Abstract
Summary
Background
Omphalitis contributes to neonatal morbidity and mortality in developing countries. Umbilical cord cleansing with antiseptics might reduce infection and mortality risk, but has not been rigorously investigated.
Methods
In our community-based, cluster-randomised trial, 413 communities in Sarlahi, Nepal, were randomly assigned to one of three cord-care regimens. 4934 infants were assigned to 4·0% chlorhexidine, 5107 to cleansing with soap and water, and 5082 to dry cord care. In intervention clusters, the newborn cord was cleansed in the home on days 1−4, 6, 8, and 10. In all clusters, the cord was examined for signs of infection (pus, redness, or swelling) on these visits and in follow-up visits on days 12, 14, 21, and 28. Incidence of omphalitis was defined under three sign-based algorithms, with increasing severity. Infant vital status was recorded for 28 completed days. The primary outcomes were incidence of neonatal omphalitis and neonatal mortality. Analysis was by intention-to-treat. This trial is registered with Clinicaltrials.gov, number NCT00109616.
Findings
Frequency of omphalitis by all three definitions was reduced significantly in the chlorhexidine group. Severe omphalitis in chlorhexidine clusters was reduced by 75% (incidence rate ratio 0·25, 95% CI 0·12−0·53; 13 infections/4839 neonatal periods) compared with dry cord-care clusters (52/4930). Neonatal mortality was 24% lower in the chlorhexidine group (relative risk 0·76 [95% CI 0·55−1·04]) than in the dry cord care group. In infants enrolled within the first 24 h, mortality was significantly reduced by 34% in the chlorhexidine group (0·66 [0·46−0·95]). Soap and water did not reduce infection or mortality risk.
Interpretation
Recommendations for dry cord care should be reconsidered on the basis of these findings that early antisepsis with chlorhexidine of the umbilical cord reduces local cord infections and overall neonatal mortality.
Introduction
Most of the 4 million neonatal deaths every year are in low-income and middle-income countries.1 Infections account for an estimated 1·44 million (36%) deaths, and about half of deaths in regions with high neonatal mortality rates.2 Contamination of the umbilical cord can lead to omphalitis, characterised by pus, abdominal erythema, or swelling. Pathogens can enter the bloodstream through the patent vessels of the newly cut cord and lead to rapid demise, even in the absence of overt signs of cord infection.3 Hygienic delivery and postnatal-care practices are widely promoted as important interventions to reduce risk of omphalitis and death.4 There are few specific data, however, on omphalitis incidence and little evidence for optimum cord-care practices to prevent cord infections and mortality in the community, so better studies are urgently needed.
Investigators in hospital-based studies in developing countries have described the characteristics of omphalitis,5–9 and reported a range of incidence estimates (2−77 per 1000 hospital-born infants).7,8 In the community, where infectious challenge is higher and many cases go unrecognised, risk could be higher. A review of omphalitis in Oman9 noted that the incidence rate in home-delivered neonates was 5·9 times higher than in hospital births.
Community-based case-control studies have focused on risk factors for neonatal tetanus,10–17 and provide some evidence that topical antiseptics on the cord are protective.11,17,18 In settings where neonatal tetanus has been eliminated but unhygienic cord practices continue to place newborn babies at risk, topical antiseptics might protect against infection.19
In developed countries, many studies20–27 have shown that single or repeated antiseptic applications to the cord can substantially reduce bacterial colonisation. The link between cord colonisation and infection, however, has not been firmly established,28,29 prompting a trend towards dry cord care. WHO promotes dry cord care for developing countries, although notes that antiseptics might benefit infants in settings where harmful substances are traditionally applied.3 The current WHO recommendations for dry cord care, however, are based on inadequate data and could be inappropriate in areas with high omphalitis risk.
Of the numerous potential topical antimicrobials for cord care (eg, ethanol, silver sulfadiazine, triple dye, gentian violet, chlorhexidine, povidone iodine), chlorhexidine seems to be a favourable choice. It has broad-spectrum activity against gram-positive and gram-negative organisms, an extensive safety record, strong binding potential that results in residual effectiveness, and low cost.30 Chlorhexidine use substantially reduces bacterial colonisation of the cord stump28 and may be associated with reduced superficial skin infections.21,22,31 Chlorhexidine is currently included in the WHO Essential Drug List and can be maintained in non-alcohol aqueous solutions. Furthermore, WHO recommends that chlorhexidine is the preferred agent if an antiseptic is to be used on the cord, for example in settings in which it might be strategic to use antiseptic applications to the cord to discourage the use of dung or other unclean substances.3 We therefore aimed to investigate whether chlorhexidine applied to the cord prevents omphalitis and reduces mortality.
Methods
Settings and population
This double-masked, placebo-controlled, cluster-randomised, community-based trial was done by the Nepal Nutrition Intervention Project, Sarlahi (NNIPS), between November, 2002, and March, 2005. The NNIPS surveillance area consisted of 30 village development committees, each encompassing nine government-defined geopolitical units (wards), which we further divided into sectors on the basis of population. In every sector, a local female worker provided interventions to 50−100 households. All infants born after Nov 17, 2002, in the 413 actively monitored sectors were eligible for enrolment in the cord cleansing trial. This study was approved by the Nepal Health Research Council and the Committee on Human Research of the Johns Hopkins Bloomberg School of Public Health.
Procedures
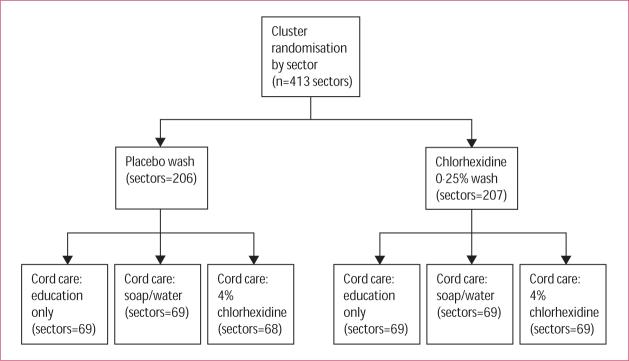
Sectors (clusters) were randomised with a computerised random number generator, with assignment blocked on tertiles of sector-wise infant mortality risk estimated with data from a previous NNIPS study.32 This cord cleansing trial was nested within a study of the effect of full-body skin cleansing with antiseptic on neonatal mortality. In that trial, newborns were given a single full-body wipe with either 0·25% chlorhexidine or placebo solution immediately after birth.33 In each skin cleansing group (0·25% chlorhexidine or placebo) in the main trial, sectors were randomised to one of three cord-care regimens (umbilical stump cleansing with 4·0% chlorhexidine, cleansing with soap and water, or dry cord care only; figure 1). In all clusters, educational messages were provided to all pregnant women about hygienic cord-care practices (eg, hand-washing, sterile cutting or tying of the cord, avoiding potentially harmful topical applications such as dung, mud, or ash, and keeping the cord dry and clean). A cluster-randomised design was chosen to keep to a minimum the chance of misallocation of the interventions and to use the established field staff and logistical channels for delivery of the intervention.
