kg. All experimental procedures were approved by the Animal Care and Use Committees of the National Eye Institute, the New York State Psychiatric Institute, and Columbia University in compliance with the Public Health Service Guide for the Care and Use of Laboratory Animals. The monkeys were kept unrestrained in single or paired caging environments. Periodically they were allowed to spend time in a large “playcage” with toys and climbing devices. They were first taught to participate in the pole-and-collar method of transfer from cage to primate chair, and then to sit in a primate chair and accept food and liquid reward. They were then prepared surgically for chronic psychophysical experimentation. Under ketamine/isoflurane general anesthesia using aseptic surgical technique, each monkey was implanted with a plastic head holder for restraint of the head during experimental sessions and subconjunctival eye coils, by which eye position was measured using the magnetic search coil technique (Judge, Richmond, & Chu, 1980). The plastic device was anchored in an acrylic cap, which in turn was connected to titanium screws affixed to the skull. The animals were allowed to recover fully from surgery before any experimentation was performed. Animal weights and health status were carefully monitored, and fluid supplements were given as necessary.
2.2. Behavioral paradigms
Each monkey had controlled access to fluids and received most of its daily fluid intake during behavioral sessions.
Monkeys S and W. The experiment was conducted in a dark room using a projection screen with background luminance <1 cd/m2. During a testing session, the monkey sat in a primate chair facing a tangent screen that was 58 cm away for Monkey S and either 58 cm or 72 cm away for Monkey W, depending on the booth that was used. The positions of stimuli were scaled appropriately for these two distances and Monkey W’s performance did not differ between booths. The monkey’s head was restrained but it was free to move its arms and legs. Visual stimuli were back-projected onto the screen from an LCD projector (Hitachi CP-X275).
The task, a variation of the double-step task (Hallett & Lightstone, 1976), is shown in Fig. 1. The trial began when the monkey fixated the initial fixation point (FP1, white square, 0.1° in width, 180 cd/m2 in luminance), which was presented 12° to the left or right (chosen pseudorandomly before the beginning of each trial) of the center of the screen. At a random time between 950 ms and 1550 ms after the monkey began to fixate it, FP1 would disappear and an identical second fixation point, FP2, would appear in the center of the screen. This was the monkey’s cue to make the first saccade. At some time (varied randomly from trial to trial) between 300 ms before the disappearance of FP1 and 700 ms after it, the target stimulus (red square, 0.5° in width, 25 cd/m2 in luminance) was flashed on-screen for approximately 100 ms. The actual presentation time for the target averaged 99.5 ms, with a standard deviation of 10.9 ms. The target’s horizontal (±18° or ±6°) and vertical (±7°) positions were chosen pseudorandomly at the beginning of each trial. The monkey had to remember the target location but withhold from making a saccade to it until instructed to do so. If the monkey made its initial saccade to the target rather than to FP2, the trial was terminated and the monkey received no reward. FP2 remained illuminated for a total of 1000 ms. After FP2 was extinguished, the monkey had to make a saccade to the remembered location of the target flash. If the monkey’s saccade landed within an acceptable window (±6° vertically and ±6° horizontally) and its eyes remained there for 100 ms, the target reappeared and the monkey received a liquid reward. Each combination of FP1 location and target location, of which there were 16, comprised what will henceforth be referred to as a trial type.
Fig. 1.
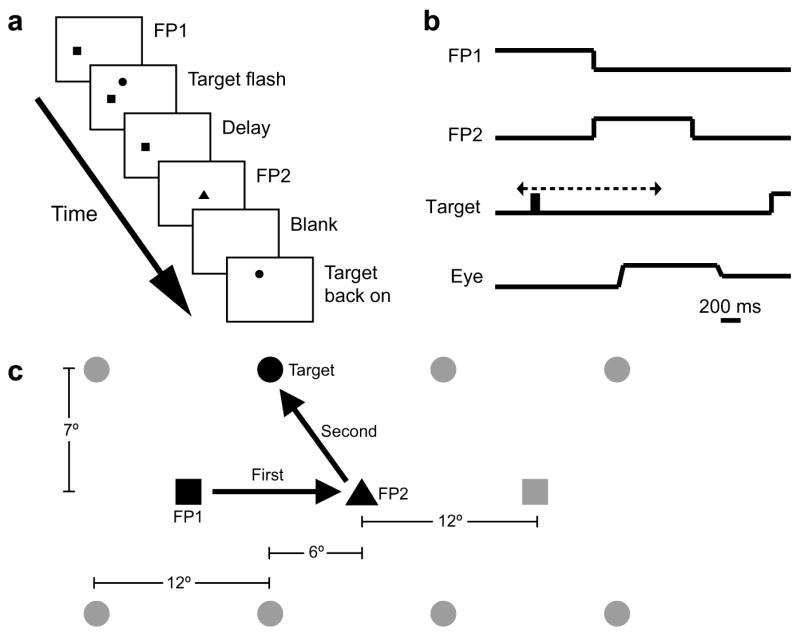
Behavioral task. (a) Double-step task. The trial began when the monkey looked at an eccentrically-located initial fixation point, FP1. At a random time between 950 ms and 1550 ms after the monkey began to fixate it, FP1 disappeared and an identical second fixation point, FP2, appeared in the center of the screen. This was the monkey’s cue to make the first saccade. At some time (varied randomly from trial to trial) between 300 ms before the disappearance of FP1 (shown in b) and 700 ms after it, the target stimulus was flashed on-screen for approximately 100 ms. The monkey continued to fixate FP2 for 1000 ms, at which point it disappeared. He then made a saccade to the remembered location of the target flash. If his saccade fell within the appropriate window and he remained there for 100 ms, the target was turned back on, and he received a liquid reward. For Monkey R, FP2 disappeared as soon as the target had flashed and the monkey had completed the first saccade. (c) Configuration of stimuli for Monkeys S and W. FP1 (illustrated by the squares) appeared at ±12° horizontally and 0° vertically. FP2 (illustrated by the triangle) always appeared at the center of the screen. The target was flashed at −18°, −6°, +6°, or +18° horizontally and ±7° vertically. For Monkey R, FP1 was presented at ±20° horizontally and 0° vertically, FP2 was at the center of the screen, and the target was flashed at ±10° horizontally and +10° vertically. The specific configuration of stimuli on any given trial was chosen pseudorandomly at the beginning of that trial.
Because Monkey S was used to test various aspects of our experimental procedure, including the design of our task, she spent approximately two months training on variations of the double-step task prior to data collection. Monkey W received ten days of training prior to data collection. Prior to this, Monkey W had been trained on a memory-guided saccade task that required a manual response (Krishna, Steenrod, Bisley, Sirotin, & Goldberg, 2006).
Monkey R. The general organization of the experiment was the same for Monkey R as for Monkeys S and W, but with changes in the following parameters. Monkey R sat 57 cm from a tangent screen. Stimuli were dimly projected LED spots positioned by a pair of General Scanning mirror galvanometers controlled by the analog output of the computer. The mirrors had a transit time of 8 ms for 20° movements, and the LEDs were blanked for the duration of the mirror movement. FP1’s location was ±20° horizontally and 0° vertically from the center of the screen. The target location was ±10° horizontally and +10° vertically from the center of the screen. This resulted in a total of four trial types. The target was flashed between 500 ms before and 750 ms after the disappearance of FP1. The eye window was ±9° horizontally and ±9° vertically. Monkey R had to reach and remain within the window for at least 10 ms before receiving a reward. Online saccade detection software as well as post-hoc analysis confirmed that the monkey was not rapidly moving its eye through the window, but made a saccade that terminated within it.
