Abstract
The basal ganglia-thalamo-cortical loop is an important neural circuit that regulates motor control. A key parameter that the nervous system regulates is the level of force to exert against an object during tasks such as grasping. Previous studies indicate that the basal ganglia do not exhibit increased activity with increasing amplitude of force, although these conclusions are based mainly on the putamen. The present study used functional magnetic resonance imaging to investigate which regions in the basal ganglia, thalamus, and motor cortex display increased activity when producing pinch-grip contractions of increasing force amplitude. We found that the internal portion of the globus pallidus (GPi) and subthalamic nucleus (STN) had a positive increase in percent signal change with increasing force, whereas the external portion of the globus pallidus, anterior putamen, posterior putamen, and caudate did not. In the thalamus we found that the ventral thalamic regions increase in percent signal change and activation volume with increasing force amplitude. The contralateral and ipsilateral primary motor/somatosensory (M1/S1) cortices had a positive increase in percent signal change and activation volume with increasing force amplitude, and the contralateral M1/S1 had a greater increase in percent signal change and activation volume than the ipsilateral side. We also found that deactivation did not change across force in the motor cortex and basal ganglia, but that the ipsilateral M1/S1 had greater deactivation than the contralateral M1/S1. Our findings provide direct evidence that GPi and STN regulate the amplitude of force output. These findings emphasize the heterogeneous role of individual nuclei of the basal ganglia in regulating specific parameters of motor output.
INTRODUCTION
An important goal of motor systems neuroscience has been to characterize how neural activity in the brain mediates movement parameters such as force and velocity (Ashe 1997; Evarts 1968; Todorov 2000; Turner and Anderson 1997) and movement direction (Georgopoulos 1986). Three brain areas that have a pivotal role in controlling these motor output parameters are the motor cortex, thalamus, and basal ganglia. Together, the motor cortex, thalamus, and basal ganglia constitute the motor loop that, when impaired, can introduce abnormalities in the ability to generate force and reduce the capacity to move. Although it has been shown that the control of force is regulated by additional regions such as the parietal cortex and cerebellum (Dai et al. 2001; Dettmers et al. 1995; Ehrsson et al. 2000; Vaillancourt et al. 2003), the purpose of the current investigation is to focus on specific regions in the basal ganglia-thalamo-cortical loop to determine how these circuits scale in neuronal activation with the level of force output.
Currently, it is unclear whether neuronal activation in specific nuclei of the basal ganglia scales with increasing force amplitude. This is surprising because studies in nonhuman primates have demonstrated that neuronal firing rate is correlated with movement amplitude and/or movement velocity in many of the pallidal (contralateral, internal, and external) neurons tested (Georgopoulos et al. 1983; Turner and Anderson 1997). In humans, two positron emission tomography (PET) studies have demonstrated an increase in regional cerebral blood flow (rCBF) in the bilateral basal ganglia with increasing movement amplitude (Desmurget et al. 2004; Turner et al. 2003). Also, scaling grip force to the task-appropriate level is abnormally high in diseases that affect the human basal ganglia such as Huntington’s disease (Gordon et al. 2000), Parkinson’s disease (Nowak et al. 2005a), and dystonia (Nowak et al. 2005b). However, studies that have examined how activation in the brain scales with the level of force amplitude have either not found (Dettmers et al. 1995; Muley et al. 2001) or not reported (Dai et al. 2001; Ehrsson et al. 2000) that the basal ganglia increase in activation across levels of force. Therefore the role of the specific basal ganglia nuclei in scaling the force amplitude level has yet to be defined.
Several studies have also investigated how neuronal activation in the motor cortex scales with increasing force amplitude. For instance, electrophysiological studies in nonhuman primates have documented a direct relation between neuronal discharge rates in multiple regions of the contralateral motor cortex and exerted force amplitude (Evarts 1968; Hepp-Reymond et al. 1978, 1999). Studies using functional magnetic resonance imaging (fMRI) in humans have also confirmed the relation between increasing neuronal activation and increasing amplitude of force in the contralateral primary motor/somatosensory (M1/S1) cortices and supplementary motor area (SMA), and also indicated that the contralateral premotor cortex may increase in activation with the amplitude of force (Dai et al. 2001; Ehrsson et al. 2000; Thickbroom et al. 1998). However, there are only a small number of studies that have investigated the role of the ipsilateral motor cortex in force production. Some studies suggest that the ipsilateral motor cortex operates in a manner similar to that of the contralateral motor cortex and scales in activation with increasing levels of force (Dai et al. 2001; Dettmers et al. 1995; Svoboda et al. 2002). Nevertheless, what remains unclear is how the pattern of activation in the ipsilateral hemisphere changes across a range of force levels and how this pattern of change compares to the pattern of change in the contralateral motor cortex.
This study had three main objectives. First, we examined activation and deactivation of specific nuclei of the basal ganglia including caudate, putamen, external and internal portions of the globus pallidus (GPe and GPi, respectively), and subthalamic nucleus (STN) to determine whether each region scales with the level of force generation. We also examined each hemisphere of the basal ganglia to determine whether the same force scaling patterns in the basal ganglia occurred ipsi-and contralaterally. Second, we examined activation and deactivation in specific regions of the thalamus to determine which regions scale with the amplitude of force. Third, we determined how activation and deactivation in the ipsilateral and contralateral M1/S1 change with increased levels of force. In addition to these three objectives, correlation analyses were used to identify significant relations between activation patterns and force amplitude, rate of force, and duration of force.
METHODS
Subject selection
Twelve right-handed subjects with corrected or normal vision participated in the study (6 male and 6 female, ages 20–35 yr). Each of the subjects was naïve to the purpose of the experiment and the subjects did not have a neurological disorder. Before the experiment, each subject provided informed consent to all procedures, which were approved by the local Institutional Review Board and were in accord with the Declaration of Helsinki. One male subject was not included in the analysis due to excessive head motion that correlated with the task.
Force data acquisition
Subjects used their right hand (thumb, first, and second fingers) to produce isometric force against a custom grip device. The grip device was constructed from nonmetallic material (polycarbonate) to allow for its use inside the magnetic resonance environment. When prompted, subjects pinched the apparatus producing force that was transmitted to a connected plastic tube (35 ft.). The water-filled tube ran into an Entran pressure transducer (EPX-N13-250P) located outside the fMRI environment. When the transducer sensed the increase in hydraulic pressure, the pressure signal was amplified by a custom-built amplifier. Data were digitized at 100 Hz from the amplifier by a PCMCI National Instruments A/D board. At each sampling interval, the output from the pressure transducer was displayed to the subject using a visual feedback system (Vaillancourt et al. 2003). The feedback was projected using a parallax biofeedback system (Thulborn and Shen 1999) through a mirror located 35 cm from the subject’s eyes. The force output was displayed at a refresh rate of 60 Hz and a resolution of 640 × 480 pixels.
Experimental design
The task sequence for the fMRI experiment used a block design paradigm (Fig. 1A). The experiment required subjects to repeat the force task at five different amplitudes during five different scans. Before scanning, each subject participated in a 1-h training session outside the scanner to minimize motor learning effects when inside the scanner. Once in the fMRI environment, the individual’s maximum voluntary contraction (MVC) was calculated. The subjects were asked to sustain a contraction of maximum force for three consecutive 5-s trials. Each trial was separated by a period of rest. The MVC was calculated as the average force during the sustained maximum force contraction. The force level requirement for each of the five functional scans was set to 5, 20, 40, 60, or 80% of the subjects’ collected MVC.
FIG. 1.
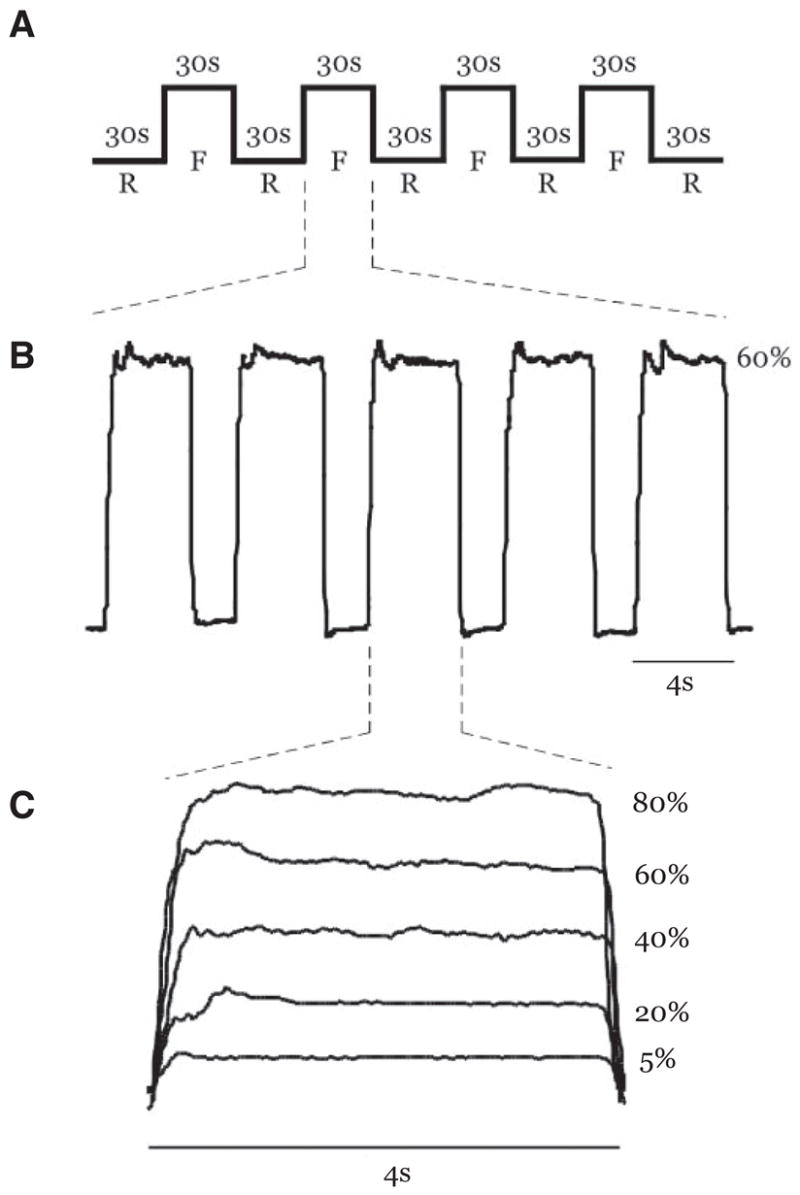
A: schematic description of the task repeated at each force level (F, force blocks; R, rest blocks). B: actual force trace from a single subject during the 60% maximum voluntary contraction (MVC) force level. Five 4 s contractions completed within a single force block are shown. C: actual force trace from a single subject performing a contraction at 5, 20, 40, 60, and 80% MVC during 5 different trials. All contractions were 4 s long.