Figure 1.
Two by three factorial design of the trial
Solutions with a concentration of 4·0% free chlorhexidine were prepared by diluting 20% chlorhexidine digluconate (Medichem SA, Barcelona, Spain) to the appropriate concentration with purified water (equivalent to a concentration of 7·1% for the total digluconate salt). The soap and water cleansing solution was prepared by diluting Ivory Liqui-Gel (Proctor and Gamble, Cincinnati, Ohio, USA), a mild cleansing agent suitable for newborn skin, with purified water (1:30). The perfume used in the commercial cleanser was added to the chlorhexidine solution to make the smell of the solutions indistinguishable. The intervention solutions were prepared in Kathmandu, Nepal, and packaged in identical opaque plastic bottles for use in the study setting. Study investigators, analysts, project field workers, and participants were masked to the chlorhexidine and to the soap and water treatments.
6 months into pregnancy, women were approached for enrolment by the local female worker. Study procedures were explained and oral informed consent obtained. All women received iron and folic acid supplementation (90 days), deworming (albendazole 400 mg), vitamin A supplementation every week (7000 retinol equivalents), and a locally manufactured clean delivery kit, which included soap, a sterile blade and tie, a small plastic disc, and a plastic sheet. A government-supported distribution programme for maternal tetanus toxoid provides routine immunisation in Sarlahi district; coverage of this programme was supplemented by our project workers who provided tetanus toxoid to women who were not fully immunised at enrolment.
After delivery, the local worker implemented the assigned skin cleansing treatment33 and notified their area supervisor, a nonmedical project worker, who subsequently visited the newborn baby up to 11 times: days 1, 2, 3, 4, 6, 8, 10, 12, 14, 21, and 28. In clusters assigned to chlorhexidine or soap and water cleansing, parents received educational messages about clean cord care and cord cleansing with the assigned solution at each visit within the first 10 days of life. After washing his or her hands with soap and water, the worker moistened a cotton ball with solution and gently dabbed the umbilical cord stump. A second similarly soaked cotton ball was used to gently cleanse the base of the stump and the skin immediately around the base. Before initiating the study, all workers were standardised through repeated training sessions to deliver this simple intervention uniformly. In the control clusters (dry cord care), educational messages regarding clean cord care were provided, but the unbilical cord stump was not cleansed. An infant was defined as enrolled if their mother gave consent to participate, the infant was alive at first visit, and the first visit took place within the first 10 days of life.
All data were obtained at the individual or household level, not the level of randomised clusters. Household level data, collected during a census immediately before the study, included socioeconomic variables such as caste, ethnic group, and ownership of materials, livestock, or both. Data were also collected on birth history, parental literacy, and occupation.
At each home visit, workers recorded signs of umbilical cord infection including pus and redness (inflammation), swelling (oedema), or both, of the cord stump and skin at the base of the stump. Redness and swelling was categorised into four gradations (none, mild, moderate, or severe). Mild redness or swelling was defined as limited to the cord stump only; moderate was defined as less than 2 cm extension onto the abdominal skin at the base of the cord stump; and severe was defined as spreading noticeably (• 2 cm) outward from the base of the stump. Workers referred caretakers to the nearest government health post if redness or swelling was moderate or severe, or if all three signs of infection happened concurrently. Materials applied to the cord in the previous 24 h were recorded at each visit.
On days 1 and 14, field workers administered questionnaires to identify neonatal care practices and potential risk factors for cord infection. The day 1 questionnaire focused on delivery and immediate newborn care practices (eg, use of clean delivery kit, bathing and massage practices, cord applications, breastfeeding) and other variables including birth weight, sex, and caretaker hand-washing. The day 14 questionnaire measured duration and frequency of these and other newborn care practices since birth.
The primary outcomes for the cord cleansing intervention study were incidence of neonatal omphalitis and neonatal mortality. Combinations of the recorded signs and severity levels were used to define cord infection under three algorithms: moderate redness extending to the abdominal skin at the base of the cord stump; redness as above with pus, or severe redness extending further than 2 cm from the base with or without pus; and severe redness extending further than 2 cm from the base, with pus.34 The vital status (alive or had died) of each child was recorded at each household visit.
Statistical analysis
The cord cleansing trial was designed to detect a minimum 25% relative reduction in incidence of cord infection, given 80% power, and 5% two-sided type 1 error. The expected omphalitis rate in the dry cord-care group of 10·5 per 100 livebirths was estimated from previously obtained maternal reports of pus on the umbilical cord stump (Christian P, personal communication). Including a potential design effect of 2·0, these parameters resulted in a required sample size of 3800 per group (11400 total infants). This amount was lower than the overall number of infants required for the neonatal mortality outcome of the skin cleansing trial in which this study was nested (17 000 total infants).
Individual infant data on cord infection signs recorded during multiple home visits for all infants were combined and assessed for positive status according to the infection algorithms. Infection was defined for every child as one or more cord assessments meeting the algorithm. Incident cases were defined only for infants who were negative at first visit; thus, infants meeting the definition at the first visit were excluded from the analysis for that algorithm. Person-time was expressed as 28-day (or neonatal) periods; exposure for each child was calculated as the time from first visit to time of first positive infection status, death, or discharge from the study (28 days). Incidence-density of infection was estimated by dividing the total incident cases by overall person-time, and was expressed as incident cases per 100 neonatal periods.
Neonatal mortality was expressed as deaths per 1000 livebirths. Cause of death was identified by verbal autopsy with a computer-generated algorithmic approach defined previously.35 Infection and mortality outcomes were estimated overall, and stratified by time of first intervention (early: • 24 h vs late: • 24 h). Effect of treatment on infection and mortality was also examined separately by the full-body cleansing group to assess potential effect modification. The assessment of potential effect modifiers was prespecified but the study was not originally powered to detect interaction.