2.3. Data collection
Behavioral paradigms were controlled by a PC running the REX data acquisition system under the QNX real-time operating system (Hays, Richmond, & Optican, 1982). For Monkeys S and W, stimulus presentation times were measured using a photoprobe. This was not necessary for Monkey R, as the LEDs had very short, consistent latencies and the stimulus presentation times registered by REX were therefore reliable. Horizontal and vertical eye position signals were measured using a search coil system and sampled at a rate of 1 kHz. For Monkeys S and W, these signals were low-pass filtered with a cutoff of 120 Hz (Kron-Hite Model 3384) prior to analog-to-digital conversion. Monkeys S and W’s eye position signals were then corrected using an interpolation algorithm. Each day, sixty-four different fixation points arranged in a square matrix were presented to the monkey in a pseudorandom order until roughly five samples had been collected at each point. These data were used to produce a square matrix of local gain and offset values. Each time the eye position was sampled, the four closest points in the matrix were found. These points were weighted by their distance from the measured eye position, then a weighted mean of these gain and offset values was used to convert the measured eye position into an estimate of the true eye position. Uncorrected eye position and behavioral indicators were saved on magnetic disk for off-line analysis, with a sample rate of 1 kHz.
2.4. Data analysis
Data were prepared in a format readable by the MATLAB software package (The MathWorks), and final data analyses were performed using MATLAB. Saccades were detected by using velocity and amplitude threshold criteria and were visually verified by the investigator.
The monkeys had some systematic day-to-day variation in performance, resulting in slightly different mean saccadic endpoints to the same targets on different days. To minimize the effect of this variation we took advantage of the fact that the second saccades in trials with very early and very late flashes could be considered memory-guided saccades with and without an intervening saccade, respectively (i.e. traditional double-step saccades and traditional memory-guided saccades). These saccades were in general quite accurate, and usually had similar mean errors, although there was less variance among trials with very late flashes. Thus, we standardized each day’s data by calculating, for each trial type, the mean horizontal eye position at the end of the second saccade in trials with very late target flashes. We then subtracted this value from the measured horizontal eye position at the end of the second saccade of every trial of the same type collected on the same day. This yielded a standardized measure of horizontal error that could be pooled across sessions. In total, 4 sessions’ worth of data were collected from Monkey R, 9 from Monkey S, and 14 from Monkey W.
For Monkeys S and W, trials in which the second saccade was in the wrong vertical direction were excluded. From the remaining trials, those in which either the horizontal or vertical error of the second saccade was more than 2 standard deviations from the mean for its trial type were also excluded. This resulted in the exclusion of 1098 out of 36806 trials from these two animals. Because of the smaller number of trials from Monkey R (2195 total trials) and because he never made errors in vertical direction, we did not remove outliers from his data.
To determine the peak mislocalization for a given trial type, we first calculated the saccade to stimulus interval (SSI) for every trial, defined as the time from the onset of the initial saccade to the disappearance of the target for the second saccade. The trials were then grouped by SSI into 20 ms bins. The bin with the largest mean error was designated as the peak error bin.
Our initial examination of the data identified four trial types in which large errors were present even with very early target flashes (see Fig. 4 for an example). These were objectively identifiable by the fact that the peak error, relative to either very early or very late target flashes, was less than the difference in error between very early and very late flash target flashes. Except where otherwise noted, these four trial types were excluded from further analysis.
Fig. 4.
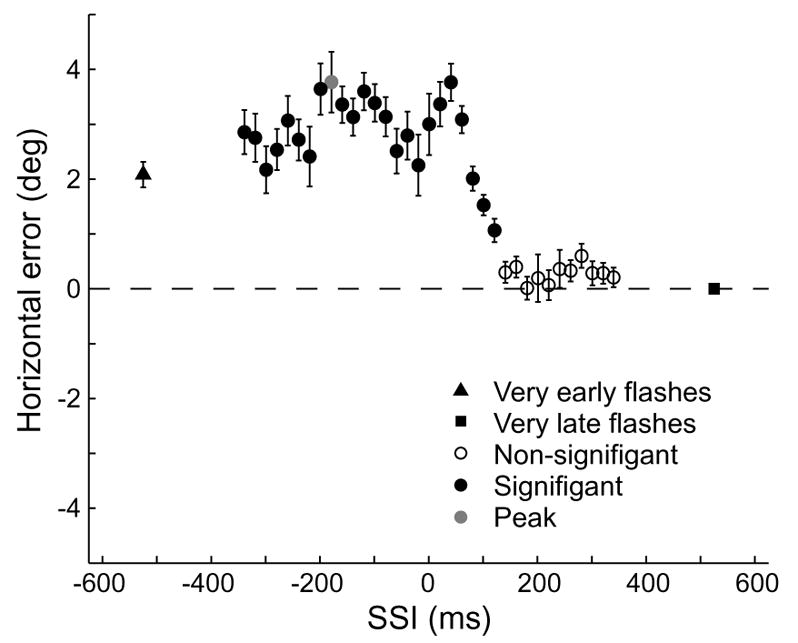
Trial types in which the typical pattern of mislocalization was not observed. Although there is significant error in the peri-saccadic period, it also extends backwards to include very early target flash times. This pattern was most commonly seen in Monkey W, who received less extensive training on the task.