As shown in Fig. 1A, each functional scan consisted of alternating 30-s rest and force blocks. Rest blocks were always positioned at the beginning and end of the sequence. Therefore individual scans consisted of five rest blocks and four force blocks containing five contractions each (Fig. 1B). During rest blocks, the subjects fixated on a stationary red target and white cursor but did not produce force. Figure 1B displays an expanded view of an individual 30-s force block. In each of the force blocks, subjects were cued to produce 4-s force contractions separated by 2 s of rest. During force blocks, the target would switch to green to cue the subjects to start producing force. When the target was green, the white cursor could be vertically displaced from its resting position with respect to the level of force generated by the subject and collected through the A/D board. Each force block required subjects to complete five isometric contractions. Figure 1C shows a single contraction event from the same subject at each of the five force levels that occurred in the five separate fMRI scans. In total, the subjects completed 20 isometric contractions at each of the 5, 20, 40, 60, and 80% MVC force levels over a time period of 4 min and 30 s per scan. The order of the scans was randomized for each subject.
Force data analysis
The force data were analyzed using custom algorithms in MAT-LAB. We calculated the mean amplitude of force, mean rate of force, and mean duration of force during each 4 s contraction for each subject. The mean force during each 4 s contraction was calculated after removing the first and last 500 ms, which focused the mean estimate during the steady-state phase of the contraction. The rate of force production was calculated as the peak of the first derivative of force during the first second of each contraction. The duration of force was calculated as the difference between the onset and offset of force of each individual contraction. For any particular contraction, the onset of force was the point in time after the start of the 4-s contraction periods at which the subject reached 5% of the peak force. The offset of force was marked as the time point at which force dropped to <5% of the peak force reached during the contraction.
Twenty values for the mean force, rate, and duration of force were calculated per scan for each subject. These 20 values were averaged and then analyzed using a separate one-factor (level of force) repeated-measures ANOVA for each dependent measure (Statistica, v6.1). Further investigation of the behavioral data was completed using Tukey’s HSD post hoc test.
MRI data acquisition
Magnetic resonance images were collected using a volume head coil inside a 3-Tesla MR Scanner (GE Healthcare 3T94 Excite 2.0). The subjects lay supine in the scanner while performing the force task. The subject’s head was stabilized using adjustable padding and then fitted with the projector-visor system for displaying visual feedback. The functional images were obtained using a T2*-sensitive, single shot, gradient-echo, echo-planar pulse sequence (echo time 25 ms; repeat time 2,500 ms; flip angle 90°; field of view 200 mm2; imaging matrix 64 × 64; 42 axial slices at 3-mm thickness; 0-mm gap between slices). The high-resolution anatomical scans were obtained using a T1-weighted fSPGR (fast spoiled gradient echo) pulse sequence (echo time 1.98 ms; repeat time 9 ms; flip angle 25°; field of view 230 mm2; imaging matrix 256 × 256; 120 contiguous slices at 1.5 mm thickness).
fMRI data analysis
AFNI, the public domain software (http://afni.nimh.nih.gov/afni/), was used to process and analyze the fMRI data sets. First, we will describe methods for head motion analysis. Next, we will describe the region of interest (ROI) analysis. Finally, we will explain the methods used to construct group maps for visual presentation purposes.
HEAD MOTION ANALYSIS AND MOTION CORRECTION
After importing the acquired data, motion detection and correction functions were applied to each functional time series. The head displacement in all data sets for all included subjects (n = 11) was <1 mm (one third of our voxel size) in any direction. We used a direction × force level (3 × 5) ANOVA for statistical analysis. There was no significant difference in average displacement across direction [F(2,20) = 0.19, P = 0.82]. Also, there was no significant difference in average displacement across all force levels [F(4,40) = 0.97, P = 0.43] and no significant force × direction interaction [F(8,80) = 1.43, P = 0.20].
STATISTICAL ANALYSIS OF REGIONS OF INTEREST
Percentage signal change and activation volume were the dependent variables used in the ROI analysis. To acquire percent signal change data, we first calculated the mean activation in each voxel for both rest and force blocks across each functional time series. The mean percent signal change for each voxel was calculated as the difference between the mean signal during the force blocks and the mean signal during the rest blocks divided by the mean signal during the rest blocks and then multiplied by 100. Therefore the output data at this level of analysis represented the percent signal change in each voxel. Resultant data sets were thresholded to include only voxels with a positive percent signal change located within a region of interest. In addition, we separately thresholded the percent signal change maps to include only negative voxels, and we performed the ROI analysis separately on these negative percent signal change voxels (deactivations).
The ROI analysis examined percent signal change and activation volume in the bilateral basal ganglia, the bilateral cortical areas, and the bilateral thalamus. These ROIs were drawn on a single Talairach-transformed anatomical image to form a template mask, and this template was overlaid on each subject’s Talairach-transformed functional image. The cortical ROIs included M1/S1, SMA, pre-SMA, and both ventral and dorsal premotor cortices (PMv and PMd, respetively) and these were based on the Human Motor Area Template (HMAT) created from a comprehensive meta-analysis of motor areas (Mayka et al. 2006). The mask is publicly available (http://mcl.mvsc.uic.edu).
In the basal ganglia, the regions included the caudate, anterior putamen, posterior putamen, external and internal portions of the globus pallidus (GPe and GPi, respectively), and subthalamic nucleus (STN). These ROIs are the same as those used in previous work (Vaillancourt et al. 2004, 2007). Anatomical guidelines from previously published literature were used to help identify each basal ganglia nucleus (Yelnik 2002). The centroid coordinate in Talairach space for the left-side ROIs are listed for each region (3dclust in AFNI).
Caudate nucleus (x = −11, y = 9, z = 11) is a curved structure with the rostral head being more voluminous than the body rostrally (Yelnik 2002). It can be identified up to the level of the top of the ventricles. The medial border of the caudate nucleus is defined by the frontal horn or body of the lateral ventricle and the lateral edge by the anterior limb of the internal capsule (Ifthikharuddin et al. 2000).
Putamen (x = −24, y = 2, z = 4) is limited medially on inferior sections by the globus pallidus and on more superior levels by the internal capsule (Ifthikharuddin et al. 2000). Anteriorly, the anterior limb of the internal capsule separates the putamen from the caudate. Laterally, it is limited by the external capsule. The anterior and posterior parts of the putamen were differentiated on a slice-by-slice basis using the anterior border of the thalamus and the posterior border of the caudate as the dividing line.
Globus pallidus is limited medially by the posterior limb of the internal capsule and laterally by the putamen (Ifthikharuddin et al. 2000). It is divided into the globus pallidus internal portion (GPi) (x = −16, y = −4, z = 2) and the globus pallidus external portion (GPe) (x = −20, y = −4, z = 4). The GPe lies lateral to the GPi and is almost twice as large (Yelnik 2002).
STN (x = −11, y = −14, z = −3) lies ventral to the thalamus, medial to the peduncular portion of the internal capsule, and lateral and caudal to the hypothalamus. It is lateral to the red nucleus and dorsolateral to the substantia nigra in the coronal plane (or anteromedial in the axial plane) (Dormont et al. 2004). The size of the STN may be smaller than reported in the Talairach and Tournoux atlas, particularly in the medial–lateral direction (Richter et al. 2004).
The thalamic ROIs included the ventrolateral (VL), ventroanterior (VA), lateral ventroposterior (VPl), medial ventroposterior (VPm), and centromedial (CM) nuclei. Descriptions of each of these ROIs follow.
VA nucleus (x = −10, y = −6, z = 8) is the most anterior component of the ventral thalamic nuclei, lying rostral to the VL nucleus. It is lateral to the AV nucleus in the superior portion of the thalamus in the axial plane, but it extends medially to the internal capsule in the inferior portion of the thalamus (Wiegell et al. 2003).
VL nucleus (x = −12, y = −12, z = 10) lies caudal to the VA nucleus in the axial plane on the ventral edge of the thalamus, medial to the internal capsule. It is lateral to the dorsomedial (DM) nucleus and extends from the superior portion of the thalamus to the inferior boundary. The VL nucleus is bordered rostrally by the VA nucleus and caudally by the VPl nucleus of the thalamus.
VPl nucleus (x = −18, y = −19, z = 5) and VPm nucleus (x = −14, y = −19, z = 5). The ventral posterior nucleus of the thalamus is split into lateral and medial portions. The lateral portion of the ventral posterior nucleus is medial to the internal capsule and lateral to the ventral medial posterior nucleus. Both nuclei lay inferior to the lateral posterior nucleus and extend to the interior edge of the thalamus.
CM nucleus (x = −8, y = −19, z = 3) is bordered medially by the caudal edge of the DM nucleus and laterally by the VPm nucleus of the thalamus. The CM nucleus is also inferior to the superior portion of the DM nucleus and the VPm nucleus and extends to the inferior edge of the thalamus. The caudal edge of the CM nucleus borders the posterior nucleus of the thalamus.
Next, we quantified the average percent signal change and activation volume within the aforementioned ROIs. The average percent signal change was calculated across all positive (or negative) percent signal change voxels within an ROI. Percentage signal change of each individual ROI was calculated at 5, 20, 40, 60, or 80% MVC for each individual subject, separately for activations and deactivations. In calculating the activation volume, we first used a general linear model (3Ddeconvolve, AFNI) to regress the voxel time-series data to a simulated hemodynamic response function for the force and rest blocks included in each of the five MVC force level scans. This level of analysis resulted in a regression coefficient for each voxel with an associated P value. The activation volume was calculated by counting the number of significantly activated voxels that had a t-value >3 (P < 0.005).
The mean percent signal change data and activation volume for individual subjects were analyzed using separate repeated-measures ANOVAs for each ROI. The within-subjects repeated factors were hemisphere (left or right) and target force level (5, 20, 40, 60, and 80%). Separate one-way ANOVAs were performed for each hemisphere of the regions that showed a significant force × hemisphere interaction. A paired t-test was performed at each force level to determine the locus of the interaction between each hemisphere of GPe. All statistical tests were evaluated as significant if the P value was <0.05.