Sector-level clustering was accounted for with generalised estimating equations.36 Potential confounding of outcome-treatment relations was examined with multivariable models. Analysis followed an intention-to-treat approach in participating infants, irrespective of the actual treatment received. No formal stopping rules were used. On recommendation of the data safety monitoring board, the trial was ended on March 8, 2005, to provide all infants with the beneficial interventions. Infants less than 10 days old on this date were right-censored; all other infants were followed-up for 28 days. Analyses were done with Stata version 8.0.
Role of the funding source
The sponsor had no role in study design, data collection, data analysis, data interpretation, or the writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
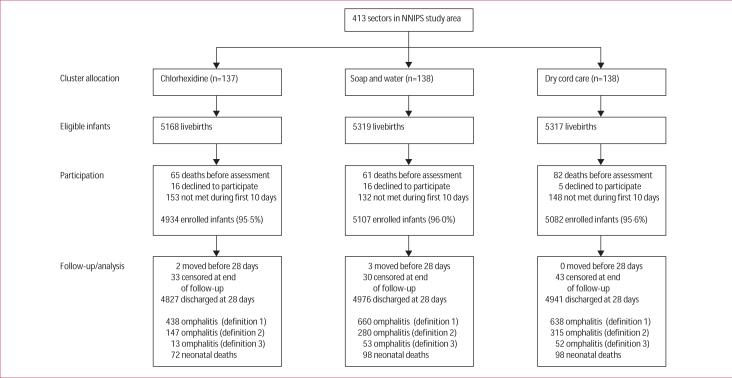
Between Nov 18, 2002, and March 8, 2005, 15 804 infants were born in the study area and were eligible for enrolment in the cord cleansing trial (figure 2). Of these, 208 (1·3%) infants died before implementation of the intervention, 37 (0·2%) mothers declined to participate, and 436 (2·8%) eligible infants were not met during the first 10 days of life. A total of 15 123 newborn babies were enrolled in the chlorhexidine (N=4934), soap and water (N=5107), and dry cord care (N=5082) clusters.
Figure 2.
Study flow diagram
Socioeconomic, household, and maternal characteristics and newborn-care-seeking behaviours including the use of clean cord practices (eg, hand washing, cord cutting, and cord applications) were well balanced across the groups (table 1). In the intervention groups maternal literacy was slightly higher and more infants were born to families originating from the hills (Pahadi) than the plains region of Nepal (Madeshi). The clean delivery kits were almost universally used; 97·8% of households used a new blade to cut the cord. Although common in all groups, mustard oil applications to the cut cord were slightly higher in the dry cord-care group. Other substances (non-study antiseptics, 7·5%; ash, 2·4%; and mud, 0·7%) were applied less frequently, and skin-to-skin care was uniformly low (• 5%). Half (49%) of all infants were breastfed within the first 12 h, and 81% were fed colostrum. Overall prevalence of low birthweight (• 2500 g) was 29·9%.
Table 1.
Baseline socioeconomic, household, and newborn care characteristics by treatment group
|
Chlorhexidine |
Soap/water |
Dry cord care |
||||
|---|---|---|---|---|---|---|
| |
N |
% |
N |
% |
N |
% |
| Number of clusters | 137 | 138 | 138 | |||
| Number of infants per cluster (mean, SD) | 36·0 | (18·2) | 37·0 | (16·5) | 36·8 | (15·7) |
| Total number of infants | 4934 | 5107 | 5082 | |||
| Sex | ||||||
| Boy | 2552 | (52%) | 2586 | (51%) | 2614 | (51%) |
| Girl | 2382 | (48%) | 2521 | (49%) | 2468 | (49%) |
| Caste | ||||||
| Brahmin | 292 | (6%) | 381 | (8%) | 322 | (6%) |
| Chetri | 352 | (7%) | 369 | (7%) | 269 | (5%) |
| Vaiysha | 3024 | (62%) | 3121 | (62%) | 3287 | (66%) |
| Shudra | 722 | (15%) | 666 | (13%) | 651 | (13%) |
| Muslim/other | 464 | (10%) | 464 | (9%) | 470 | (9%) |
| Ethnic group | ||||||
| Hills (Pahadi) | 1637 | (33%) | 1502 | (30%) | 1279 | (25%) |
| Plains (Madeshi) | 3261 | (67%) | 3559 | (70%) | 3769 | (75%) |
| Electricity | ||||||
| No electricity | 3675 | (76%) | 3840 | (77%) | 3750 | (75%) |
| Electricity available | 1175 | (24%) | 1158 | (23%) | 1246 | (25%) |
| Radio/television | ||||||
| Neither | 3015 | (62%) | 3115 | (62%) | 3048 | (61%) |
| Radio only | 958 | (20%) | 1009 | (20%) | 1034 | (21%) |
| Television only | 400 | (8%) | 386 | (8%) | 425 | (9%) |
| Both | 476 | (10%) | 485 | (10%) | 486 | (10%) |
| Maternal literacy | ||||||
| No | 3605 | (73%) | 3802 | (74%) | 3795 | (75%) |
| Yes | 1325 | (27%) | 1302 | (26%) | 1285 | (25%) |
| Tetanus toxoid coverage (number received in previous 2 years) | ||||||
| None | 517 | (10%) | 528 | (10%) | 581 | (11%) |
| One | 2052 | (42%) | 2019 | (40%) | 2076 | (41%) |
| Two or more | 2361 | (48%) | 2556 | (50%) | 2422 | (48%) |
| Delivery place | ||||||
| Home | 3643 | (76%) | 3648 | (74%) | 3668 | (74%) |
| Maiti (maternal home) | 696 | (15%) | 775 | (16%) | 834 | (17%) |
| Health post/clinic | 162 | (3%) | 196 | (4%) | 146 | (3%) |
| Hospital | 221 | (5%) | 272 | (5%) | 248 | (5%) |
| Outdoors/other | 49 | (1%) | 63 | (1%) | 50 | (1%) |
| Birth assistant washed hands | ||||||
| No | 1746 | (38%) | 1837 | (39%) | 1823 | (39%) |
| Yes | 2795 | (62%) | 2889 | (61%) | 2908 | (61%) |
| Birthweight | ||||||
| • 2500 g | 1413 | (29%) | 1523 | (30%) | 1527 | (30%) |
| • 2500 g | 3463 | (71%) | 3494 | (70%) | 3489 | (70%) |
| Breastfeeding initiation time (h) | ||||||
| None | 20 | (• 1%) | 25 | (• 1%) | 23 | (• 1%) |
| 0·0−11·9 | 2308 | (52%) | 2123 | (47%) | 2100 | (46%) |
| 12·0−23·9 | 293 | (7%) | 361 | (8%) | 321 | (7%) |
| 24·0−47·9 | 602 | (14%) | 681 | (15%) | 672 | (15%) |
| 48·0−71·9 | 864 | (19%) | 945 | (21%) | 1057 | (23%) |
| • 72 | 350 | (8%) | 428 | (9%) | 376 | (8%) |
| Colostrum given to infant | ||||||
| No | 784 | (18%) | 926 | (20%) | 847 | (19%) |
| Yes | 3656 | (82%) | 3649 | (80%) | 3715 | (81%) |
| Mother/infant skin-to-skin care | ||||||
| No | 4241 | (96%) | 4329 | (95%) | 4363 | (96%) |
| Yes | 195 | (4%) | 226 | (5%) | 178 | (4%) |
| Cut cord with new blade | ||||||
| No | 103 | (2%) | 125 | (3%) | 88 | (2%) |
| Yes | 4543 | (98%) | 4652 | (97%) | 4696 | (98%) |
| Home applications to the cord Mustard oil | ||||||
| No | 2557 | (52%) | 2704 | (53%) | 2363 | (46%) |
| Yes | 2377 | (48%) | 2403 | (47%) | 2719 | (54%) |
| Home antiseptics | ||||||
| No | 4602 | (93%) | 4697 | (92%) | 4685 | (92%) |
| Yes | 332 | (7%) | 410 | (8%) | 397 | (8%) |
| Mud | ||||||
| No | 4906 | (99%) | 5075 | (99%) | 5031 | (99%) |
| Yes | 28 | (1%) | 32 | (1%) | 51 | (1%) |
| Ash | ||||||
| No | 4807 | (97%) | 4978 | (97·5%) | 4969 | (98%) |
| Yes | 127 | (3%) | 129 | (3%) | 113 | (2%) |
Data are number (%) unless otherwise stated.