3. Results
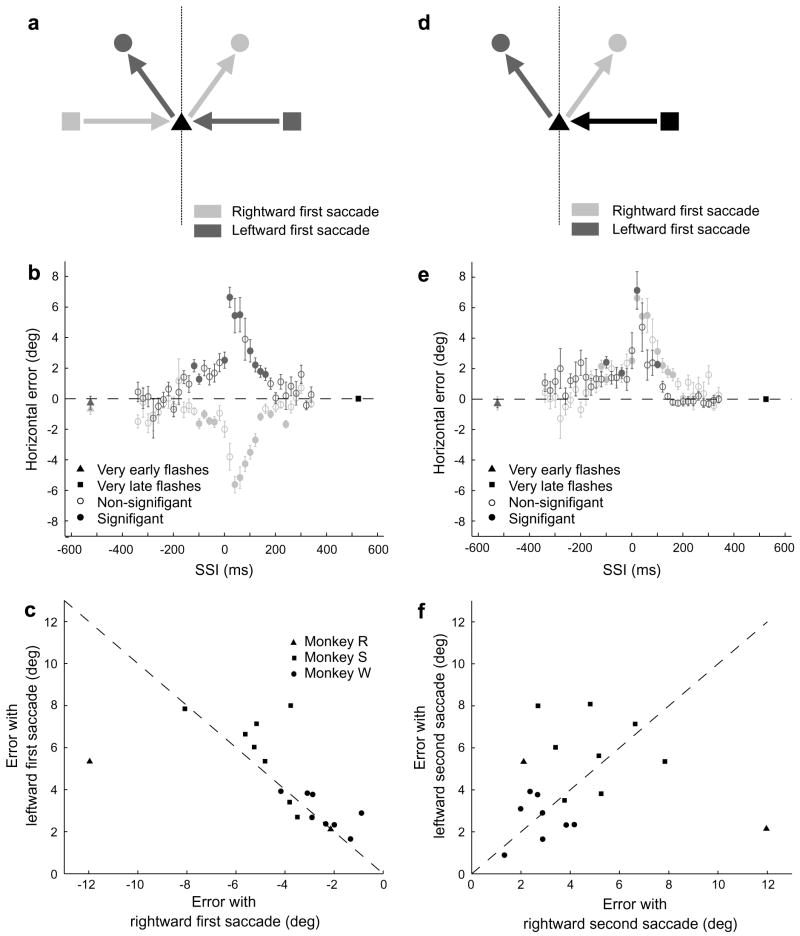
Monkeys consistently mislocalized a target when it was flashed around the time of saccade onset. A typical result can be seen in Fig. 2a, which shows saccadic error on individual trials plotted as a function of saccade-stimulus interval (SSI), the interval between the beginning of the initial saccade and the time that the target disappeared. A negative SSI implies that the target was only present before the saccade. Because the target was present for 100 ms on average, an SSI of 100 ms implies that the target appeared as the saccade began, an SSI greater than 120 ms implies that 95% of the time the target appeared after the saccade began, and an SSI greater than 170 ms implies that the target appeared after the saccade ended. In this example Monkey S made an initial rightward saccade, and the target was flashed at −18° horizontally and −7° vertically. The error in this case was to the left, in the direction opposite that of the first saccade (anti-directional mislocalization). Fig. 2b shows the same data grouped into 20 ms bins. The direction of the first saccade determined the direction of mislocalization. The set of all possible stimulus locations in our experiment was symmetrical about the vertical mid-line (see Fig. 1c), so that reflecting the stimulus configuration of any one of our trial types (Fig. 3a, light grey) across that line yielded another of our trial types (Fig. 3a, dark grey). Trial types that were paired in this way tended to produce a similar pattern of error, but in opposite directions, within a given subject. An example of one such pairing is shown in Fig. 3b, taken from Monkey S. The data in light grey are from trials in which the monkey made an initial rightward saccade and in which the target was flashed at −6° horizontally and +7° vertically. For those in dark grey the first saccade was leftward, and the target was flashed at +6° horizontally and +7° vertically. Without exception, trial types that began with a rightward saccade produced leftward mislocalization, whereas those that began with a leftward saccade produced rightward mislocalization. When we compared peak errors from every paired trial type in all subjects (Fig. 3c), we found a significant correlation between pairs (slope = −0.50, 95% CI −0.82 to −0.17; R2 = 0.40). If the single point from Monkey R at (−12°, 5°) is excluded, the slope improves to −0.97 (95% CI −1.344 to −0.6036) and the R2 improves to 0.68. Note that every trial type was included in this analysis.
Fig. 2.
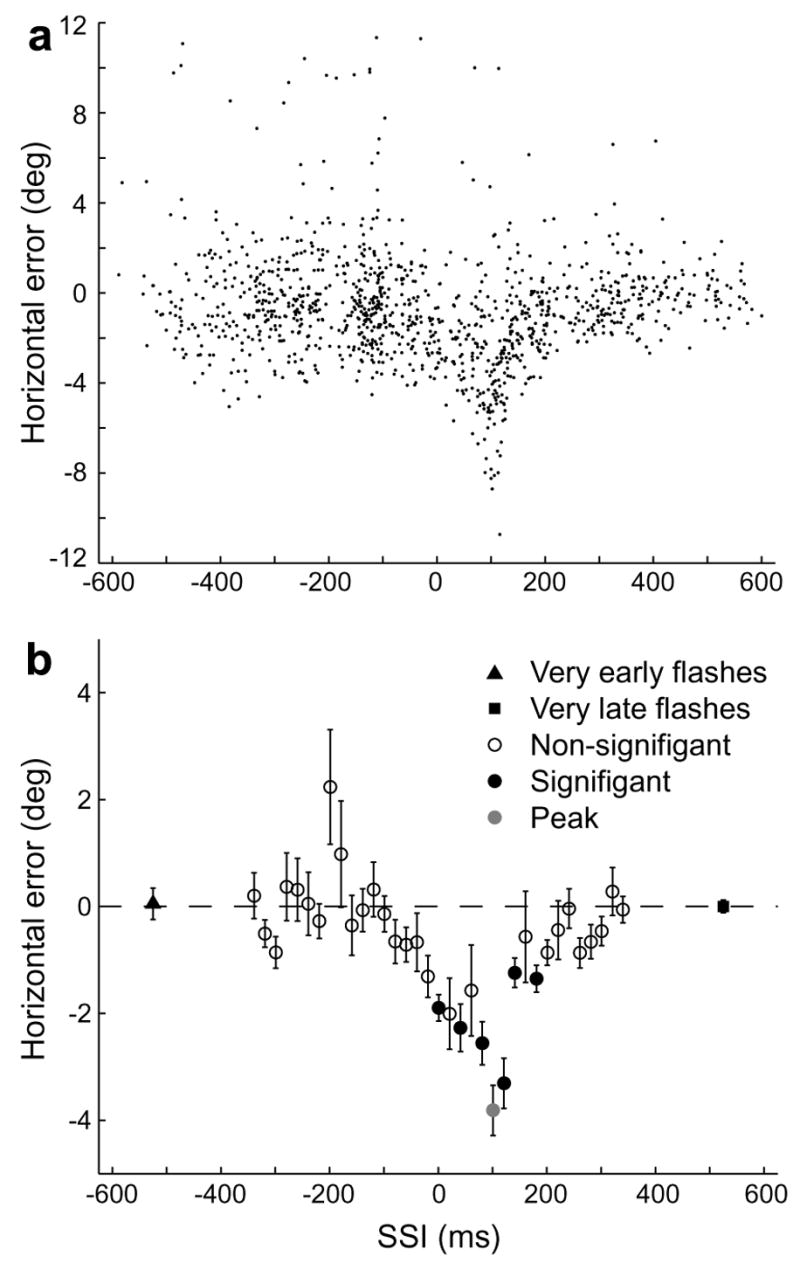
Performance by an animal on a single trial type. (a) Horizontal errors at the end of the second saccade are plotted against the saccade to stimulus interval (SSI), defined as the time from the onset of the first saccade until the disappearance of the target. In this example FP1 was at −12° horizontally, and the target was flashed at −18 horizontally and −7° vertically. (b) Binned error. The data from A are shown grouped by SSI into 20 ms bins, with the value of each bin being equal to the mean error of the individual trials in that bin. Bars indicate SEMs. Filled circles represent a significant difference from the error in trials with very late target flashes (MATLAB’s t-test of samples from populations with unequal variances, p < 0.05, Bonferroni-corrected). The bin with the largest error (peak error bin) is shown in grey. Negative horizontal errors represent errors to the left.
Fig. 3.
Comparison of error in trial types with symmetrical stimulus configurations. (a) Diagram of the spatial relationship between paired trial types. In trial types with a rightward first saccade (light grey), the animal makes an initial saccade from the FP1 location (square) on the left to FP2 (black triangle), and then to the target (circle), which in this example is on the upper right. Its symmetrical partner (dark grey) can be produced by reflecting the stimulus positions across the dotted vertical line. (b) Performance of one animal on two trial types paired in the manner diagrammed in a. Negative horizontal errors represent errors to the left. As in a, light grey represents a trial type with a rightward first saccade, and dark grey represents a trial type with a leftward first saccade. Note both the similarity in the magnitude of error and the overall symmetrical shape of the curves. (c) Plot of all pairs in all three animals. Most pairs are nearly equivalent in magnitude. In every case, the error is in the direction opposite the saccade (anti-directional). (d) Spatial relationship between trial types paired according to second saccade direction. In these pairings, the FP1 location was the same within a pair, but the target locations were symmetrical across the vertical midline. (e) Performance of one animal on two trial types paired in the manner diagrammed in a. Although the magnitude of error was similar for both members of this pair, that was not always the case. (f) Plot of all pairs in all three animals. There is no correlation of the magnitude of errors between pairs.