The positive percent signal change data in each ROI were further analyzed using a correlation analysis between each ROI and the three kinetic parameters under investigation (force, rate of change of force, duration of force). All correlation analyses were computed using data from the left hemisphere only. The group mean percent signal change data at each force level provided five data points for comparison within each ROI. The Pearson product moment coefficient of correlation (r) was calculated between the group mean percent signal change in each ROI and each kinetic parameter (Statistica, 6.1). A correlation was considered statistically significant when P < 0.05.
VOXELWISE GROUP ANALYSIS FOR ROI PRESENTATION
We constructed group maps of GPi, STN, SMA, M1/S1, and thalamus for visualization of activation within the ROIs. We first normalized the signal in each scan of each subject by dividing the instantaneous signal in each voxel at each point in the time series by the mean signal in that voxel for each subject’s scan. This process was repeated for each scan for each subject. Therefore at the end of this process, the blood-oxygen-level– dependent (BOLD) signal in each functional data set for each subject was normalized around a baseline value of 100. After this, we applied a Gaussian filter to the resultant data sets [full-width-half-maximum (FWHM) at 5 mm]. Then, we used a general linear model (3Ddeconvolve, AFNI) to regress the time-series data to a simulated hemodynamic response function for the force and rest blocks included in each of the five MVC force level scans. We then performed a group analysis of each force level scan. In the group analysis, we first used a mixed-effect two-way ANOVA with the respective MVC force level as the fixed factor and the subject as a random factor. All group maps were then masked using the ROIs generated a priori, to present only those voxels that were contained in the ROIs that we used for our hypothesis testing. The maps were thresholded so that all included voxels had a t-value >3 (P < 0.005, uncorrected). The uncorrected statistical threshold was used for the group analysis because we had specific hypotheses regarding the basal ganglia nuclei. The analysis using uncorrected P values can be more informative when studying small brain targets that produce weak BOLD signals like the basal ganglia regions (Turner et al. 2003; Vaillancourt et al. 2004; Wu and Hallett 2005). The thresholded masks were coregistered to an individual anatomical map in Talairach space.
To construct the three-dimensional (3D) functional surface maps of the cortex, the data sets were corrected for type I error by Monte Carlo simulation (AFNI alphasim, http://afni.nimh.nih.gov/afni/doc/manual/AlphaSim/). The cortical activation maps were thresholded at t = 3 with an activation cluster minimum of 500 μl to give a significantly low probability for type I error (P < 0.01, corrected). The data were then masked to display only those voxels within M1/S1 or SMA. The corrected functional maps were coregistered to a Talairach version of the N27 brain data set (MNI, http://www.bic.mni.mcgill.ca; UCLA, http://www.loni.ucla.edu). The surface maps displaying cortical data were created using the AFNI surface mapper, SUMA (http://afni.nimh.nih.gov/afni).
RESULTS
Behavioral performance
As shown in Fig. 2, subjects effectively completed the force-production task by producing force close to the percentage required in each of the five conditions. The average force production for the 5%-MVC condition was greater than that required by the task. Also, the mean force production during the 60%-MVC and 80%-MVC conditions was lower than the target level established. Nevertheless, the ANOVA showed that the main effect of force was highly significant across the target force levels [F(4,44) = 573.79, P < 0.01]. A Tukey’s HSD post hoc test indicated that mean force at each force level was different between all other levels (all values of P were <0.01).
FIG. 2.
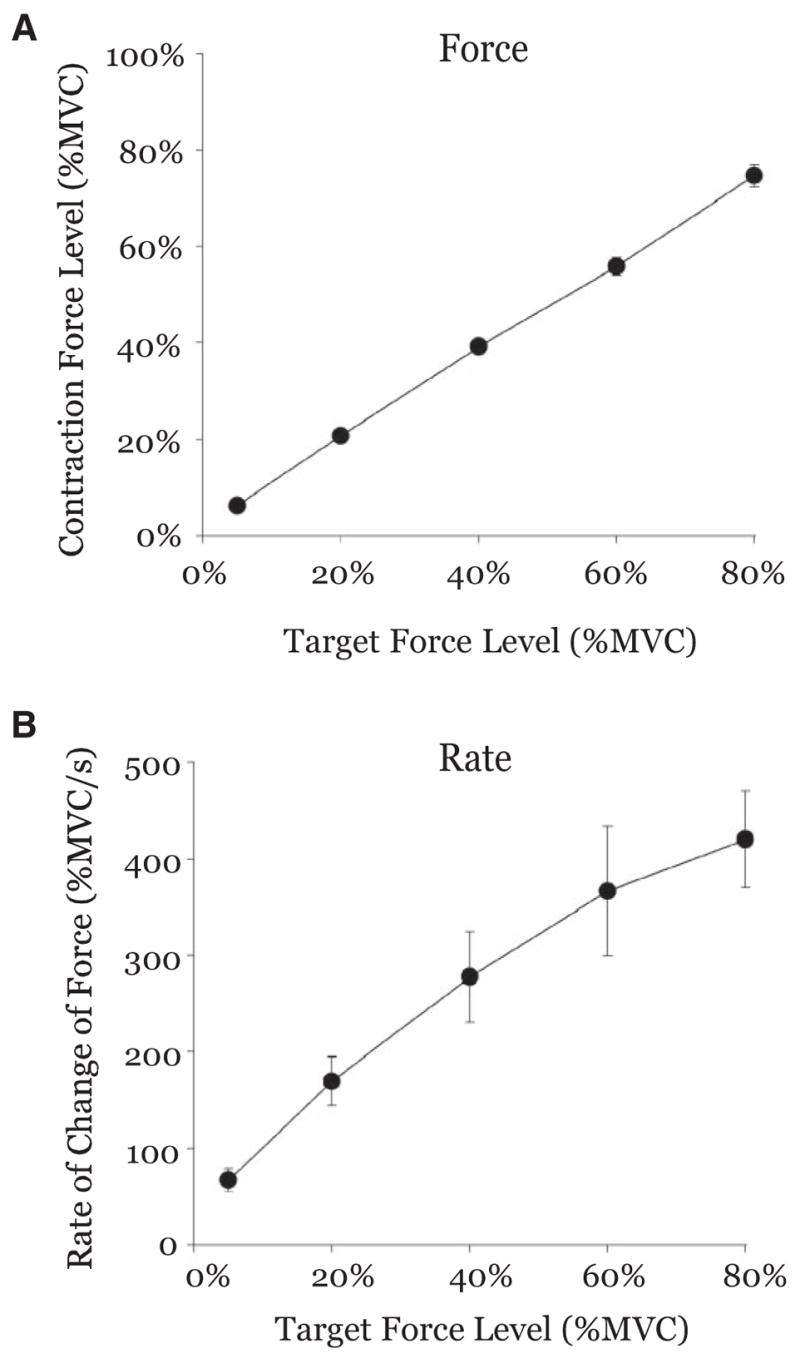
Group mean force output (A) and rate of change of force (B) are plotted against the target force level used in each trial. Mean force output was slightly higher than the target for the 5% condition and slightly lower than the target for the 80% condition. Nevertheless, the subjects were able to effectively produce force close to the target force level during each of the 5 conditions. Between-subject variance was larger for the rate of change of force than the amplitude of force.
Statistical analysis of the rate of force showed significant scaling across all force levels [F(4,40) = 28.339, P < 0.01]. Figure 2B shows that the lowest rate of force of 66.65%-MVC/s occurred at the 5%-MVC force level. During the 20, 40, and 60% force levels, the rate of force increased to 169.37%-, 277.59%-, and 366.33%-MVC/s, respectively. The highest rate of force production was 420.37%-MVC/s during the 80%-MVC force level. Tukey’s HSD post hoc test indicated that the rate of change of force was different between 5% compared with the 40, 60, and 80% conditions, and 20% was different compared with the 60 and 80% conditions. It is also important to note that Fig. 2B shows a much greater between-subject variance for the rate of change of force, compared with the low between-subject variance in Fig. 2A for the amplitude of force.
Further statistical tests confirmed that the mean duration of force did not increase significantly across increasing force levels [F(4,40) = 1.553, P = 0.20]. The duration of force was 3.96, 4.00, 4.00, 3.99, and 4.01 s during the 5, 20, 40, 60, and 80% target force levels, respectively.
Functional activation: basal ganglia
GPI
Figure 3 shows mean group activation maps and results from the ROI analysis of percent signal change versus force for GPi. As shown in the voxelwise group analysis, there was activation in GPi at each force level shown in Fig. 3A except there was no significant activation on the right hemisphere at 20%. The ROI analysis for GPi showed an increase in the mean percent signal change with increasing amplitudes of force (Fig. 3B), and statistical analysis confirmed this effect (Table 1). Figure 3C depicts the individual subject changes in percent signal change across levels of force. The hemisphere and force × hemisphere effects were not significant (Table 1).
FIG. 3.