Number and timing of home visits per child and effective treatment coverage were equivalent for the three treatment groups (table 2). About two-thirds of all infants were first visited and assessed for cord infection signs within 24 h after birth (median 18·3 h). For each definition of omphalitis, the total number of incident cases, overall person-time contributed, and incidence-density rate of cord infection is shown in table 3 for each group. Under the first definition (redness extending to the skin at the base of the umbilical stump), the risk of cord infection was 32% lower in infants who were treated with chlorhexidine than in those who received dry cord care (incidence rate ratio [IRR]=0·68, 95% CI 0·58−0·80). For the second definition (pus with moderate or severe redness, or severe redness alone), the infection risk in the chlorhexidine group was 54% lower than in those in the dry cord-care group (0·46, 0·36−0·59). Under the strictest definition of omphalitis (severe redness with pus), risk was reduced by 75% (0·25, 0·12−0·53). The design effects for the three definitions of omphalitis were 1·79, 1·60, and 1·43, respectively.
Table 2.
Intervention coverage by treatment group
| |
Chlorhexidine (n=4934) |
Soap/water (n=5107) |
Dry cord care (n=5082) |
|---|---|---|---|
| Household visits* | |||
| Mean (SD) | 9·5 (1·8) | 9·5 (1·8) | 9·5 (1·8) |
| Median (IQR) | 10 (9 − 11) | 10 (9 − 11) | 10 (9−11) |
| Intervention visits† | |||
| Mean (SD) | 5·9 (1·5) | 5·8 (1·5) | 5·8 (1·4) |
| Median (IQR) | 6 (5 − 7) | 6 (5 − 7) | 6 (5−7) |
| Effective coverage‡ | 0·84 | 0·83 | 0·84 |
| Time to first visit (h, median [IQR]) | 18·0 (9·0−29·9) | 18·7 (9·7−32·5) | 18·9 (9·5−31·0) |
| First visit* 24 h (n [%]) | 3134 (63·5%) | 3144 (61·6%) | 3179 (62·6%) |
Maximum possible 11.
Maximum possible 7.
Effective coverage is defined as the total number of treatments received divided by the total number of possible treatments during the first 10 days of life (days 1−10).
Table 3.
Umbilical cord infection by treatment group
| |
Infants |
Cases |
Person-time* |
Rate† |
IRR (95% CI)‡ |
|---|---|---|---|---|---|
| Algorithm 1: Moderate or severe redness | |||||
| Chlorhexidine 4·0% | 4703 | 438 | 4236·1 | 10·3 | 0·68 (0·58−0·80) |
| Soap/water | 4884 | 660 | 4214·4 | 15·7 | 1·03 (0·87−1·22) |
| Dry cord care | 4859 | 638 | 4195·6 | 15·2 | 1·00 |
| Algorithm 2: Moderate or severe redness, with pus, or severe redness alone | |||||
| Chlorhexidine 4·0% | 4883 | 147 | 4675·8 | 3·1 | 0·46 (0·36−0·59) |
| Soap/water | 5029 | 280 | 4701·6 | 6·0 | 0·88 (0·69−1·12) |
| Dry cord care | 5021 | 315 | 4651·9 | 6·8 | 1·00 |
| Algorithm 3: Severe redness with pus | |||||
| Chlorhexidine 4·0% | 4930 | 13 | 4839·4 | 0·3 | 0·25 (0·12−0·53) |
| Soap/water | 5096 | 53 | 4962·3 | 1·1 | 1·01 (0·58−1·77) |
| Dry cord care | 5076 | 52 | 4929·6 | 1·1 | 1·00 |
Person-time expressed as neonatal periods (28 infant-days).
Incidence expressed as infections per 100 neonatal periods.
Generalised estimating equations (Poisson distribution, log link function) were used to adjust point estimates and CIs for the cluster-randomised design.
There was no evidence for a protective benefit of soap and water. The reduction in risk for the chlorhexidine-treated compared with dry cord-care infants was greater for infants enrolled early (• 24 h after birth) for each definition (table 4), reaching an 87% (69−93%) reduction in incidence of infection as defined by algorithm 3. Moreover, early initiation of treatment was needed, especially for prevention of the most severe form of omphalitis There was no evidence for interaction between the intervention used in the skin cleansing trial and those used in this study.
Table 4.