The direction of the second saccade had no impact on either the magnitude or direction of error. This is suggested by the fact that the mislocalization was always in the direction opposite that of the first saccade, regardless of the second saccade’s direction. When we paired trial types whose FP1 location was the same and whose target locations were reflections of one another across the vertical midline (Fig. 3d), we found that the pair always produced errors in the same direction (Fig. 3f). The example in Fig. 3e compares two trial types from Monkey S, both of which began with a leftward saccade. Although the target was flashed at +6° horizontally and +7° vertically for the light grey data points and at −6° horizontally and +7° vertically for the dark grey, a rightward mislocalization was present in both cases. Although the magnitude of error was approximately equal between these two cases, this was not the case for all pairs. When we compared peak errors, corrected for the expected direction of error, from every paired trial type across subjects (Fig. 3f), we found no significant correlation between pairs (slope = −0.08, 95% CI −0.37 to 0.52; R2 = 0.008). As above, we included every trial type in this analysis. Even when the single point from Monkey R at (12°, 2°) was excluded, the correlation was not significant (slope = 0.53, 95% CI −0.09 to 1.15; R2 = 0.18).
Although in most cases there was a strong, sustained peri-saccadic mislocalization, two out of the three monkeys failed to produce this pattern in a small number of trial types. In four of the thirty-six trial types, the error extended backwards in time to include trials with very early target flashes. An example of this pattern can be seen in Fig. 4, taken from Monkey W. In this trial type, FP1 was located at 12° horizontally and 0° vertically and the target was flashed at −18° horizontally and −7° vertically. Monkey W produced this pattern in three of the four trial types with the largest eccentricities. Otherwise, there was no pattern to the trial types that were excluded, either within or between subjects.
Errors of the targeting saccade did not result from errors of the initial saccade. Fig. 5 shows the initial fixations and the first and second saccade endpoints for the data in Fig. 2. Saccades to targets that were flashed very early (SSIs < −350 ms, black) or very late (SSIs > 350 ms, light grey), which were accurate, are compared to saccades to targets flashed around the time of peak error (SSIs 91–111 ms, red). Panel a shows the results from individual trials; the mean values are presented in panel b. The fixations and the endpoints of the first saccades overlap, but there is a significant overshoot for the second saccades to stimuli that appeared closer in time to the first saccades. There was no significant error in the first saccade in any of the thirty-two trial types included in subsequent analyses.
Fig. 5.
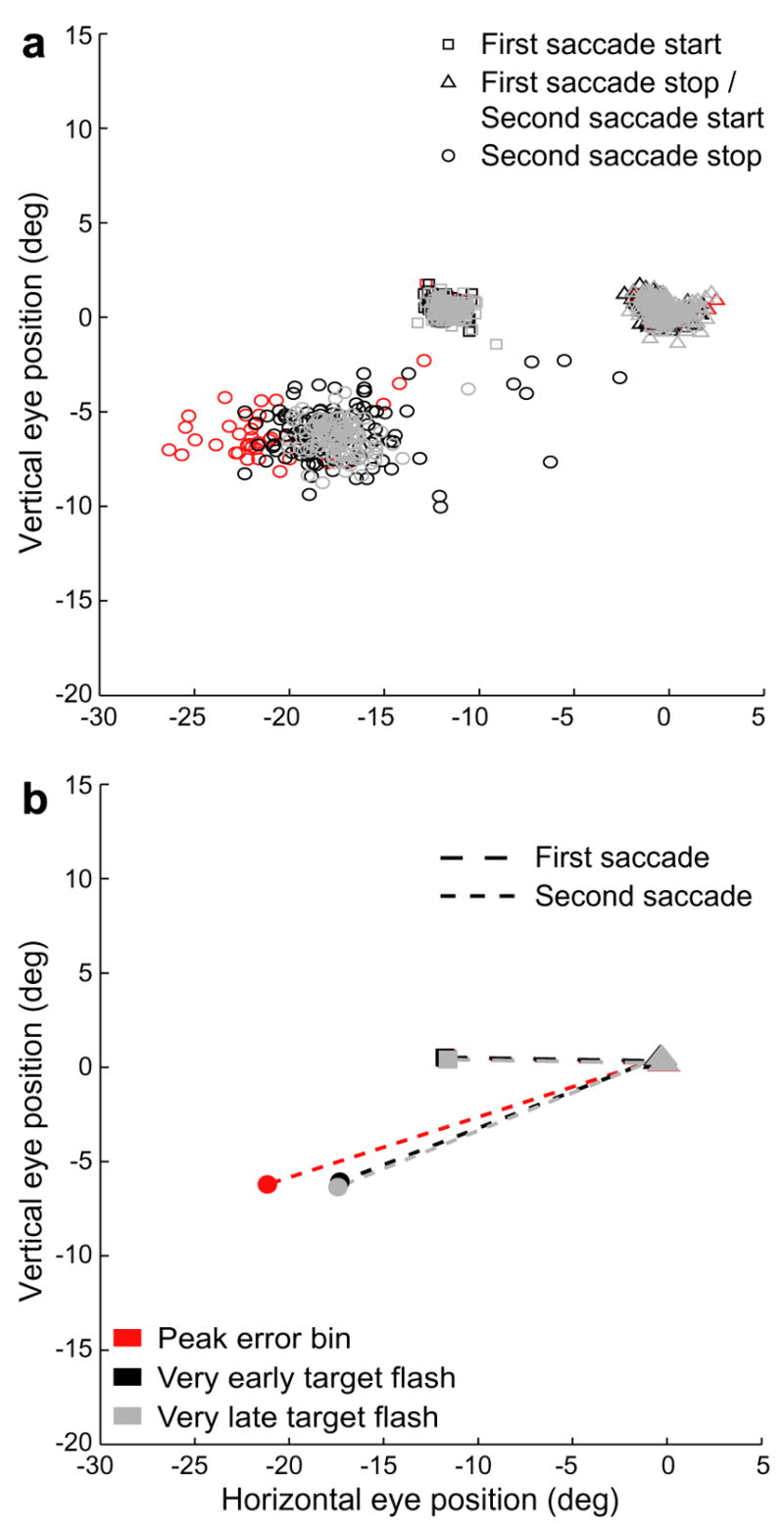
Comparison of saccadic endpoints in the peak error bin with those of trials with very early and very late target flashes. (a) Eye positions at the beginning (squares) and end (triangles) of the first saccade and the beginning (triangles) and end (circles) of the second saccade for the trial type shown in Fig. 2. Trials in which the target was flashed very early or very late are shown in black and light grey, respectively. Trials from the peak error bin are shown in red. (b) Sample means for the data shown in a. Error bars, representing SEMs of the horizontal position, are smaller than the symbols representing the means.