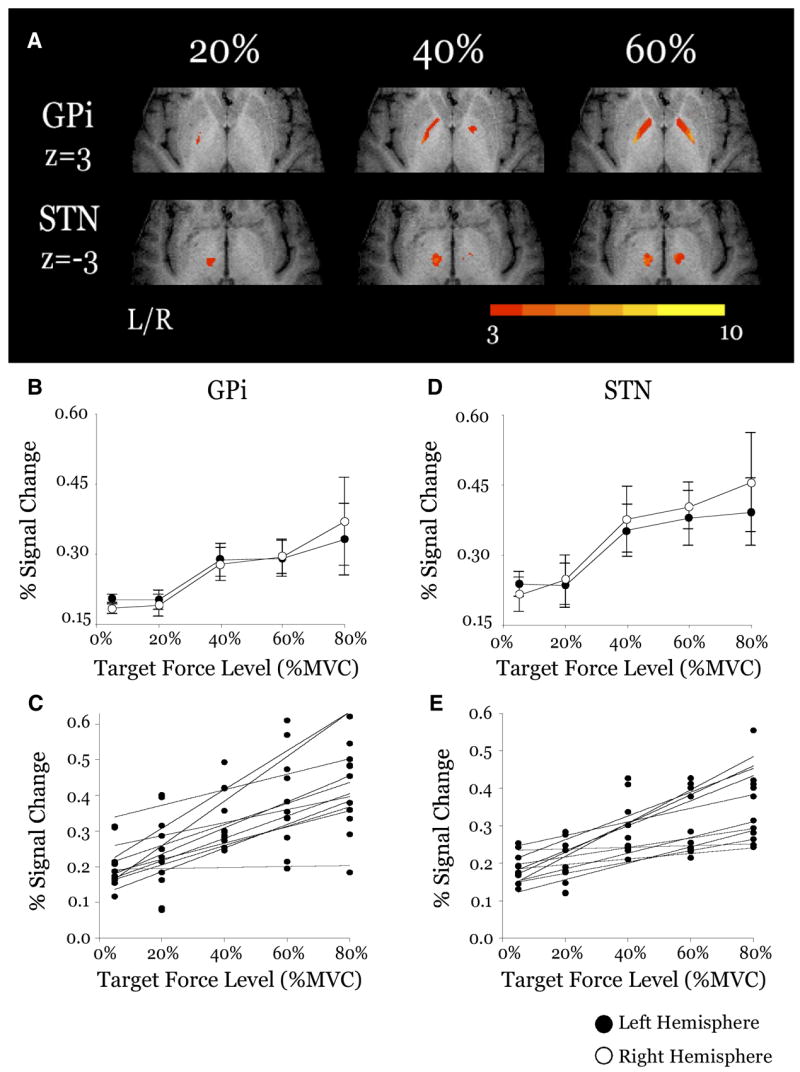
Results from the group analysis and region of interest (ROI) analysis for the force scaling nuclei of the basal ganglia: internal portion of the globus pallidus (GPi) and subthalamic nucleus (STN). A: axial slices from the 20, 40, and 60% MVC conditions. Each slice has been masked to focus on a specific region. Activation in each slice is overlaid on the same anatomical brain in Talairach space. Group activation threshold is P < 0.005, uncorrected. Color bar ranges from t = 3 to t = 10. B: plot of group mean percent signal change vs. target force level for the contralateral (filled circles) and ipsilateral (open circles) hemispheres of GPi. C: plots of individual subjects (n = 11) percent signal change vs. target force level for the contralateral hemisphere of GPi. D: plot of group mean percent signal change vs. target force level for the contralateral (filled circles) and ipsilateral (open circles) hemispheres of STN. E: plots of individual subjects (n = 11) percent signal change vs. target force level for the contralateral hemisphere of STN.
TABLE 1.
Statistical results from ROI analysis
| Region | Force | Hemisphere | Interaction |
|---|---|---|---|
| M1/S1 | <0.01 | <0.01 | <0.01 |
| SMA | 0.03 | 0.34 | 0.86 |
| Pre-SMA | 0.20 | 0.86 | 0.73 |
| PMv | 0.34 | 0.04 | 0.82 |
| PMd | 0.19 | 0.13 | 0.30 |
| GPi | 0.03 | 0.81 | 0.31 |
| STN | 0.04 | 0.43 | 0.75 |
| GPe | 0.30 | <0.01 | 0.04 |
| Caudate | 0.37 | 0.14 | 0.95 |
| Anterior putamen | 0.63 | 0.78 | 0.70 |
| Posterior putamen | 0.21 | <0.01 | 0.52 |
| VA thalamus | 0.02 | 0.28 | 0.21 |
| VL thalamus | 0.02 | 0.45 | 0.41 |
| VPl thalamus | <0.01 | <0.01 | 0.01 |
| VPm thalamus | 0.02 | <0.01 | 0.03 |
| CM thalamus | 0.30 | <0.01 | 0.19 |
Bold indicates significance P < 0.05.
STN
Figure 3D also shows group activation maps and results from the ROI analysis of percent signal change versus force for the STN. Figure 3A shows there was significant activation in the left STN at each force level and in the right STN at the 40 and 60% force levels. The ROI analysis showed an increase in activation in the STN with increasing amplitudes of force (Fig. 3D, Table 1), and Fig. 3E shows the individual subject trends for STN. The hemisphere effect and the force × hemisphere interaction were not significant for STN (Table 1).
PUTAMEN
For the statistical ROI analysis we divided the putamen into anterior and posterior segments. The mean percent signal change did not increase across the five force levels (Fig. 4, A and B), and the statistical analysis confirmed that neither segment of the putamen had a significant main effect of force (Table 1). There was a significant hemisphere effect in the posterior putamen with greater activation in the contralateral (left) hemisphere than in the ipsilateral (right) hemisphere. Interestingly, the hemisphere effect in the anterior putamen was not significant (Table 1). Neither segment of the putamen exhibited a significant force × hemisphere interaction.
FIG. 4.

Results from the statistical ROI analysis for the nonforce scaling nuclei of the basal ganglia: anterior putamen (A), posterior putamen (B), caudate (C), and external portion of the globus pallidus (GPe, D). All plots depict the group mean percent signal change vs. target force level in each ROI. There were no significant changes in the group mean percent signal change with target force level in any of these ROIs (all values of P were >0.05).
CAUDATE
Figure 4C also shows that the mean percent signal change in the caudate did not increase with increasing amplitudes of force, and this was confirmed in the statistical analysis (Table 1). Similarly, both the hemisphere effect and interaction were not significant in the caudate.
GPE
Figure 4D shows percent signal change data for GPe, and statistical analysis confirmed that the main effect of force was not significant for GPe (Table 1). The main effect of hemisphere was significant, with the left hemisphere having higher activation than the right. The force × hemisphere interaction was also significant (Table 1). Because there was a significant interaction in GPe, we separately analyzed the main effect of force in the contralateral (left) and ipsilateral (right) GPe. The main effect of force was not significant in both the left (P = 0.18) and the right (P = 0.43) GPe. A paired t-test completed at each force level revealed that the 40% condition was the locus of the interaction because there was highly significant difference between the activation in the two hemispheres during this force level (P < 0.01) and a nonsignificant difference during all other force levels (all values of P were >0.05).
Thus the main finding was that GPi and STN appear to be the only basal ganglia structures that reliably scale in activation intensity with increasing amplitudes of force. The results further indicate that GPe, anterior putamen, posterior putamen, and caudate did not scale with the level of force.
Functional activation: thalamus
VENTROLATERAL AND VENTRAL ANTERIOR NUCLEI
Figure 5A depicts the mask used in the ROI analysis. Figure 5B demonstrates significant activation at the 20, 40, and 60% force levels. In addition, we show an increase in the mean percent signal change in VL and VA thalamus as exerted force increases (Fig. 5C). Statistical analysis of the mean percent signal change indicated that there was a highly significant main effect of force in the VL nucleus of the thalamus (Table 1). However, both the hemisphere effect and the force × hemisphere interaction were not significant. Similarly, there was a highly significant main effect of force in the VA nucleus of the thalamus; however, both the hemisphere effect and the interaction effect were not significant (Table 1).
FIG. 5.
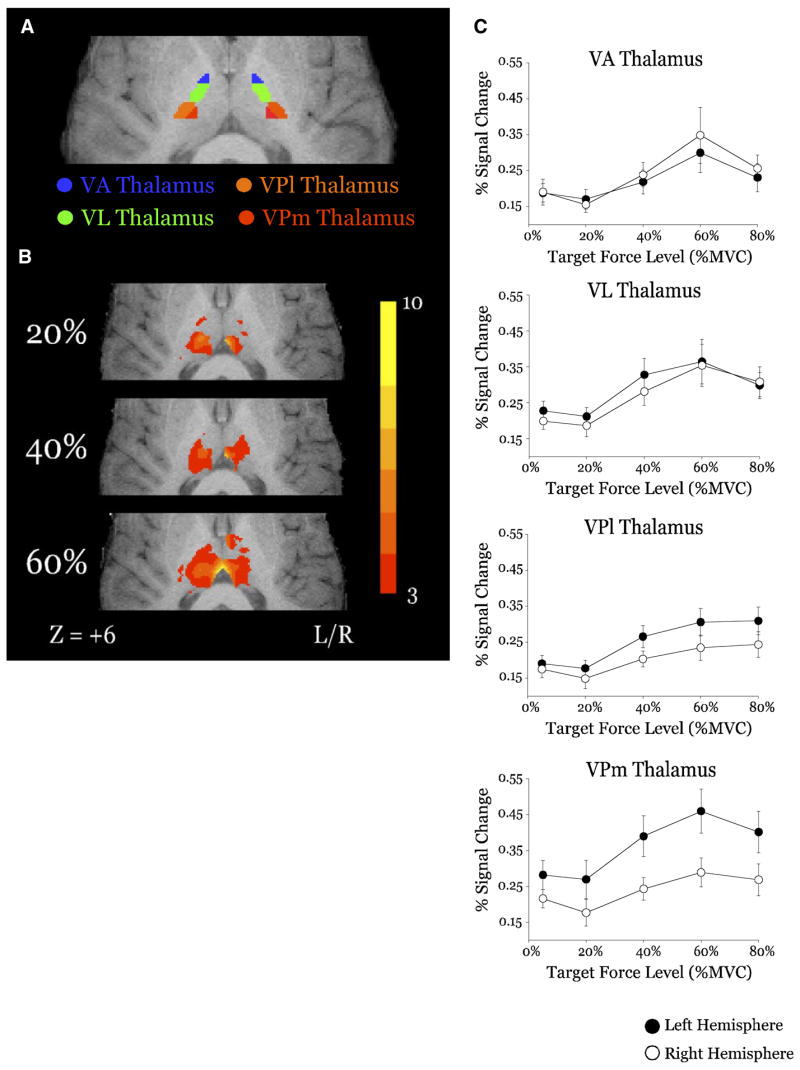
Results from the group analysis and ROI analysis for the force scaling ROIs of the thalamus: ventroanterior (VA), ventrolateral (VL), lateral ventroposterior (VPl), and medial ventroposterior (VPm). A: location of mask ROIs for VA (blue), VL (green), VPl (orange), and VPm (red) (z = +6). B: group mean activation from the 20, 40, and 60% MVC conditions in each for VA, VL, VPl, and VPm. Activation on each surface (A, B) is overlaid on the same anatomical brain in Talairach space. Color bar ranges from t = 3 to t = 10. C: plots of group mean percent signal change vs. target force level for the contralateral (filled circles) and ipsilateral (open circles) hemispheres of VA, VL, VPl, and VPm thalamus.
VENTROPOSTERIOR LATERAL AND MEDIAL NUCLEI
The ventroposterior caudal portion of the thalamus was separated into lateral (VPl) and medial (VPm) portions for analysis (Fig. 5A). Mean percent signal change in these regions scales with the level of exerted force (Fig. 5C, Table 1). Both the VPl and VPm had significant hemisphere effects and interaction effects as well.