Umbilical cord infection by treatment group stratified by timing of intervention and skin cleansing trial intervention
|
Timing of intervention |
||
|---|---|---|
| |
Early intervention (• 24 h) |
Late intervention (• 24 h) |
| Algorithm 1: Moderate or severe redness | ||
| Chlorhexidine 4.0% | 0.65 (0.56−0.78) | 0.70 (0.49−1.01) |
| Soap/water | 1.00 (0.84−1.18) | 1.32 (0.93−1.87) |
| Algorithm 2: Moderate or severe redness, with pus, or severe redness alone | ||
| Chlorhexidine 4.0% | 0.42 (0.32−0.54) | 0.66 (0.40−1.09) |
| Soap/water | 0.82 (0.64−1.06) | 1.19 (0.79−1.81) |
| Algorithm 3: Severe redness with pus | ||
| Chlorhexidine 4.0% | 0.13 (0.06−0.31) | 1.06 (0.35−3.20) |
| Soap/water | 1.00 (0.55−1.82) | 1.12 (0.37−3.36) |
|
Skin cleansing trial intervention |
||
|---|---|---|
| |
Chlorhexidine 0.25% |
Placebo |
| Algorithm 1: Moderate or severe redness | ||
| Chlorhexidine 4.0% | 0.71 (0.57−0.89) | 0.64 (0.51−0.82) |
| Soap/water | 1.00 (0.78−1.27) | 1.07 (0.84−1.37) |
| Algorithm 2: Moderate or severe redness, with pus, or severe redness alone | ||
| Chlorhexidine 4.0% | 0.49 (0.35−0.67) | 0.43 (0.30−0.62) |
| Soap/water | 0.86 (0.62−1.18) | 0.90 (0.62−1.30) |
| Algorithm 3: Severe redness with pus | ||
| Chlorhexidine 4.0% | 0.37 (0.16−0.86) | 0.12 (0.03−0.58) |
| Soap/water | 1.16 (0.56−2.38) | 0.86 (0.36−2.10) |
Data are IRR (95% CI) Generalised estimating equations (Poisson distribution, log link function) were used to adjust point estimates and CIs for the cluster-randomised design.
There were 268 neonatal deaths in 15 123 enrolled infants (17·7 per 1000). Compared with the dry cord-care group, mortality risk was 24% lower in the chlorhexidine group (relative risk [RR] 0·76 [0·55−1·04]; table 5). After adjusting for ethnic group, maternal literacy, and topical mustard oil applications to the cord by caregivers in the home, the risk ratio for mortality was 0·78 (0·57−1·07). The design effect for mortality was 1·10. Of the infants enrolled early (less than 24 h after birth) there was a significant reduction in neonatal mortality (0·66 [0·46−0·95]) in those treated with chlorhexidine compared with those who received dry cord care (table 6), and the sepsis-specific risk ratio for mortality, as defined by verbal autopsy, was 0·69 (0·40−1·18). After physician review of the verbal autopsy data, no deaths in any of the groups were attributed to tetanus. No reduction in mortality was seen among those enrolled 24 h or more after birth. There was no evidence for interaction between the full-body cleansing and cord cleansing interventions.
Table 5.
Neonatal mortality by treatment group
| |
Deaths |
Births |
Rate* |
RR (95% CI) † |
|---|---|---|---|---|
| Chlorhexidine 4.0% | 72 | 4924 | 14.6 | 0.76 (0.55−1.04) |
| Soap/water | 98 | 5107 | 19.2 | 1.00 (0.76−1.31) |
| Dry cord care | 98 | 5082 | 19.3 | 1.00 |
Absolute mortality rate expressed per 1000 livebirths. †Generalised estimating equations (binomial distribution, log link function) were used to adjust point estimates and CIs for the cluster-randomised design.
Table 6.
Neonatal mortality by treatment group stratified by timing of intervention and skin cleansing trial intervention
|
Timing of intervention |
||||
|---|---|---|---|---|
|
Early intervention (• 24 h) |
Late intervention (• 24 h) |
|||
| |
Rate* |
RR (95% CI)† |
Rate* |
RR (95% CI)† |
| Chlorhexidine 4.0% | 14.4 | 0.66 (0.46−0.95) | 15.1 | 1.02 (0.54−1.92) |
| Soap/water | 20.5 | 0.95 (0.69−1.31) | 16.9 | 1.14 (0.68−1.90) |
| Dry cord care | 21.6 | 1.00 | 14.8 | 1.00 |
|
Skin cleansing trial intervention |
||||
|---|---|---|---|---|
|
Chlorhexidine 0.25% full-body wipe |
Placebo full-body wipe |
|||
| |
Rate* |
RR (95% CI)† |
Rate* |
RR (95% CI)† |
| Chlorhexidine 4.0% | 13.2 | 0.74 (0.46−1.19) | 16.1 | 0.78 (0.51−1.22) |
| Soap/water | 18.9 | 1.06 (0.71−1.60) | 19.5 | 0.95 (0.66−1.37) |
| Dry cord care | 17.8 | 1.00 | 20.6 | 1.00 |
Absolute mortality rate expressed per 1000 livebirths.
Generalised estimating equations (binomial distribution, log link function) were used to adjust point estimates and CIs for the cluster-randomised design.
Discussion
These data provide evidence that umbilical cord cleansing with chlorhexidine can markedly reduce the risk of omphalitis. The risk of cord infection was reduced by 32−75% with greater effect on more severe grades of infection, and an 87% reduction in risk of the most severe grade of omphalitis was seen in those whose treatment was initiated within the first 24 h after birth. The time to first cleansing was an important modifying factor, with stronger evidence of protection against infection in infants enrolled within 24 h of birth for all three grades of infection severity. In infants receiving 4·0% chlorhexidine applications to the cord, the risk of mortality was 24% lower than dry cord care alone. As with cord infection, reduction in mortality was greater (34%) for those treated with chlorhexidine on the first day of life. An association between early application of antiseptics and reduced odds of neonatal tetanus death compared with late or no antiseptics has been noted previously.37
Interventions to reduce exposure of the infant to bacterial colonisation and infection of the cord stump should take place at the earliest possible time, with a primary focus beyond the prevention of omphalitis. In this high mortality setting, although identifying and treating severe omphalitis must remain an important priority, visible signs of cord infection could in fact, for many infants, signal a successful immune response which limits infection to a local process. In the absence of such a response, however, acquisition of infectious pathogens might lead directly to a systemic infectious process without producing visible signs of omphalitis.