Although peak errors tended to occur with positive SSIs, some error was present even when targets were flashed entirely before the saccade. For each of the 32 trial types that met our criteria, we compared the mean horizontal error from trials in which the target was flashed immediately prior to the saccade (SSIs of −65 ms to −15 ms) with that from trials with very early target flashes (Fig. 6a). To facilitate comparison between trial types, we defined all errors in the same direction as the first saccade as negative, while those in the opposite direction were considered positive. The error in the immediate pre-saccadic period was consistently greater than that seen with very early target flashes (p ≪ 0.001, Wilcoxon rank-sum test), showing that for almost all trial types, there was some anti-directional mislocalization prior to the beginning of the saccade. This difference was not due to errors in the first saccade. When we compared first saccade errors from the same two sets of trials (Fig. 6b), we found that in only two cases were the errors from the immediately pre-saccadic interval significantly greater than those from the very early flash interval for the same trial type. Furthermore, there was no overall difference in the degree of error between the groups (p = 0.37, Wilcoxon rank-sum test).
Fig. 6.
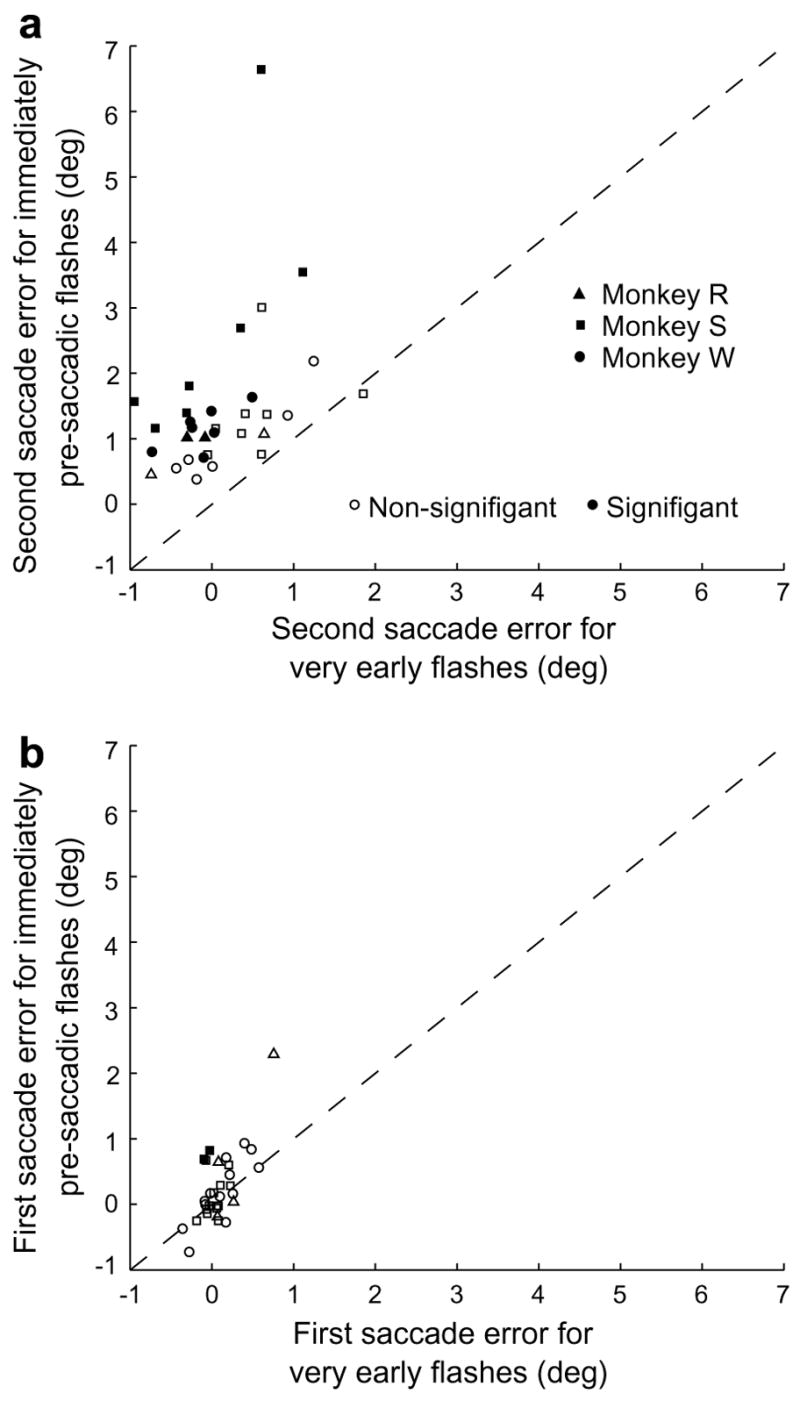
Anti-directional errors occurred for pre-saccadic target flashes. (a) Comparison of second saccade error in the immediate pre-saccadic period with that in trials with very early target flashes. The mean error in the immediate pre-saccadic period (SSIs −65 ms to −15 ms) is plotted against the mean error in trials with very early target flashes. Each data point represents a single animal’s performance on a single trial type, with thirty-two of the thirty-six trial types shown. Positive values represent errors in the direction opposite the first saccade. (b) Comparison of first saccade error in the immediate pre-saccadic period with that in trials with very early target flashes. Data points are from the same trial types as in panel a. Positive values represent hypometric first saccades.
3.1. Robustness to variation of inter-saccadic interval
Once a briefly flashed target was localized, the localization was held in memory in a stable fashion, regardless of interval between the target flash and the saccade to the spatial location of the vanished stimulus. Our data were obtained across a wide range of inter-saccadic intervals (ISIs). Monkey R was allowed to make the second saccade as soon as (1) he had completed the initial saccade and (2) the target had flashed (ISI mean 371 ms, SD 77 ms). Monkeys S and W were forced to wait at FP2 until it had been presented for the full 1000 ms giving mean ± SD ISIs of 989 ± 46 and 908 ± 43, respectively. There were no qualitative differences between the monkeys in terms of either the timing (Fig. 2b, Fig. 6a) or direction (Fig. 5b) of errors. This suggests that the mislocalization does not result from some error in the motor effector, but rather from either a perceptual error or an error in motor planning.
4. Discussion
Humans and monkeys can make accurate saccades to stimuli flashed before an intervening saccade. This finding has been used to argue that the oculomotor system can update its representation of a visual stimulus to compensate for a change in eye position. This compensation is imperfect, especially when the saccade goal flashes briefly (for 1 or 2 ms) around the time of the saccade itself (Dassonville et al., 1992, 1995; Honda, 1989, 1991, 1993, 1999; Schlag & Schlag-Rey, 1995; Sogo & Osaka, 2001; Watanabe et al., 2005). In previous studies, targets flashed before the saccade were mislocalized in the direction of the saccade, whereas those flashed immediately after the saccade were mislocalized in the opposite direction. In our experiments, we used a flashed target that remained on the screen for 100 ms. We found that the monkey mislocalized the target in the direction opposite that of the saccade for the entire perisaccadic epoch. This mislocalization was stable, and depended only on when the stimulus appeared relative to the intervening saccade, not when the monkey actually made the saccade to the target. We will discuss these findings in relation to other studies of peri-saccadic targeting and visual perception, to the effects of saccades on visually responsive neurons in the monkey brain, and to current models of peri-saccadic mislocalization.
