Abstract
Objective
mXinα, a downstream target gene of Nkx2.5 transcription factor, was shown to encode a proline-rich and Xin repeats-containing protein which localizes to the intercalated disc of adult hearts. Our previous voltage-clamp studies have shown that the ventricular myocytes of mXinα-deficient mice exhibited a significant reduction in K+ currents (Ito and IK1), L-type Ca2+ currents, and maximum diastolic potential, leading to the development of early afterdepolarization (EAD) and arrhythmias. However, changes in cationic inward currents could also contribute to the genesis of EAD and arrhythmias in mXinα-deficient mice.
Methods
The present study aims to characterize changes in Na+ currents on depolarization and transient inward currents (Iti) on repolarization. Conduction velocity (CV) on the frontal surface of ventricles were also measured and compared.
Results
Results of optical mapping on the Langendorff-perfused hearts at 37°C revealed a 36% reduction of CV in mXinα-/- ventricle. Pacing (3 Hz)-induced tachyarrhythmias were more frequently found and ventricular fibrillation (VF, 21 Hz for 5 min) occurred in one out of 8 mXinα-/- heart. When perfused at 30°C, no VF was observed in both types of preparations. Voltage-clamp study on isolated ventricular myocytes at 37°C shows increase in INa and Iti in mXinα-/- cardiomyocytes thus could explain the occurrence of re-entrant triggered arrhythmias.
Conclusion
The present results revealed that the CV was slower, but INa and Iti were increased in mXinα-/-cardiomyocytes thus were prone to reentrant triggered arrhythmias. Hypothermia could reduce the occurrence of arrhythmias.
Keywords: arrhythmia, conduction velocity, ionic currents, Xinα-deficient murine heart, temperature, voltage-clamp studies
Introduction
Ablation of mXinα gene, encoding an intercalated discs protein (1), results in cardiac hypertrophy and cardiomyopathy with a reduction in connexin 43 expression (2). Previous voltage-clamp study revealed that mXinα-deficient ventricular myocytes have markedly reduced L-type Ca2+ currents, transient outward K+ currents and barium-sensitive inward rectifier K+ currents, in agreement with some of the abnormal action potential (AP) characteristics, such as prolonged action potential (AP) and reduced maximal diastolic potential (3). However, further experiments are required to explain the ionic mechanisms, in addition to the reduced K+ currents, leading to the development of early afterdepolarizations (EADs) and slow response APs (4) observed in mXinα-/- myocytes. Changes in inward cationic currents might also contribute to EADs, slow response APs during depolarization and transient inward currents (Iti) on repolarization (5). The present study aims to characterize changes in Na+ currents, Iti and conduction velocity (CV) of the mXinα-/- murine ventricular myocardium.
Methods
Whole-cell patch-clamp techniques (6, 7) were used on ventricular myocytes isolated enzymatically from 16 to 20-week-old wild-type and mXinα-/- male mice. Inward Na+ currents were measured on depolarization for 40 ms in 22-24°C Tyrode solution containing 5 mM NaCl (133 mM NaCl was replaced by equimolar CsCl), 5 mM HEPES and 2 μM nifedipine (an ICa,L blocker). Transient inward currents (Iti) were measured on repolarization after a series of depolarizing pulses (50-3050 ms) in 37°C HEPES-Tyrode solution as described (5). Optical mapping technique was used to measure CV of murine ventricular myocardium as described previously (8). In brief, the ascending aorta was cannulated and the heart was perfused with standard oxygenated Tyrode solution (2.5 ml/min) of the following composition (in mM): NaCl 137, NaHCO3 15.5, NaH2PO4 0.7, CaCl2 1.8, KCl 4, MgCl2 1, and glucose 11.1, gassed with gas mixture (95% O2-5% CO2) to pH 7.2-7.4 (37°C). Murine heart was stained with 15 mL standard oxygenated Tyrode solution plus 30 μL of 2 mM di-4-ANEPPS in DMSO (Molecular Probe, Paisley, UK) to monitor cardiac APs and paced (3 Hz, 3 ms in duration, 3 fold threshold voltage) at the basal portion of the right ventricle from a stimulator (S88J, Grass, West Warwick, Rhode Island, USA). The murine heart was further perfused with 100 mL standard oxygenated Tyrode solution plus 25 μL of 10 μM cytochalasin D (Sigma, Missouri, USA) to block cardiac contraction. The APs were then recorded and the CV was calculated as described (8) on the frontal surface of ventricle using optical mapping method.
Statistical Analysis
Quantitative data were expressed as mean±S.E.M. Unpaired Student’s t test and Chi-square test were used for comparison of data between groups. P values less than 0.05 were considered to be statistically significant.