A similar process arises with exposure to Clostridium tetani at the umbilical cord stump, producing neonatal tetanus without concurrent signs of local cord infection.38 During the initial days of life, rapid colonisation of the moist stump tissue might lead to direct exposure of the bloodstream to pathogens, since the umbilical vessels remain patent at this time. Therefore, early application of chlorhexidine to the umbilical cord stump not only reduces omphalitis, but also reduces mortality risk by preventing exposure to pathogens that might otherwise lead to a systemic infectious process and death, with or without signs of local cord infection. The 34% difference in mortality between treatment and control infants who were reached within the first 24 h of life and the increased protective effect of early chlorhexidine treatment on omphalitis incidence lend support to this argument. This trial, the first designed to assess the effect of cord antisepsis on mortality, suggests that previous studies have focused on the less important outcome—signs that might signal local containment of cord infection—and thus, until now, the benefits of cord antisepsis have been underestimated.
There was no evidence that the full-body cleansing assignment33 modified the relation between cord-care regimen and risk of infection or mortality. Also, these data provide no evidence that applications of a non-antiseptic soap and water-cleansing solution to the cord reduces infection or mortality risk compared with a dry cord-care regimen. The soap and water regimen might transiently reduce surface bacteria via mechanical removal of loosely adherent microorganisms. For residual and cumulative effects, however, non-antibacterial agents are substantially less effective than antibacterial agents such as chlorhexidine.39–42 In this study, the soap and water cord-care regimen probably shares this characteristic inefficiency of non-medicated cleansing agents, and cannot be further recommended, whereas the residual effect of chlorhexidine cord cleansing might have significantly reduced exposure of the infant to infectious pathogens.
There were some limitations to this study. The umbilical cord stump of infants was not cultured to examine bacterial colonisation because it was not possible to include this as a corollary sign of umbilical cord infection in this rural setting. The cord-cleansing intervention and cord assessment was done by the same workers. All were masked, however, to the treatment codes. Confounding is unlikely to explain these results, since the randomisation achieved balance across the treatment clusters; slightly imbalanced variables did not confound the relative risk estimates. Selection bias was also unlikely because 96% of eligible infants participated in the study.
The predominately rural farming communities of Sarlahi district share cultural, social, and economic characteristics with a broad population in southern Asia, and thus the results might be applicable not only within the Terai region of southern Nepal, but also in northern India, Pakistan, and northwestern Bangladesh. In these and in other low-resource settings where a high proportion of women deliver at home without skilled assistance, and exposure of the newly cut umbilical cord to environmental pathogens is high, chlorhexidine cord cleansing could provide a protective benefit similar to that seen in this study. Where neonatal mortality rates are higher or neonatal tetanus remains a public health problem, or both, the intervention could have a larger effect because the proportion of deaths from umbilical sepsis might be higher. Research on the effectiveness of this intervention in communities of sub-Saharan Africa is also warranted.
Although this study provides evidence that early use of chlorhexidine will reduce omphalitis and mortality risk, further information is needed about the frequency of cord cleansing during the first week of life to confer maximum protection. For example, a more simple programmatic approach, limiting cleansing to the first 3 days of life, or possibly even the first day of life alone, could provide substantial protection against infection and mortality. Furthermore, in view of the reductions in neonatal mortality among infants receiving this simple intervention within the first day of life, investigations designed to identify optimum models for community-based delivery near the time of birth are urgently needed.
Umbilical cord cleansing with chlorhexidine is regarded to be safe.3,30 Trace levels of the compound have been detected in the blood of infants after umbilical cord cleansing43,44 without any related clinical consequence. Contact dermatitis has been reported in up to 15% of infants after placement of a 0·5% chlorhexidine impregnated dressing over a central venous catheter.45 These exceptional reactions, however, might have resulted from the circumstances in which infants of less than 28 weeks gestational age and less than 1000 g at birth had the occlusive dressing in place for more than 7 days. Despite widespread use in clinical and community settings for over 30 years, no adverse events associated with topical applications to the cord stump have been reported in neonates.
The strong safety record, low cost, and ease of implementation make cord cleansing with 4·0% chlorhexidine an ideal intervention, even for mothers, traditional birth attendants, or other people with little training who might assist with deliveries in low-resource settings. To increase coverage and ensure the earliest possible intervention time, topical antiseptics could be incorporated into clean delivery kits for use by skilled birth attendants or caretakers in low-resource settings, or implemented within comprehensive community outreach efforts to improve newborn care. Although well accepted in our study population, qualitative investigations to determine potential barriers to uptake should be included in any well-designed neonatal care programme that includes topical antiseptic applications to the cord.
These results suggest that the current WHO recommendation for dry cord care is inappropriate for many low-resource settings where the baseline risk of omphalitis and mortality associated with a portal of entry through the cord is high. Furthermore, prevention of local cord infections could be secondary to the role that cord cleansing with chlorhexidine might have in reducing the risk of early neonatal sepsis and death. Continued efforts to identify and implement efficacious, affordable, and feasible community-based interventions to reduce neonatal infections and mortality are needed in order to meet the challenge of Millennium Development Goal 4 for child survival.46
We believe that the use of 4·0% chlorhexidine for topical cord antisepsis represents an important intervention with the potential for substantial effect on public health.
Acknowledgments
All members of the Nepal Nutrition Intervention Project, Sarlahi (NNIPS) collaborated on the successful implementation of this research project. Commodity support (perfume for masking intervention groups) was provided by Procter and Gamble Company, Cincinnati, Ohio. We thank the Data and Safety Monitoring Board members, P S S Sundar Rao, Pushpa Sharma, Dharma Manandhar, and Martin Bloem. This study was supported by grants from the National Institutes of Health, National Institute of Child Health and Human Development (HD44004 and HD38753), The Bill & Melinda Gates Foundation (810−2054), and cooperative agreements between the Johns Hopkins Bloomberg School of Public Health and the Office of Heath and Nutrition, United States Agency for International Development (HRN-A-00−97−00015−00, GHS-A-00−03−000019−00). These data were presented at the XVII IEA World Congress of Epidemiology, Bangkok, Thailand, August, 2005 and the 43rd Annual Meeting of the Infectious Diseases Society of America, San Francisco, USA, October, 2005.
Footnotes
Contributors L C Mullany, J M Tielsch, G L Darmstadt, and J Katz made primary contributions to the study design, conduct, analysis, and interpretation, and to the writing of this manuscript. Subarna K Khatry, Steven C LeClerq, and Sharada Ram Shrestha contributed to the study design, field conduct, quality control, and interpretation of the results. Ramesh Adhikari participated in review of the verbal autopsies and in interpretation of the results. All authors have reviewed and approved the manuscript.
Conflict of interest statement We declare that we have no conflict of interest.