CENTROMEDIAN NUCLEUS
The centromedian (CM) nucleus of the thalamus did not have a significant effect of force. However, there was a highly significant main effect of hemisphere with the left side greater than the right (Table 1). The force × hemisphere interaction was not significant.
Therefore the overall finding is that the VA, VL, VPl, and VPm nuclei of the thalamus all increase significantly in BOLD percent signal change as individuals produce increasing amplitudes of force. Furthermore, we also found that the CM nucleus of the thalamus does not scale significantly with force.
Functional activation: motor and premotor cortex
M1/S1
Figure 6A shows group surface maps of contra- and ipsilateral M1 and S1 (M1/S1) for the 20, 40, and 60% force levels. As expected, the group analysis in contralateral M1/S1 demonstrates significant activation at each level of force. The mean percent signal change in M1/S1 increased with force (Fig. 6C), and there was a significant main effect of force (Table 1). M1/S1 also showed a significant hemisphere effect with more activation in the left hemisphere than in the right. Importantly, the force × hemisphere interaction in M1/S1 was significant (Table 1). To determine whether each hemisphere scaled with force, we conducted separate one-way ANOVAs for the right and left hemispheres. It was confirmed that the main effect of force was highly significant in both the right and left M1/S1 (both values of P were <0.01). Furthermore, visual inspection of the data in Fig. 6C indicates that the contralateral (left) M1/S1 had a greater absolute change in mean percent signal change than the ipsilateral (right) M1/S1.
FIG. 6.
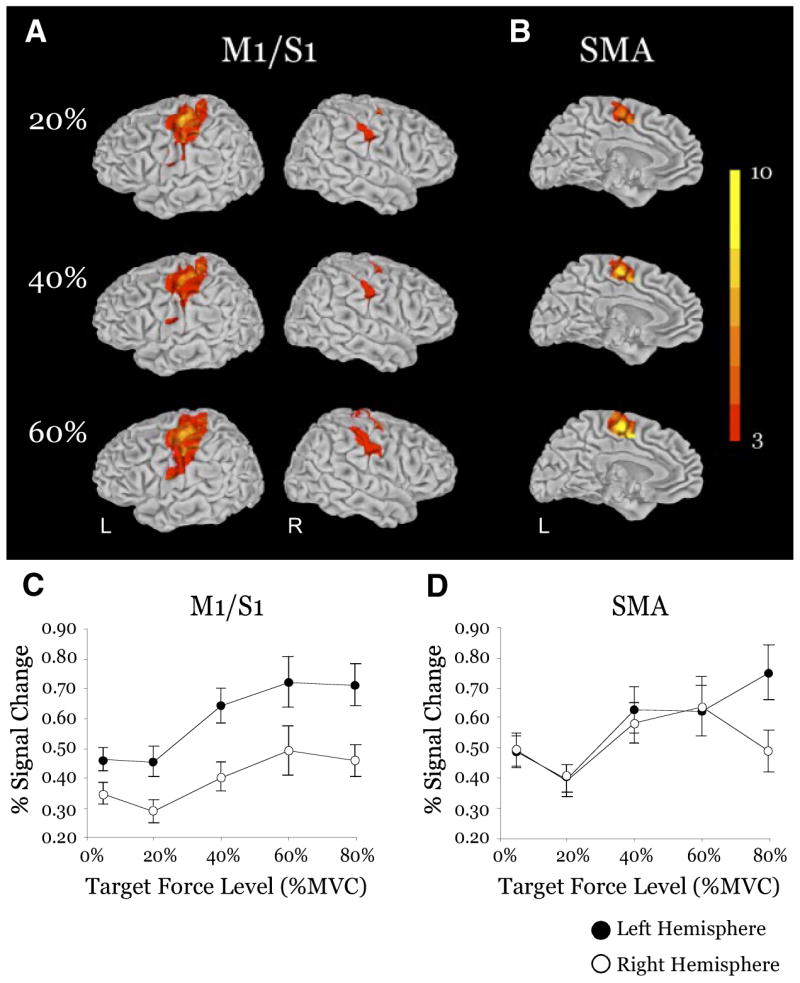
Results from the group analysis and ROI analysis for the force scaling ROIs of the motor cortex: primary motor/somatosensory (M1/S1) cortices and supplementary motor area (SMA). A: surface maps of the group mean activation from the 20, 40, and 60% MVC conditions in each hemisphere of M1/S1. Activation on each surface is overlaid on the same anatomical brain in Talairach space. Group activation threshold is P < 0.05, corrected. Color bar ranges from t = 3 to t = 10. B: surface maps of the group mean activation from the 20, 40, and 60% MVC conditions in the contralateral hemisphere of SMA. Activation on each medial surface is overlaid on the same anatomical brain in Talairach space. Group activation threshold is P < 0.05, corrected. Color bar ranges from t = 3 to t = 10. C and D: plots of group mean percent signal change vs. target force level for the contralateral (filled circles) and ipsilateral (open circles) hemispheres of M1/S1 and SMA.
SMA
There was significant activation in the SMA at each level of force (Fig. 6B). The increase in mean percent signal change across force was significant (Fig. 6D) (Table 1). SMA did not exhibit a significant hemisphere effect and there was no significant force × hemisphere interaction (Table 1).
PRE-SMA
Statistical analysis of the mean percent signal change in the pre-SMA showed that there was not a significant main effect of force (Table 1). Also, there was not a significant hemisphere effect or force × hemisphere interaction (Table 1).
PREMOTOR AREAS
The mean percent signal change in PMv indicated that there was not a significant main effect of force (Table 1). The hemisphere effect was significant (Table 1) with the left hemisphere having greater signal intensity than the right. The force × hemisphere interaction was not significant in this region (Table 1). In the PMd, the main effect of force, hemisphere, and the force × hemisphere interaction were all not significant (Table 1).
In summary, we found that M1/S1 and SMA increase in signal intensity with increased force, whereas pre-SMA, PMd, and PMv did not. We also found that both hemispheres of M1/S1 scale in activation with increasing amplitudes of force.
Linear versus nonlinear scaling
In regions where we found a main effect of force for percent signal change in Table 1, we examined the left and right hemisphere for these regions to determine whether a linear or nonlinear function provided the best fit to the data across force level. The first function we used was a linear polynomial (y = ax + y0). The second function was a sigmoid function (y = y0 + a/{1 + exp[−(x − x0)/b]}) (S-shaped curve), and this function was used because the data of Cheney and Fetz (1980) suggest that the firing rate of cortical cells follows an S-shaped pattern across torque. Also, Ashe (1997) suggested that cortical cells may be linear in specific ranges but that a more complex function is needed across a large range of forces. Finally, we used a logarithmic function [y = ln (ax + y0)] because this was suggested by Dettmers and colleagues (1996) for both PET and fMRI signals across force. With the exception of GPi, the sigmoid function explained a greater degree of variance than the linear and logarithmic functions (Table 2). In GPi, all three functions lead to similar results. Also, in left SMA the logarithmic function had a higher variance explained than the sigmoid function. Thus the average percent signal change in each region appears to be linear in middle range of static force levels, but nonlinear across a large range of static forces. This nonlinear relation is consistent with the data of cortical cells shown by Cheney and Fetz (1980) and with the review by Ashe (1997).
TABLE 2.
Correlation analysis (r2)
| Region | Linear | Sigmoid | Logarithmic |
|---|---|---|---|
| M1/S1 left | 0.86 | 0.99 | 0.78 |
| M1/S1 right | 0.69 | 0.92 | 0.52 |
| SMA left | 0.62 | 0.92 | 0.97 |
| SMA right | 0.44 | 0.78 | 0.93 |
| GPi left | 0.90 | 0.94 | 0.86 |
| GPi right | 0.95 | 0.94 | 0.98 |
| STN left | 0.87 | 0.99 | 0.85 |
| STN right | 0.95 | 0.98 | 0.57 |
| VA thalamus left | 0.48 | 0.75 | 0.48 |
| VA thalamus right | 0.50 | 0.78 | 0.51 |
| VL thalamus left | 0.51 | 0.86 | 0.52 |
| VL thalamus right | 0.73 | 0.95 | 0.74 |
| VPl thalamus left | 0.87 | 0.99 | 0.87 |
| VPl thalamus right | 0.81 | 0.94 | 0.81 |
| VPm thalamus left | 0.69 | 0.93 | 0.70 |
| VPm thalamus right | 0.63 | 0.87 | 0.64 |
Correlations between ROIs and kinetic variables
Figure 7 shows the r2 values between each ROI in the left hemisphere and two kinetic variables. In the basal ganglia, we found that GPi and STN were both significantly correlated with force amplitude and rate of change of force, whereas GPe, anterior putamen, posterior putamen, and caudate were not (Fig. 7). Although these relationships reflect the force-related increases in activation in the basal ganglia, this was not the case across the whole brain. In the thalamus, only the VPl was significantly correlated with both force amplitude and rate of change of force, whereas VPm was significantly correlated to only the rate of change of force (Fig. 7). Although the VA and VL thalamus showed significant increases in activation with increasing levels of force, they were not significantly correlated to amplitude or rate of change of force. Similarly, the M1/S1 was highly and significantly correlated to both force amplitude and rate of change of force, but the SMA, which also scaled in activation with force amplitude, was not significantly correlated to amplitude or rate of change of force (Fig. 7). The correlation between each ROI and duration of force was always low (r2 < 0.05) and nonsignificant.
FIG. 7.
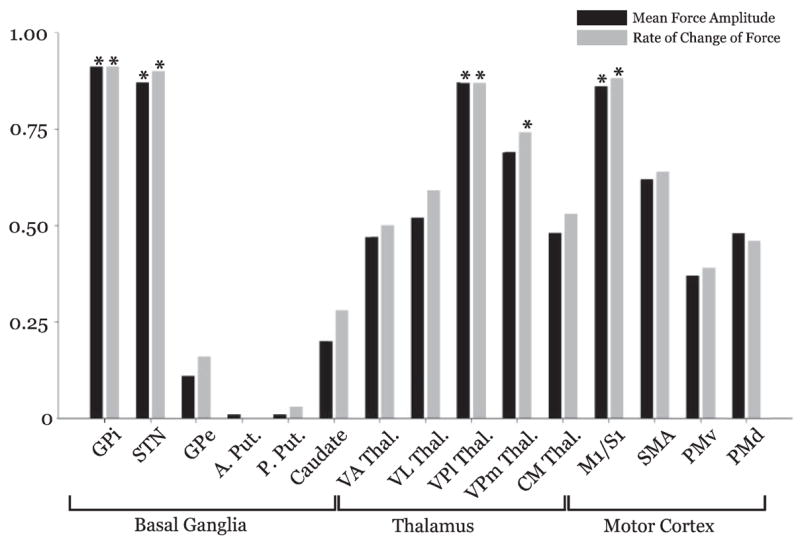
Depicts the r2 value for the correlation between each ROI (contralateral) and force amplitude (black), rate of change of force (light gray), and duration of force (dark gray). Asterisk designates a significant correlation where P < 0.05.