Results
Measurement of CV on the frontal surface of ventricle in Langendorff perfused heart
Figure 1 shows representative activation maps obtained from the frontal surface of ventricles of mXinα+/+ and mXinα-/- hearts. When paced at the base portion of the right ventricle, activation spreads progressively in the mXinα+/+ heart. In contrast, a slower activation with frequently interrupted propagation was observed in the mXinα-/- heart. The CV could be determined from these activation maps. The CV was significantly slower in mXinα-/- heart than in mXinα+/+ heart. At 37°C, the average CV calculated from 11 mXinα+/+ ventricles was 86±9 cm/s, whereas the CV from 6 mXinα-/- ventricles reduced significantly to 55±7 cm/s (p<0.05). The incidence of pacing (3 Hz)-induced ventricular tachycardia (VT) was 1 in 12 mXinα+/+ preparations, whereas the frequency (4 out of 8) of pacing-induced VT was significantly higher in mXinα-/- preparations (p<0.05). Ventricular fibrillation (VF, 21 Hz for 5 min) occurred in one out of 8 mXinα-/- ventricles but none in 12 mXinα+/+ ventricles (1/8 vs. 0/12, p>0.05). At a lower temperature (30 °C), the CV of 3 mXinα+/+ ventricles decreased to 60±14 cm/s and, interestingly, there was no significant changes in the CV of 3 mXinα-/- ventricles (56±8 cm/s). At 30°C no VF was observed in both groups of ventricles although the incidence of VT appeared higher in mXinα-/- (4/5) ventricles as compared to 1/4 in mXinα+/+ ventricles (p>0.05). Thus, a moderate hypothermia appears to reduce the occurrence of arrhythmias.
Figure 1.
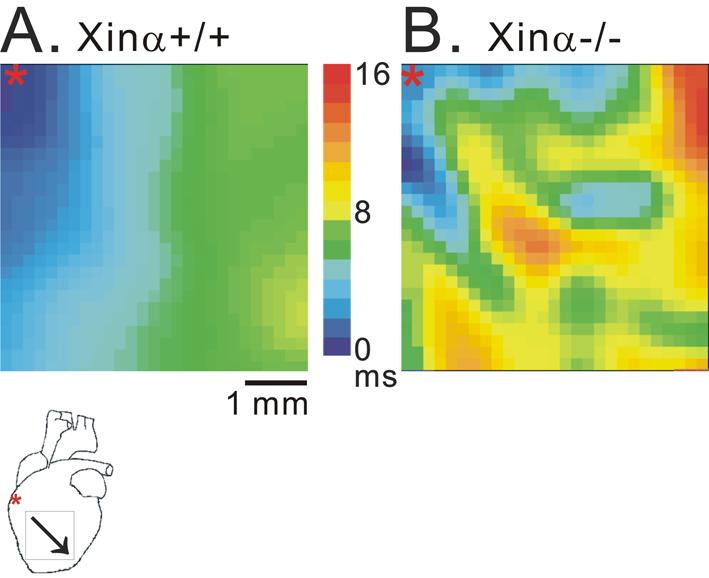
Activation maps of murine ventricles induced by 3 Hz electrical pacing at 37°C. Panel A and panel B show the activation maps of a wild-type (mXinα+/+, CV=88 cm/s) and a mXinα-deficient (mXinα-/-, CV=44 cm/s), respectively, on the frontal surface of Langendorff’s perfused ventricular myocardium. In the diagram underneath panel A, *indicates site of electrical stimulation and oblique arrow indicates direction of activation. Note the slower CV and presence of irregular conduction in the mXinα-/- heart (panel B).
CV- conduction velocity
Experiments on ventricular myocytes isolated from wild type and mXinα-/- mice
The membrane capacitance (Cm) of isolated ventricular myocytes were measured by a small hyperpolarizing step (from -50 to -55 mV) (5-7). Results revealed a 38% larger Cm (and therefore larger surface area) in mXinα-/- than in mXinα+/+ ventricular myocytes, consistent with hypertrophied mXinα-/- myocytes detected previously (2).