The idea that the location of an object in space can be calculated by adding together the retinal position of the object with an estimate of the eye’s position arising from the motor command was first proposed by von Helmholtz (1963) in 1866. Von Helmholtz originally attributed this estimate to an “effort of will,” suggesting a role for the oculomotor system in its generation, and both Sperry (1950), using the term “corollary discharge”, and von Holst and Mittelstaedt (1950), who called it “efference copy”, followed suit. Because objects are localized accurately under normal conditions, it was initially assumed that the eye position signal was veridical. However, the finding that targets flashed around the time of saccades were systematically mislocalized (Matin & Pearce, 1965) forced a revision of this model. The damped eye position model (Matin, 1976) was an attempt to incorporate the finding of peri-saccadic mislocalization in terms of a corollary discharge theory. As the name suggests, it replaces the veridical eye position signal with one that is both anticipatory and damped. The time courses of this signal and the retinal signal are depicted in Fig. 7a. The eye position signal begins to change early, before saccade onset, but it does not reach its new steady state until well after the saccade has ended. Conversely, the retinal signal is veridical, following the same time course as the saccade itself; it is therefore equal in magnitude and opposite in direction to the true eye position. This provides us with a simplified framework for understanding the mislocalization: since the value of the eye position signal required to cancel the retinal signal at any given time is equal to the true eye position at that time, the mislocalization at any given time is equal to the difference between the eye position signal and the true eye position. This can be seen in Fig. 7b, which shows a schematic representation of the eye with the relevant vectors at each of the four time points shown in panel a. For very early or very late target flashes (1 and 4, respectively), the eye position signal equals the true eye position and accordingly, there is no mislocalization. However, at time point 2, when the eye position signal leads the true eye position by an angle θpro, target flashes are mislocalized in the direction of the saccade by the same angular magnitude. At time point 3, after the saccade has started and the eye position signal trails the true eye position by θanti, target flashes are mislocalized by θanti in the direction opposite that of the saccade.
Fig. 7.
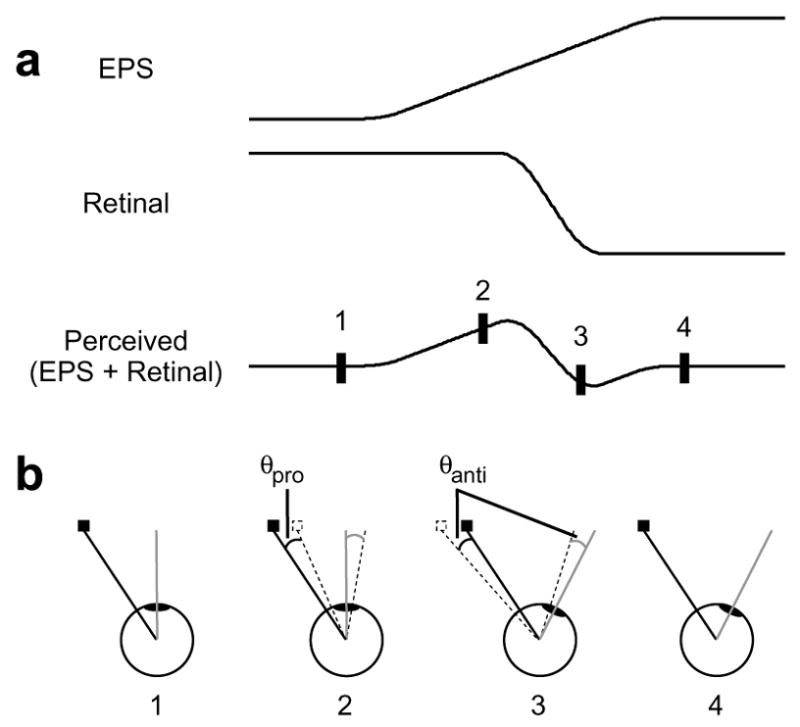
Damped eye position model of mislocalization. (a) Traditional representation. According to the model, the perceived location of a target is calculated by adding together the eye position signal (EPS) and the retinal signal. (b) Modified representation. The eye and the relevant vectors are shown for the four time points denoted in panel a. The true location of the flashed target and its retinal signal are represented by the solid square and the solid black line, respectively, and the subjective location and retinal vector are represented by the dotted silhouette and the dotted black line, respectively. The solid grey line represents the eye’s true bearing, and the dotted grey line is the bearing indicated by a damped eye position signal. Because the retinal signal is always veridical, it will exactly oppose the true bearing. The magnitude and direction of error therefore always correspond to the difference between the true bearing and the eye position signal (θpro) or (θanti).
Our results are incompatible with the damped eye position theory because all of the mislocalization was in the direction opposite that of the saccade. This was true even for targets that were flashed in the period immediately before saccade onset (Fig. 6). According to the damped eye position theory, the eye position signal should have been leading the true eye position in this period, causing mislocalization in the direction of the saccade. Anti-directional localization errors that occur after saccade onset can be explained by the damped eye position signal being exceeded in magnitude by the veridical retinal signal. However, this cannot be the case when they occur before saccade onset, since a veridical retinal signal would not have changed at this point. A damped eye position theory would require that the eye position signal must make an initial move in the direction opposite that of the saccade before changing course in mid-flight and reaching the correct new steady state. An anti-direction eye position signal does not seem physiologically plausible, and we are aware of no other data that support it.
A number of authors have addressed the importance of afferent delays in the visual system to localization (Boucher et al., 2001; Schlag & Schlag-Rey, 2002). However, afferent delays do not affect the value of the retinal signal, only the time at which the eye position signal is sampled. Pola (2004) has noted that a target flashed in the real world for a few ms may have a retinal persistence that is considerably greater than the lifetime of the target, but the models he proposes do not predict antidirectional errors in the pre-saccadic period.
It should also be noted that the effect demonstrated in the present experiment is distinct from compression mislocalization. With compression mislocalization, targets beyond the saccade goal are mislocalized in the direction opposite that of the saccade, but targets proximal to the saccade goal are mislocalized in the saccade direction. In the current experiment, all targets were mislocalized in the direction opposite that of the saccade goal; their position relative to the saccade target had no effect. This is more consistent with shift mislocalization.
As an alternative to the damped eye position theory, we propose that shift mislocalization may be related to the anticipatory remapping of receptive fields that occurs in multiple brain areas around the time of a saccade. LIP (Duhamel et al., 1992), FEF (Umeno & Goldberg, 1997), SC (Walker et al., 1995), and PRR (Snyder et al., 2000) all maintain retinotopic representations of space that are remapped in an anticipatory fashion around the time of a saccade. It has been shown in LIP that while targets flashed well in advance of a saccade are remapped accurately, targets flashed near the time of saccade onset elicit post-saccadic responses in two populations of neurons: those whose receptive fields include the location of the target flash pre-saccadically and those whose receptive fields will include the location of the target flash post-saccadically (Kusunoki & Goldberg, 2003). This period of neuronal ambiguity corresponds roughly to the period during which errors of localization are observed. Although LIP normally operates in a winner-take-all fashion to determine the focus of attention (Bisley & Goldberg, 2003) or the goal of an upcoming saccade (Ipata, Gee, Goldberg, & Bisley, 2006), ambiguous remapping represents a special case in which multiple location signals must be mapped onto a single object. An analogous situation can be produced experimentally by direct stimulation of cortex. Dual stimulation of either FEF (Robinson & Fuchs, 1969) or SC (Kustov & Robinson, 1995), both of which are interconnected with LIP and which also undergo remapping, results in averaging saccades. If a similar averaging process occurs with ambiguous remapping, it could result in the pattern of errors seen in mislocalization experiments. Note that this explanation, in contrast to damped eye position theory, does not depend on the exact nature of a corollary discharge signal (discrete versus continuous); all that matters is that there is activity in multiple populations of retinotopically mapped neurons. An advantage to this explanation is that because multiple brain regions demonstrate anticipatory remapping, it can be adapted to explain shift mislocalization across effectors.