References
- 1.Lawn JE, Cousens S, Darmstadt GL, Paul V, Martines J. Why are 4 million newborn babies dying every year? Lancet. 2004;364:2020. doi: 10.1016/S0140-6736(04)17511-9. [DOI] [PubMed] [Google Scholar]
- 2.Lawn JE, Cousens S, Zupan J. 4 million neonatal deaths: when? Where? Why? Lancet. 2005 March 2;365:891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . Care of the umbilical cord. WHO/FHE/MSM-cord care. WHO; Geneva: 1998. [Google Scholar]
- 4.Darmstadt GL, Bhutta ZA, Cousens S, Adam T, Walker N, de BL. Evidence-based, cost-effective interventions: how many newborn babies can we save? Lancet. 2005 March 12;365:977–88. doi: 10.1016/S0140-6736(05)71088-6. [DOI] [PubMed] [Google Scholar]
- 5.Faridi MM, Rattan A, Ahmad SH. Omphalitis neonatorum. J Indian Med Assoc. 1993;91:283–85. [PubMed] [Google Scholar]
- 6.Guvenc H, Aygun AD, Yasar F, Soylu F, Guvenc M, Kocabay K. Omphalitis in term and preterm appropriate for gestational age and small for gestational age infants. J Trop Pediatr. 1997;43:368–72. doi: 10.1093/tropej/43.6.368. [DOI] [PubMed] [Google Scholar]
- 7.Guvenc H, Guvenc M, Yenioglu H, Ayata A, Kocabay K, Bektas S. Neonatal omphalitis is still common in eastern Turkey. Scand J Infect Dis. 1991;23:613–16. doi: 10.3109/00365549109105186. [DOI] [PubMed] [Google Scholar]
- 8.Airede AI. Pathogens in neonatal omphalitis. J Trop Pediatr. 1992;38:129–31. doi: 10.1093/tropej/38.3.129. [DOI] [PubMed] [Google Scholar]
- 9.Sawardekar KP. Changing spectrum of neonatal omphalitis. Pediatr Infect Dis J. 2004;23:22–26. doi: 10.1097/01.inf.0000105200.18110.1e. [DOI] [PubMed] [Google Scholar]
- 10.Islam MS, Rahaman MM, Aziz KM, Munshi MH, Rahman M, Patwari Y. Birth care practice and neonatal tetanus in a rural area of Bangladesh. J Trop Pediatr. 1982;28:299–302. doi: 10.1093/tropej/28.6.299. [DOI] [PubMed] [Google Scholar]
- 11.Bennett J, Macia J, Traverso H, Banoagha S, Malooly C, Boring J. Protective effects of topical antimicrobials against neonatal tetanus. Int J Epidemiol. 1997;26:897–903. doi: 10.1093/ije/26.4.897. [DOI] [PubMed] [Google Scholar]
- 12.Bennett J, Ma C, Traverso H, Agha SB, Boring J. Neonatal tetanus associated with topical umbilical ghee: covert role of cow dung. Int J Epidemiol. 1999;28:1172–35. doi: 10.1093/ije/28.6.1172. [DOI] [PubMed] [Google Scholar]
- 13.Bennett J, Schooley M, Traverso H, Agha SB, Boring J. Bundling, a newly identified risk factor for neonatal tetanus: implications for global control. Int J Epidemiol. 1996;25:879–84. doi: 10.1093/ije/25.4.879. [DOI] [PubMed] [Google Scholar]
- 14.Traverso HP, Bennett JV, Kahn AJ, et al. Ghee applications to the umbilical cord: a risk factor for neonatal tetanus. Lancet. 1989;1:486–88. doi: 10.1016/s0140-6736(89)91378-0. [DOI] [PubMed] [Google Scholar]
- 15.Quddus A, Luby S, Rahbar M, Pervaiz Y. Neonatal tetanus: mortality rate and risk factors in Loralai District, Pakistan. Int J Epidemiol. 2002;31:648–53. doi: 10.1093/ije/31.3.648. [DOI] [PubMed] [Google Scholar]
- 16.Chai F, Prevots DR, Wang X, Birmingham M, Zhang R. Neonatal tetanus incidence in China, 1996−2001, and risk factors for neonatal tetanus, Guangxi Province, China. Int J Epidemiol. 33:551–7. doi: 10.1093/ije/dyh073. [DOI] [PubMed] [Google Scholar]
- 17.Raza SA, Akhtar S, Avan BI, Hamza H, Rahbar MH. A matched case-control study of risk factors for neonatal tetanus in Karachi, Pakistan. J Postgrad Med. 2004;50:247–51. [PubMed] [Google Scholar]
- 18.Bennett J, Breen C, Traverso H, Agha SB, Macia J, Boring J. Circumcision and neonatal tetanus: disclosure of risk and its reduction by topical antibiotics. Int J Epidemiol. 1999;28:263–66. doi: 10.1093/ije/28.2.263. [DOI] [PubMed] [Google Scholar]
- 19.Garner P, Lai D, Baea M, Edwards K, Heywood P. Avoiding neonatal death: an intervention study of umbilical cord care. J Trop Pediatr. 1994;40:24–28. doi: 10.1093/tropej/40.1.24. [DOI] [PubMed] [Google Scholar]
- 20.Alder VG, Burman D, Simpson RA, Fysh J, Gillespie WA. Comparison of hexachlorophane and chlorhexidine powders in prevention of neonatal infection. Arch Dis Child. 1980;55:277–80. doi: 10.1136/adc.55.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belfrage E, Enocksson E, Kalin M, Marland M. Comparative efficiency of chlorhexidine and ethanol in umbilical cord care. Scand J Infect Dis. 1985;17:413–20. doi: 10.3109/13813458509058783. [DOI] [PubMed] [Google Scholar]
- 22.Meberg A, Schoyen R. Bacterial colonization and neonatal infections. Effects of skin and umbilical disinfection in the nursery. Acta Paediatr Scand. 1985;74:366–71. doi: 10.1111/j.1651-2227.1985.tb10985.x. [DOI] [PubMed] [Google Scholar]
- 23.Speck WT, Driscoll JM, Polin RA, O'Neill J, Rosenkranz HS. Staphylococcal and streptococcal colonization of the newborn infant: effect of antiseptic cord care. Am J Dis Child. 1977;131:1005–08. doi: 10.1001/archpedi.1977.02120220071012. [DOI] [PubMed] [Google Scholar]
- 24.Speck WT, Driscoll JM, O'Neil J, Rosenkranz HS. Effect of antiseptic cord care on bacterial colonization in the newborn infant. Chemotherapy. 1980;26:372–76. doi: 10.1159/000237929. [DOI] [PubMed] [Google Scholar]