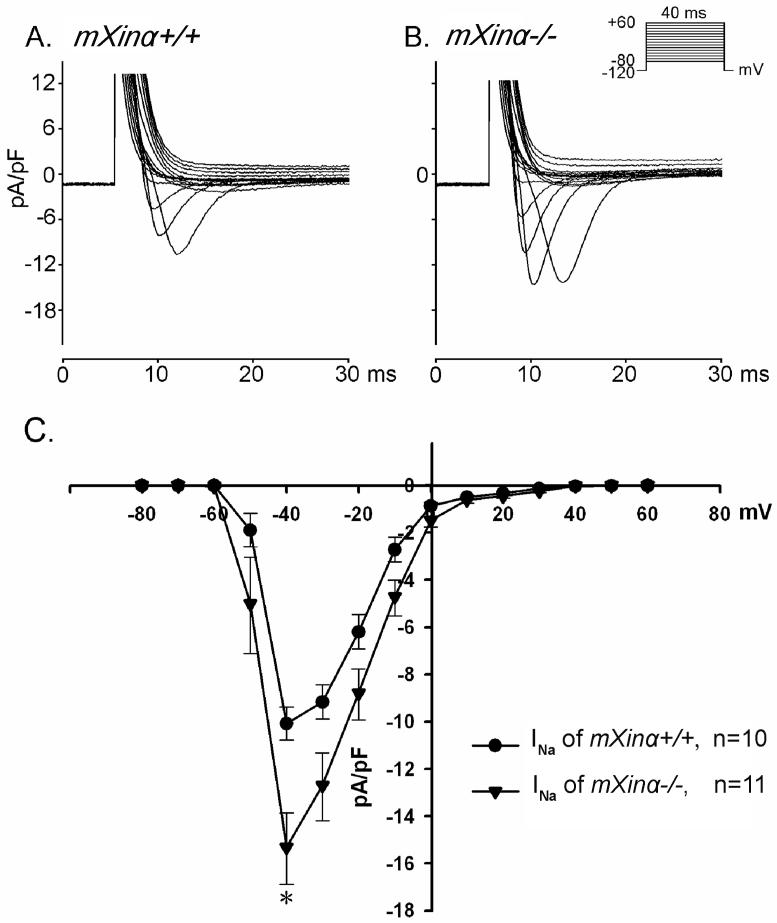
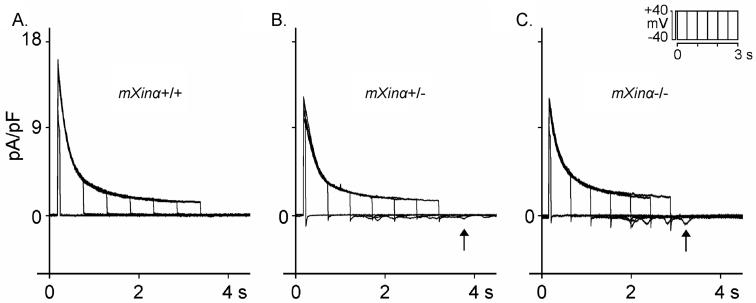
We determined further the Na+ inward currents (INa) in ventricular myocytes perfused in 22-24°C solution. Extracellular [Na+]o was reduced to 5 mM in the presence of 133 mM Cs+, 5 mM HEPES and 2 μM nifedipine. As shown in Figure 2, INa inward currents on depolarization was significantly larger in mXinα-/- than in mXinα+/+ ventricular myocyte. In 37°C HEPES-Tyrode solution (containing 137 mM [Na+]o), on repolarization to the holding potential (-40 mV) after prolonged depolarizing pulses (for 1050-3050 ms) (see Figure 3), the average oscillatory transient inward currents (Iti) after depolarizing pulse of 3050 ms (5) was 0.488±0.081 pA/pF for 28 mXinα-/- ventricular myocytes. For 27 mXinα+/+ myocytes, similar clamp protocol induced significantly smaller Iti (average values 0.203±0.059 pA/pF, p<0.05). Also average Iti in the 27 mXinα+/- (0.508±0.103 pA/pF) were significantly larger than the 27 mXinα+/+ myocytes (p<0.05).
Figure 2.
Sodium inward currents (INa) in a wild-type (mXinα+/+) (panel A) and a mXinα-deficient (mXinα-/-) (panel B) ventricular myocytes. Clamp protocol is shown at right upper corner. Panel C shows current-voltage relationships in the two groups of myocytes. n, number of myocytes tested. Values are mean±S.E.M. p<0.05 - differences are significant between groups by paired Student’s t test.
Figure 3.
Transient inward currents (Iti) induced on repolarization after depolarizing pulses of increasing durations (50, 550, 1050, 1550, 2050, 2550 and 3050 ms) applied once every 10 s on Iti (indicated by upward arrows after depolarizing pulse duration of 3050 ms). Superimposed current traces were recorded in wild-type (mXinα+/+, panel A), heterozygous (mXinα+/-, panel B) and homozygous (mXinα-/-, panel C) ventricular myocytes. Clamp protocol is shown at right upper corner.
Results of these patch-clamp studies indicate that both INa currents induced on depolarization and the arrhythmogenic Iti currents generated on repolarization to the holding potential were increased in mXinα-/- and mXinα+/- hearts.