Activation volume in regions of interest
Because it is possible that the volume of activation may change without an effect of percent signal change, we also examined the activation volume changes across force and hemisphere. Table 3 shows the statistical analysis for how the activation volume scaled with the level of force and across hemisphere in each region. The M1/S1, VPl thalamus, and VPm thalamus had a significant increase in activation volume with the level of force. In addition, the activation volume was greater in the left hemisphere compared with the right hemisphere in M1/S1, PMd, anterior putamen, VPl thalamus, VPm thalamus, and CM thalamus.
TABLE 3.
Activation volume
| Region | Force | Hemisphere | Interaction |
|---|---|---|---|
| M1/S1 | <0.01 | <0.01 | 0.25 |
| SMA | 0.08 | 0.23 | 0.72 |
| Pre-SMA | 0.43 | 0.10 | 0.67 |
| PMv | 0.59 | 0.08 | 0.23 |
| PMd | 0.19 | 0.04 | <0.01 |
| GPi | 0.13 | 0.06 | 0.28 |
| STN | 0.18 | 0.38 | 0.83 |
| GPe | 0.87 | 0.56 | 0.22 |
| Caudate | 0.74 | 0.41 | 0.39 |
| Anterior putamen | 0.26 | <0.01 | 0.56 |
| Posterior putamen | 0.57 | 0.53 | 0.78 |
| VA thalamus | 0.32 | 0.48 | 0.91 |
| VL thalamus | 0.10 | 0.14 | 0.60 |
| VPl thalamus | 0.03 | <0.01 | 0.03 |
| VPm thalamus | 0.04 | <0.01 | 0.27 |
| CM thalamus | 0.145 | <0.01 | 0.18 |
Bold indicates significance P < 0.05.
Deactivation scaling across force and hemisphere
Because deactivation of the basal ganglia-thalamo-cortical regions may also occur with increased force, we performed a separate analysis of the voxels that had a negative signal change in the comparison of force versus rest. Table 4 shows the statistical analysis for how the voxels with deactivation (negative percent signal change) varied with the level of force and hemisphere. We found that only the VPm thalamus had significant changes in deactivation with the level of force. The significant effect in VPm was due to a greater negative percent signal change with increased force. Also, the ipsilateral M1/S1 and PMd had greater deactivation than the contralateral M1/S1 and PMd, respectively, and this difference did not change across force.
TABLE 4.
Deactivation
| Region | Force | Hemisphere | Interaction |
|---|---|---|---|
| M1/S1 | 0.10 | 0.05 | 0.63 |
| SMA | 0.12 | 0.82 | 0.85 |
| Pre-SMA | 0.28 | 0.13 | 0.89 |
| PMv | 0.21 | 0.06 | 0.57 |
| PMd | 0.22 | 0.05 | 0.73 |
| GPi | 0.10 | 0.57 | 0.95 |
| STN | 0.22 | 0.90 | 0.38 |
| GPe | 0.33 | 0.10 | 0.70 |
| Caudate | 0.73 | 0.17 | 0.13 |
| Anterior putamen | 0.30 | 0.22 | 0.30 |
| Posterior putamen | 0.33 | 0.08 | 0.26 |
| VA thalamus | 0.82 | 0.77 | 0.70 |
| VL thalamus | 0.36 | 0.23 | 0.96 |
| VPl thalamus | 0.08 | 0.06 | 0.16 |
| VPm thalamus | 0.03 | 0.09 | 0.30 |
| CM thalamus | 0.43 | 0.03 | 0.35 |
Bold indicates significance P < 0.05.
DISCUSSION
Our study provides direct evidence using BOLD fMRI that specific nuclei of the basal ganglia and thalamus increase in percent signal change with the amplitude and rate of change of force. In addition, we have shown that distinct scaling patterns of percent signal change and activation volume exist in the contralateral and ipsilateral hemispheres of M1/S1 during the control of force amplitude (rate). We will discuss these findings for the control of force amplitude and rate in the context of previous human and nonhuman primate work that have implicated the basal ganglia, thalamus, and cortex in the control of several parameters (i.e., velocity, displacement) of movement and force (Dai et al. 2001; Georgopoulos et al. 1983; Turner and Anderson 1997; Turner et al. 2003; Vaillancourt et al. 2004). In addition, our finding that both rate and force amplitude covaried and that the GPi and STN scaled positively with both force and rate of change of force differs from prior work from our laboratory where an inverse relation between both GPi and STN and the rate of change of force was found (Vaillancourt et al. 2004). We further discuss that this difference in findings is actually consistent when interpreted in the context of previous models that have suggested that the CNS has different strategies and patterns of activation when controlling the rate of change of force versus force amplitude.
Force amplitude–dependent changes
Several electrophysiological studies investigating single-cell discharge in the monkey GP (internal and external segments) (Georgopoulos et al. 1983; Turner and Anderson 1997) and STN (Georgopoulos et al. 1983) during arm movements have found that some neurons increase in discharge rate and other neurons decrease in discharge rate with increased movement amplitude. Despite the variability observed in the positive or negative discharge rate and movement amplitude, the consistent result in these studies was the discharge rate scaled with movement amplitude. In humans, a PET imaging study found that the putamen had an increased rCBF during isometric force contractions compared with that of rest (Dettmers et al. 1995), but the blood flow changes did not scale with the amplitude of force. Two imaging studies using PET have shown that the bilateral globus pallidus/putamen has an increased rCBF during increased movement amplitude (Turner et al. 1998) and velocity (Turner et al. 2003). This raises questions because movement amplitude and velocity covary with force and our findings indicate that activation in the putamen does not scale with force. However, because the studies by Turner and colleagues used PET, and the spatial resolution of PET is not as robust as that of fMRI (van Eimeren and Siebner 2006; Volkow et al. 1997), it was difficult to clearly distinguish activity in putamen from the globus pallidus. Moreover, other imaging studies have not found that the activation in the basal ganglia scales with the level of force during isometric contractions. Thus our findings are the first to implicate the bilateral GPi and STN in the control of force amplitude in humans.
In addition, we have found that the GPe, putamen, and caudate do not significantly scale in BOLD percent signal change or in activation volume with the level of force. Pope and colleagues (2005) recently showed that alternating between 16 and 8 N of force compared with producing 12 N of force repetitively resulted in an increase in activation in the putamen. Because the average level of force was the same between the alternating and repetitive force conditions, the activation these authors observed in the putamen was likely due to the subjects switching between levels of force. Our findings in GPe and the striatum could also be perceived as being in contrast to the two PET studies that found an increased rCBF in the bilateral putamen with movement velocity and extent (Turner et al. 1998, 2003). However, these two studies examined single joint movements and not isometric contractions and the task may lead to different results. For instance, when moving the limb to a longer distance the duration over which the muscle is active will increase, and therefore the duration of the muscle contraction could influence how the activation in the basal ganglia scales with movement amplitude. This explanation is consistent with the results from another study using fMRI in humans, which demonstrated that BOLD activation in the GPe, putamen, and caudate scales with increasing the duration of force during isometric contractions (Vaillancourt et al. 2004). Moreover, in the current study duration was held constant and these same nuclei did not scale in either percent signal change or activation volume with the level of force, suggesting that GPe, putamen, and caudate may code for the duration of the contraction.
In addition, we investigated the BOLD activation in several thalamic ROIs that have been previously related to motor areas in the basal ganglia and motor cortex. Several anatomical studies using multiple tracers in monkeys indicate that thalamic projections to the basal ganglia and motor cortex originate predominantly in the ventrolateral nuclei of the thalamus (VPl, VL, and VA) (Herrero et al. 2002; Holsapple et al. 1991; Kultas-Ilinsky et al. 1997; Kuypers et al. 1980; Rouiller et al. 1999; Schell and Strick 1984; Strick 1976b). One study using horseradish peroxidase injected into the arm representation of M1 also demonstrated sparse neuronal labeling in the CM nucleus of the thalamus (Strick 1975). These anatomical connections have been further supported by findings from functional studies that demonstrate functional connectivity within the basal ganglia-thalamo-cortical loop. For instance, a study using single-cell recording in the ventral thalamus of nonhuman primates demonstrated that microstimulation applied in the GPi resulted in the suppression of neural discharge in the VApc (parvocellularis), VLo (pars oralis), and VLc (pars caudalis) of the thalamus (Buford et al. 1996). It was also determined that microstimulation in the VPl and VLc resulted in a palpable or visible motor response (Buford et al. 1996). Although several electrophyiological studies have demonstrated movement-related changes in neural activity in the ventral thalamus (Anderson and Turner 1991; Forlano et al. 1993; Ivanusic et al. 2005; Strick 1976a), none reported a relation between discharge rate and either movement amplitude, velocity, or force. Imaging studies of the thalamus as a single ROI using PET and fMRI in humans have shown increased activation during increases in movement velocity and force amplitude (Dettmers et al. 1995; Ehrsson et al. 2000). A study using PET found increased rCBF in the VA and VL nuclei of the thalamus when humans produce movements with increased velocity (Turner et al. 1998). However, no study has directly investigated activation patterns in individual thalamic nuclei that are related to force. Our findings support the converging evidence from previous studies that the ventral thalamic nuclei (VA, VL, VP) are related to movement in human and nonhuman primates. Furthermore, our observation that activation in the CM nucleus is not significantly related to force amplitude control is consistent with the lack of dense anatomical connections to the motor cortex (Strick 1975) and the lack of electrophysiological evidence in the literature.