Previous studies of peri-saccadic mislocalization have used human subjects almost exclusively, which raises the possibility that our results are simply due to interspecies differences. However, Dassonville et al. (1992) compared the performance of a monkey (Macaca nemestrina) to that of four human subjects on a modified double-step task and found no qualitative differences in the pattern of mislocalization. The monkey, like the humans, mislocalized targets that were flashed just before saccade onset in the direction of the saccade and mislocalized targets that were flashed post-saccadically in the opposite direction. It is also possible that the forced pause between saccades for Monkeys S and W altered the results for these animals. However, Monkey R was not forced to wait between saccades but still showed anti-directional mislocalization for presaccadic target flashes.
Another possible reason for the differing results between this and previous experiments is the presence of visual cues. Dassonville et al. (1995) have noted that the presence of visual cues led to a reduction in the magnitude of shift mislocalization. However, the only qualitative change in mislocalization that has been shown to depend on visual cues is the presence or absence of compression (Lappe et al., 2000). Although visual references, in the form of objects in the room and FP2, were available during the experiment, we did not observe compression parallel to the saccade.
The long duration of the target flashes used in this experiment might also have contributed to the effects seen here. Our experiment employed a flash duration of 100 ms, as opposed to the flash duration of 2 ms or less used in most previous studies (Dassonville et al., 1992, 1995; Honda, 1989, 1991, 1999; Schlag & Schlag-Rey, 1995; Sogo & Osaka, 2001; Watanabe et al., 2005). A number of studies have examined mislocalization with longer stimulus presentations (Honda, 2006; Schlag & Schlag-Rey, 1995; Watanabe et al., 2005). These studies have shown that a long-duration stimulus can be localized accurately under the same conditions that produce mislocalization of a stimulus that is briefly presented. These results account for our continued perception of a stable world, despite making multiple saccades per second. However, Watanabe et al. (2005) found that when they presented a 100 ms duration stimulus, they observed only pro-directional mislocalization. The reasons for this are unclear, though it may be related to their use of a flickering stimulus, as opposed to our continuously lit stimulus. Although a 500 Hz flicker such as the one used in their experiment is above the threshold for flicker-fusion, it can be differenti-ated from a continuously lit stimulus intra-saccadically, both by the appearance of a phantom array, in contrast to a smear (Hershberger, 1987), and by its greater perceived length (Noritake, Kazai, Terao, & Yagi, 2005). It is therefore possible that there is a difference in the way these two stimuli are processed extra-retinally.
Here we have presented evidence that errors of localization do occur under the same conditions that produce ambiguous remapping in LIP. These errors cannot be explained by a damped eye position theory, and we propose that they may result from the ambiguous remapping itself. In order to develop a coherent model, future experiments should detail the remapping of LIP and related areas. One question of particular relevance is whether ambiguous neuronal responses can be induced with briefly flashed stimuli. Additionally, ambiguous remapping has only been demonstrated in LIP; it should be determined whether this phenomenon occurs in other retinotopically-mapped regions.
Acknowledgments
These experiments began preliminarily at the Laboratory of Sensorimotor Research of the National Eye Institute (monkey R). We are grateful to the staff of the National Eye Institute for assistance in the early phases of these experiments: Drs. James Raber and Ginger Tansey for veterinary care; Dr. John McClurkin for display programming; Thomas Ruffner and Altah Nichols for machining; Lee Jensen for electronics; Art Hays for computer systems; Brian Keegan for technical assistance; and Becky Harvey and Jean Steinberg for facilitating everything. The experiments were completed at the Keck-Mahoney Center for Brain and Behavior at Columbia University, where they were supported by grants from the James S. McDonnell Foundation, the United States National Eye Institute, (1 R01 EY014978-01 and 1 R24 EY015634-01), and the Whitehall, Keck, Dana, and Kavli foundations. We are grateful to G. Duncan for electronic and computer support, to Y. Pavlova for dedicated animal maintenance, to Drs. M. Osman and G. Asfaw for veterinary care, and to L. Palmer for facilitating everything.
References
- Awater H, Burr D, Lappe M, Morrone MC, Goldberg ME. Effect of saccadic adaptation on localization of visual targets. Journal of Neurophysiology. 2005;93(6):3605–3614. doi: 10.1152/jn.01013.2003. [DOI] [PubMed] [Google Scholar]
- Awater H, Krekelberg B, Lappe M. Peri-saccadic visual stimulation changesperceived target positions. Perception. 2000;29(Suppl):8. [Google Scholar]
- Bisley JW, Goldberg ME. Neuronal activity in the lateral intraparietal area and spatial attention. Science. 2003;299(5603):81–86. doi: 10.1126/science.1077395. [DOI] [PubMed] [Google Scholar]
- Bockisch CJ, Miller JM. Different motor systems use similar damped extraretinal eye position information. Vision Research. 1999;39(5):1025–1038. doi: 10.1016/s0042-6989(98)00205-3. [DOI] [PubMed] [Google Scholar]
- Boucher L, Groh JM, Hughes HC. Afferent delays and the mislocalization of perisaccadic stimuli. Vision Research. 2001;41(20):2631–2644. doi: 10.1016/s0042-6989(01)00156-0. [DOI] [PubMed] [Google Scholar]
- Burr DC, Morrone MC, Ross J. Separate visual representations for perception and action revealed by saccadic eye movements. Current Biology. 2001;11(10):798–802. doi: 10.1016/s0960-9822(01)00183-x. [DOI] [PubMed] [Google Scholar]
- Dassonville P, Schlag J, Schlag-Rey M. Oculomotor localization relies on a damped representation of saccadic eye displacement in human and nonhuman primates. Visual Neuroscience. 1992;9(3–4):261–269. doi: 10.1017/s0952523800010671. [DOI] [PubMed] [Google Scholar]
- Dassonville P, Schlag J, Schlag-Rey M. The use of egocentric and exocentric location cues in saccadic programming. Vision Research. 1995;35(15):2191–2199. doi: 10.1016/0042-6989(94)00317-3. [DOI] [PubMed] [Google Scholar]
- Duhamel JR, Colby CL, Goldberg ME. The updating of the representation of visual space in parietal cortex by intended eye movements. Science. 1992;255(5040):90–92. doi: 10.1126/science.1553535. [DOI] [PubMed] [Google Scholar]
- Hallett PE, Lightstone AD. Saccadic eye movements towards stimuli triggered by prior saccades. Vision Research. 1976;16(1):99–106. doi: 10.1016/0042-6989(76)90083-3. [DOI] [PubMed] [Google Scholar]
- Hays AV, Richmond BJ, Optican LM. A UNIX-based multiple process system for real-time data acquisition and control. WESCON. 1982;2:1–10. [Google Scholar]