- 25.Wald ER, Snyder MJ, Gutberlet RL. Group B beta-hemolytic streptococcal colonization. Acquisition, persistence, and effect of umbilical cord treatment with triple dye. Am J Dis Child. 1977;131:178–80. doi: 10.1001/archpedi.1977.02120150060011. [DOI] [PubMed] [Google Scholar]
- 26.Watkinson M, Dyas A. Staphylococcus aureus still colonizes the untreated neonatal umbilicus. J Hosp Infect. 1992;21:131–6. doi: 10.1016/0195-6701(92)90032-h. [DOI] [PubMed] [Google Scholar]
- 27.Janssen PA, Selwood BL, Dobson SR, Peacock D, Thiessen PN. To dye or not to dye: a randomized, clinical trial of a triple dye/alcohol regime versus dry cord care. Pediatrics. 2003;111:15–20. doi: 10.1542/peds.111.1.15. [DOI] [PubMed] [Google Scholar]
- 28.Mullany LC, Darmstadt GL, Tielsch JM. Role of antimicrobial applications to the umbilical cord in neonates to prevent bacterial colonization and infection: a review of the evidence. Pediatr Infect Dis J. 2003;22:996–1002. doi: 10.1097/01.inf.0000095429.97172.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zupan J, Garner P, Omari AA. Topical umbilical cord care at birth. Cochrane Database Syst Rev. 2004;3:CD001057. doi: 10.1002/14651858.CD001057.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Denton GW. Chlorhexidine. In: Block SS, editor. Disinfection, sterilization, and preservation. 5th edn. Lippencott Williams & Wilkens; Philadelphia: 2001. pp. 321–26. [Google Scholar]
- 31.Seeberg S, Brinkhoff B, John E, Kjellmer I. Prevention and control of neonatal pyoderma with chlorhexidine. Acta Paediatr Scand. 1984;73:498–504. doi: 10.1111/j.1651-2227.1984.tb09961.x. [DOI] [PubMed] [Google Scholar]
- 32.Christian P, West KP, Khatry SK, et al. Effects of maternal micronutrient supplementation on fetal loss and infant mortality: a cluster-randomized trial in Nepal. Am J Clin Nutr. 2003;78:1194–202. doi: 10.1093/ajcn/78.6.1194. [DOI] [PubMed] [Google Scholar]
- 33.Tielsch JM, Darmstadt GL, Mullany LC, Khatry SK, Katz J, LeClerq SC. Newborn skin cleansing with a dilute chlorhexidine solution reduces neonatal mortality in southern Nepal: a community-based, randomized trial; Countdown to 2015: Tracking Progress in Child Survival Conference; London, UK. 2005.Dec 13−14, [Google Scholar]
- 34.Mullany LC, Darmstadt GL, Katz J, et al. Development of clinical sign-based algorithms for community-based assessment of omphalitis. Arch Dis Child Fetal Neonatal Ed. 2006;91:F99–104. doi: 10.1136/adc.2005.080093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freeman JV, Christian P, Khatry SK, et al. Evaluation of verbal autopsy using physician review versus algorithm-based cause of death assignment in Sarlahi, Nepal. Paediatr Perinatal Epidemiol. 2005;19:322–31. doi: 10.1111/j.1365-3016.2005.00652.x. [DOI] [PubMed] [Google Scholar]
- 36.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 37.Parashar UD, Bennett JV, Boring JR, Hlady WG. Topical antimicrobials applied to the umbilical cord stump: a new intervention against neonatal tetanus. Int J Epidemiol. 1998;27:904–08. doi: 10.1093/ije/27.5.904. [DOI] [PubMed] [Google Scholar]
- 38.Gurkan F, Bosnak M, Dikici B, et al. Neonatal tetanus: a continuing challenge in the southeast of Turkey: risk factors, clinical features and prognostic factors. Eur J Epidemiol. 1999;15:171–74. doi: 10.1023/a:1007500109522. [DOI] [PubMed] [Google Scholar]
- 39.Faoagali J, Fong J, George N, Mahoney P, O'Rourke V. Comparison of the immediate, residual, and cumulative antibacterial effects of Novaderm R,* Novascrub R,* Betadine Surgical Scrub, Hibiclens, and liquid soap. Am J Infect Control. 1995;23:337–43. doi: 10.1016/0196-6553(95)90263-5. [DOI] [PubMed] [Google Scholar]
- 40.Ayliffe GA, Babb JR, Davies JG, Lilly HA. Hand disinfection: a comparison of various agents in laboratory and ward studies. J Hosp Infect. 1988;11:226–43. doi: 10.1016/0195-6701(88)90101-6. [DOI] [PubMed] [Google Scholar]
- 41.Montville R, Chen Y, Schaffner DW. Risk assessment of hand washing efficacy using literature and experimental data. Int J Food Microbiol. 2002;73:305–13. doi: 10.1016/s0168-1605(01)00666-3. [DOI] [PubMed] [Google Scholar]
- 42.Doebbeling BN, Stanley GL, Sheetz CT, et al. Comparative efficacy of alternative hand-washing agents in reducing nosocomial infections in intensive care units. N Engl J Med. 1992;327:88–93. doi: 10.1056/NEJM199207093270205. [DOI] [PubMed] [Google Scholar]
- 43.Aggett PJ, Cooper LV, Ellis SH, McAinsh J. Percutaneous absorption of chlorhexidine in neonatal cord care. Arch Dis Child. 1981;56:878–80. doi: 10.1136/adc.56.11.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnsson J, Seeberg S, Kjellmer I. Blood concentrations of chlorhexidine in neonates undergoing routine cord care with 4% chlorhexidine gluconate solution. Acta Paediatr Scand. 1987;76:675–76. doi: 10.1111/j.1651-2227.1987.tb10544.x. [DOI] [PubMed] [Google Scholar]
- 45.Garland JS, Alex CP, Mueller CD, et al. A randomized trial comparing povidone-iodine to a chlorhexidine gluconate-impregnated dressing for prevention of central venous catheter infections in neonates. Pediatrics. 2001;107:1431–36. doi: 10.1542/peds.107.6.1431. [DOI] [PubMed] [Google Scholar]
- 46. [July 1, 2005];United Nations. General Assembly 56th Session. Road map toward the implementation of the United Nations millennium declaration. United Nations. 2001 http://www.un.org/millenniumgoals.