Previous studies have demonstrated movement-related changes in neural activation in the contralateral M1/S1. Early electrophysiological studies in nonhuman primates have shown that the firing rate of pyramidal tract neurons in the contralateral motor cortex was positively related to the amplitude of force produced (Cheney and Fetz 1980; Evarts 1968). Further studies using PET and fMRI in humans have repeatedly shown increased neural activation in contralateral M1 with the level of force during isometric contractions (Dai et al. 2001; Dettmers et al. 1995, 1996; Ehrsson et al. 2000). Although these studies provide a clear description of neural activation in contralateral M1, few studies have examined activation patterns across force in ipsilateral M1. One study using EEG in humans demonstrated coherence in motor-related potentials in contra- and ipsilateral M1 during sustained isometric finger extension (Svoboda et al. 2002). Also, studies using fMRI and PET have shown that neural activation increases in ipsilateral M1 with the amplitude of force when using a pinch- or power-grip (Dai et al. 2001; Dettmers et al. 1996; Ehrsson et al. 2000; Peck et al. 2001). Despite these recent findings, it has remained unclear how the pattern of neural activation in the contralateral and ipsilateral M1 change relative to each other across force amplitude.
Our data indicate that the contralateral M1/S1 has a greater level of BOLD percent signal change and activation volume than the ipsilateral M1/S1 at all of the force amplitudes tested (Fig. 6, Tables 1, 2, and 3). We also found that the rate of increase in the BOLD response was greater for the contralateral M1 compared with the ipsilateral M1. Because several studies using fMRI in humans have found increased activity in the ipsilateral motor and premotor cortex during precision-grip tasks when compared with power-grip tasks (Cramer et al. 2002; Dai et al. 2001; Ehrsson et al. 2000; Takasawa et al. 2003), it has been hypothesized that ipsilateral activation during precision-grip tasks reflects the additional need for sensitive somatosensory control (Ehrsson et al. 2000). Therefore it is possible that the nature of the pinch-grip task used in the current experiment enhanced our ability to detect a significant increase in activation in ipsilateral M1/S1.
Our data also extend prior anatomical work in nonhuman primates. An anterograde transport study in rhesus monkeys showed that 18% of the projections from the right M1 terminate ipsilaterally (and 82% contralaterally) in the intermediate zone of the spinal cord (cervical enlargement) and in portions of the ventral horn (Dum and Strick 1996; Picard and Strick 1996). Both sides of M1 have been shown to connect directly to the motoneurons essential for independent finger movement and precision-grip in nonhuman primates (Kuypers et al. 1980). Although the observed differences in BOLD activation are consistent with this anatomical description, the anatomy does not fully explain the role of the ipsilateral projections in the control of force. For example, it is possible that the ipsilateral M1 could increase in BOLD activation only at very high force levels, once the activation in contralateral M1 reached a ceiling. Thus this study is important in showing that ipsilateral M1 increased in BOLD percent signal change and activation volume across force (rate of change of force), albeit with a different slope than that of the contralateral M1. Both the greater slope across force and greater overall level of activation in the contralateral M1/S1 compared with those of the ipsilateral M1/S1 could be related to transcallosal inhibition through interhemispheric inhibition (Ferbert et al. 1992; Gerloff et al. 1998). This hypothesis is also consistent with our finding that the ipsilateral M1/S1 had greater deactivation compared with that of the contralateral M1/S1 (Table 4). It is also possible that ipsilateral projections by the corticospinal tract explain these interhemispheric differences and that the ipsilateral M1/S1 regulates a smaller number of motor units that control force. Because the two mechanisms are hypothesized to occur through distinct populations of motor cortical neurons both mechanisms could also occur simultaneously to explain the current findings in the ipsilateral motor cortex (Lee et al. 2007).
Force amplitude and rate of change of force: implications for basal ganglia function
The current findings have shown that the percent signal change in GPi and STN is proportional to both the force amplitude and the rate of change of force, when the duration of force was held constant. However, the findings for the rate of change of force are in contrast to prior work in our laboratory, which demonstrates that the activation in GPi and STN is inversely related to the rate of change of force (Vaillancourt et al. 2004). We propose that this discrepancy may be attributed to the differences in the strategy the subjects use in the two tasks. For instance, the current study required subjects to reach the target force “as fast as possible” and the explicit instruction was to produce force at the target force level. Thus we did not explicitly instruct the subjects to control the rate of change of force, yet this parameter naturally covaried with the level of force (Fig. 2B). On the other hand, the task used in the previous study (Vaillancourt et al. 2004) required subjects to explicitly control the rate of change of force by reaching the same target force amplitude over different periods of time. During the task, when the force increased slowly during a ramp contraction that lasted 3 s this resulted in the greatest activation in GPi and STN compared with a fast ballistic contraction that lasted a few hundred milliseconds. That study also showed that other basal ganglia regions such as the caudate, putamen, and GPe all increased in activation with the duration of the force contraction, although these nuclei did not covary with the rate of change of force. The current study extends this result by showing that caudate, putamen, and GPe did not scale in activation across force when the duration of the contraction was constant. Thus the converging evidence supports the hypothesis that the duration of force is coded by basal ganglia nuclei including the caudate, putamen, and GPe, whereas GPi and STN code the amplitude of force and the rate of change of force. Moreover, we suggest that depending on whether the subject regulates the rate of change of force implicitly or explicitly will determine the positive or negative scaling pattern for the activation in GPi and STN. The larger between-subject variance for the rate of change of force compared with the amplitude of force (Fig. 2, A and B) is also consistent with the interpretation that subjects were explicitly regulating the amplitude of force rather than the rate in the current study.
In conclusion, although movement parameters (i.e., velocity, extent) have been a frequent topic for study in human and nonhuman primates, the roles of the basal ganglia, thalamus, and motor cortex in scaling the amplitude of force have yet to be defined. The current study used neuroimaging techniques in humans to reveal three novel findings: 1) the GPi and STN scale positively in activation with force amplitude (rate); 2) the VA, VL, VPl, and VPm nuclei of the thalamus scale positively in activation with force amplitude (rate); and 3) the contralateral and ipsilateral M1/S1 scale positively with increasing force amplitude (rate), and the contralateral M1/S1 had a greater overall increase than did the ipsilateral side in percent signal change. These findings support a complex relationship between the basal ganglia and force output contractions that vary in amplitude, duration, and rate. We proposed a hypothesis that distinct nuclei in the basal ganglia regulate the duration of force and the magnitude of force, and that the pattern of change in GPi and STN depends on whether the task requires implicit or explicit control of the rate of change of force. Further experimentation will be required to thoroughly evaluate this hypothesis and construct a functional model that characterizes both the force scaling and ramplike behavior of the basal ganglia.
Acknowledgments
We thank the staff at the Center for Magnetic Resonance Research at University of Illinois at Chicago for contributions in data collection.
GRANTS
This research was supported in part by National Institute of Neurological Disorders and Stroke Grants R01-NS-52318, R01-NS-28127, and R01-NS-40902.
References
- Anderson ME, Turner RS. Activity of neurons in cerebellar-receiving and pallidal-receiving areas of the thalamus of the behaving monkey. J Neurophysiol. 1991;66:879–893. doi: 10.1152/jn.1991.66.3.879. [DOI] [PubMed] [Google Scholar]
- Ashe J. Force and the motor cortex. Behav Brain Res. 1997;87:255–269. doi: 10.1016/s0166-4328(97)00752-3. [DOI] [PubMed] [Google Scholar]
- Buford JA, Inase M, Anderson ME. Contrasting locations of pallidal-receiving neurons and microexcitable zones in primate thalamus. J Neurophysiol. 1996;75:1105–1116. doi: 10.1152/jn.1996.75.3.1105. [DOI] [PubMed] [Google Scholar]
- Cheney PD, Fetz EE. Functional classes of primate corticomotoneuronal cells and their relation to active force. J Neurophysiol. 1980;44:773–791. doi: 10.1152/jn.1980.44.4.773. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Weisskoff RM, Schaechter JD, Nelles G, Foley M, Finklestein SP, Rosen BR. Motor cortex activation is related to force of squeezing. Hum Brain Mapp. 2002;16:197–205. doi: 10.1002/hbm.10040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai TH, Liu JZ, Sahgal V, Brown RW, Yue GH. Relationship between muscle output and functional MRI-measured brain activation. Exp Brain Res. 2001;140:290–300. doi: 10.1007/s002210100815. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Grafton ST, Vindras P, Grea H, Turner RS. The basal ganglia network mediates the planning of movement amplitude. Eur J Neurosci. 2004;19:2871–2880. doi: 10.1111/j.0953-816X.2004.03395.x. [DOI] [PubMed] [Google Scholar]
- Dettmers C, Connelly A, Stephan KM, Turner R, Friston KJ, Frackowiak RS, Gadian DG. Quantitative comparison of functional magnetic resonance imaging with positron emission tomography using a force-related paradigm. Neuroimage. 1996;4:201–209. doi: 10.1006/nimg.1996.0071. [DOI] [PubMed] [Google Scholar]
- Dettmers C, Fink GR, Lemon RN, Stephan KM, Passingham RE, Silbersweig D, Holmes A, Ridding MC, Brooks DJ, Frackowiak RS. Relation between cerebral activity and force in the motor areas of the human brain. J Neurophysiol. 1995;74:802–815. doi: 10.1152/jn.1995.74.2.802. [DOI] [PubMed] [Google Scholar]