- Hershberger W. Saccadic eye movements and the perception of visual direction. Perception Psychophysics. 1987;41(1):35–44. doi: 10.3758/bf03208211. [DOI] [PubMed] [Google Scholar]
- Honda H. Perceptual localization of visual stimuli flashed during saccades. Perception Psychophysics. 1989;45(2):162–174. doi: 10.3758/bf03208051. [DOI] [PubMed] [Google Scholar]
- Honda H. The time courses of visual mislocalization and of extraretinal eye position signals at the time of vertical saccades. Vision Research. 1991;31(11):1915–1921. doi: 10.1016/0042-6989(91)90186-9. [DOI] [PubMed] [Google Scholar]
- Honda H. Saccade-contingent displacement of the apparent position of visual stimuli flashed on a dimly illuminated structured background. Vision Research. 1993;33(5–6):709–716. doi: 10.1016/0042-6989(93)90190-8. [DOI] [PubMed] [Google Scholar]
- Honda H. Visual mislocalization produced by a rapid image displacement on the retina: examination by means of dichoptic presentation of a target and its background scene. Vision Research. 1995;35(21):3021–3028. doi: 10.1016/0042-6989(95)00108-c. [DOI] [PubMed] [Google Scholar]
- Honda H. Modification of saccade-contingent visual mislocalization by the presence of a visual frame of reference. Vision Research. 1999;39(1):51–57. doi: 10.1016/s0042-6989(98)00134-5. [DOI] [PubMed] [Google Scholar]
- Honda H. Achievement of transsaccadic visual stability using presaccadic and postsaccadic visual information. Vision Research. 2006;46(20):3483–3493. doi: 10.1016/j.visres.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Ipata AE, Gee AL, Goldberg ME, Bisley JW. Activity in the lateral intraparietal area predicts the goal and latency of saccades in a free-viewing visual search task. Journal of Neuroscience. 2006;26(14):3656–3661. doi: 10.1523/JNEUROSCI.5074-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Research. 1980;20(6):535–538. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
- Kaiser M, Lappe M. Perisaccadic mislocalization orthogonal to saccade direction. Neuron. 2004;41(2):293–300. doi: 10.1016/s0896-6273(03)00849-3. [DOI] [PubMed] [Google Scholar]
- Krishna BS, Steenrod SC, Bisley JW, Sirotin YB, Goldberg ME. Reaction times of manual responses to a visual stimulus at the goal of a planned memory-guided saccade in the monkey. Experimental Brain Research. 2006;173(1):102–114. doi: 10.1007/s00221-006-0370-5. [DOI] [PubMed] [Google Scholar]
- Kustov AA, Robinson DL. Modified saccades evoked by stimulation of the macaque superior colliculus account for properties of the resettable integrator. Journal of Neurophysiology. 1995;73(4):1724–1728. doi: 10.1152/jn.1995.73.4.1724. [DOI] [PubMed] [Google Scholar]
- Kusunoki M, Goldberg ME. The time course of perisaccadic receptive field shifts in the lateral intraparietal area of the monkey. Journal of Neurophysiology. 2003;89(3):1519–1527. doi: 10.1152/jn.00519.2002. [DOI] [PubMed] [Google Scholar]
- Lappe M, Awater H, Krekelberg B. Postsaccadic visual references generate presaccadic compression of space. Nature. 2000;403(6772):892–895. doi: 10.1038/35002588. [DOI] [PubMed] [Google Scholar]
- Matin L. Saccades and extraretinal signals for visual direction. In: Monty A, Senders JW, editors. Eye movements and psychological processes. New York: Erlbaum; 1976. pp. 205–219. [Google Scholar]
- Matin L, Pearce DG. Visual perception of direction for stimuli flashed during voluntary saccadic eye movement. Science. 1965;148:1485–1488. doi: 10.1126/science.148.3676.1485. [DOI] [PubMed] [Google Scholar]
- Morrone MC, Ross J, Burr DC. Apparent position of visual targets during real and simulated saccadic eye movements. Journal of Neuroscience. 1997;17(20):7941–7953. doi: 10.1523/JNEUROSCI.17-20-07941.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noritake A, Kazai K, Terao M, Yagi A. A continuously lit stimulus is perceived to be shorter than a flickering stimulus during a saccade. Spatial Vision. 2005;18(3):297–316. doi: 10.1163/1568568054089384. [DOI] [PubMed] [Google Scholar]
- Pola J. Models of the mechanism underlying perceived location of a perisaccadic flash. Vision Research. 2004;44(24):2799–2813. doi: 10.1016/j.visres.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Robinson DA, Fuchs AF. Eye movements evoked by stimulation of frontal eye fields. Journal of Neurophysiology. 1969;32(5):637–648. doi: 10.1152/jn.1969.32.5.637. [DOI] [PubMed] [Google Scholar]
- Ross J, Morrone MC, Burr DC. Compression of visual space before saccades. Nature. 1997;386(6625):598–601. doi: 10.1038/386598a0. [DOI] [PubMed] [Google Scholar]
- Ross J, Morrone MC, Goldberg ME, Burr DC. Changes in visual perception at the time of saccades. Trends in Neuroscience. 2001;24(2):113–121. doi: 10.1016/s0166-2236(00)01685-4. [DOI] [PubMed] [Google Scholar]
- Schlag J, Schlag-Rey M. Illusory localization of stimuli flashed in the dark before saccades. Vision Research. 1995;35(16):2347–2357. doi: 10.1016/0042-6989(95)00021-q. [DOI] [PubMed] [Google Scholar]
- Schlag J, Schlag-Rey M. Through the eye, slowly: delays and localization errors in the visual system. Nature Reviews Neuroscience. 2002;3(3):191–215. doi: 10.1038/nrn750. [DOI] [PubMed] [Google Scholar]
- Snyder LH, Batista AP, Andersen RA. Saccade-related activity in the parietal reach region. Journal of Neurophysiology. 2000;83(2):1099–1102. doi: 10.1152/jn.2000.83.2.1099. [DOI] [PubMed] [Google Scholar]
- Sogo H, Osaka N. Perception of relation of stimuli locations successively flashed before saccade. Vision Research. 2001;41(7):935–942. doi: 10.1016/s0042-6989(00)00318-7. [DOI] [PubMed] [Google Scholar]
- Sogo H, Osaka N. Effects of inter-stimulus interval on perceived locations of successively flashed perisaccadic stimuli. Vision Research. 2002;42(7):899–908. doi: 10.1016/s0042-6989(02)00014-7. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. A pathway in primate brain for internal monitoring of movements. Science. 2002;296(5572):1480–1482. doi: 10.1126/science.1069590. [DOI] [PubMed] [Google Scholar]
- Sperry RW. Neural basis of the spontaneous optokinetic response produced by visual inversion. Journal of Comparative and Physiological Psychology. 1950;43(6):482–489. doi: 10.1037/h0055479. [DOI] [PubMed] [Google Scholar]
- Umeno MM, Goldberg ME. Spatial processing in the monkey frontal eye field. I. Predictive visual responses. Journal of Neurophysiology. 1997;78(3):1373–1383. doi: 10.1152/jn.1997.78.3.1373. [DOI] [PubMed] [Google Scholar]
- von Helmholtz H. Handbuch der Physiologischen Optik (1866) In: Southall JPC, editor. A treatise on physiological optics. New York: Dover; 1963. [Google Scholar]
- von Holst E, Mittelstaedt H. Das Reafferenzprinzip (Wechselwirkung zwischen Zentralnervensystem und Peripherie) Naturwissenschaften. 1950;37:464–476. [Google Scholar]
- Walker MF, Fitzgibbon EJ, Goldberg ME. Neurons in the monkey superior colliculus predict the visual result of impending saccadic eye movements. Journal of Neurophysiology. 1995;73(5):1988–2003. doi: 10.1152/jn.1995.73.5.1988. [DOI] [PubMed] [Google Scholar]
- Watanabe J, Noritake A, Maeda T, Tachi S, Nishida S. Perisaccadic perception of continuous flickers. Vision Research. 2005;45(4):413–430. doi: 10.1016/j.visres.2004.09.010. [DOI] [PubMed] [Google Scholar]