Discussion
The present results, together with our previous report (3), clearly show that mXinα-/- mouse myocytes exhibit increased Na+ inward currents, reduced transient outward K+ currents and inwardly rectifying K+ currents in concomitant with prolonged AP and slower CV. These changes in electrophysiological properties of ventricular myocytes likely render mice being prone to develop EAD and VF. The enhanced Iti in mXinα-/- ventricular myocytes is in contrast to the depressed Iti observed in ventricular myocytes of dilated myopathic Syrian hamster (5). Thus the underlying cellular mechanisms for hypertrophied mXinα-/- myocardium (2, 9) were different from that of dilated cardiomyopathic hamster model (5, 10). We will incorporate research techniques used in the laboratory of Roshchevsky (11) in our joint research works in the future.
Acknowledgements
The present studies were supported by grants NSC95-2320-B016-013 (CIL), NSC95-2923-B016-001-MY2 (CIL, YCC), NSC-RFBR 050490586 (MPR) from National Science Council, MMH-95103 from the Mackay Memorial Hospital, Taipei, Taiwan, R.O.C., and a grant RO1 HL075015 from the NIH (JJCL), U.S.A.
References
- 1.Wang DZ, Reiter RS, Lin JL, Wang Q, Williams HS, Krob SL, et al. Requirement of a novel gene, Xin, in cardiac morphogenesis. Development. 1999;126:1281–94. doi: 10.1242/dev.126.6.1281. [DOI] [PubMed] [Google Scholar]
- 2.Gustafson-Wagner EA, Lin JLC, Sinn H, Wang DZ, Reiter RS, Yang B, et al. Targeted deletion of mXinα gene, encoding an intercalated disc protein, leads to cardiac hypertrophy (Abstract) AHA Scientific Sessions 2006; 2006 Nov 12-15; Chicago, IL, USA; Circulation. 2006;114(Suppl):II–54. [Google Scholar]
- 3.Cheng CP, Loh YX, Lin CI, Lai YJ, Chen YC, Sytwu HK, et al. In: Kimchi A, editor. Electrophysiological characteristics of ventricular myocytes of Xinα-deficient mice; Advances in Heart Disease. Proceedings of the 12th World Congress on Heart Disease; Vancouver, Canada; s.r.l. Bolongna, Italy; MEDIMOND. 2005 July 16-19.2005. pp. 25–9. [Google Scholar]
- 4.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 5.Wu SH, Chen YC, Higa S, Lin CI. Oscillatory transient inward currents in ventricular myocytes of healthy versus myopathic Syrian hamster. Clin Exp Pharmacol Physiol. 2004;31:668–76. doi: 10.1111/j.1440-1681.2004.04082.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen YJ, Chen SA, Chen YC, Yeh HI, Chan P, Chang MS, et al. Rapid atrial pacing increases the arrhythmogenic activity of single cardiomyocytes from pulmonary veins: implication in initiation of atrial fibrillation. Circulation. 2001;104:2849–54. doi: 10.1161/hc4801.099736. [DOI] [PubMed] [Google Scholar]
- 7.Chen YJ, Chen SA, Chen YC, Yeh HI, Chang MS, Lin CI. Electrophysiology of single cardiomyocytes isolated from rabbit pulmonary veins: implication in initiation of focal atrial fibrillation. Basic Res Cardiol. 2002;97:26–34. doi: 10.1007/s395-002-8384-6. [DOI] [PubMed] [Google Scholar]
- 8.Baker LC, London B, Choi BR, Koren G, Salama G. Enhanced dispersion of repolarization and refractoriness in transgenic mouse hearts promotes reentrant ventricular tachycardia. Circ Res. 2000;86:396–407. doi: 10.1161/01.res.86.4.396. [DOI] [PubMed] [Google Scholar]
- 9.Pashmforoush M, Lu JT, Chen H, St. Amand T, Kondo R, Pradervand S, et al. Nkx2-5 pathways and congenital heart disease: loss of ventricular myocyte lineage specification leads to progressive cardiomyopathy and complete heart block. Cell. 2004;117:373–86. doi: 10.1016/s0092-8674(04)00405-2. [DOI] [PubMed] [Google Scholar]
- 10.Homburger F. Myopathy of hamster dystrophy: history and morphologic aspects. Ann NY Acad Sci. 1979;317:1–7. [PubMed] [Google Scholar]
- 11.Roshchevsky MP, Chudorodova SL, Shmakov DN, Roshchevskaya IM. The cardioelectric field on the pig body surface during initial atrial activity. Doklady Biol Sci. 2005;402:561–2. doi: 10.1007/s10630-005-0079-9. [DOI] [PubMed] [Google Scholar]