- Dormont D, Ricciardi KG, Tande D, Parain K, Menuel C, Galanaud D, Navarro S, Cornu P, Agid Y, Yelnik J. Is the subthalamic nucleus hypointense on T2-weighted images? A correlation study using MR imaging and stereotactic atlas data. Am J Neuroradiol. 2004;25:1516–1523. [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL. Spinal cord terminations of the medial wall motor areas in macaque monkeys. J Neurosci. 1996;16:6513–6525. doi: 10.1523/JNEUROSCI.16-20-06513.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrsson HH, Fagergren A, Jonsson T, Westling G, Johansson RS, Forssberg H. Cortical activity in precision- versus power-grip tasks: an fMRI study. J Neurophysiol. 2000;83:528–536. doi: 10.1152/jn.2000.83.1.528. [DOI] [PubMed] [Google Scholar]
- Evarts EV. Relation of pyramidal tract activity to force exerted during voluntary movement. J Neurophysiol. 1968;31:14–27. doi: 10.1152/jn.1968.31.1.14. [DOI] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlano LM, Horne MK, Butler EG, Finkelstein D. Neural activity in the monkey anterior ventrolateral thalamus during trained, ballistic movements. J Neurophysiol. 1993;70:2276–2288. doi: 10.1152/jn.1993.70.6.2276. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP. On reaching. Annu Rev Neurosci. 1986;9:147–170. doi: 10.1146/annurev.ne.09.030186.001051. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP, DeLong MR, Crutcher MD. Relations between parameters of step-tracking movements and single cell discharge in the globus pallidus and subthalamic nucleus of the behaving monkey. J Neurosci. 1983;3:1586–1598. doi: 10.1523/JNEUROSCI.03-08-01586.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerloff C, Cohen LG, Floeter MK, Chen R, Corwell B, Hallett M. Inhibitory influence of the ipsilateral motor cortex on responses to stimulation of the human cortex and pyramidal tract. J Physiol. 1998;510:249–259. doi: 10.1111/j.1469-7793.1998.249bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AM, Quinn L, Reilmann R, Marder K. Coordination of prehensile forces during precision grip in Huntington’s disease. Exp Neurol. 2000;163:136–148. doi: 10.1006/exnr.2000.7348. [DOI] [PubMed] [Google Scholar]
- Hepp-Reymond M, Kirkpatrick-Tanner M, Gabernet L, Qi HX, Weber B. Context-dependent force coding in motor and premotor cortical areas. Exp Brain Res. 1999;128:123–133. doi: 10.1007/s002210050827. [DOI] [PubMed] [Google Scholar]
- Hepp-Reymond MC, Wyss UR, Anner R. Neuronal coding of static force in the primate motor cortex. J Physiol (Paris) 1978;74:287–291. [PubMed] [Google Scholar]
- Herrero MT, Barcia C, Navarro JM. Functional anatomy of thalamus and basal ganglia. Childs Nerv Syst. 2002;18:386–404. doi: 10.1007/s00381-002-0604-1. [DOI] [PubMed] [Google Scholar]
- Holsapple JW, Preston JB, Strick PL. The origin of thalamic inputs to the “hand” representation in the primary motor cortex. J Neurosci. 1991;11:2644–2654. doi: 10.1523/JNEUROSCI.11-09-02644.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ifthikharuddin SF, Shrier DA, Numaguchi Y, Tang X, Ning R, Shibata DK, Kurlan R. MR volumetric analysis of the human basal ganglia: normative data. Acad Radiol. 2000;7:627–634. doi: 10.1016/s1076-6332(00)80579-6. [DOI] [PubMed] [Google Scholar]
- Ivanusic JJ, Bourke DW, Xu ZM, Butler EG, Horne MK. Cerebellar thalamic activity in the macaque monkey encodes the duration but not the force or velocity of wrist movement. Brain Res. 2005;1041:181–197. doi: 10.1016/j.brainres.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kultas-Ilinsky K, Reising L, Yi H, Ilinsky IA. Pallidal afferent territory of the Macaca mulatta thalamus: neuronal and synaptic organization of the VAdc. J Comp Neurol. 1997;386:573–600. doi: 10.1002/(sici)1096-9861(19971006)386:4<573::aid-cne5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Kuypers HG, Bentivoglio M, Catsman-Berrevoets CE, Bharos AT. Double retrograde neuronal labeling through divergent axon collaterals, using two fluorescent tracers with the same excitation wavelength which label different features of the cell. Exp Brain Res. 1980;40:383–392. doi: 10.1007/BF00236147. [DOI] [PubMed] [Google Scholar]
- Lee H, Gunraj C, Chen R. The effects of inhibitory and faclitatory intracortical circuits on interhemispheric inhibition in the human motor cortex. J Physiol. 2007;580:1021–1032. doi: 10.1113/jphysiol.2006.126011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayka MA, Corcos DM, Leurgans SE, Vaillancourt DE. Three-dimensional locations and boundaries of motor and premotor cortices as defined by functional brain imaging: a meta-analysis. Neuroimage. 2006;31:1453–1474. doi: 10.1016/j.neuroimage.2006.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muley SA, Strother SC, Ashe J, Frutiger SA, Anderson JR, Sidtis JJ, Rottenberg DA. Effects of changes in experimental design on PET studies of isometric force. Neuroimage. 2001;13:185–195. doi: 10.1006/nimg.2000.0676. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Rosenkranz K, Topka H, Rothwell JC. Disturbances of grip force behaviour in focal hand dystonia: evidence for a generalised impairment of sensory-motor integration. J Neurol Neurosurg Psychiatry. 2005b;76:953–959. doi: 10.1136/jnnp.2004.043943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak DA, Topka H, Tisch S, Hariz M, Limousin P, Rothwell JC. The beneficial effects of subthalamic nucleus stimulation on manipulative finger force control in Parkinson’s disease. Exp Neurol. 2005a;193:427–436. doi: 10.1016/j.expneurol.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Peck KK, Sunderland A, Peters AM, Butterworth S, Clark P, Gowland PA. Cerebral activation during a simple force production task: changes in the time course of the haemodynamic response. Neuroreport. 2001;12:2813–2816. doi: 10.1097/00001756-200109170-00012. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex. 1996;6:342–353. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- Pope P, Wing AM, Praamstra P, Miall RC. Force related activations in rhythmic sequence production. Neuroimage. 2005;27:909–918. doi: 10.1016/j.neuroimage.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Richter EO, Hoque T, Halliday W, Lozano AM, Saint-Cyr JA. Determining the position and size of the subthalamic nucleus based on magnetic resonance imaging results in patients with advanced Parkinson disease. J Neurosurg. 2004;100:541–546. doi: 10.3171/jns.2004.100.3.0541. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Tanne J, Moret V, Boussaoud D. Origin of thalamic inputs to the primary, premotor, and supplementary motor cortical areas and to area 46 in macaque monkeys: a multiple retrograde tracing study. J Comp Neurol. 1999;409:131–152. doi: 10.1002/(sici)1096-9861(19990621)409:1<131::aid-cne10>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Schell GR, Strick PL. The origin of thalamic inputs to the arcuate premotor and supplementary motor areas. J Neurosci. 1984;4:539–560. doi: 10.1523/JNEUROSCI.04-02-00539.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick PL. Multiple sources of thalamic input to the primate motor cortex. Brain Res. 1975;88:372–377. doi: 10.1016/0006-8993(75)90402-3. [DOI] [PubMed] [Google Scholar]
- Strick PL. Activity of ventrolateral thalamic neurons during arm movement. J Neurophysiol. 1976a;39:1032–1044. doi: 10.1152/jn.1976.39.5.1032. [DOI] [PubMed] [Google Scholar]
- Strick PL. Anatomical analysis of ventrolateral thalamic input to primate motor cortex. J Neurophysiol. 1976b;39:1020–1031. doi: 10.1152/jn.1976.39.5.1020. [DOI] [PubMed] [Google Scholar]
- Svoboda J, Sovka P, Stancak A. Intra- and inter-hemispheric coupling of electroencephalographic 8–13 Hz rhythm in humans and force of static finger extension. Neurosci Lett. 2002;334:191–195. doi: 10.1016/s0304-3940(02)01070-4. [DOI] [PubMed] [Google Scholar]
- Takasawa M, Oku N, Osaki Y, Kinoshita H, Imaizumi M, Yoshikawa T, Kimura Y, Kajimoto K, Sasagaki M, Kitagawa K, Hori M, Hatazawa J. Cerebral and cerebellar activation in power and precision grip movements: an H2 15O positron emission tomography study. J Cereb Blood Flow Metab. 2003;23:1378–1382. doi: 10.1097/01.WCB.0000091258.83091.C2. [DOI] [PubMed] [Google Scholar]
- Thickbroom GW, Phillips BA, Morris I, Byrnes ML, Mastaglia FL. Isometric force-related activity in sensorimotor cortex measured with functional MRI. Exp Brain Res. 1998;121:59–64. doi: 10.1007/s002210050437. [DOI] [PubMed] [Google Scholar]
- Thulborn KR, Shen GX. An integrated head immobilization system and high-performance RF coil for fMRI of visual paradigms at 1.5 T. J Magn Reson. 1999;139:26–34. doi: 10.1006/jmre.1999.1748. [DOI] [PubMed] [Google Scholar]
- Todorov E. Direct cortical control of muscle activation in voluntary arm movements: a model. Nat Neurosci. 2000;3:391–398. doi: 10.1038/73964. [DOI] [PubMed] [Google Scholar]
- Turner RS, Anderson ME. Pallidal discharge related to the kinematics of reaching movements in two dimensions. J Neurophysiol. 1997;77:1051–1074. doi: 10.1152/jn.1997.77.3.1051. [DOI] [PubMed] [Google Scholar]
- Turner RS, Desmurget M, Grethe J, Crutcher MD, Grafton ST. Motor subcircuits mediating the control of movement extent and speed. J Neurophysiol. 2003;90:3958–3966. doi: 10.1152/jn.00323.2003. [DOI] [PubMed] [Google Scholar]
- Turner RS, Grafton ST, Votaw JR, Delong MR, Hoffman JM. Motor subcircuits mediating the control of movement velocity: a PET study. J Neurophysiol. 1998;80:2162–2176. doi: 10.1152/jn.1998.80.4.2162. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Mayka MA, Thulborn KR, Corcos DM. Subthalamic nucleus and internal globus pallidus scale with the rate of change of force production in humans. Neuroimage. 2004;23:175–186. doi: 10.1016/j.neuroimage.2004.04.040. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Thulborn KR, Corcos DM. Neural basis for the processes that underlie visually guided and internally guided force control in humans. J Neurophysiol. 2003;90:3330–3340. doi: 10.1152/jn.00394.2003. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Yu H, Mayka MA, Corcos DM. Role of the basal ganglia and frontal cortex in selecting and producing internally guided force pulses. Neuroimage. 2007;36:793–803. doi: 10.1016/j.neuroimage.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eimeren T, Siebner HR. An update on functional neuroimaging of parkinsonism and dystonia. Curr Opin Neurol. 2006;19:412–419. doi: 10.1097/01.wco.0000236623.68625.54. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Rosen B, Farde L. Imaging the living human brain: magnetic resonance imaging and positron emission tomography. Proc Natl Acad Sci USA. 1997;94:2787–2788. doi: 10.1073/pnas.94.7.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegell MR, Tuch DS, Larsson HBW, Wedeen VJ. Automatic segmentation of thalamic nuclei from diffusion tensor magnetic resonance imaging. Neuroimage. 2003;19:391–401. doi: 10.1016/s1053-8119(03)00044-2. [DOI] [PubMed] [Google Scholar]
- Wu T, Hallett M. A functional MRI study of automatic movements in patients with Parkinson’s disease. Brain. 2005;128:2250–2259. doi: 10.1093/brain/awh569. [DOI] [PubMed] [Google Scholar]
- Yelnik J. Functional anatomy of the basal ganglia. Mov Disord. 2002;17(Suppl 3):S15–S21. doi: 10.1002/mds.10138. [DOI] [PubMed] [Google Scholar]


